Remarkable progress in unveiling chronic lymphocytic leukemia (CLL) biology and its definitive tendency towards chemo-resistance has set concrete evidence for the hypothesis that tumor cells are not stand alone, but constitute a complex network that contribute to the process of tumorigenesis[1]. The tumor “complexity” involves the presence of multiple cell types and several secretory molecules which collectively constitute the “tumor associated stroma” and soluble factors such as chemokines, cytokines and or growth factors[2] (Figure 1). The production of these molecules potentially activates numerous cell signaling cascades that ultimately shift the paradigm towards anti-apoptotic mechanisms in CLL.
Figure 1.
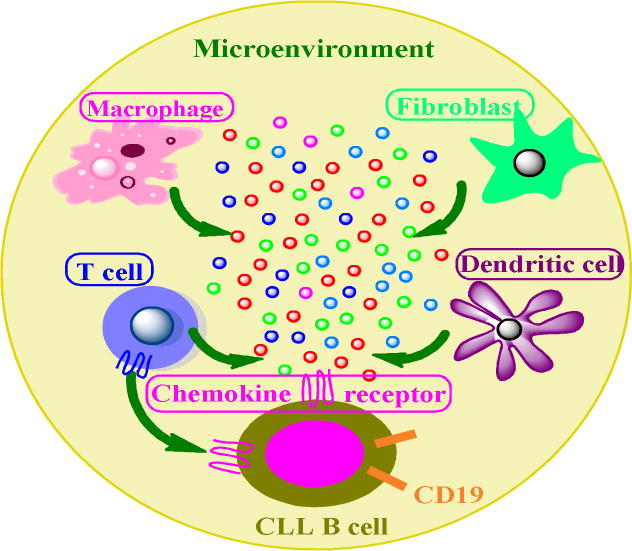
The tumor associated microenvironment constitutes multiple cell types that release several chemokines and cytokines. These soluble factors in turn attract the chemokine receptors expressed on B cell surface and regulate their activation and migration.
Chemokines are a family of small cytokines with the ability to induce directed chemotaxis in the nearby responsive cells by activating surface receptors that are seven transmembrane-domain G-protein-coupled receptors. In this regard, chemokines are termed chemo-attractants serving as signal lights in trafficking T and B cells for their development and maturation, migration and function. Approximately 50 human chemokines, the majority C-C and C-X-C family members, are currently identified and their sustained role in the motility of distinct cell types has been recognized and reviewed [3]. More importantly, developing lymphocytes differentially express chemokine receptors in order to modulate their chemotactic sensitivity to certain B and T cell chemokines, which essentially enable them to localize at particular microenvironments or niches in lymphoid tissues. Within such a complex environment, any form of inappropriate interpretation by cells may be deleterious, leading to their activation, inappropriate trafficking and survival. Taken together, chemokines and their receptors appear to play a crucial role in directing the movement of mononuclear cells throughout the body, stimulating their adaptive immune response and contributing to the pathogenesis of a variety of diseases including cancer. For this reason, chemokine receptors are endorsed as most important drug targets and many clinical trials involving chemokine-receptor antagonists are currently under investigation [4].
The most thoroughly characterized C-C chemokine is monocyte chemoattractant protein 1 (MCP-1), termed “chemokine ligand CCL2” which is commonly secreted by cells such as macrophages, fibroblasts, endothelial cells and tumor cells. Chemokine CCL2 attracts many cell types including monocytes, dendritic cells, memory T cells, and basophils as they all carry its cognitive receptor CCR2. Several reports show that CCL2 is highly expressed in malignant cells of both solid tumors as well as hematological malignancies[5]. Mechanistic studies have demonstrated that CCL2 has a regulatory role on molecular mechanisms such as autophagy and B-cell receptor pathways [6,7]. In particular, the robust expression of CCL2 in CLL cells in the bone marrow microenvironment has been established previously [8]. Furthermore, the plasma levels of other T-cell chemokines such as macrophage inflammatory protein, (MIP-1α or CCL3, whose receptors are CCR1 and CCR5), MIP-1β (CCL4, whose receptor is CCR5) have been identified and shown to be elevated in CLL when compared to normal subjects and their plasma concentrations were strongly associated with other established prognostic markers [9,10]. In addition, the homeostatic trafficking of B cells is regulated in part by the C-X-C chemokines CXCL12 (SDF-1, whose receptor is CXCR4) and CXCL13 (BCA-1, BLC, whose receptor is CXCR5) via interactions with their receptors commonly expressed on resting B cells. While CCL3 and CCL4 attract monocytes and lymphocytes, CXCL12 and CXCL13 released by nurse like cells are specific chemo-attractants of B-cells.
