Abstract
Background
Despite the observed association between diabetes mellitus and hepatocellular carcinoma (HCC), little is known about the effect of diabetes duration prior to HCC diagnosis and whether some diabetes medications reduced the risk of HCC development.
Aim
We aimed at determining the association between HCC risk and diabetes duration and type of diabetes treatment.
Methods
A total of 420 HCC patients and 1104 healthy controls were enrolled in an ongoing hospital-based case-control study. We used multivariate logistic regression models to adjust for HCC risk factors.
Results
The prevalence of diabetes mellitus was 33.3% in HCC and 10.4% in the control group, yielding an adjusted odds ratio (AOR) and 95% confidence interval (CI) of 4.2 (3.0-5.9). In 87% of cases, diabetes was present prior to HCC diagnosis yielding an AOR of 4.4 (95%CI, 3.0-6.3). Compared to patients with a diabetes duration of 2-5 years, the estimated AORs (95% CI) for those with a diabetes duration of 6-10 years and those with diabetes duration > 10 years were 1.8 (0.8-4.1) and 2.2 (1.2-4.8) respectively. In respect to diabetes treatment, the AORs (95% CI) were 0.3 (0.2-0.6), 0.3 (0.1-0.7), 7.1 (2.9-16.9), 1.9 (0.8-4.6), and 7.8 (1.5-40.0) for those treated with biguanides, thiazolidinediones, sulfonylureas, insulin, and dietary control respectively.
Conclusions
Diabetes increases HCC risk, and such risk is correlated with long duration of diabetes. Relying on dietary control and treatment with sulfonylureas or insulin conferred the highest magnitude of HCC risk, while biguanides or thiazolidinediones treatment was associated with 70% HCC risk-reduction among diabetics.
Keywords: diabetes mellitus, metformin, sulfonylurea, cirrhosis, HCC
INTRODUCTION
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and inadequate secretion of or receptor insensitivity to endogenous insulin, 1 it is a major public health problem and the fifth leading cause of death in the United States. 2,3 This high death rate is partially due to the high incidence of renal and heart diseases among patients with diabetes mellitus. 3,4 In addition, diabetes is associated with increased risks of colon, kidney, and pancreatic cancers. 5
Because the liver plays a crucial role in glucose metabolism, it is not surprising that diabetes mellitus is an epiphenomenon of many chronic liver diseases such as chronic hepatitis, fatty liver, liver failure, and cirrhosis. The association between diabetes mellitus and hepatocellular carcinoma (HCC) has been reported by cohort 6-9 and case-control studies.10-12. Although such an association could be related to the underlying chronic liver diseases that preceded the development of HCC,13-16 there are several lines of evidence suggesting that diabetes is in fact an independent risk factor for HCC development. This evidence includes 1) results from review and meta-analysis reports concluding that diabetes is a risk factor of HCC; 4,17-19 2) findings that the positive association between diabetes and HCC is independent from underlying cirrhosis and chronic liver diseases; 11,16 3) findings that the association is positively correlated with disease duration; 12,20,21 4) demonstration of the synergistic interaction between diabetes and other HCC risk factors; 6,10,12 5) findings of HCC recurrence after liver resection and transplantation among patients with diabetes; 22,23 6) suggestion of a biological plausibility that underlies the association between diabetes and HCC; 18,19,24 and 7) the observation of risk of HCC development among patients with type 1 diabetes mellitus.10
Because diabetes mellitus is a complication of many chronic liver diseases and because transient hyperglycemia can be a symptom of metastatic tumors or side effect of chemotherapy intake,25 detailed information about patients’ duration of diabetes mellitus prior to HCC development may be crucial for properly studying the association between diabetes and HCC. Moreover, it is not known whether diabetes control reduces the risk of HCC or whether specific regimen of diabetes confers high risk for HCC developing. Therefore, we embarked on a case-control study to address these questions after controlling for established HCC risk factors.
Materials and Methods
Study design and population
The current investigation is part of an ongoing hospital-based case-control study that was approved by the institutional review board at The University of Texas M. D. Anderson Cancer Center. Written informed consent for participation was obtained from each study participant. Detailed description of cases and controls were previously reported.26-28 Case patients were recruited from the population of patients with newly diagnosed HCC who were evaluated and treated at the M. D. Anderson Cancer Center gastrointestinal medical oncology and surgical oncology outpatient clinics. The inclusion criteria were as follows: pathologically confirmed diagnosis of HCC, U.S. residency, and the ability to communicate in English. The exclusion criteria were the presence of other types of primary liver cancer (such as cholangiocarcinoma or fibrolamellar hepatocarcinoma), unknown primary tumors, and concurrent or past history of cancer at another organ site.
From January 2000 through July 2008, 652 patients with suspected HCC were identified, 518 of whom were eligible for this study. We enrolled 420 eligible patients with HCC; 98 eligible patients (18.9%) were not recruited because of patient refusal, patient sickness, or inadequate time to complete the interview. Statistical analyses indicated that the eligible patients who were not recruited did not differ from the recruited patients in terms of demographic, epidemiologic, or clinical factors (retrieved from patients’ medical records).
