Abstract
AIM: To explore the efficacy of PCI-24781, a broad-spectrum, hydroxamic acid-derived histone deacetylase inhibitor, in the treatment of gastric cancer (GC).
METHODS: With or without treatment of PCI-24781 and/or cis-diamminedichloroplatinum (CDDP), GC cell lines were subjected to functional analysis, including cell growth, apoptosis and clonogenic assays. Chromatin immunoprecipitation and luciferase reporter assays were used to determine the interacting molecules and the activity of the enzyme. An in vivo study was carried out in GC xenograft mice. Cell culture-based assays were represented as mean ± SD. ANOVA tests were used to assess differences across groups. All pairwise comparisons between tumor weights among treatment groups were made using the Tukey-Kramer method for multiple comparison adjustment to control experimental-wise type I error rates. Significance was set at P < 0.05.
RESULTS: PCI-24781 significantly reduced the growth of the GC cells, enhanced cell apoptosis and suppressed clonogenicity, and these effects synergized with the effects of CDDP. PCI-24781 modulated the cell cycle and significantly reduced the expression of RAD51, which is related to homologous recombination. Depletion of RAD51 augmented the biological functions of PCI-24781, CDDP and the combination treatment, whereas overexpressing RAD51 had the opposite effects. Increased binding of the transcription suppressor E2F4 on the RAD51 promoter appeared to play a major role in these processes. Furthermore, significant suppression of tumor growth and weight in vivo was obtained following PCI-24781 treatment, which synergized with the anticancer effect of CDDP.
CONCLUSION: These data suggest that RAD51 potentiates the synergistic effects of chemotherapy with PCI-24781 and CDDP on GC.
Keywords: Chemotherapy, Combination, Gastric cancer, Histone deacetylase inhibitor, Homologous recombination
Core tip: This is the first study to show that PCI-24781 synergizes with the chemotherapeutic effect of cis-diamminedichloroplatinum in gastric cancer in vivo and in vitro, and PCI-24781-induced RAD51 repression may be one of the mechanisms. PCI-24781 could be a potential drug and novel therapeutic strategy for the treatment of gastric cancer.
INTRODUCTION
Gastric cancer (GC) is one of the most aggressive malignancies, especially in South Asia. It ranks as the second leading cause of cancer mortality in China[1]. Despite curative surgery and postoperative adjuvant therapy, nearly 60% of patients succumb to the disease[2,3]. While the utility of classical chemotherapy agents has been thoroughly explored, advances have been slow, and the efficacy of these agents has reached a plateau[4]. Recent studies have suggested that histone deacetylase inhibitors may be attractive anticancer drugs because histone deacetylases (HDACs) are frequently upregulated in cancers, and these drugs can be less toxic to patients[5-7]. Among these HDAC inhibitors (HDACis), suberoylanilide hydroxamic acid (SAHA, Vorinostat) was first approved by the US Food and Drug Administration for the treatment of T-cell lymphoma. In vitro chemosensitivity of gastric adenocarcinomas to SAHA has been reported[8]. Similarly, PCI-24781 (Pharmacyclics, Inc.), a broad-spectrum, hydroxamic acid-derived HDACi currently being evaluated in phase I clinical trials[9], has shown significant anticancer activity in soft tissue sarcomas[10] and gallbladder carcinomas[11], as well as other tumor cell lines including colon carcinomas[12], glioblastomas[13], breast cancers[14], and bone sarcomas[15]. However, information about the efficacy of PCI-24781 in the treatment of gastric cancer is limited.
Despite HDACis showing promise as single agents, several recent studies have suggested that the optimal use of HDACis is likely in combination with other chemotherapeutic agents[10,15,16]. Cis-diamminedichloroplatinum (CDDP) is a classic chemotherapeutic drug frequently used in GC treatment. CDDP exerts its effect mainly by causing DNA damage. However, DNA damage can be repaired through homologous recombination (HR) or through non-homologous end joining[17], which can lead to chemotherapy resistance. HR usually occurs during and shortly after DNA replication during the S and G2 phases of the cell cycle, when sister chromatids are more easily available[18]. RAD51 is a key protein involved in and is regarded as a biomarker for homologous recombination[19]. Studies have suggested that RAD51, which highly correlates with GC[20], is involved in the repair of DNA double-strand breaks (DSBs) produced by CDDP and other platinum agents[21]. Adimoolam et al[22] also confirmed that PCI-24781 could decrease RAD51 expression and suppress HR in colon tumor cells. Thus, in this study we aimed to evaluate the efficacy of PCI-24781 on GC and its combinational effect of chemotherapy with CDDP and elucidate underlying mechanisms to find out whether RAD51 is involved in the process.
MATERIALS AND METHODS
Cell culture
The human GC cell line AGS (most common gastric adenocarcinoma), HGC27 (undifferentiated carcinoma with high malignancy) was obtained from the Committee of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). GC cell lines were cultured in RPMI 1640 medium (Gibco), supplemented with 10% fetal calf serum (HyClone, Logan, UT, United States) at 37 °C under a humidified atmosphere of 5% CO2. The HDAC inhibitor PCI-24781 was purchased from Pharmacyclics and dissolved in DMSO to create stock solutions.
Measurement of cell growth
Cell growth assays were done using CellTiter96 cell proliferation assay kit (Promega)[23]. Cells were plated at concentrations of 2 × 103 cells per well in 96-well plates. The next day, cells were treated with either 0.1% DMSO as control or different concentrations of PCI-24781 or/and CDDP for 48 h. Absorbance was measured at a wave length of 490 nm. Drug concentrations required to inhibit cell growth by 50% (IC50) were determined by interpolation of dose-response curves. Isobologram analysis was introduced to evaluate the synergistic effect[24]. Briefly, when the combination is synergistic, the data points from the combination will be depicted at the left side of the curve that generated by IC50 of two drugs, while the combination is antagonistic when these points are at the right side of the curve.
Apoptosis assay
Apoptosis was measured using flow cytometry (FCM) stained with Annexin V as well as Quantum Dot Probing cleaved Caspase-3[25]. As a standard, DMSO or drug treated cells were stained with Annexin V and Qdot conjugation before subjected to flow cytometry or fluorescent microscopy. Fluorescent positive rates were counted five randomly selective areas and analyzed independently by three reviewers.
Clonogenic assay
GC cells were treated in culture dishes with 0.1% DMSO (control), PCI-24781 (0.25 μmol/L), CDDP (2.5 μmol/L) or a combination of PCI-24781 plus CDDP for 24 h. One hundred cells per well were replated, then allowed to grow in corresponding drug-added media for 10 d, then stained with a 6% glutaraldehyde, 0.5% crystal violet solution for 30 min. Staining solution was decanted from each well and cells were washed with deionized H2O. Individual colonies retaining staining solution were counted.
