Abstract
Objective
The aims of this study were to implement a patient-administered checklist designed to identify endometrial cancer patients at elevated risk for Lynch syndrome; measure subsequent genetic counseling and testing; and identify differences between those who attended genetic counseling and those who did not.
Methods
We developed a 4-item yes/no checklist of personal and family history risk factors for Lynch syndrome-associated endometrial cancer and recommended referral for genetic counseling for patients meeting any of the criteria. Retrospective chart review was performed to determine subsequent genetic counseling and testing outcomes over a 15 month period.
Results
6/387 (1.6%) endometrial cancer patients tested positive for a Lynch syndrome mutation. 4/24 (17%) of endometrial cancer patients who met referral criteria and attended genetic counseling tested positive. 38/70 (55%) of patients who met referral criteria were not seen for genetic counseling. Patients who were diagnosed with endometrial cancer at younger ages, who had primary surgery at our institution, or who met more than one referral criteria were more likely to be seen for genetic counseling.
Conclusions
Endometrial cancer patients who met referral criteria and attended genetic counseling comprised a population enriched for Lynch syndrome. This approach allowed Lynch syndrome evaluation resources to be targeted to a population of patients that is high risk and interested in the information. The referral rate of at-risk patients needs to be improved, and allocating resources towards this goal could increase the identification of Lynch syndrome while avoiding some of the pitfalls of universal screening.
Introduction
The average lifetime risk of endometrial cancer in the United States is 2.6%, making it the most common invasive gynecologic cancer.[1] A small but significant percentage (about 2%–3%) of endometrial cancer is attributable to Lynch syndrome, a hereditary cancer predisposition syndrome that significantly increases risk of colorectal, endometrial, and other cancers. The identification of Lynch syndrome in an endometrial cancer patient has important benefits both for her and for at-risk relatives, who can take advantage of cancer risk reduction strategies.
The optimal way to screen colorectal and endometrial cancer patients for Lynch syndrome is an area of active discussion. Universal immunohistochemistry (IHC) and/or microsatellite instability analysis (MSI) of colorectal cancers has been adopted by many NCI-designated comprehensive cancer centers[4]. The EGAPP working group concluded that there is moderate certainty that assessing all newly diagnosed colorectal cancer patients for Lynch syndrome with a series of genetic tests (including tumor studies) would provide moderate population benefit via at-risk relatives undergoing increased screening and thus decreasing their cancer risk[5]. Some advocate for universal screening of all endometrial cancer patients by IHC and/or MSI. Hampel et al. found the prevalence of Lynch syndrome in a general population sample of 562 endometrial cancer patients to be 2.3% (95% CI 1.3% – 4.0%), and advocate for universal screening as a feasible approach[6, 7]. Moline et al. recently described their experiences implementing endometrial tumor MSI/IHC screening at a single institution through a stepwise process culminating in universal screening, and report that they found universal screening to be a practical approach.[8]
Universal screening has the clear advantage of the potential to detect all Lynch syndrome - associated endometrial cancers. However, universal screening also has costs and limitations. While offering BRCA1 and BRCA2 genetic testing to all high grade serous ovarian cancer patients has gained wide acceptance in light of the relatively high (15% – 22%) prevalence of mutations in this population[9–13], the Lynch syndrome prevalence of 2%–3% in unselected endometrial and colorectal cancer patients leaves room for debate. There are the obvious direct costs of the tumor studies themselves; a universal approach means that a lot of tumor studies are done for a relatively small increase in yield as compared to targeting a high-risk population. A recent study of universal tumor mismatch repair screening in colorectal cancer probands reported that 78/82 mutations would have been found using a selective approach, and in order to identify the 4 missed mutations, an additional 1277 tumor studies needed to be done[14].