Following these findings, Burgess et al, in this issue of Leukemia and Lymphoma, demonstrate that CCL2 and CXCL2 (MIP2α, whose receptor is CXCR2) levels are high in CLL, predominantly in the presence of bone marrow microenvironment and their expression appears to correlate with sustained survival [11]. This observation is consistent with a recent report from Schulz et al in 2010, who demonstrated that CCL2 expression is associated with survival of primary CLL cells in vitro [8]. These results further support the finding that expression of chemokines CCL3 and CCL4 are linked with a high risk for disease progression in CLL [9,10]. Although this study reproduces and supports some of previously published findings, it certainly provides important insights into CLL biology. In this regard, the experiments elaborating the concept that CLL cell survival is primarily influenced by the presence of accessory cells, most importantly by the secretion of chemokines rather than by cell-cell interaction, is a key illustration of this phenomenon. Using several different approaches, the authors established that cytokines CCL2, CXCL2, IL8 and IL6 are high in CLL compared to healthy controls and these are even more elevated in the presence of bone marrow stromal cells, demonstrating that cytokines may serve as potential targets for inhibition in CLL. Both T cells and macrophages, presented in higher proportions in the initial cultures, subsequently decreased in numbers thereafter, providing evidence for the fact that they differentiate into nurse like cells and contribute to CLL cell survival. Simply adding cytokines to purified B cells did not improve survival, but the presence of accessory cells was necessary for the sustained survival, is indeed an interesting finding. The molecular modulations induced by exogenous cytokines primarily increase the survival of cancer cells but not normal cells implies that cancer cells probably have a distinct signature of cytokines that is expressed differentially in comparison to normal cells.
Although these findings highlight salient features of the malignant cell and its microenvironment, this study essentially leads us to ask many other questions. While CCL2 and CXCL2 expression have been shown to be significant, the global chemokine CXCL12 and its respective receptor CXCR4 expressed in different cancer cells, does not exhibit any significance in this model, which is surprising. Although in vitro systems greatly mimic what happens in CLL in vivo, only a few types of accessory cells were examined here and the studies of other cancer – associated cell types that partially promote angiogenesis, inflammation and provide growth signals could be useful and add significant contribution to further understanding this complex system. How these cell types could affect the cancer cell phenotype or genotype and its associated microenvironment still needs to be addressed. Additionally, these experiments represent only a single microenvironment, the bone marrow, and if the same holds true for other types of microenvironments such as the spleen, lymph nodes and other extra-nodal sites is certainly unknown. Finally, investigating the functional relevance of chemokines in molecular and pathological events and their regulatory role in the activation of cell signaling pathways should certainly reveal related anti-apoptotic proteins and potentially significant biomarkers in CLL. All of the latter may well be of importance for planning future novel approaches to therapy in this disease.
Acknowledgments
This work was supported in part by grants CA81534 and CA136411 (Lymphoma SPORE) from Department of Health and Human Services and US/European Alliance of CLL Global Research Foundation.
References
- 1.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, Sivina M, Wierda WG, Estrov Z, Keating MJ, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–50. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 4.Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319–26. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 5.Mazur G, Wrobel T, Butrym A, Kapelko-Slowik K, Poreba R, Kuliczkowski K. Increased monocyte chemoattractant protein 1 (MCP-1/CCL-2) serum level in acute myeloid leukemia. Neoplasma. 2007;54:285–9. [PubMed] [Google Scholar]
- 6.Lebrecht A, Grimm C, Lantzsch T, Ludwig E, Hefler L, Ulbrich E, Koelbl H. Monocyte chemoattractant protein-1 serum levels in patients with breast cancer. Tumour Biol. 2004;25:14–7. doi: 10.1159/000077718. [DOI] [PubMed] [Google Scholar]
- 7.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008;283:25057–73. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz A, Toedt G, Zenz T, Stilgenbauer S, Lichter P, Seiffert M. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica. 2010;96:408–16. doi: 10.3324/haematol.2010.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, Rosenwald A. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113:3050–8. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, LaPushin R, Xiao L, Huang X, Werner L, et al. CCL3 (MIP-1alpha) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood. 2010;117:1662–9. doi: 10.1182/blood-2010-09-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess M, Gill D, Mollee P, Cheung C, Chambers L, Knopp L, Ravindranath K, Minhas G, McMillian N. CCL2 and CXCL2 enhance the survival of primary chronic lymphocytic leukaemia cells in vitro. Leukemia and Lymphoma. 2012 doi: 10.3109/10428194.2012.672735. [DOI] [PubMed] [Google Scholar]