Protein extraction and immunoblotting
The procedures were as previously described[26,27]. Briefly, frozen tissue samples were solubilized in lysis buffer, containing 7 mol/L urea, 2 mol/L thiourea, 2% CHAPS, 0.1 mol/L DTT, 0.1% NP40, 40 mmol/L Tris-HCl, using a polytron homogenizer following centrifugation (100000 g) for 30 min at 4 °C. For cell lines, lysates were harvested by centrifugation (12000 rpm) at 4 °C for 15 min. The supernatants were separated, and protein concentration was assessed by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). After heating for 10 min at 100 °C, equal amounts of denatured protein were resolved by 12% SDS-PAGE and electrophoretically transferred on to PVDF membranes. The blots were probed with primary antibodies. After completion, membranes were stripped and reprobed with β-actin antibody as a control. The bands were visualized using the enhanced chemiluminescence detection system (GE Healthcare, United States) and the intensity was quantified by densitometry.
Reverse transcription polymerase chain reaction and real-time quantitative polymerase chain reaction
The procedure was slightly modified according to our previous report[28]. The expression of mRNA in GC cells with different treatment was assessed with reverse transcription polymerase chain reaction (RT-PCR) using 0.5 μg of total RNA extracted by an RNeasy kit (Qiagen, Hilden, Germany). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was chosen as an endogenous standard. Primer sets were constructed by Invitrogen as follows: GAPDH Forward AATCCCATCACCATCTTCCAG and Reverse AAATGAGCCCCAGCCTTC. RAD51 Forward GGGAGAA TCACTTAAGCCTGG and Reverse CTGTTTACTTGCCCTCTGAAATG. Reaction conditions were: 1 cycle of 95 °C for 10 min, followed by 35 cycles at 95 °C for 1 min for denaturation, 55 °C for 1 min for annealing, 72 °C for 1 min for extension, and finally 1 cycle of 72 °C for 5 min. Gene-specific amplification was analyzed on 1% agarose gel and visualized by ethidium bromide staining method, and then performed in real-time quantitative PCR using a Light Cycler Real-time Detection System (Roche, Switzerland). All reactions were carried out with SYBR Green Master (Roche) according to the manufacturer’s protocol. Reaction conditions were similar to those described above, except that the first cycle of 95 °C was 3 min, followed by 45 cycles of each temperature for 30 s for each reaction. The Ct (threshold cycle) value of each sample was calculated, and the relative mRNA expression was normalized to the GAPDH value.
Manipulation of RAD51
The procedures were similar to those described previously[28]. Commercial siRNA targeting RAD51 reagent was used for knocking down its expression. For the overexpression system, human RAD51 cDNA was cloned into pcDNA3.1 (Invitrogen) to yield plasmid pcDNA-RAD51. Cells with or without transfection were subjected to treatment of DMSO, PCI-24781, DDP or combination. Then the expressions of RAD51 and cell apoptosis were evaluated by Western blotting or flow cytometry respectively.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays[29] were performed using ChIP assay kits (Upstate Technology) according to the instructions. Appropriate treated GC cells were fixed in 1% formaldehyde and incubated in 0.125 mol/L glycine to stop cross-linking, then washed and recovered, followed by lysis in SDS buffer. Lysates were sonicated, yielding genomic DNA fragments with a bulk size of 200-1000 bp before centrifugation. Supernatants were diluted and precleared with salmon sperm DNA/protein G-agarose. Lysates were immunoprecipitated with relevant antibodies. Antibody-nucleoprotein complex mixtures were incubated overnight and recovered by incubation with 60 μL salmon sperm DNA/protein G-agarose for 1 h at 4 °C. One hundred-microliter aliquots were reserved from negative control (no antibody) samples before washes; these aliquots were processed in parallel with eluted samples and used as input DNA. Beads were washed five times, and nucleoprotein complexes were eluted from protein G-agarose beads in immunoprecipitation elution buffer. Cross-links were reversed by adding 4 μL 5 mol/L NaCl and incubating overnight at 65 °C, followed by 1.5-h digestion with RNAse A and proteinase K at 50 °C. DNA fragments were recovered by phenol/chloroform extraction and ethanol precipitation and analyzed by qPCR.
Luciferase reporter assay
RAD51 promoter sequence (Entrez GeneID 5888, from -403 to +63 bp) was cloned into pGL3 and then transfected into HGC27 or AGS cells using Fugene 6 transfection reagent (Roche) according to the manufacturer’s instructions. The RAD51 reporter construct was further mutated at the E2F binding site using Stratagene Quick Change mutagenesis kit (Stratagene) per the manufacturer’s instruction and used for transfection as above. Cells were harvested after incubation with 0.1% DMSO or PCI-27481 (0.5 μmol/L) for 24 h. Luciferase assays were done using dual-luciferase assay reagents (Promega) and assessed with a luminometer.
Immunostaining analysis
For in vivo tumor samples, immunohistochemistry PCNA (Sigma) for cell proliferation and TUNEL assay (Sigma) for cell apoptosis were used. Staining distribution (% positive stained tumor cells) and intensity (0 = no staining, 1 = low, 2 = high) counts were evaluated and scored by three independent reviewers. Cells stained with FK2 and RAD51 antibodies after IR or drugs were examined by confocal laser scanning microscopy (CLSM). Cells stained with FK2 (Life technologies) and RAD51 (Santa Cruz Biotechnology) antibodies after ionizing radiation (IR) or drugs were examined by CLSM.
In vivo therapeutic studies
All animal procedures and care were approved by the Hospital Animal Care and Usage Committee according to NIH “Guide for the Care and Use of Laboratory Animals.” Animal models were utilized as previously described[30]. Viable HGC27 cells (1 × 106/0.1 mL HBSS/mouse) were injected into the flank (sc) of 6-wk-old female SCID mice (n = 40/experiment), growth was measured twice weekly. When average tumor volumes reached about 100 mm3, the mice were assigned to treatment of either vehicles (negative control, n = 8), PCI-24781 (50 mg/kg per day × 5 d/wk, ip; according to company recommendations and previous study[10]; n = 8), cis-diamminedic-hloroplatinum (10 mg/kg per day × 5 d/wk, ip; as a positive control[31,32]; n = 8). The mice were followed for tumor size and body weight and sacrificed 6 wk later. Tumors were resected, weighed, and frozen for detection of RAD51 expression or fixed in paraformaldehyde and paraffin-embedded for immunohistochemical studies.
Statistical analysis
Cell culture-based assays were represented as mean ± SD. ANOVA tests were used to assess differences across groups. Tumor volume was logarithmically transformed for further statistical analyses. A linear mixed model was used to assess the effect of treatment on tumor growth over time and a linear regression model for tumor weights. All pairwise comparisons between tumor weights among treatment groups were made using the Tukey-Kramer method for multiple comparison adjustment to control experimental-wise type I error rates. Significance was set at P < 0.05.