The control subjects were healthy and genetically unrelated family members (i.e., spouses and in-laws) of patients at M. D. Anderson who had cancers other than liver, gastrointestinal, lung, or head and neck cancer. The reason for excluding family members and spouses of patients with these cancers as controls was to prevent the introduction of selection bias connected with shared environmental and genetic factors that are highly associated with HCC, e.g., alcohol consumption, diabetes mellitus, smoking, family history of cancer, and hepatitis virus infection.
The eligibility criteria for controls were the same as those for patients, except for having a cancer diagnosis. Control subjects were recruited from various diagnostic radiology clinics of M.D. Anderson, where cancer patients and their companions are sent to receive the initial cancer diagnosis or treatment follow-up examination. A short structured questionnaire was used to screen for potential controls on the basis of the eligibility criteria. Analysis of the answers received on the short questionnaire indicated that 83.6% of those questioned agreed to participate in clinical research. A comparison of those recruited as controls and those who refused to participate in the research revealed no significant differences in age, sex, race/ethnicity, educational level, personal history of cancer, or the accompanied patient’s type of cancer.
We sought to confirm the control subjects’ reasons for coming to the hospital with cancer patients and whether these reasons could have been related to the risk factors for HCC. We found that the underlying causes for the controls’ companionship were care and altruism. Moreover, all spouses of patients with other cancers who served as control subjects reported that they would have chosen to be referred to M.D. Anderson if they had been diagnosed with cancer during the same time period because they tended to share the same family physician, had the same health insurance coverage, and lived in the same geographic location. All of the above mentioned results indicated that the patients and controls had the same catchments, which further supported the idea that the control subjects were representative of the M. D. Anderson population from which HCC patients were selected.29-31 Total of 1286 eligible control subjects were ascertained in the current study. However 172 control subjects were excluded due to limited blood samples for testing hepatitis B virus (HBV) and HCV markers. Extra 10 control subjects were excluded for living outside the United States. Total of 1104 control subjects were analyzed in this study.
HCC patients and controls were recruited simultaneously and were personally interviewed for approximately 25–30 minutes. No proxy interviews were conducted. The interviewers used a structured and validated questionnaire 32 to collect information on demographic features and HCC risk factors, such as personal smoking history, alcohol consumption, medical history, occupational history, and family history of cancer. The definitions used for smokers, alcohol drinkers, and individuals with a family history of cancer were previously reported. 26-28
Diabetes mellitus
Each participant was questioned about his or her prior history of diabetes mellitus, the type of diabetes (insulin-treated or non–insulin-treated), the age at diagnosis, and the duration of each type of diabetes. Subjects with a history of diabetes were questioned about medications used for diabetes control and the duration of treatment. Oral antidiabetics used were classified into biguanides (e.g. metformin), sulfonylureas (e.g. glyburide, glipizide), and thiazolidinediones (e.g. siglitazone). 33
Hepatitis virus infection
Blood samples from cases and controls were tested for hepatitis B virus (HBV) and hepatitis C virus (HCV). HCV antibodies, hepatitis B surface antigen (HBsAg), and antibodies to hepatitis B core (HBc) antigen were detected by use of a third-generation enzyme-linked immunosorbent assay (ELISA) (Abbott Laboratories, North Chicago, IL). Positive results prompted repeated confirmatory ELISA testing.
Statistical methods
Stata software (Stata Corp, College Station, TX) was used for statistical analysis. Univariate analysis was done using the χ2 or Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables. To test for the association between diabetes and HCC, we performed multivariable unconditional logistic regression analyses using all variables significant at p < .05 in the univariable analyses and have a confounding effect on the association between diabetes and HCC. To determine the association between HCC development and diabetes duration and diabetes treatment, we performed restricted analysis among diabetic cases and controls. For each factor, we calculated the adjusted odds ratio (AOR) and 95% confidence interval (CI), using maximum likelihood estimation. All odds ratios (ORs) were adjusted for age, sex, race, education level, cigarette smoking, alcohol consumption, diabetes mellitus, family history of cancer, and HBV/HCV infection. The final model was chosen on the basis of biological plausibility and the lowest −2 log likelihood function.
RESULTS
The baseline demographic characteristics of patients and controls are summarized in table 1. Most study subjects were non-Hispanic white men; the men-to-women ratio was 2.5 to 1 for HCC patients. Case patients were slightly older than control subjects, with a mean difference of 3 years (95% CI 2 to 5; the mean [± standard error (SE)] ages were 63 ± .6 years for HCC patients and 60 ± .3 years for controls.
Table 1.
