Abstract
Objective
To develop a method to quantify displacement of pelvic structures during contraction of the pelvic floor muscles from transperineal ultrasound images in men and investigate the reliability of the method between days.
Methods
Ten healthy male volunteers (aged 28 – 41 years) attended two separate data collection sessions. Ultrasound images were recorded during voluntary pelvic floor muscle contractions in cine-loop (video) format with the transducer aligned in the mid-sagittal plane on the perineum. Five anatomical points were defined to represent contraction from striated urethral sphincter (SUS), levator ani (LA) and bulbocavernosus (BC) muscles. Displacement of each point was calculated between the relaxed and contracted-state images. Intra-class correlation coefficient (ICC) values were calculated from displacement data to assess reliability of the method between days.
Results
Displacements of the five anatomical points closely matched predictions based on anatomical considerations of the male pelvic musculature. ICC values for displacement data calculated from 1, 2 and 3 repetitions ranged between 0.82–0.95 for ICC (2,1), 0.85–0.97 for ICC (2,2) and 0.86–0.97 for ICC (2,3), respectively.
Conclusions
The new method reliably calculates displacements of points previously validated for females (Ano-rectal junction and bladder base) in addition to new measures of muscle actions (SUS and BC) specific to men. Future use might include assessment of clinical populations to understand how these displacements relate to symptoms of incontinence.
Keywords: Perineal ultrasound imaging, male continence, striated urethral sphincter, urinary incontinence
1. Introduction
When the striated muscles of the male pelvic floor contract, movement of the mid-urethra, urethra-vesical junction (UVJ), bulb of the penis (BU) and ano-rectal junction (ARJ) can be observed in a 2D ultrasound image generated with the transducer placed on the perineum and aligned in the mid-sagittal plane. Although 2D ultrasound imaging can reliably quantify movements of the UVJ and ARJ related to levator ani contraction in females,1, 2 anatomical differences between genders necessitate development and validation of new methods specific for the male pelvic floor using the transperineal imaging approach. More invasive approaches have been used in men to quantify movement of single structures (transrectal ultrasound imaging for ARJ and transurethral for striated urethral sphincter [SUS] muscle contraction).3–5 Transperineal ultrasound imaging has the potential to quantify displacement of multiple structures that can influence the urethra concurrently.
This paper describes a new method to quantify displacement of different pelvic structures simultaneously that are anatomically related to urethral movement during voluntary contractions of the pelvic floor muscles in men. Measurements of displacement of specific anatomical structures related to the urethra were measured and related to the actions that were predicted, based on anatomy and biomechanics, to be caused by specific muscles with potential to influence continence. These muscles included SUS, bulbocavernosus (BC) and levator ani. Displacement of each structure and consideration of the possible relative contribution of each to maintenance of continence has not been measured in men.
Movements caused by contraction of levator ani (quantified as displacement of ARJ and UVJ) were based on those described and validated for women, but quantified here for the first time in men, although measurement of levator plate and ano-rectal angle has been reported previously.6 Movement caused by contraction of the SUS (quantified as dorsal displacement of mid-urethra) and BC (quantified by compression of the BU) were also investigated and have not been described previously. The study also investigated the reliability of each measure in men between days.
2. Material and Methods
2.1 Participants
Ten healthy men (28–41 years of age) with no history of urological or neurological disorders volunteered. The institutional Medical Research Ethics Committee approved the study and participants provided informed written consent.