RESULTS
PCI-24781 suppressed human gastric cancer cell function, synergizing the effects of CDDP
The efficacy of PCI-24781 in the treatment of gastric cancer was evaluated by cell growth, apoptosis and clonogenic assays. GC cell growth was abrogated by pretreatment with PCI-24781 (IC50 = 0.35 μmol/L in HGC27, 0.31 μmol/L in AGS) or CDDP (IC50 = 8.00 μmol/L in HGC27, 7.28 μmol/L in AGS) in a dose-dependent manner (Figure 1A-D). Isobologram analysis revealed a synergistic effect when the two treatments were combined (Figure 1E, F). Furthermore, low dose treatment (0.2 μmol/L PCI-24781 and 2.5 μmol/L CDDP) increased the apoptotic cell ratio (Annexin V positive by FCM) from 14% ± 3.9% for PCI-24781 and 16% ± 2.5% for the CDDP to 50% ± 3.6% (Figure 2A). Quantum dot probing for cleaved caspase-3 also showed similar results (Figure 2B). In addition, the clonogenicity was dramatically impaired when cells were treated with the combination therapy (8 ± 2) compared to cells treated with PCI-24781 (20 ± 4.2), CDDP (22 ± 3.5) or untreated cells (57 ± 5.6) (Figure 2C, D).
Figure 1.
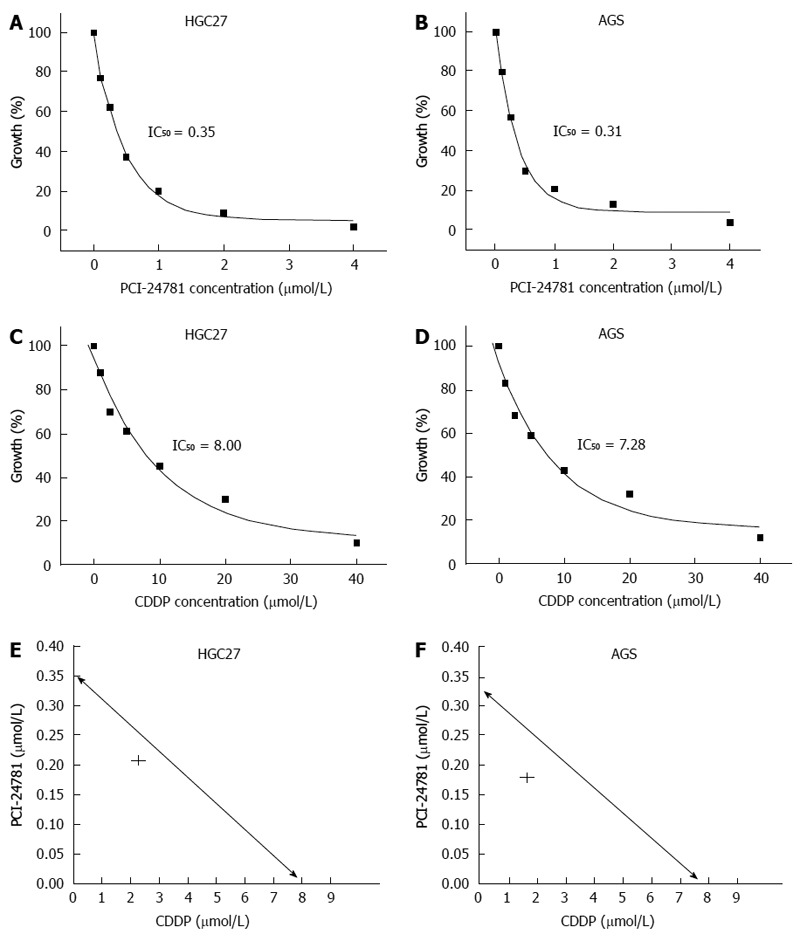
PCI-24781 suppressed gastric cancer cell growth and synergized with the effect of cis-diamminedichloroplatinum. The gastric cell lines HGC27 and AGS were plated at densities of 2 × 103 cells per well in 96-well plates and were subjected to cell growth assays. The cells were incubated with various concentrations of PCI-24781 and/or cis-diamminedichloroplatinum (CDDP) for 48 h, and the inhibition ratio was determined (IC50). Cell growth assays showed the significant cell growth inhibition of the GC cells in response to PCI-24781 (A, B) and CDDP (C, D) treatment. Isobologram analysis was used to evaluate the synergistic effect. The data point (IC50) from the combination treatment is shown on the left side of the curve and was generated by IC50 of the two drugs; this suggests that the combination treatment (concentration constant ratio PCI-24781:CDDP = 1:10) as synergistic (E, F).
Figure 2.
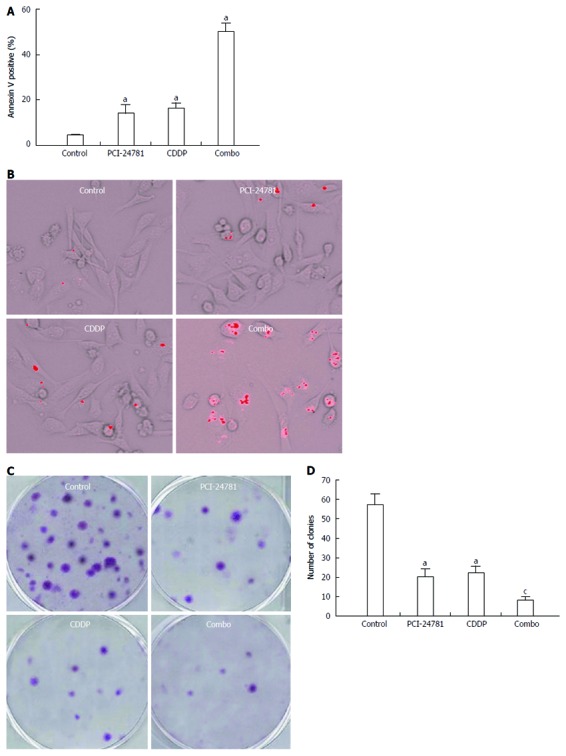
PCI-24781 synergizes with the cis-diamminedichloroplatinum-induced gastric cancer cell apoptosis and clonogenic inhibition. A, B: After appropriate treatment [PCI-24781, cis-diamminedichloroplatinum (CDDP) and combination] for 48 h, the apoptosis of the HGC27 cells was measured using flow cytometry of annexin V labeled cells (A) and the quantum dot analysis of cleaved caspase-3 (B). The number of apoptotic cells was remarkably increased in the PCI-24781- or CDDP-treated groups, with a synergistic effect in the combination treated group; C, D: PCI-24781 or CDDP partially abrogates the colony formation capacity of the GC cells (10 d). The combination treatment exhibited a synergistic effect. Combo: Combination treatment. aP < 0.05 vs the control; cP < 0.05 vs PCI-24781 or CDDP.
PCI-24781 modulated the cell cycle and genes related to DNA damage and repair
To explore how PCI-24781 synergized with CDDP, we further checked whether PCI-24781 could modulate the cell cycle and affect the DNA damage repair mechanism. Indeed, treatment with 0.5 μmol/L PCI-24781 for 24 h resulted in a decreased number of cells in S phase and a G2 cell cycle arrest (Figure 3A), which is consistent with a previous report[10]. The increased sub-G1 population also indicated more apoptotic cells. Meanwhile, we found the expression of RAD51, one of the most important mediators of HR, was decreased in a time-dependent manner at both the mRNA and protein levels (Figure 3B, C). We then tried to determine what role the PCI-24781-induced reduction of RAD51 plays in the DNA damage conditions. It has been well documented that DSBs elicit a signaling cascade that modifies the chromatin surrounding the break, first by ATM-dependent phosphorylation and then by chromatin ubiquitination[33]. Here, we utilized classic IR-induced DNA damage to test the potential effect of PCI-24781 on HR. Both ubiquitin conjugation on the chromatin, as tagged by an anti-FK2 antibody, and RAD51-containing subnuclear repair foci were visible in HGC27 cells 16 h after radiation exposure. With pretreatment of a low dose of PCI-24781 (0.1 μmol/L), which did not result in significant apoptosis, for 24 h, the number of DNA damage foci (FK2 positive) significantly increased while the number of repair foci (RAD51 positive) decreased markedly (Figure 3D, E). These data suggested that HR-related RAD51 may play a central role in the potency of PCI-24781.