Prevalence of diabetes mellitus by participants’ characteristics
| Demographic Variables | HCC Patients | Controls | p | Prevalence of diabetes |
p * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=420 | (%) | N=1104 | (%) | Cases N =140 |
(%) | Controls N=115 |
(%) | |||
| Sex | .001 | |||||||||
| 299 | 71.2 | 636 | 57.6 | 112 | 37.5 | 78 | 12.3 | <0.0001 | ||
| Male | ||||||||||
| 121 | 28.8 | 468 | 42.4 | 28 | 23.1 | 37 | 7.9 | <0.0001 | ||
| Female | ||||||||||
| Age (years) | .001 | |||||||||
| 15 | 3.6 | 50 | 4.5 | 1 | 6.7 | 1 | 2.0 | 0.4 | ||
| ≤40 | ||||||||||
| 46 | 10.9 | 181 | 16.4 | 5 | 10.9 | 14 | 7.7 | 0.3 | ||
| 41-50 | ||||||||||
| 119 | 28.3 | 336 | 30.4 | 33 | 27.7 | 34 | 10.1 | <0.0001 | ||
| 51-59 | ||||||||||
| 117 | 27.9 | 358 | 32.4 | 55 | 47.0 | 39 | 10.9 | <0.0001 | ||
| 60-69 | ||||||||||
| 123 | 29.3 | 179 | 16.2 | 46 | 37.4 | 27 | 15.1 | <0.0001 | ||
| ≥70 | ||||||||||
| Ethnicity | .001 | |||||||||
| 294 | 70.0 | 973 | 88.1 | 92 | 31.3 | 92 | 9.5 | <0.0001 | ||
| Non-Hispanic white | ||||||||||
| 56 | 13.3 | 84 | 7.6 | 32 | 57.1 | 14 | 16.7 | <0.0001 | ||
| Hispanics | ||||||||||
| 40 | 9.5 | 39 | 3.5 | 11 | 27.5 | 6 | 15.4 | 0.1 | ||
| African Americans | ||||||||||
| 30 | 7.1 | 8 | 0.7 | 5 | 16.7 | 3 | 37.5 | 0.2 | ||
| Asians | ||||||||||
| Educational level | .001 | |||||||||
| 198 | 47.1 | 316 | 28.6 | 67 | 33.8 | 39 | 12.3 | <0.0001 | ||
| ≤ High school | ||||||||||
| 94 | 22.4 | 287 | 26.0 | 32 | 34.0 | 26 | 9.1 | <0.0001 | ||
| Some colleges | ||||||||||
| 128 | 30.5 | 501 | 45.4 | 41 | 32.0 | 50 | 10.0 | <0.0001 | ||
| ≥ College degree | ||||||||||
| State of residency | .5 | |||||||||
| 308 | 73.3 | 809 | 73.3 | 106 | 34.4 | 90 | 11.1 | <0.0001 | ||
| TX, LA, AK, NM,OK † | ||||||||||
| 112 | 26.7 | 295 | 26.7 | 34 | 30.4 | 25 | 8.5 | <0.0001 | ||
| Other states | ||||||||||
P value for the difference in diabetes prevalence between cases and controls in each characteristic
States of Texas, Louisiana, Arkansas, New Mexico, and Oklahoma
Table 2 shows that the prevalences of hepatitis virus infection (detected by anti-HCV, HBsAg, or anti-HBc), cigarette smoking, alcohol consumption, and family history of cancer were significantly higher for cases than for controls. Our previous reports from the same population indicated that each factor is an independent risk factor for HCC development. 26-28
Table 2.
Prevalence of diabetes mellitus by HCC risk factors
| Demographic Variables | HCC Patients | Controls | p | Prevalence of diabetes | P* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N =140 | (%) | N=1104 | (%) | Cases N =140 |
(%) | Controls N=115 |
(%) | |||
| Hepatitis Virus | <0.0001 | |||||||||
| None | 232 | 55.2 | 1066 | 96.6 | 94 | 40.5 | 109 | 10.2 | <0.0001 | |
| Anti-HCV+ | 94 | 22.4 | 6 | 0.5 | 22 | 23.4 | 2 | 33.3 | 0.6 | |
| HBsAg+/ Anti-HBc+ | 30 | 7.1 | 4 | 0.4 | 4 | 13.3 | 1 | 25.0 | 0.5 | |
| HBsAg−/ Anti-HBc+ | 24 | 5.7 | 25 | 2.3 | 10 | 41.7 | 2 | 8 | 0.006 | |
| Both HCV and HBV | 40 | 9.5 | 3 | 0.3 | 10 | 25.0 | 1 | 33.3 | 0.7 | |
| Cigarette smoking | <0.0001 | |||||||||