2.2 Predicted motion of pelvic structures based on anatomical/biomechanical considerations
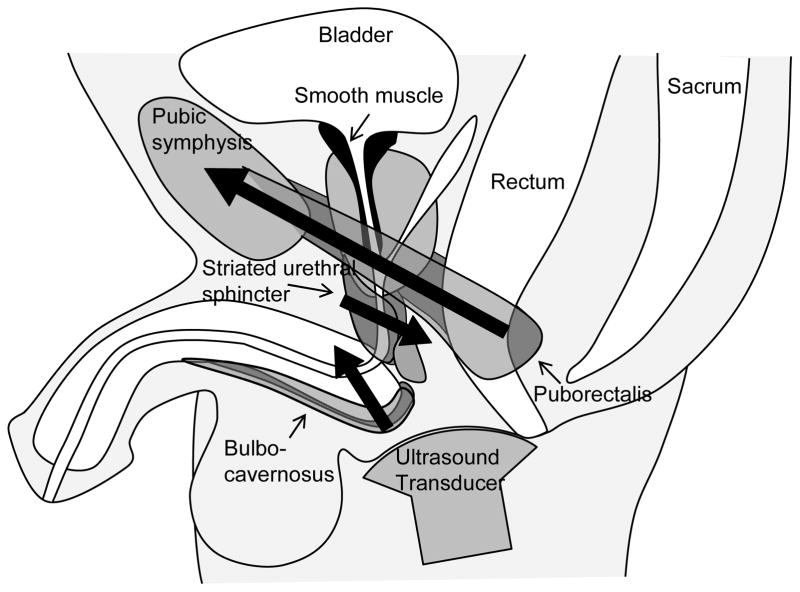
The SUS is an omega-shaped muscle with fibres anterior and lateral to the urethra and a posterior insertion to the perineal body.7 Contraction of SUS is predicted to compress the urethra against the perineal body, generating motion/urtheral compression in dorsal direction. The puborectalis muscle of the levator ani group originates from the body of the pubis to form a sling looping behind the ARJ. Similar to the action described in females,8 contraction of this muscle should displace the ARJ and UVJ in an anterior/superior direction. Fibres of the BC muscle insert to the dorsal surface of the corpus spongiosum and compress both the bulb of the penis and distal urethra.9 This action is thought to expel any urine remaining in the urethra at the completion of micturition.9 Contraction of each of these muscles (and movement of their adjacent structures) is likely to influence male urinary continence (Fig. 1).
Fig. 1.
Anatomical depiction of the male pelvic floor and associated structures viewed in the midsagittal plane with an ultrasound transducer placed on the perineum. Bold arrows indicate hypothesized direction of movement during voluntary muscle contraction based on anatomy.
2.3 Transperineal ultrasound image acquisition
A curved linear array ultrasound transducer (frequency: 7.0 MHz [M7C]; Logiq9 ultrasound, GE Healthcare, Australia) was enclosed in a rubber sheath (ultrasound gel transmission medium) to image pelvic structures. Participants voided their bladder and consumed 450 ml water 1 hr before testing to standardise bladder volume. Ultrasound images were recorded with participants seated upright (back rest at 70°) and legs extended. Data were recorded as a cine-loop and exported in video format (.MPG, frame rate 30 Hz). Frames with optimal image quality for evaluation of the relaxed and contracted state were identified and captured as .JPG images (640 × 480 pixels) for analysis.
2.4 Procedure
US data were collected during maximal voluntary contractions of the pelvic floor muscles on two different days by a single experienced operator. During each contraction the participant was instructed to take a breath in and out. Image collection commenced at the end of expiration of a quiet breath to standardize intra-abdominal pressure. The participant was then instructed to maximally contract the pelvic floor muscles as if they were attempting to stop the flow of urine mid-stream, and data collection was ceased once a steady state of maximal contraction was achieved. The procedure was repeated three times with 60 s rest between each effort. Image quality was optimised at the time of collection to include all points required for analysis in a single image to facilitate efficient analysis and prevent trial rejection.
2.5 Magnetic resonance imaging (MRI)
In order to further validate the measures of movement of structures of the male pelvic floor with voluntary pelvic floor muscle activation made with transperineal US imaging, measures were compared with those made with magnetic resonance imaging. One participant from the main sample underwent an additional session in which MRI images were recorded at rest and during a maximal pelvic floor muscle contraction in a 1.5T scanner (Siemens AG; Medical Solutions, Germany). For this measurement the participant lay supine with a pillow under the knees. A sagittal localizing sequence was performed to optimise capture of the bladder and entire urethral length, followed by a rapid image capture sequence (images captured every 3 s) at the optimal location in the rested and contracted state. Procedures for bladder volume standardization, instruction of muscle contraction, and image selection for analysis were identical to those described for ultrasound imaging.