Figure 3.
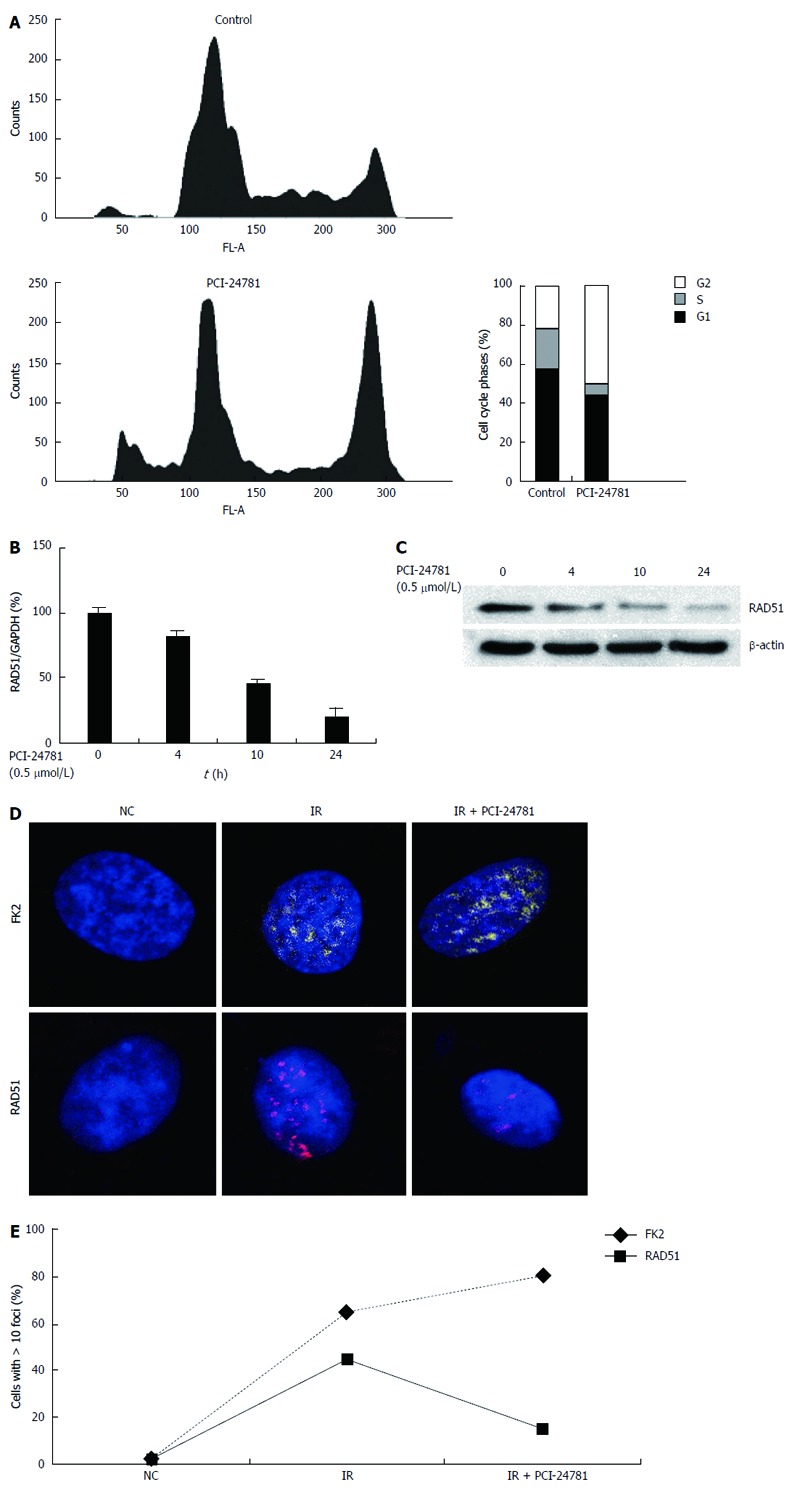
PCI-24781 modulates the genes related to DNA damage and repair. A: Propidium iodide/fluorescence activated cell sorting analysis showed the effect of the histone deacetylase inhibitor (0.5 μmol/L PCI-24781, 24 h) on HGC27 cell cycle progression. A significant (P < 0.05) reduction in the number of S phase cells and a G2 arrest was observed; The increased sub-G1 population also indicated more apoptotic cells; B, C: Treatment with 0.5 μmol/L PCI-24781 induced a time-dependent decrease in RAD51, both at the mRNA and protein levels; D, E: The confocal laser scanning microscopy immunofluorescence images show HGC27 cells stained with an anti-FK2 antibody (DNA damage marker) and an anti-RAD51 antibody (homologous recombination marker) after pretreatment with 0.1 μmol PCI-24781 for 24 h, followed by 5 Gy irradiation (IR); images were captured 16 h after irradiation. PCI-24781 notably increased the amount of DNA damage-induced ubiquitin conjugation on chromatin (FK2 positive) and suppressed RAD51 foci formation. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
RAD51 participates in the effects of PCI-24781, CDDP and the combination treatment
RAD51 dysfunction may be a key event leading to genomic instability and tumorigenesis[34]. To validate the role of RAD51 in the potency of PCI-24781, we manipulated the expression of RAD51 in gastric cancer cells. When RAD51 was depleted (Figure 4A, B), we observed a significant increase in the number of apoptotic cells in the PCI-24781- or CDDP-treated cells, with the highest ratio in the combination treatment (Figure 4C). In contrast, overexpression of RAD51 suppressed the pro-apoptotic effects of PCI-24781 and CDDP (Figure 4D-F). These data suggested an essential role of RAD51 in the anti-GC effect of PCI-24781, CDDP and combination treatment.
Figure 4.