| No | 126 | 30 | 582 | 52.7 | 42 | 33.3 | 57 | 9.8 | <0.0001 | |
| Yes † | 294 | 70 | 522 | 47.3 | 98 | 33.3 | 58 | 11.1 | <0.0001 | |
| Smoking quantity | 0.007 | |||||||||
| ≤ 20 pack years | 115 | 27.4 | 258 | 23.4 | 35 | 30.4 | 23 | 8.9 | <0.0001 | |
| > 20 pack years | 176 | 41.9 | 264 | 23.9 | 62 | 35.2 | 35 | 13.3 | <0.0001 | |
| Alcohol consumption | <0.0001 | |||||||||
| No | 137 | 32.6 | 485 | 43.9 | 52 | 38.0 | 55 | 11.3 | <0.0001 | |
| Yes ‡ | 283 | 67.4 | 619 | 56.1 | 88 | 31.1 | 60 | 9.7 | <0.0001 | |
| Alcohol quantity | <0.001 | |||||||||
| < 60 ml ethanol/day | 192 | 45.7 | 551 | 49.9 | 60 | 31.3 | 53 | 9.6 | <0.0001 | |
| ≥ 60 ml ethanol/day | 89 | 21.2 | 65 | 5.9 | 27 | 30.3 | 7 | 10.8 | 0.003 | |
| Family history of cancer | <0.0001 | |||||||||
| No | 132 | 31.4 | 355 | 32.2 | 48 | 36.4 | 44 | 12.4 | <0.0001 | |
| Yes § | 264 | 62.9 | 740 | 67 | 82 | 31.1 | 71 | 9.6 | <0.0001 | |
| Liver cancer (first-degree) | 24 | 5.7 | 9 | 0.8 | 10 | 41.7 | 0 | 0 | <0.0001 | |
P value for the difference in diabetes prevalence between cases and controls in each stratum of the risk factor
Duration of smoking was missing for three HCC cases
Duration of drinking was missing for two HCC cases and 3 controls
Any cancer in first- and second-degree relatives
Total of 140 HCC patients (33.3%) and 115 controls (10.4%) recalled prior history of diabetes mellitus which conferred a four-fold increase in HCC risk when compared with nondiabetic individuals (p =.001); AOR =4.2 (3.0-5.9), Figure 1. The prevalence of diabetes mellitus stratified by demographic characteristics (Table 1) and HCC risk factors (Table 2) was significantly higher in cases than in controls. The significant risk of HCC development among patients with diabetes mellitus was observed for both men (AOR 5.2; 95% CI 3.3 to 8.3; p < .001] and women (AOR 3.2; 95% CI 1.6 to 6; p = .001].
Figure 1.
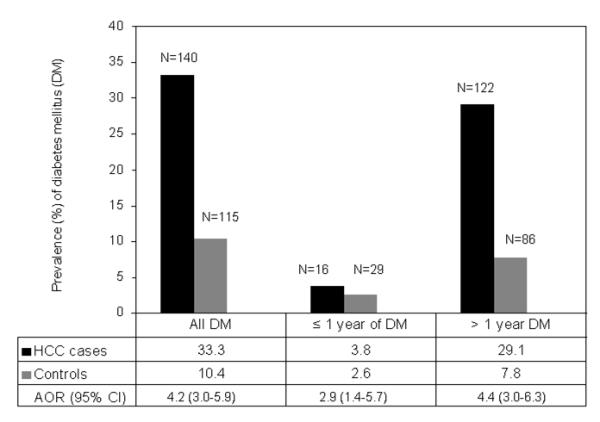
The prevalence of diabetes mellitus (DM) in cases (n=420) and controls (n=1104) and AOR for the association between HCC development and diabetes according to duration of diabetes (all, ≤ 1 year, and > 1 year). ORs were adjusted for the confounding effect of age, sex, race, educational level, cigarette smoking, alcohol drinking, HCV, HBV, and family history of cancer; using unconditional multivariable logistic regression analyses. Duration of diabetes was missing from two HCC patients.
To ensure that diabetes was not induced by the cancer, analysis of the association between diabetes and HCC risk was restricted to those who were diagnosed with diabetes more than 1 year prior to HCC diagnosis or prior to control recruitment (122 cases and 86 controls; Figure 1); AORs and 95% CIs were 4.4 (3.0 to 6.3) for all subjects, 5.2 (3.3 to 8.3) for men, and 3.5 (1.7 to 7.1) for women.
Table 3 presented results of restricted analyses among diabetic cases and controls. The estimated AORs and 95% CIs of developing HCC were 1.8 (0.8-4.1) for patients diagnosed with diabetes 6 to 10 years prior to HCC diagnosis and 2.2 (1.2-4.8) for those with a duration >10 years.
Table 3.