2.6 Image analysis
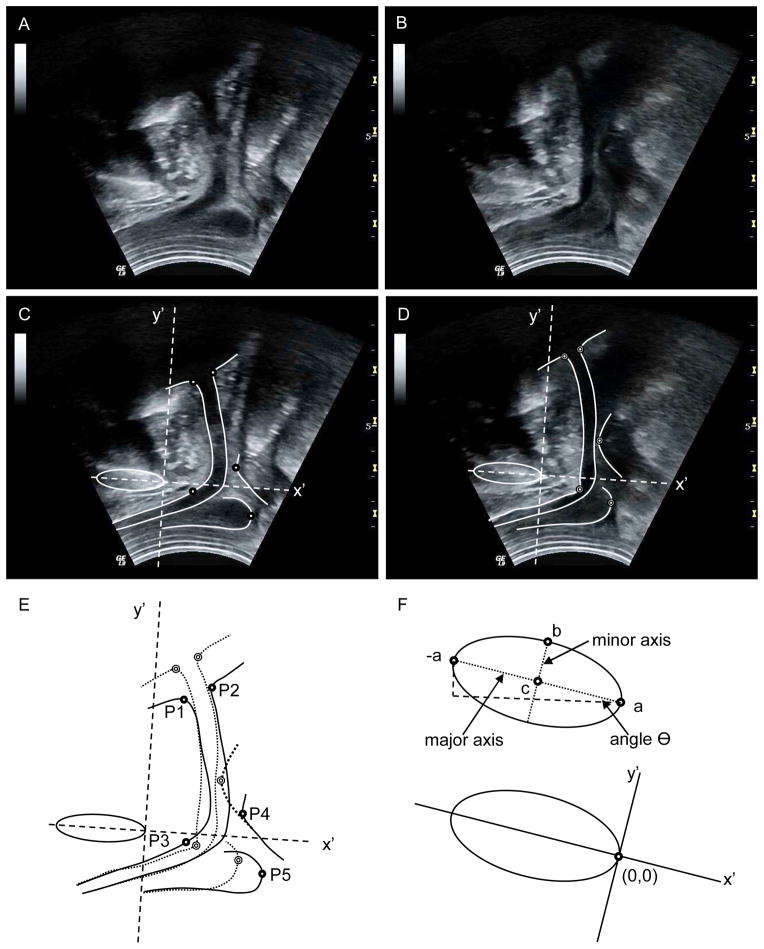
A graphical user interface was developed using Matlab software (r2010b, The Mathworks, USA) for US and MRI image analysis. A common axis system was used to calculate displacement of pelvic structures between images, with the origin aligned to the posterior aspect of the pubic symphysis (PS).1, 8 The PS was identified and approximated by an ellipse based on points selected by the user (“a”, “-a” and “b”, Fig. 2F). The slope of the major axis determined the inclination, Θ. A new image axis system was defined by the ellipse (Fig. 2F) and coordinates of other points in the image (xn,yn) were redefined relative to the new axes by subtracting the position of the new origin (xo,yo) and multiplying with a rotation matrix:
| [Equation 1] |
Fig. 2.
Transperineal ultrasound images made in the (A) relaxed and (B) contracted states. (C, D) Borders and points of interest superimposed on the US images. (E) Overlaid borders of the two images; dark circles indicate the points of interest in the relaxed state and light circles from the contracted state. (F) Ellipse defined by user-selected points “-a”, “a” and “b” with coordinate system used for image analysis based at origin “a”.
A spline function was used to identify the ventral and dorsal borders of the urethra (including UVJ), the inferior/dorsal border of BU, and the ventral border of ARJ (Fig. 2C–D). The function plotted a curve-fitting line in real time (based on user-selected points) with resolution 1 point/mm to accurately overlay the structure border and provide visual feedback to the user.