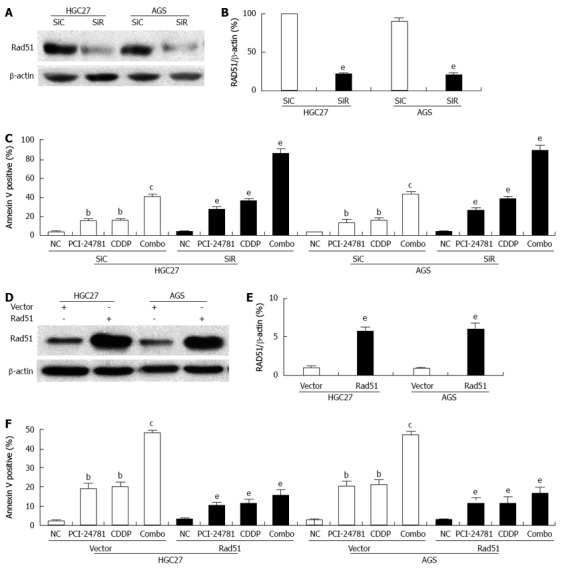
RAD51 mediates the effect of PCI-24781. A, B: siRNA targeting RAD51 dramatically decreased the expression of RAD51; C: Depletion of RAD51 significantly increased the apoptotic cells ratio both in the PCI-24781- and cis-diamminedichloroplatinum (CDDP)-treated cells. A synergistic effect was observed in the combination treatment; D, E: For the overexpression system, human RAD51 cDNA was cloned into pcDNA3.1 to yield the pcDNA-RAD51 plasmid. The empty vector served as a control. The cells transfected with pcDNA-RAD51 showed a notable increase in RAD51 expression; F: Overexpression of RAD51 significantly decreased the apoptotic cells ratio both in the PCI-24781- and CDDP-treated cells. A synergistic effect was observed in the combination treatment. SiC: siRNA-control; SiR: siRNA-RAD51; Combo: Combination treatment. bP < 0.01 vs the control; cP < 0.05 vs PCI-24781 or CDDP; eP < 0.05 vs SiC or vector.
PCI-24781 induces RAD51 transcriptional repression
To determine the mechanism by which PCI-24781 decreases the expression of RAD51, we pretreated the cells with actinomycin D for 30 min to block mRNA transcription and then added PCI-24781 to the medium. RAD51 mRNA was monitored via quantitative RT-PCR, and we found no significant difference in the half-lives (6-8 h) of the mRNA from the PCI-24781-treated and untreated samples, suggesting that PCI-24781 does not directly induce RAD51 mRNA degradation. Thus, we proposed that PCI-24781 treatment might result in RAD51 transcription repression. The results showed that treatment with PCI-24781 for 24 h significantly decreased the association of RNA polymerase II (Poly II) and slightly increased the association of acetylated histones H3 and H4 with the RAD51 gene (Figure 5A, B). To investigate the translational activity of the promoter, a RAD51 luciferase reporter, which contained the -403 to +63 RAD51 promoter region, was transiently transfected into GC cells. As shown in Figure 5C, PCI-24781 treatment for 24 h resulted in a significant suppression of RAD51 transcription (P < 0.05). Because -50 Luc was previously reported to regulate the transcription of RAD51, which contains an E2F binding-site[35], a mutation in this region was introduced to identify whether this cis element mediated the aforementioned repressive effect. Indeed, the PCI-24781-induced luciferase reduction in the wild-type promoter construct was abolished when this element was mutated (Figure 5D). We also observed a significant increase in E2F4 binding, whereas slightly decreased E2F1 binding to the RAD51 promoter was observed in response to PCI-24781 treatment (Figure 5E, F); however, there was no significant difference in the levels of these two proteins (data not shown). To elucidate the role of E2F4 in this process, siRNA knockdown was introduced. When E2F4 was depleted (Figure 5G), RAD51 transcription increased approximately 1.5-fold, and the PCI-24781-induced repression was abolished (Figure 5H, I), indicating that E2F4 mediates the efficacy of PCI-24781 as a transcription repressor of RAD51. These data suggested that PCI-24781 transcriptionally represses the expression of RAD51 mainly by increasing E2F4 binding to the promoter of the RAD51 gene, which accounts for its anti-GC effect.
Figure 5.
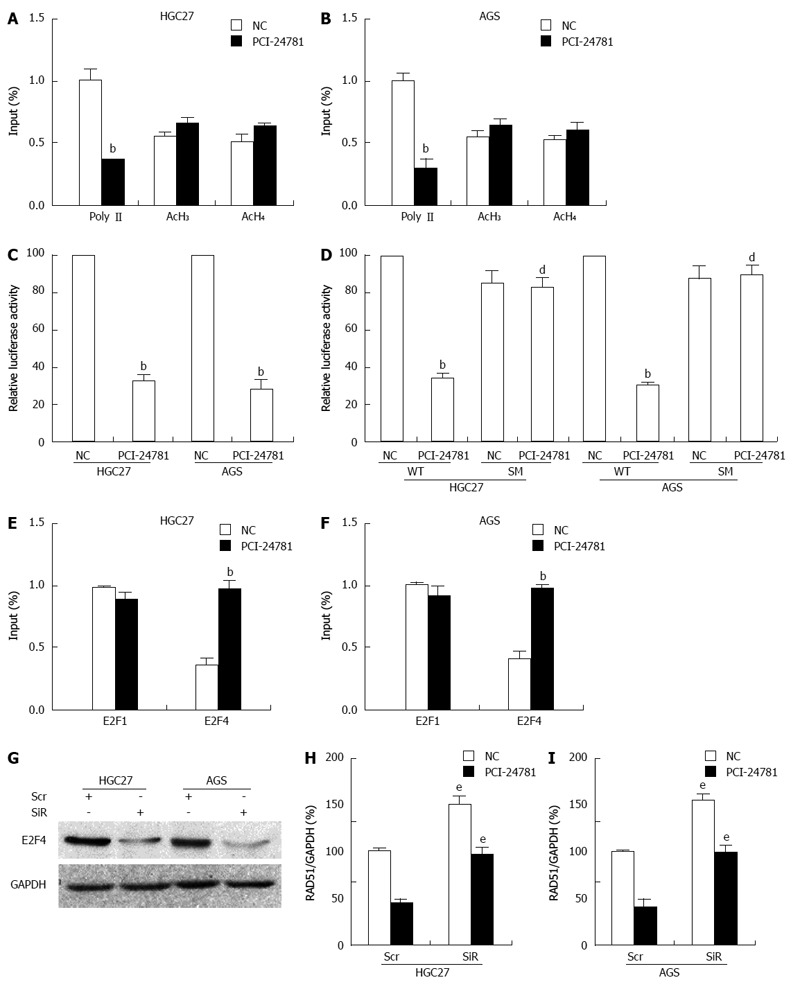
PCI-24781 induces RAD51 transcriptional repression. A, B: Chromatin immunoprecipitation assays showed a decrease in Polymerase II binding to the transcribed region of the proximal RAD51 gene in gastric cancer (GC) cells in response to treatment with 0.5 μmol/L PCI-24781 for 24 h; binding of acetylated histones 3 and 4 to the RAD51 region was slightly enhanced. No significant nonspecific IgG binding was observed, and input DNA was used as a loading control; C: Luciferase assays demonstrated that treatment with 0.5 μmol/L PCI-24781 for 24 h resulted in the suppression of RAD51 promoter activity; D: Mutation of the E2F binding site located within the first 50 bp of the RAD51 promoter construct abrogated the aforementioned repression; E, F: ChIP analysis showed significantly enhanced E2F4 binding and slightly decreased E2F1 binding to the cis element in the RAD51 promoter in response to PCI-24781 treatment (0.5 μmol/L for 24 h); G: siRNA targeting E2F4 dramatically decreased the expression of E2F4; H, I: Depletion of E2F4 significantly increased the expression of RAD51 and reversed the PCI-24781-induced decrease in RAD51 expression. SM: E2F binding-site mutation; Scr: Scramble siRNA; SiR: siRNA-E2F4. bP < 0.01 vs the control; dP < 0.01 vs wild-type (WT); eP < 0.05 vs Scr.