An under-recognized limitation of universal tumor MSI/IHC is that, as with all screening tests, the positive predictive value depends on the prevalence of the condition in the population being screened[15]. When tumor studies are performed in a high-risk population, the positive predictive value (as measured by identification of a pathogenic germline mutation that confirms Lynch syndrome and allows for the predictive genetic testing of relatives from which much of the benefit of identifying Lynch syndrome accrues) is quite high. Positive predictive values of 82% – 85% have been reported for tumor studies performed in high risk colorectal and endometrial cancer populations[16–18]. In contrast, the yield of positive germline genetic test results from recent studies of tumor screening applied to broader populations has been disappointingly low, ranging from 29% – 40%, even though these studies first ruled out the known sporadic causes of MSI-H tumors (MLH1 promoter hypermethylation and/or presence of BRAF mutation)[8, 19–21]. In other words, it is more likely that these patients with abnormal tumor studies will receive an uninformative germline genetic test result, than a positive result. Whether the population of patients with abnormal tumor studies and no germline mutation identified have cryptic Lynch syndrome mutations, a different hereditary syndrome, other somatic causes for the MSI-H phenotype, or a combination of these, is not yet known, and this poses a significant clinical challenge in managing both patients and their possibly at-risk relatives[21, 22].
An alternative to universal screening is to target Lynch syndrome evaluation to endometrial cancer patients with personal and/or family histories suggestive of Lynch syndrome. Any such initial screen will necessarily result in missing some Lynch syndrome cases, given the variable presentation of the syndrome as well as reports of Lynch syndrome identified in endometrial cancer patients with no suggestive personal or family history[6, 8]. However, this must be weighed against the costs and limitations of universal screening outlined above, particularly in the context of endometrial cancer where there are not established prognostic or therapeutic implications of the MSI-H phenotype[23] as there are for colorectal cancer[24].
Given the costs and limitation of universal screening, we chose to study a targeted screening approach. The purpose of our study was to implement a simple, patient-administered checklist designed to identify a sub-population of endometrial cancer patients at elevated risk for Lynch syndrome, among those presenting for management at a gynecologic oncology clinic; measure subsequent genetic counseling and testing; and identify differences between those who attended genetic counseling and those who did not.
Methods
We developed a 4-item yes/no checklist of risk factors for Lynch syndrome-associated endometrial cancer (Figure 1). We used the SGO Lynch syndrome referral guidelines as a starting point[25], then modified them to be specific to patients with endometrial cancer and also to simplify and broaden them. We purposefully avoided using previously established, more stringent criteria such as the revised Amsterdam criteria[26], because they have already been shown to have low sensitivity[6]. We did not place any restriction on the patients’ age at diagnosis of endometrial cancer, since Lynch syndrome-associated endometrial cancer is often diagnosed after age 50.[6, 8, 27] We chose not to include Lynch syndrome cancers other than colorectal and endometrial, given their relative rarity versus how much more complex they would have made the checklist. We did continue to encourage clinicians to refer based on clinical judgment even when the screening criteria were not met (for example, a young non-obese woman with no family history, or a woman with a family history of multiple cancers).
Figure 1.

Lynch syndrome screening questions for endometrial cancer patients
The patient completed this checklist as part of the routine paperwork for their first visit to the clinic. The checklist form also asked whether patients had already undergone Lynch syndrome genetic testing. Patients answering “yes” to at least one of the four items were considered at risk. Clinicians were encouraged to discuss genetic counseling referral with their at-risk patients. In addition, a one page patient education document regarding genetic counseling referral was developed and routinely distributed to all at-risk patients; this document also includes information about accessing local genetic counseling, as many of our patients are not local to our institution. Lynch syndrome evaluation (generally beginning with tumor studies) was undertaken only for patients who presented for genetic counseling and agreed to undergo Lynch syndrome testing. Genetic counseling services are provided within our gynecologic oncology clinic and appointments are generally coordinated with other followup visits to the clinic, in order to increase patient convenience.
Medical records were reviewed for patients seen during a 15 month period during which the checklist was administered (September 2009 – December 2010), to determine whether patients subsequently received genetic counseling and testing, as well as for demographics. Answers to the screening questions were also abstracted from the medical record, so that this could be compared to patient responses. Statistical comparisons between patients attending genetic counseling and those who did not were performed using Fishers exact test for discrete variables and 2 sample t-tests for continuous variables. The study was approved by our institutional review board.