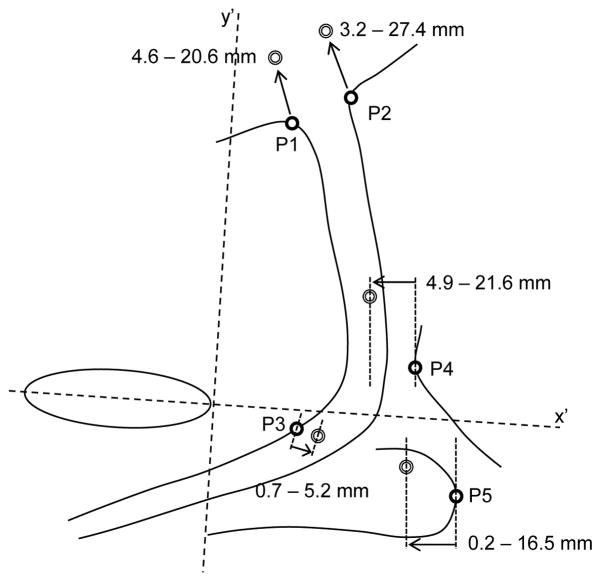
Five anatomical points (P1–P5 in Fig. 2E) were identified to quantify displacement of UVJ (ventral [VU] and dorsal [DU]), SUS, ARJ and BU. The ventral and dorsal positions of the UVJ were defined as the inflection points of the respective borders, nearest to the bladder (P1 and P2).1 The point of VU that underwent greatest displacement due to SUS contraction was identified as the posterior-most point on VU in quadrant IV of the axis system (Cartesian system) from the contracted-state image (P3). A line from origin to P3 was calculated to approximate the SUS contraction vector. The intersection of this line with VU (relaxed state) was used as the initial position. The point on ARJ used to quantify its displacement was defined as the ventral-most point in the x′ plane (P4).1 Likewise, displacement of BU was calculated from movement of the dorsal-most point in the x′ plane (P5).
Statistical analysis
To assess the reliability of the data (from US imaging) between days and the whether reliability was affected by the number of repetitions over which data were averaged, intra-class correlations (ICC) of type (2,1), (2,2) and (2,3) were calculated as described by Shrout and Fleiss10 and Hopkins11 using displacement values from one, two and three consecutive repetitions, respectively. Typically ICC of 0.8–0.9 are considered to reflect “good” reliability, and values >0.9 reflect “high” reliability.12, 13 The standard error of the measurement (SEM), also referred to as the typical error, was calculated.11
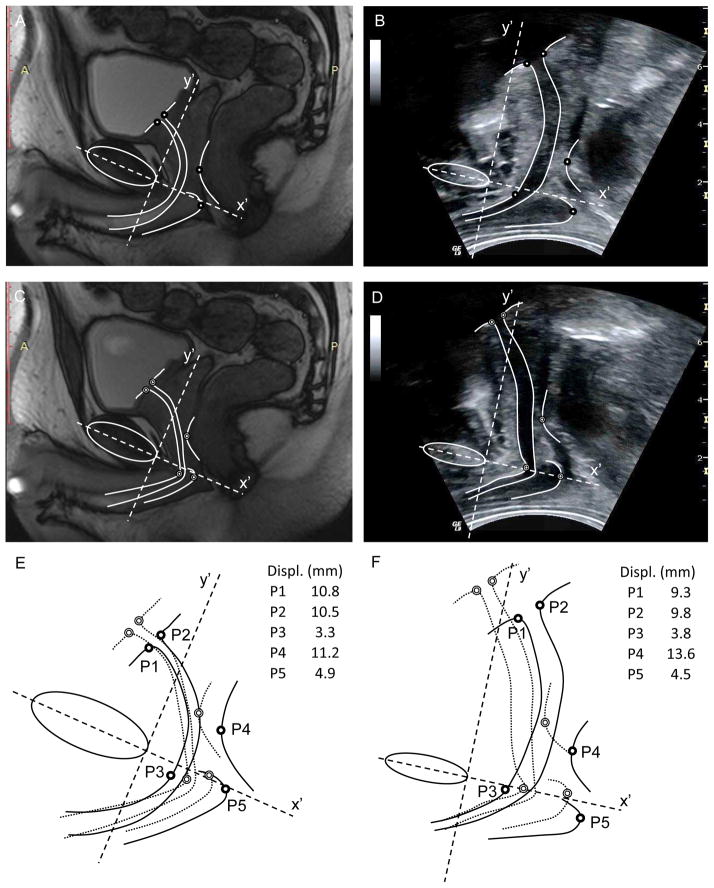
Displacement data calculated from ultrasound and MRI images with the same analysis method applied to both images are both presented for participant #8 in Table 1 for comparison. Displacements calculated from MRI and US imaging at each anatomical point (P1–P5) were both averaged over three repetitions.
Table 1.