PCI-24781 synergized with the anti-GC effects of CDDP in vivo
To explore the anti-tumor effect of PCI-24781 in vivo, we used SCID mice with hypodermal gastric cancer. A linear mixed model was used to assess tumor growth (tabulated as the log-transformed tumor volume) across the treatment groups over time, and a linear regression model was used to assess the tumor weights. Therapy was initiated after the tumor was established (initial tumor sizes of control, PCI-24781, CDDP and combinational treatment were 112 ± 1.1, 104 ± 1.1, 100 ± 1.0 and 107 ± 1.2 mm3, respectively). Both PCI-24781 and CDDP treatment significantly repressed tumor growth indicated as reduction in the volume of tumor (Figure 6A) and decreased tumor weight versus the control mice (Figure 6B; P < 0.05). The average group tumor weights at the termination of the study were 2.01 ± 0.57 g for the control, 1.26 ± 0.35 g for the PCI-24781-treated, 1.36 ± 0.34 g for the CDDP-treated, and 0.68 ± 0.27 g for the combination mice (Figure 6B). The largest reduction of tumor growth and weight were obtained with the combination treatment. Immunohistochemical analysis revealed that the synergy decreased tumor cell proliferation and enhanced cell apoptosis, which might account for this outcome (Figure 6C-F). In the combination therapy group, cell proliferation (PCNA positive ratio) dropped to 22% ± 4.5% compared to the 85% ± 6.2% found in the control group, 57% ± 3.6% in the PCI-24781 alone group, and 60% ± 8.2% in the CDDP alone group; additionally, apoptosis (TUNEL positive ratio) increased to 52% ± 4.1% compared to the 5% ± 1.2% for the control group, 26% ± 3.3% for the PCI-24781 alone group, and 24% ± 3.1% for the CDDP alone group. These data suggested that PCI-24781 could synergize with the anti-GC effects of CDDP in vivo. Additionally, consistent with the in vitro data, PCI-24781 also inhibited the expression of RAD51 in vivo.
Figure 6.

PCI-24781 synergized with the chemotherapeutic effect of cis-diamminedichloroplatinum in a xenograft model. A, B: Tumor growth curves showed a significant difference between the PCI-24781- or cis-diamminedichloroplatinum (CDDP)-treated mice and the negative control. PCI-24781 or CDDP also dramatically decreased the tumor weight. The combination treatment obtained the smallest and lightest tumors compared to the single treatment or negative control groups. Immunohistochemical analysis of the GC xenograft specimens demonstrated the significantly decreased tumor proliferation (C, D; PCNA positive) and increased apoptosis (E, F; TUNEL positive) in the PCI-24781- or CDDP-treated tumors, with a synergistic effect in the combination treatment. CDDP: Cis-diamminedichloroplatinum; Combo: Combination treatment. aP < 0.05 vs the control; cP < 0.05 vs PCI-24781 or CDDP. Bar = 10 μm.
DISCUSSION
In this study, we provided the first evidence that the HDACi PCI-24781 synergizes with the therapeutic effects of CDDP in GC. PCI-24781-induced downregulation of RAD51, which closely relates with HR and DNA repair mechanism, may potentiate these effects.
HDACis are a new class of anticancer therapeutics whose mechanisms have yet to be elucidated in GC. Previous studies have demonstrated the antitumor effect of PCI-24781 on leukemia[36], lymphoma[37], glioblastoma[13], and malignant peripheral nerve sheath tumors[38], as well as showing its adjuvant chemo-function in multidrug-resistant sarcoma cell lines[16], bone sarcoma cells[15], and soft tissue sarcomas[10]. However, evidence is limited concerning its effect in GC. In vitro, we found that PCI-24781 could abrogate GC cell growth, suppress clonogenicity and enhance cell apoptosis (Figures 1 and 2). These effects were greatly enhanced when combined with CDDP treatment. We also confirmed that PCI-24781 treatment resulted in a significant reduction of RAD51, as well as a drastic suppression of DNA damage-induced ubiquitin conjugation on chromatin and RAD51 foci formation (Figure 3). This suggested that PCI-24781 may affect HR by modulating RAD51. The results from Adimoolam et al[22] robustly support this concept. They found that level of RAD51 was reduced to 20% after 24 h treatment with PCI-24781. They transfected DRAA8/CHO cells with the I-SceI-expressing plasmid to monitor recombination frequency. Results showed that the rate of HR dropped from 0.72% to 0.27% upon the addition of 2.0 μmol/L PCI-24781 6 h after transfection. Furthermore, PCI-24781 could inhibit expression of RAD51 both in vitro and in vivo (Figure 3B, C). Depletion of RAD51 augmented the apoptotic effect of PCI-24781, CDDP and the combination treatment, whereas overexpressing RAD51 had the opposite effects (Figure 4). In agreement with these findings, studies have reported that defects in either recombination mediators or co-mediators, including BRCA1 and BRCA2, lead to impaired HR[39-41], which can be genetically complemented by overexpression of RAD51[42]. Conversely, administration of a RAD51 inhibitor disrupts homologous recombination[43]. Consistent with in vitro data, PCI-24781 also exerted comparable anti-GC effects with the conventional chemotherapeutic CDDP in vivo. Combination treatment of PCI-24781 and CDDP gained most favorable therapeutic outcomes (Figure 6).
The exact mechanism of the HDACi-induced decreased gene expression remains uncertain. The decreased expression may be due to HDACi-induced transcriptional repression and/or decreased mRNA stability[44,45]. Alternatively, HDACis may induce the acetylation of non-histone proteins, including several transcription factors, thereby altering their function and potentially negatively affecting oncogenic target gene transcription[5]. In this study, PCI-24781 appeared to transcriptionally repress RAD51 rather than affect its mRNA stability. Although a slight increase in the association of acetylated histones H3 and H4 and RAD51 was observed, which may promote transcription, the significant decrease in Pol II association seemed to play the dominant negative role here (Figure 5A, B). Luciferase and site mutation assays also revealed that the E2F binding-site in the RAD51 promoter was essential for potency of PCI-24781 (Figure 5E, F). Researchers have indicated that E2F4, as a transcription repressor, may specifically inhibit RAD51 transcription in response to hypoxia[46], whereas E2F1 is a positive transcriptional regulator[47]. We also observed that the binding of E2F4 to the RAD51 promoter significantly increased, while the binding of E2F1 slightly decreased. Depletion of E2F4 using siRNA led to an increase in RAD51 expression and reversed the PCI-24781-induced decrease in RAD51 (Figure 5G-I). Overall, our findings suggest an anti-GC role of PCI-24781 and the potential mechanism.
To the best of our knowledge, this is the first study to show that PCI-24781 synergizes with the chemotherapeutic effect of CDDP in gastric cancer in vivo and in vitro, and PCI-24781-induced RAD51 repression may be one of the mechanisms. PCI-24781 could be a potential drug and novel therapeutic strategy for the treatment of gastric cancer.