Association between diabetes duration/ treatment and risk of HCC development
| Diabetes Variables | HCC Patients | Controls | AOR (95% CI)* | P value | ||
|---|---|---|---|---|---|---|
| N = 122 | % | N = 86 | % | |||
| Duration of diabetes (years) | ||||||
| 2-5 | 30 | 24.6 | 33 | 38.4 | 1 (reference) | |
| 6-10 | 38 | 31.1 | 23 | 26.7 | 1.8 (0.8-4.1) | 0.2 |
| > 10 | 54 | 44.3 | 30 | 34.9 | 2.2 (1.2-4.8) | 0.04 |
| Age at diabetes diagnosis (years) | ||||||
| ≥ 50 | 83 | 68.0 | 57 | 66.3 | 1 (reference) | |
| <50 | 39 | 32.0 | 29 | 33.7 | 1.5 (0.7-3.4) | 0.3 |
| Diabetes treatment | ||||||
| Oral treatment | 0.009 | |||||
| Nonusers | 32 | 26.2 | 11 | 12.8 | 1 (reference) | |
| Users | 90 | 73.8 | 75 | 87.2 | 0.3 (0.1-0.7) | |
| Insulin treatment | 0.1 | |||||
| Nonusers | 95 | 77.9 | 73 | 84.9 | 1 (reference) | |
| Users | 27 | 22.1 | 13 | 15.1 | 1.9 (0.8-4.6) | |
| Diet only | 0.01 | |||||
| Nonusers | 106 | 86.9 | 84 | 97.7 | 1 (reference) | |
| Users | 16 | 13.1 | 2 | 2.3 | 7.8 (1.5-40.0) | |
| Type of oral treatment | ||||||
| Biguanide | ||||||
| Nonusers | 78 | 63.9 | 32 | 37.2 | 1 (reference) | |
| Users | 44 | 36.1 | 54 | 62.8 | 0.3 (0.2-0.6) | <0.001 |
| Sulfonylureas | ||||||
| Nonusers | 75 | 61.5 | 58 | 67.4 | 1 (reference) | |
| Users | 47 | 38.5 | 10 | 11.6 | 7.1 (2.9-16.9) | <0.001 |
| Thiazolidinediones | ||||||
| Nonusers | 116 | 95.1 | 70 | 81.4 | 1 (reference) | |
| Users | 6 | 4.9 | 16 | 18.6 | 0.3 (0.1-0.7) | 0.01 |
AOR=adjusted odds ratio for the confounding effect of age, sex, race, educational level, cigarette smoking, alcohol drinking, HCV, HBV, and family history of cancer; using unconditional multivariable logistic regression analyses
Among patients who had diabetes for more than a year, most subjects were considered to have type 2 diabetes mellitus and were on an oral antidiabetic regimen, yielding an inverse association with HCC for all subjects (AOR 0.3; p < .0009). A total of 16 HCC case patients and 2 control subjects with diabetes reported relying on diet alone to control diabetes, yielding a significantly higher risk of HCC development (AOR 7.8; 95% CI , 1.4-40). The majority of diabetic patients on oral antidiabetic regimens received agents in the biguanide and sulfonylurea classes. The AORs (95% CIs) for HCC association with biguanide use were 0.3 (0.1-0.7) for all subjects, 0.3 (0.1-0.7) for men, and 0.2 (0.1-0.9) for women. Only 6 HCC patients and 16 controls received thiazolidinedione-class agents, which showed 70% risk reduction in HCC development (Table 3). Use of the sulfonylurea class of oral antidiabetics had a much higher association with HCC development; the AORs (95% CIs) were 7.1 (2.9-16.9) for all subjects, 5.3 (1.9-14.2) for men, and 12.3 (1.6-96.9) for women. Moreover, insulin use was associated with risk for HCC development as compared to the use of oral modalities, however, the association was not statistical significant, P=0.1.
We found no significant association between early onset of diabetes diagnosis (age < 50 years) and risk of HCC development. Moreover, we found no correlations between duration of diabetes and individuals’ age or types of treatment in this study population.
DISCUSSION
Results from the current study suggested that the magnitude of association between diabetes and HCC increased as duration of diabetes increased and with specific antidiabetic treatment. A notable finding is that the use of sulfonylurea drugs (such as glyburide) among diabetics revealed a 7-fold increase in HCC risk compared to non users. Moreover, diabetic patients who were treated with exogenous insulin were at a higher risk for HCC development as compared to non-insulin treatment group; however, such elevated risk was not statistically significant. On the other hand, insulin-sensitizing agents such as biguanides (including metformin) and thiazolidinediones are alternative options for treating obese patients with diabetes mellitus or patients with underlying NAFLD or NASH. In the current study, the use of metformin or thiazolidinediones was associated with 70% risk reduction of HCC as compare to the use of insulin or sulfonylureas.
The above findings of the elevated risk associated with the use of insulin or sulphonylureas and the reduced risk associated with the use of biguanide (metformin) are in agreement with newly published study by Donadon et al among Italian patients with cirrhosis and HCC 34 who reported significant increased risk for HCC among diabetic patients treated with insulin and sulphonylureas (OR=2.99, 95% CI 1.34-6.65) and reduced HCC risk among diabetic patients treated with metformin (OR=0.33, 95% CI 0.1-0.7). Moreover, Bowker et al 35 reported that patients with type 2 diabetes exposed to sulphonylureas and exogenous insulin had a significant risk of cancer-related mortality compared with patients exposed to metformin. Both studies came in agreement with an earlier report by Evans et al 36 who observed lower incidence of cancer among diabetic patients treated with metformin as compared to other diabetes treatments. Interestingly, such risk reduction was associated with duration and dosage of metformin treatment.