Mean displacement of each pelvic floor structure with maximal voluntary contraction
| UVJ (ventral) (mm) | UVJ (dorsal) (mm) | ARJ (mm) | BU (mm) | SUS (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Subject | day 1 | day 2 | day 1 | day 2 | day 1 | day 2 | day 1 | day 2 | day 1 | day 2 |
| 1 | 20.6 | 18.2 | 27.4 | 25.1 | 21.6 | 17.6 | 13.9 | 12.2 | 1.5 | 1.6 |
| 2 | 12.9 | 15.8 | 11.2 | 13.4 | 12.3 | 11.1 | 7.4 | 8.1 | 1.8 | 2.1 |
| 3 | 11.6 | 8.9 | 13.2 | 9.9 | 13.5 | 12.6 | 3.5 | 3.7 | 2.5 | 2.6 |
| 4 | 11.2 | 9.1 | 11.8 | 9.6 | 12.3 | 12.9 | 5.7 | 4.6 | 3.6 | 4.5 |
| 5 | 5.8 | 5.3 | 5.9 | 6.3 | 8.7 | 7.3 | 3.3 | 4.6 | 5.2 | 5.1 |
| 6 | 4.6 | 5.2 | 3.2 | 5.7 | 4.9 | 5.7 | 4.1 | 3.0 | 4.6 | 4.4 |
| 7 | 12.0 | 11.7 | 10.7 | 10.3 | 10.1 | 9.6 | 0.2 | 0.4 | 1.4 | 1.6 |
| 8* | 10.8(9.3) | 11.2 | 10.5(9.8) | 10.5 | 11.2(13.6) | 13.3 | 4.9(4.5) | 7.2 | 3.3(3.8) | 3.6 |
| 9 | 8.2 | 6.9 | 8.0 | 5.5 | 8.9 | 7.3 | 2.2 | 5.9 | 3.1 | 3.1 |
| 10 | 17.1 | 17.5 | 18.7 | 17.2 | 18.1 | 15.7 | 16.5 | 11.6 | 1.0 | 0.7 |
|
| ||||||||||
| ICC (2,1) (SEM) | 0.88 (1.8) | 0.86 (2.6) | 0.83 (2.3) | 0.82 (2.2) | 0.95 (0.3) | |||||
|
| ||||||||||
| ICC (2,2) (SEM) | 0.95 (1.1) | 0.94 (1.6) | 0.93 (1.2) | 0.85 (1.7) | 0.97 (0.3) | |||||
|
| ||||||||||
| ICC (2,3) (SEM) | 0.94 (1.2) | 0.95 (1.4) | 0.92 (1.2) | 0.86 (1.7) | 0.97 (0.2) | |||||
Denotes subject that participated in an additional magnetic resonance imaging investigation. Results of this investigation shown in parentheses.
UVJ – urethra-vesical junction, ARJ – ano-rectal junction, BU – bulb of the penis, SUS – striated urethral sphincter
ICC – intra-class correlation, SEM – standard error of the measure
3. Results
Displacements of the five anatomical points closely matched our predictions of movement based on anatomical/biomechanical considerations. Figure 3 shows the direction of motion from a representative participant and the range for the group. Intra-class correlation coefficients for between days ranged from 0.82 to 0.95 for ICC (2,1), 0.85 to 0.97 for ICC (2,2) and 0.86 to 0.97 for ICC (2,3). Although the repeatability between days of most measures was “good” (0.8–0.9) when a single measure was analysed, the repeatability of all measures, except BU, was “high” when two or three images were used. ICC and displacement data are shown in Table 1. Displacement data measured from US and MRI from participant #8 show values of similar order of magnitude (Table 1) with comparison of the images shown in Figure 4.
Fig. 3.
Pelvic structures are shown for a relaxed image with points used for displacement analyses highlighted (P1–P5). Dark circles indicate resting position and light circles indicate position during maximal voluntary contraction. Arrows highlight the direction and amplitude of movement of each point of interest for a representative participant (same participant as Fig. 2). Range of displacements for the group is shown for each measure.
Fig. 4.
MRI (A, C) and transperineal ultrasound (B, D) images from participant #8 in the (A, B) relaxed and (C, D) contracted states. (C, D) Borders and points of interest superimposed on the US images. (E, F) Overlaid borders of the two images; dark circles indicate the points of interest in the relaxed state and light circles from the contracted state.