COMMENTS
Background
Gastric cancer (GC) is one of the most aggressive malignancies, especially in South Asia. It ranks as the second leading cause of cancer mortality in China. Despite curative surgery and postoperative adjuvant therapy, nearly 60% of patients succumb to the disease. Recent studies have suggested that histone deacetylase (HDAC) inhibitors may be attractive anticancer drugs. PCI-24781, a broad-spectrum, hydroxamic acid-derived HDAC inhibitor (HDACi), shown significant anticancer activity in various tumors including soft tissue sarcomas, gallbladder carcinomas, colon carcinomas, glioblastomas, breast cancers, and bone sarcomas. However, information about the efficacy of PCI-24781 in the treatment of gastric cancer is limited.
Research frontiers
Despite HDACis showing promise as single agents, several recent studies have suggested that the optimal use of HDACis is likely in combination with other chemotherapeutic agents, like cis-diamminedichloroplatinum (CDDP). In the area of cancer chemotherapy, the research hotspot is discovering and revealing mechanisms of new drugs with or without combining conventional chemotherapeutic agent, by which authors are hoping to improve effectiveness of chemotherapy and simultaneously reduce its adverse reactions.
Innovations and breakthroughs
In this study, authors provide the first evidence that the HDACi PCI-24781 synergizes with the therapeutic effects of CDDP in GC. Not only in GC cell lines but also in xenograft mouse model, PCI-24781 shows decent suppressive effect. When combining with conventional agent CDDP, chemotherapy gains synergistic benefit. To one step further, they attempt to reveal the potential underlying mechanisms. Previous studies demonstrated that DNA damage can be repaired through homologous recombination or through non-homologous end joining, which can lead to chemotherapy resistance. RAD51 is a key protein involved in and is regarded as a biomarker for HR. Interestingly, they found that PCI-24781 can down-regulate RAD51 in a time and dose dependent fashion; RAD51 potentiates synergistic effects of chemotherapy with PCI-24781 and CDDP on gastric cancer.
Applications
The study results suggest that PCI-24781 could be a potential drug and novel therapeutic strategy for the treatment of gastric cancer.
Terminology
Homologous recombination: Homologous recombination is a type of genetic recombination in which nucleotide sequences are exchanged between two similar or identical molecules of DNA. It is most widely used by cells to accurately repair harmful breaks that occur on both strands of DNA, known as double-strand breaks. Non-homologous end joining is a pathway that repairs double-strand breaks in DNA. It typically utilizes short homologous DNA sequences called microhomologies to guide repair. These microhomologies are often present in single-stranded overhangs on the ends of double-strand breaks.
Peer review
The manuscript reported a series of evaluations for the efficacy of PCI-24781 on gastric cancer. Each experiment is well designed.
Footnotes
Supported by National Natural Science Foundation of China, No. 30973395, No. 81172337, No. 31271444 and No. 81201726; Municipal Medicine Science and Technology Foundation of Guangzhou, No. 201102A212012; Science and Technology Development Program of Guangdong, No. 2012B031800115; and Science Novel Program of Guangdong Education Department, No. A2003165
P- Reviewer: Matsuo Y, Syam AF S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Wang CH
References
- 1.Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437–447. doi: 10.6004/jnccn.2010.0033. [DOI] [PubMed] [Google Scholar]
- 2.Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692–698. doi: 10.1002/jso.22017. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS. Gastric cancer--new therapeutic options. N Engl J Med. 2006;355:76–77. doi: 10.1056/NEJMe068121. [DOI] [PubMed] [Google Scholar]
- 4.Yoong J, Michael M, Leong T. Targeted therapies for gastric cancer: current status. Drugs. 2011;71:1367–1384. doi: 10.2165/11592530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 6.Gediya LK, Khandelwal A, Patel J, Belosay A, Sabnis G, Mehta J, Purushottamachar P, Njar VC. Design, synthesis, and evaluation of novel mutual prodrugs (hybrid drugs) of all-trans-retinoic acid and histone deacetylase inhibitors with enhanced anticancer activities in breast and prostate cancer cells in vitro. J Med Chem. 2008;51:3895–3904. doi: 10.1021/jm8001839. [DOI] [PubMed] [Google Scholar]
- 7.Tarasenko N, Nudelman A, Tarasenko I, Entin-Meer M, Hass-Kogan D, Inbal A, Rephaeli A. Histone deacetylase inhibitors: the anticancer, antimetastatic and antiangiogenic activities of AN-7 are superior to those of the clinically tested AN-9 (Pivanex) Clin Exp Metastasis. 2008;25:703–716. doi: 10.1007/s10585-008-9179-x. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SN, Roh SA, Cho DH, Kim MB, Hyun YL, Ro S, Kim BS, Kim SY, Kim YS, Kim JC. In vitro chemosensitivity of gastric adenocarcinomas to histone deacetylase inhibitors, compared to established drugs. Hepatogastroenterology. 2010;57:657–662. [PubMed] [Google Scholar]
- 9.Lassen U, Molife LR, Sorensen M, Engelholm SA, Vidal L, Sinha R, Penson RT, Buhl-Jensen P, Crowley E, Tjornelund J, et al. A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br J Cancer. 2010;103:12–17. doi: 10.1038/sj.bjc.6605726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G, Zhu QS, Bornmann WG, McConkey DJ, Pollock RE, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res. 2009;15:3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T, Connolly K, Ruffino L, Ajiki T, Lueckgen A, DiGiovanni J, Kiguchi K. The therapeutic effect of histone deacetylase inhibitor PCI-24781 on gallbladder carcinoma in BK5.erbB2 mice. J Hepatol. 2012;57:84–91. doi: 10.1016/j.jhep.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JM, Huang S, Tougeron D, Sinicrope FA. MSH3 mismatch repair protein regulates sensitivity to cytotoxic drugs and a histone deacetylase inhibitor in human colon carcinoma cells. PLoS One. 2013;8:e65369. doi: 10.1371/journal.pone.0065369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh MM, Manton CA, Bhat KP, Tsai WW, Aldape K, Barton MC, Chandra J. Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro Oncol. 2011;13:894–903. doi: 10.1093/neuonc/nor049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Assar O, Mantoni T, Lunardi S, Kingham G, Helleday T, Brunner TB. Breast cancer stem-like cells show dominant homologous recombination due to a larger S-G2 fraction. Cancer Biol Ther. 2011;11:1028–1035. doi: 10.4161/cbt.11.12.15699. [DOI] [PubMed] [Google Scholar]