Results
During the study time period, 387 endometrial cancer patients were seen for consultation through our gynecologic oncology center. Demographics are summarized in Table 1; the majority of patients were not local to our institution and/or did not have their primary endometrial cancer surgery at our institution. 91% of patients showed concordance between screening positive or negative for genetic counseling referral based on their checklist answers, as compared to answers to the screening questions derived from their medical records.
Table 1.
Study population demographics
| Mean (SD) | Range | |
|---|---|---|
| Age at endometrial cancer diagnosis | 58.59 (11.78) | 18 – 89 |
| BMI | 32.08 (9.61) | 16.24 – 77.3 |
| N | % | |
| Race | ||
| Asian | 15 | 4% |
| Black | 41 | 11% |
| Hispanic | 54 | 14% |
| White | 270 | 70% |
| Other | 7 | 2% |
| Local (lives within 50 miles) | ||
| No | 289 | 75% |
| Yes | 98 | 25% |
| Primary Surgery at this institution | ||
| No | 243 | 63% |
| Yes | 144 | 37% |
| Endometrial Cancer Grade | ||
| 1 | 55 | 14% |
| 2 | 167 | 44% |
| 3 | 161 | 42% |
| Unknown | 4 | |
| Endometrial Cancer Stage | ||
| 1 | 157 | 51% |
| 2 | 25 | 8% |
| 3 | 78 | 26% |
| 4 | 46 | 15% |
| Unknown | 81 | |
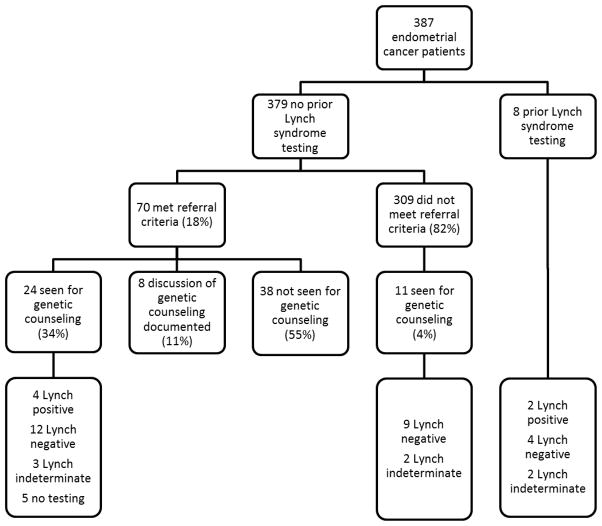
Figure 2 describes the screening, genetic counseling, and genetic testing outcomes for the study population. Eight patients had prior Lynch syndrome genetic testing at another institution. Of the remaining 379, 18% met our referral criteria, and of those, 34% received genetic counseling at our institution. An additional 11% did not receive genetic counseling at our institution, but there was clear documentation that Lynch syndrome and the option of genetic counseling was discussed with the patient. The remaining 55% of patients meeting referral criteria were neither seen for genetic counseling at our institution, nor had written documentation of discussion of genetic counseling referral.
Figure 2.
Screening, genetic counseling, and genetic testing outcomes for endometrial cancer patients
4/24 (17%) of at-risk patients presenting for genetic counseling at our institution tested positive for Lynch syndrome. 4% of patients not meeting referral criteria were also seen for genetic counseling, and none tested positive for a Lynch syndrome mutation. Table 2 specifies how many patients met which referral criteria; 77% met one criteria and 23% met more than one. Of the total 6 patients who tested positive for Lynch syndrome, 4 had MSH2 mutations, 1 had an MLH1 mutation, and 1 had a PMS2 mutation. Table 2 also specifies how many patients meeting each criteria were seen for genetic counseling, as well as how many tested positive. Patients were significantly more likely to test positive if they met more than one screening criteria, or if they had a family history of Lynch syndrome mutation. In addition, we compared demographics between patients who tested positive vs. negative, and found that mean age at diagnosis was significantly younger in those that tested positive (44.5 Lynch positive vs. 55.6 Lynch negative, p = 0.0282). No other significant demographic differences were found.