The results of our study are consistent with the notion that the biological mechanism for liver-cell damage induced by type 2 diabetes mellitus involves insulin resistance and hyperinsulinemia. 4,37 HCC development related to hyperinsulinemia can be mediated through inflammation, cellular proliferation, inhibition of apoptosis, and mutation of tumor suppressor genes.4 Increased insulin levels lead to reduced liver synthesis and blood levels of insulin growth factor–binding protein-1 (IGFBP-1), which may contribute to increased bioavailability of insulin-like growth factor-1 (IGF-1), the promotion of cellular proliferation, and the inhibition of apoptosis. 38 Insulin also binds to the insulin receptor and activates its intrinsic tyrosine kinase, leading to phosphorylation of insulin receptor substrate-1 (IRS-1). 39 Both IGF-1 and IRS-1have been overexpressed in tumor cells.40 Overexpression of IRS-1 has been associated with the prevention of apoptosis mediated by transforming growth factor–β. 41 In addition, insulin is associated with lipid peroxidation and increased oxidative stress and the generation of reactive oxygen species, which may contribute to DNA mutation. In fact, lipid peroxidation has been implicated in the upregulation of peroxidation of proinflammatory cytokines, which has been involved in p53 tumor suppressor gene mutations. 42
Metformin can reduce blood glucose in diabetic patients, predominantly through reduction of hepatic gluconeogenesis and glycogenolysis. 33,43,44 It also increases the insulin-stimulated glucose uptake in the skeletal muscles, suppresses oxidation of fatty acids, and reduces triglyceride levels in patients with hypertriglyceridemia. All of these effects may contribute to reducing hyperinsulinemia, improving hepatic insulin resistance, reducing steatosis, improving liver enzymes, and reducing body weight.
Although the molecular mechanisms of metformin’ antidiabetic activity have yet to be fully identified, experimental studies on ob/ob mice indicated that the key role of metformin may be related to decreased hepatic expression of tumor necrosis factor–alpha (TNF-α), a cytokine that promotes insulin resistance. 45 The beneficial effect of metformin treatment among patients with NAFLD was assessed by small-scale trials; improvement in liver enzymes, steatosis, and fibrosis was seen. 46-48 However, a recently reported study by Haukeland and colleague 49 indicated that metformin treatment for six months was not better than placebo in terms of improving liver histology in patients with NAFLD. Even though, body weight and metabolic profile improved significantly.
Unlike metformin, the use of sulfonylureas is associated with weight gain, hyperinsulinemia, and hepatotoxicity. 50 Therefore, it may not be the appropriate diabetes treatment for patients with underlying chronic liver diseases, obesity, or insulin resistance because of possible exacerbation of the underlying NAFLD or NASH observed in these patients and possible acceleration of HCC development. 51
Although the mechanism for the anti-neoplastic activity of metformin is not fully understood, there is substantial evidence suggesting that metformin suppress cellular proliferation and protein synthesis with AMP-independent protein kinase activation in both malignant and nonmalignant cells. 52,53 A recent review by Cazzaniga M et al 54 reported that such AMPK actions may be mediated by multiple pathways including up-regulation of the P53 and reduction of Cyclin D1 levels which may eventually lead to anti-proliferative effect.
Although the intake of thiazolidinediones was significantly associated with reduced risk of HCC, however only 6 HCC patients with diabetes recalled using this medication, which may not enough to conclude the protective effect of thiazolidinediones treatment on HCC development.
In this study, cases were pathologically-confirmed HCC patients who were newly diagnosed and prospectively enrolled in the study where both cases and controls were personally and simultaneously interviewed, using a structured-validated questionnaire. Control subjects were selected to represent the study population from which cases were selected. To ensure the accuracy of our data, subjects with a history of diabetes were asked about the duration of their disorder, their age at diagnosis, and their treatment exposure. Questions of prior history of diabetes mellitus along with other chronic medical conditions were part of a long list of questions where study subjects were blinded for the current study hypothesis and its specific aims. It is reasonable to assume that subjects who had received a definite diagnosis and had been treated could accurately report their prior history of medical conditions and recalled the condition duration. Upon reviewing the medical records of HCC patients, we found no discrepancy between interview information and patients’ records. In fact, there is strong evidence supporting the reliability and validity of self-reported diabetes mellitus where agreement between self reported disease diagnosis and medical conditions was observed. 55-57 It is partially attributable to patients’ awareness of diabetes complications and the importance to monitor blood sugar during treatment. Therefore, it is not surprising that patients with diabetes mellitus tend to remember the name of exposed medications with and without therapeutic response during their lifetime.
Our study did have some limitations. Overweight and obesity may have a confounding effect for the observed association between diabetes and HCC and might modulate the anti-diabetic treatment selection. Nevertheless, we have collected information about subjects’ weight prior to HCC diagnosis or prior to control ascertainment. Such data was initiated in 2004, and is available for 184 HCC patients and 648 controls. Results indicated that the mean of body mass index (BMI) at early age (between age 20 and age 40) ± SE was significantly larger in HCC patients (24.06 ± 0.3) than in controls (23.04 ± 0.1), P=0.001. However, adjustment for the effect of prior BMI did not meaningfully change the observed significant association between diabetes and HCC, the estimated OR (95%C) was 3.8 (2.3-6.1).