- 15.Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X, Mankin H, Duan Z. Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemother Pharmacol. 2011;67:439–446. doi: 10.1007/s00280-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X, Mankin H, Duan Z. Histone deacetylase inhibitor PCI-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer Res. 2011;31:1115–1123. [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz-Galván S, López-Saavedra A, Jackson SP, Huertas P, Cortés-Ledesma F, Aguilera A. Competing roles of DNA end resection and non-homologous end joining functions in the repair of replication-born double-strand breaks by sister-chromatid recombination. Nucleic Acids Res. 2013;41:1669–1683. doi: 10.1093/nar/gks1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano MA, Li Z, Dangeti M, Musich PR, Patrick S, Roginskaya M, Cartwright B, Zou Y. DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to facilitate homologous recombination DNA repair. Oncogene. 2013;32:2452–2462. doi: 10.1038/onc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Lipfert J, Sanchez H, Wyman C, Dekker NH. Structural and torsional properties of the RAD51-dsDNA nucleoprotein filament. Nucleic Acids Res. 2013;41:7023–7030. doi: 10.1093/nar/gkt425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poplawski T, Arabski M, Kozirowska D, Blasinska-Morawiec M, Morawiec Z, Morawiec-Bajda A, Klupińska G, Jeziorski A, Chojnacki J, Blasiak J. DNA damage and repair in gastric cancer--a correlation with the hOGG1 and RAD51 genes polymorphisms. Mutat Res. 2006;601:83–91. doi: 10.1016/j.mrfmmm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, Orelli BJ, Bishop DK, Hirano S, Ohzeki M, et al. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- 22.Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci USA. 2007;104:19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He WL, Li YH, Yang DJ, Song W, Chen XL, Liu FK, Wang Z, Li W, Chen W, Chen CY, et al. Combined evaluation of centromere protein H and Ki-67 as prognostic biomarker for patients with gastric carcinoma. Eur J Surg Oncol. 2013;39:141–149. doi: 10.1016/j.ejso.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 25.Le Gac S, Vermes I, van den Berg A. Quantum dots based probes conjugated to annexin V for photostable apoptosis detection and imaging. Nano Lett. 2006;6:1863–1869. doi: 10.1021/nl060694v. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhang M, Li Y, Liu S, Ping S, Wang J, Ning F, Xie F, Li C. Simvastatin inhibits the additive activation of ERK1/2 and proliferation of rat vascular smooth muscle cells induced by combined mechanical stress and oxLDL through LOX-1 pathway. Cell Signal. 2013;25:332–340. doi: 10.1016/j.cellsig.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Liu S, Zhang Z, Xu Q, Xie F, Wang J, Ping S, Li C, Wang Z, Zhang M, et al. RAGE mediates accelerated diabetic vein graft atherosclerosis induced by combined mechanical stress and AGEs via synergistic ERK activation. PLoS One. 2012;7:e35016. doi: 10.1371/journal.pone.0035016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Li Y, Zhang Z, Xie F, Xu Q, Huang X, Huang J, Li C. α1-Adrenergic receptors mediate combined signals initiated by mechanical stretch stress and norepinephrine leading to accelerated mouse vein graft atherosclerosis. J Vasc Surg. 2013;57:1645–1656, 1656.e1-3. doi: 10.1016/j.jvs.2012.09.061. [DOI] [PubMed] [Google Scholar]
- 29.Zha L, Wang Z, Tang W, Zhang N, Liao G, Huang Z. Genome-wide analysis of HMGA2 transcription factor binding sites by ChIP on chip in gastric carcinoma cells. Mol Cell Biochem. 2012;364:243–251. doi: 10.1007/s11010-012-1224-z. [DOI] [PubMed] [Google Scholar]
- 30.Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306:171–179. doi: 10.1016/j.canlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Máthé A, Komka K, Forczig M, Szabó D, Anderlik P, Rozgonyi F. The effect of different doses of cisplatin on the pharmacokinetic parameters of cefepime in mice. Lab Anim. 2006;40:296–300. doi: 10.1258/002367706777611514. [DOI] [PubMed] [Google Scholar]
- 32.Mohammad RM, Banerjee S, Li Y, Aboukameel A, Kucuk O, Sarkar FH. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer. 2006;106:1260–1268. doi: 10.1002/cncr.21731. [DOI] [PubMed] [Google Scholar]
- 33.Nakada S, Tai I, Panier S, Al-Hakim A, Iemura S, Juang YC, O’Donnell L, Kumakubo A, Munro M, Sicheri F, et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]
- 34.Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasselbach L, Haase S, Fischer D, Kolberg HC, Stürzbecher HW. Characterisation of the promoter region of the human DNA-repair gene Rad51. Eur J Gynaecol Oncol. 2005;26:589–598. [PubMed] [Google Scholar]
- 36.Rivera-Del Valle N, Gao S, Miller CP, Fulbright J, Gonzales C, Sirisawad M, Steggerda S, Wheler J, Balasubramanian S, Chandra J. PCI-24781, a Novel Hydroxamic Acid HDAC Inhibitor, Exerts Cytotoxicity and Histone Alterations via Caspase-8 and FADD in Leukemia Cells. Int J Cell Biol. 2010;2010:207420. doi: 10.1155/2010/207420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhalla S, Balasubramanian S, David K, Sirisawad M, Buggy J, Mauro L, Prachand S, Miller R, Gordon LI, Evens AM. PCI-24781 induces caspase and reactive oxygen species-dependent apoptosis through NF-kappaB mechanisms and is synergistic with bortezomib in lymphoma cells. Clin Cancer Res. 2009;15:3354–3365. doi: 10.1158/1078-0432.CCR-08-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez G, Torres K, Liu J, Hernandez B, Young E, Belousov R, Bolshakov S, Lazar AJ, Slopis JM, McCutcheon IE, et al. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2011;71:185–196. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12:587–598. doi: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- 40.Mao Z, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalev P, Simicek M, Vazquez I, Munck S, Chen L, Soin T, Danda N, Chen W, Sablina A. Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition. Cancer Res. 2012;72:6414–6424. doi: 10.1158/0008-5472.CAN-12-1667. [DOI] [PubMed] [Google Scholar]
- 42.Schild D, Wiese C. Overexpression of RAD51 suppresses recombination defects: a possible mechanism to reverse genomic instability. Nucleic Acids Res. 2010;38:1061–1070. doi: 10.1093/nar/gkp1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Budke B, Kalin JH, Pawlowski M, Zelivianskaia AS, Wu M, Kozikowski AP, Connell PP. An optimized RAD51 inhibitor that disrupts homologous recombination without requiring Michael acceptor reactivity. J Med Chem. 2013;56:254–263. doi: 10.1021/jm301565b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong Y, Dowdy SC, Podratz KC, Jin F, Attewell JR, Eberhardt NL, Jiang SW. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 2005;65:2684–2689. doi: 10.1158/0008-5472.CAN-04-2843. [DOI] [PubMed] [Google Scholar]
- 45.Ocker M, Schneider-Stock R. Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int J Biochem Cell Biol. 2007;39:1367–1374. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Bindra RS, Glazer PM. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 2007;26:2048–2057. doi: 10.1038/sj.onc.1210001. [DOI] [PubMed] [Google Scholar]
- 47.Radhakrishnan SK, Feliciano CS, Najmabadi F, Haegebarth A, Kandel ES, Tyner AL, Gartel AL. Constitutive expression of E2F-1 leads to p21-dependent cell cycle arrest in S phase of the cell cycle. Oncogene. 2004;23:4173–4176. doi: 10.1038/sj.onc.1207571. [DOI] [PubMed] [Google Scholar]