Table 2.
Genetic counseling referral criteria summary
| N (%) | Seen for genetic counseling | Lynch positive | |
|---|---|---|---|
| Met criteria overall | 70 | 24 | 4 |
| Personal history colorectal cancer | 5 (7%) | 4 | 1 |
| First degree relative with colorectal or uterine cancer | 53 (76%) | 18 | 3 |
| Relative with colorectal cancer or uterine cancer under 50 | 28 (40%) | 12 | 4 |
| Family history Lynch syndrome mutation * | 3 (4%) | 3 | 3 |
| Met only one criteria ** | 54 (77%) | 14 | 0 |
| Met more than one criteria ** | 16 (23%) | 10 | 4 |
Significantly more likely to test positive if this criteria met (p=0.0071)
Significantly more likely to test positive if more than one vs. only one criteria met (p=0.0192)
An additional 7 patients had indeterminate Lynch syndrome results, in that they were either found to have a variant of uncertain significance and/or had tumor studies suggestive of Lynch syndrome and no clearly deleterious germline mutation identified. 5 patients who met referral criteria were seen for genetic counseling and did not undergo any molecular Lynch syndrome evaluation (neither tumor studies nor genetic testing), due to full risk assessment indicating relatively low risk of Lynch syndrome, patient/family not interested in the information, concerns regarding cost, or a combination of these factors.
Table 3 compares the characteristics of patients who met referral criteria and attended genetic counseling, to those of patients who met referral criteria and did not attend genetic counseling. Race and BMI did not differ significantly with regard to attendance at genetic counseling. Patients who attended genetic counseling were significantly younger at diagnosis (mean age 53 for those who attended vs. 59 for those who did not, p = 0.042), were more likely to have had primary surgery at our institution (p = 0.035), and were more likely to meet more than one referral criteria (p = 0.0090). 8/15 (53%) local patients attended genetic counseling and 16/55 (29%) non-local patients attended genetic counseling; this difference is not statistically significant.
Table 3.
Comparison of endometrial cancer patients seen for genetic counseling vs. those not seen
| Seen | Not seen | P value | |
|---|---|---|---|
| N | 24 | 46 | |
| Mean age (SD) at endometrial cancer diagnosis | 53.3 (12.7) | 58.5 (11.3) | 0.042 |
| Mean BMI (SD) | 33.7 (8.6) | 30.2 (9.9) | 0.93 |
| Race | 0.11 | ||
| Asian | 1 | 0 | |
| Black | 3 | 5 | |
| Hispanic | 5 | 4 | |
| White | 14 | 37 | |
| Other | 1 | 0 | |
| Local (lives within 50 miles) | 0.076 | ||
| No | 16 | 39 | |
| Yes | 8 | 7 | |
| Primary surgery at this institution | 0.035 | ||
| No | 10 | 31 | |
| Yes | 14 | 15 | |
| Met referral criteria | 0.0090 | ||
| One | 14 | 40 | |
| More than one | 10 | 6 | |
Discussion
At least 6/387 (1.6%) of endometrial cancer patients presenting to our institution during the study period had Lynch syndrome. The subset of endometrial cancer patients meeting referral criteria and subsequently presenting for genetic counseling was enriched for Lynch syndrome, with 4/24 (17%) testing positive. Of all patients who met referral criteria (without reference to whether they presented for genetic counseling and testing or not), 4/70 (5.7%) tested positive. Patients’ answers to the screening questions were highly reflective of answers derived from their medical records but were not 100% concordant; clinician review of patient-supplied answers is therefore recommended.