4. Comment
The method described here calculates displacement of pelvic structures that have been validated for females (UVJ and ARJ movement) in addition to two new measures (displacement of the mid-urethra related to SUS and BU movement) with potential to influence male pelvic floor function. Although displacement of a single pelvic structure from US imaging has been described in men,14 the combination of displacement measures demonstrated here has potential to provide a more complete understanding of male pelvic floor function with high utility for assessment of function. Reliability analysis demonstrated high ICC values for all analyses between days, and displacement measured from transperineal US images were similar to those measured from MRI for one participant. Further validation should include concurrent measurement of pressure and electromyographic recordings to test the relationship between the observed displacements, activation of the muscles proposed to be related to the generation of the motion and the relationship to urethral pressure changes. Limitations include the use of a single operator, potential variation in force used to hold the probe against the perineum, and a modest sample size, however, none of these issues compromised the repeatability of the measures.
Although the reliability demonstrated in the present study from single measures (first repetition) were “good” between days (ICC (2,1)), the ICC values indicated “high” repeatability (>0.9) with lower typical error when data were averaged over 2 trials. There was no additional benefit from averaging over three images, at least when the measures were made by an experienced examiner.
Identification of anatomical landmarks was facilitated by the use of the cine-loop. Evaluation of this dynamic recording enabled unambiguous identification of structures in both relaxed and contracted-state frames, enabled selection of an optimal frame in which all of the structures could be clearly identified, and reduced the potential for experimenter error when attempting to freeze the image during the data collection at the point of maximal displacement.
Conclusions
The new method allows concurrent investigation of displacements of points previously validated for females in addition to new measures (SUS and BC) with potential to influence male pelvic floor function.
Acknowledgments
Funding for this study was provided by the National Health and Medical Research Council of Australia and the Australian Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peng Q, et al. 2D Ultrasound image processing in identifying responses of urogenital structures to pelvic floor muscle activity. Annals of Biomedical Engineering. 2006;34:477–93. doi: 10.1007/s10439-005-9059-3. [DOI] [PubMed] [Google Scholar]
- 2.Constantinou CE. Dynamics of female pelvic floor function using urodynamics, ultrasound and Magnetic Resonance Imaging (MRI) European Journal of Obstetrics & Gynecology and Reproductive Biology. 2009;144:S159–S65. doi: 10.1016/j.ejogrb.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constantinou CE, et al. Displacement sequence and elastic properties of anterior prostate/urethral interface during micturition of spinal cord injured men. Ultrasound in Medicine and Biology. 2002;28:1157–63. doi: 10.1016/s0301-5629(02)00505-7. [DOI] [PubMed] [Google Scholar]
- 4.Strasser H, et al. Transurethral ultrasound: evaluation of anatomy and function of the rhabdosphincter of the male urethra. J Urol. 1998;159:100–4. doi: 10.1016/s0022-5347(01)64025-4. discussion 04–5. [DOI] [PubMed] [Google Scholar]
- 5.Strasser H, et al. Three-dimensional transrectal ultrasound of the male urethral rhabdosphincter. World J Urol. 2004;22:335–8. doi: 10.1007/s00345-004-0416-x. [DOI] [PubMed] [Google Scholar]
- 6.Davis SN, et al. Use of pelvic floor ultrasound to assess pelvic floor muscle function in Urological Chronic Pelvic Pain Syndrome in men. J Sex Med. 2011;8:3173–80. doi: 10.1111/j.1743-6109.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- 7.Strasser H, et al. Anatomy and innervation of the rhabdosphincter of the male urethra. Prostate. 1996;28:24–31. doi: 10.1002/(SICI)1097-0045(199601)28:1<24::AID-PROS4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Lovegrove Jones RC, et al. Mechanisms of pelvic floor muscle function and the effect on the urethra during a cough. European Urology. 2010;57:1101–10. doi: 10.1016/j.eururo.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorey G. Are erectile and ejaculatory dysfunction associated with postmicturition dribble? Urologic nursing. 2003;23:42–5. 48–52. [PubMed] [Google Scholar]
- 10.Shrout PE, et al. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins WG. Measures of reliability in sports medicine and science. Sports Medicine. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson G, et al. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–38. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Vincent WJ. Statistics in kinesiology. 3. Champaign, IL: Human Kinetics; 2005. pp. xvipp. 311pp. 194–197. [Google Scholar]
- 14.Nahon I, et al. Assessing Muscle Function of the Male Pelvic Floor using Real Time Ultrasound. Neurourology and Urodynamics. 2011;30:1329–32. doi: 10.1002/nau.21069. [DOI] [PubMed] [Google Scholar]