Although obesity is a risk factor for diabetes mellitus and HCC, obesity is not necessarily present in patients with NAFLD; a significant portion of patients with NAFLD have a normal body weight. 58 Therefore, it is not surprising that the association between diabetes and HCC is not confounded by obesity in the current study and other studies. 6,7
We also noted that most patients with diabetes were treated with oral antidiabetic drugs, implicating type 2 diabetes. Although we do not know why some patients with type 2 diabetes received insulin treatment, it is possible that insulin was given to some patients for whom safety and efficacy considerations favor its use as the drug of choice, for example, patients with severe hepatic or renal impairment. It may also indicate that the diabetes was severe or that some patients required insulin therapy, either as monotherapy or in conjunction with oral antidiabetic therapy, to maintain long-term glycemic control. However, we lacked information about fasting blood glucose, diabetes complications, and glycosylated hemoglobin (HgA1c) to identify the average plasma glucose concentration over prolonged periods of time. This information is crucial to explain whether severity of diabetes is correlated with duration and type of treatment and why some diabetic patients with dietary control are at high risk for HCC development. Future large cohort studies among diabetic patients with detailed information about family history of diabetes, type of diabetes, diabetes treatment, response to diabetes therapy, diabetes-related complications and clinicopathological changes in liver tissues may reveal the explanation for the relationship between diabetes and HCC development reported by case-control studies.
The preliminary finding of this study may indicate that choosing an appropriate and safe treatment for diabetes mellitus is critical in patients with underlying liver diseases. The need for developing specific guidelines for treating diabetic patients with underlying liver diseases—with consideration of subjects’ BMI and whether they have NAFLD or NASH—is warranted . Such guidelines should outline appropriate and safe treatment for these patients, with the ultimate goal of preventing progressive liver disease and HCC development. Future studies should be aimed at investigating the preventive role of metformin on HCC development.
Acknowledgments
Supported by: National Institutes of Health grants R03 ES11481 (to M.H.) and CA106458-01 (to M.H.)
Reference List
- 1.Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol.Appl.Pharmacol. 2007;225:214–20. doi: 10.1016/j.taap.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 3.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 4.Harrison SA. Liver disease in patients with diabetes mellitus. J.Clin.Gastroenterol. 2006;40:68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am.J.Epidemiol. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 6.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–21. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 7.Lai MS, Hsieh MS, Chiu YH, Chen TH. Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43:1295–302. doi: 10.1002/hep.21208. [DOI] [PubMed] [Google Scholar]
- 8.Adami HO, Chow WH, Nyren O, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J.Natl.Cancer Inst. 1996;88:1472–7. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 9.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J.Natl.Cancer Inst. 1997;89:1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 10.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–13. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 11.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–17. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 13.Ohki T, Tateishi R, Sato T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin.Gastroenterol.Hepatol. 2008;6:459–64. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Torisu Y, Ikeda K, Kobayashi M, et al. Diabetes mellitus increases the risk of hepatocarcinogenesis in patients with alcoholic cirrhosis: A preliminary report. Hepatol.Res. 2007;37:517–23. doi: 10.1111/j.1872-034X.2007.00077.x. [DOI] [PubMed] [Google Scholar]
- 15.El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology. 2002;122:1822–8. doi: 10.1053/gast.2002.33650. [DOI] [PubMed] [Google Scholar]
- 16.Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–62. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin.Gastroenterol.Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Bell DS, Allbright E. The multifaceted associations of hepatobiliary disease and diabetes. Endocr.Pract. 2007;13:300–12. doi: 10.4158/EP.13.3.300. [DOI] [PubMed] [Google Scholar]
- 19.Tolman KG, Fonseca V, Tan MH, Dalpiaz A. Narrative review: hepatobiliary disease in type 2 diabetes mellitus. Ann.Intern.Med. 2004;141:946–56. doi: 10.7326/0003-4819-141-12-200412210-00011. [DOI] [PubMed] [Google Scholar]
- 20.Yu MC, Tong MJ, Govindarajan S, Henderson BE. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J.Natl.Cancer Inst. 1991;83:1820–6. doi: 10.1093/jnci/83.24.1820. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 22.Komura T, Mizukoshi E, Kita Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am.J.Gastroenterol. 2007;102:1939–46. doi: 10.1111/j.1572-0241.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda Y, Shimada M, Hasegawa H, et al. Prognosis of hepatocellular carcinoma with diabetes mellitus after hepatic resection. Hepatology. 1998;27:1567–71. doi: 10.1002/hep.510270615. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Shaheen NJ. Diabetes and hepatocellular carcinoma: associations, biologic plausibility, and clinical implications. Gastroenterology. 2005;129:1132–4. doi: 10.1053/j.gastro.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 25.Poulson J. The management of diabetes in patients with advanced cancer. J.Pain Symptom.Manage. 1997;13:339–46. doi: 10.1016/s0885-3924(96)00326-0. [DOI] [PubMed] [Google Scholar]