Patients who had primary surgery at our institution were more likely to have been seen by us for genetic counseling; this group of patients likely had multiple return visits to our institution and therefore more opportunities to be referred and seen for genetic counseling. This difference suggests that ease of access plays a role in whether patients attend genetic counseling, underscoring the importance of maximizing access to genetic counseling in diverse practice settings. Patients diagnosed at younger ages or meeting more than one referral criteria were more likely to attend genetic counseling, implying that the patients at highest risk for Lynch syndrome were more likely to attend genetic counseling. Among those patients who underwent Lynch syndrome testing, those who were younger at endometrial cancer diagnosis, met more than one referral criteria, or had a family history of a Lynch syndrome mutation, were more likely to test positive, consistent with established hereditary cancer risk factors.
We could not find documentation of attendance at genetic counseling or discussion of the option of genetic counseling for 55% of at-risk patients. Possible reasons for non-attendance at genetic counseling include that the referral was not discussed with the patient, the patient declined referral, the patient was overwhelmed by coping with their cancer at the time the referral was suggested, and/or the patient subsequently received genetic counseling elsewhere. Similarly, 57% referral to genetic counseling was reported by Backes et al. for endometrial cancer patients with abnormal tumor studies identified through universal screening[28]. Moline et al. reported a higher rate of genetic counseling referral of endometrial cancer patients with abnormal tumor studies, with a high level of genetic counselor involvement in communicating the tumor study results to patients and providers[8]. Improving the genetic counseling referral rate for high risk patients is an important goal that will require the provision of additional personnel and training resources, and could be facilitated through harnessing the potential of electronic health records to capture and assess family history information.
5/24 (21%) of at-risk endometrial cancer patients presenting for genetic counseling did not undergo any Lynch syndrome testing, for a variety of reasons. Similarly, Backes et al. also found that some endometrial cancer patients with abnormal tumor studies via universal screening actively declined further analysis[28]. This suggests that not all at-risk endometrial cancer patients desire Lynch syndrome evaluation, which impacts both the yield and acceptability of universal screening.
Given the low prevalence of Lynch syndrome in unselected endometrial cancer patients, the high incremental cost per additional mutation found by universal screening, and the high rate of indeterminate results when tumor screening studies are applied to an unselected population, we believe that a well-designed and well-implemented program of tumor screening studies targeted to a high risk endometrial cancer patient population who has received pretest genetic counseling could be a viable and perhaps even superior alternative to universal screening. This is also supported by a cost-effectiveness analysis performed by our group, which found IHC triage of women with endometrial cancer at any age who have at least one first degree relative with a Lynch-associated cancer to be cost-effective, while a universal screening strategy had a much less favorable incremental cost-effectiveness ratio[29]. While the cost-benefit analysis of universal tumor screening for colorectal cancer may differ from that for endometrial cancer given the possibility of treatment and prognostic implications of the MSI-H tumor phenotype, it is also worth noting that neither EGAPP nor a recent cost-effectiveness analysis compared universal colorectal cancer tumor screening to a family history-based approach[5, 30]. Rather, both dismissed a family history-based approach at the outset. While it would take resources to successfully collect and interpret family history information and maximize referral of at-risk patients, so too would the performance and follow-up of universal tumor studies.
As the landscape of cancer genetics continues to evolve, the question of how best to identify endometrial cancer patients at risk for Lynch syndrome should continue to be revisited. For example, work is ongoing to identify other causes for the MSI-H phenotype. Sourrouille et al. were able to identify double somatic hits and somatic mosaicism in some colorectal cancer patients with abnormal tumor studies but normal genetic test results[31]. If additional clinical tests become available that could clarify the status of patients who are tumor study positive but germline negative, this could alter the risk-benefit ratio of universal screening. If Lynch syndrome genetic testing drops enough in price such that it could be done as the first step instead of tumor studies, this would also prompt re-evaluation of the best approach.
Conclusion
Endometrial cancer patients screening positive on our checklist and subsequently presenting for genetic counseling comprised a population enriched for Lynch syndrome mutations. This approach allowed Lynch syndrome evaluation resources to be targeted to a population of patients that is both high risk and expressed interest in receiving the information. The referral rate of at-risk patients needs to be improved, and allocating resources towards this goal could increase the identification of Lynch syndrome while avoiding some of the pitfalls of universal screening.