- 26.Hassan MM, Spitz MR, Thomas MB, et al. Effect of different types of smoking and synergism with hepatitis C virus on risk of hepatocellular carcinoma in American men and women: Case-control study. Int.J.Cancer. 2008;123:1883–91. doi: 10.1002/ijc.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan MM, Spitz MR, Thomas MB, et al. The association of family history of liver cancer with hepatocellular carcinoma: A case-control study in the United States. J.Hepatol. 2009;50:334–41. doi: 10.1016/j.jhep.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan MM, Kaseb A, Li D, et al. Association between hypothyroidism and hepatocellular carcinoma: a case-control study in the United States. Hepatology. 2009;49:1563–70. doi: 10.1002/hep.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am.J.Epidemiol. 1992;135:1042–50. doi: 10.1093/oxfordjournals.aje.a116398. [DOI] [PubMed] [Google Scholar]
- 30.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am.J.Epidemiol. 1992;135:1029–41. doi: 10.1093/oxfordjournals.aje.a116397. [DOI] [PubMed] [Google Scholar]
- 31.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. Am.J.Epidemiol. 1992;135:1019–28. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 32.Spitz MR, Fueger JJ, Newell GR. The development of a comprehensive, institution-based patient risk evaluation program: II. Validity and reliability of questionnaire data. Am.J.Prev.Med. 1988;4:188–93. [PubMed] [Google Scholar]
- 33.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 34.Donadon Valter, Balbi Massimiliano, Ghersetti Michela, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–11. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 36.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bugianesi E. Review article: steatosis, the metabolic syndrome and cancer. Aliment.Pharmacol.Ther. 2005;22(Suppl 2):40–3. doi: 10.1111/j.1365-2036.2005.02594.x. [DOI] [PubMed] [Google Scholar]
- 38.Moore MA, Park CB, Tsuda H. Implications of the hyperinsulinaemia-diabetes-cancer link for preventive efforts. Eur.J.Cancer Prev. 1998;7:89–107. [PubMed] [Google Scholar]
- 39.Alexia C, Fallot G, Lasfer M, Schweizer-Groyer G, Groyer A. An evaluation of the role of insulin-like growth factors (IGF) and of type-I IGF receptor signalling in hepatocarcinogenesis and in the resistance of hepatocarcinoma cells against drug-induced apoptosis. Biochem.Pharmacol. 2004;68:1003–15. doi: 10.1016/j.bcp.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26:598–604. doi: 10.1002/hep.510260310. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka S, Wands JR. Insulin receptor substrate 1 overexpression in human hepatocellular carcinoma cells prevents transforming growth factor beta1-induced apoptosis. Cancer Res. 1996;56:3391–4. [PubMed] [Google Scholar]
- 42.Hu W, Feng Z, Eveleigh J, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–9. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 43.Ong CR, Molyneaux LM, Constantino MI, Twigg SM, Yue DK. Long-term efficacy of metformin therapy in nonobese individuals with type 2 diabetes. Diabetes Care. 2006;29:2361–4. doi: 10.2337/dc06-0827. [DOI] [PubMed] [Google Scholar]
- 44.Donnelly LA, Doney AS, Hattersley AT, Morris AD, Pearson ER. The effect of obesity on glycaemic response to metformin or sulphonylureas in Type 2 diabetes. Diabet.Med. 2006;23:128–33. doi: 10.1111/j.1464-5491.2005.01755.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat.Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 46.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–4. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 47.Nair S, Diehl AM, Wiseman M, Farr GH, Jr., Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment.Pharmacol.Ther. 2004;20:23–8. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 48.Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment.Pharmacol.Ther. 2004;19:537–44. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 49.Haukeland JW, Konopsk Z, Loberg EM, et al. A randomized, placebo controlled trial with metformin in patients with NAFLD. Hepatology. 2008;48:334A. [Google Scholar]
- 50.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann.Intern.Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 51.ston-Mourney K, Proietto J, Morahan G, Andrikopoulos S. Too much of a good thing: why it is bad to stimulate the beta cell to secrete insulin. Diabetologia. 2008;51:540–5. doi: 10.1007/s00125-008-0930-2. [DOI] [PubMed] [Google Scholar]
- 52.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin.Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveras-Ferraros C, Vazquez-Martin A, Menendez JA. Genome-wide inhibitory impact of the AMPK activator metformin on [kinesins, tubulins, histones, auroras and polo-like kinases] M-phase cell cycle genes in human breast cancer cells. Cell Cycle. 2009;8:1633–6. doi: 10.4161/cc.8.10.8406. [DOI] [PubMed] [Google Scholar]
- 54.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol.Biomarkers Prev. 2009;18:701–5. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 55.Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J.Am.Geriatr.Soc. 2004;52:123–7. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 56.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am.J.Public Health. 1989;79:1554–6. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanaugh KL, Merkin SS, Plantinga LC, Fink NE, Sadler JH, Powe NR. Accuracy of patients’ reports of comorbid disease and their association with mortality in ESRD. Am.J.Kidney Dis. 2008;52:118–27. doi: 10.1053/j.ajkd.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–9. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]