Footnotes
Conflict of interest statement: The authors have no relevant financial relationships to disclose.
References
- 1.National Cancer Institute. Endometrial Cancer Prevention (PDQ) February 15, 2013 August 1, 2013 Available from: http://www.cancer.gov/cancertopics/pdq/prevention/endometrial/HealthProfessional.
- 2.Buchanan EM, Weinstein LC, Hillson C. Endometrial cancer. Am Fam Physician. 2009;80(10):1075–80. [PubMed] [Google Scholar]
- 3.Pfeiffer RM, et al. Risk Prediction for Breast, Endometrial, and Ovarian Cancer in White Women Aged 50 y or Older: Derivation and Validation from Population-Based Cohort Studies. PLoS Med. 2013;10(7):e1001492. doi: 10.1371/journal.pmed.1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beamer LC, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(10):1058–63. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampel H, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66(15):7810–7. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 7.Hampel H, et al. Comment on: Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2007;67(19):9603. doi: 10.1158/0008-5472.CAN-07-2308. [DOI] [PubMed] [Google Scholar]
- 8.Moline J, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecologic oncology. 2013;130(1):121–6. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Pal T, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 10.Risch HA, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121(2):353–7. doi: 10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Alsop K, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(21):2654–63. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreira L, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA : the journal of the American Medical Association. 2012;308(15):1555–65. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennekens CH, Buring JE, Mayrent SL. Epidemiology in medicine. 1. Boston: Little, Brown; 1987. p. xv.p. 383. [Google Scholar]
- 16.Lu KH, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25(33):5158–64. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 17.Poynter JN, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer epidemiology biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2008;17(11):3208–15. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stormorken AT, et al. Immunohistochemistry identifies carriers of mismatch repair gene defects causing hereditary nonpolyposis colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(21):4705–12. doi: 10.1200/JCO.2005.05.180. [DOI] [PubMed] [Google Scholar]
- 19.Heald B, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(10):1336–40. doi: 10.1200/JCO.2012.45.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward RL, Hicks S, Hawkins NJ. Population-based molecular screening for lynch syndrome: implications for personalized medicine. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(20):2554–62. doi: 10.1200/JCO.2012.46.8454. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Soler M, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144(5):926–932. e1. doi: 10.1053/j.gastro.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 22.Boland CR. The mystery of mismatch repair deficiency: lynch or lynch-like? Gastroenterology. 2013;144(5):868–70. doi: 10.1053/j.gastro.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karamurzin Y, Rutgers JK. DNA mismatch repair deficiency in endometrial carcinoma. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2009;28(3):239–55. doi: 10.1097/PGP.0b013e31818d8fe6. [DOI] [PubMed] [Google Scholar]
- 24.Legolvan MP, Taliano RJ, Resnick MB. Application of molecular techniques in the diagnosis, prognosis and management of patients with colorectal cancer: a practical approach. Human pathology. 2012;43(8):1157–68. doi: 10.1016/j.humpath.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Lancaster JM, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecologic oncology. 2007;107(2):159–62. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Vasen HF, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 27.Leenen CH, et al. Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer </= 70 years. Gynecologic oncology. 2012;125(2):414–20. doi: 10.1016/j.ygyno.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Backes FJ, et al. Endometrial cancer patients and compliance with genetic counseling: room for improvement. Gynecologic oncology. 2011;123(3):532–6. doi: 10.1016/j.ygyno.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Kwon JS, et al. Testing women with endometrial cancer to detect Lynch syndrome. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(16):2247–52. doi: 10.1200/JCO.2010.32.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mvundura M, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genetics in medicine : official journal of the American College of Medical Genetics. 2010;12(2):93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 31.Sourrouille I, et al. Somatic mosaicism and double somatic hits can lead to MSI colorectal tumors. Familial Cancer. 2013;12(1):27–33. doi: 10.1007/s10689-012-9568-9. [DOI] [PubMed] [Google Scholar]