Abstract
AIM: To investigate a simple noninvasive scoring system for predicting liver cirrhosis in nonalcoholic fatty liver disease (NAFLD) patients.
METHODS: A total of 1048 patients with liver-biopsy-confirmed NAFLD were enrolled from nine hepatology centers in Japan (stage 0, 216; stage 1, 334; stage 2, 270; stage 3, 190; stage 4, 38). The weight and height of the patients were measured using a calibrated scale after requesting the patients to remove their shoes and any heavy clothing. Venous blood samples were obtained in the morning after the patients had fasted overnight for 12 h. Laboratory evaluation was performed in all patients. Statistical analysis was conducted using SPSS version 12.0. Continuous variables were expressed as mean ± SD.
RESULTS: The optimal cutoff value of platelet count, serum albumin, and aminotransferase/alanine aminotransferase ratio (AAR) was set at < 15.3 104/μL, < 4.0 g/dL, and > 0.9, respectively, by the receiver operating characteristic curve. These three variables were combined in an unweighted sum (platelet count = 1 point, serum albumin = 1 point, AAR = 1 point) to form an easily calculated composite score for predicting cirrhosis in NAFLD patients, called the PLALA (platelet, albumin, AAR) score. The diagnosis of PLALA ≥ 2 had sufficient accuracy for detecting liver cirrhosis in NAFLD patients.
CONCLUSION: The PLALA score may be an ideal scoring system for detecting cirrhosis in NAFLD patients with sufficient accuracy and simplicity to be considered for clinical use.
Keywords: Nonalcoholic fatty liver disease, Cirrhosis, Fibrosis, Platelet, Albumin, Alanine aminotransferase ratio
Core tip: Nonalcoholic fatty liver disease (NAFLD) is an important cause of chronic and progressive liver injury. We aimed to develop a simple noninvasive scoring system for predicting liver cirrhosis in NAFLD patients. These three variables were combined in an unweighted sum [platelet count = 1 point, serum albumin = 1 point, aminotransferase (AST)/alanine aminotransferase (ALT) ratio = 1 point] to form an easily calculated composite score, called the PLALA (platelet, albumin, AST/ALT ratio) score. The diagnosis of PLALA ≥ 2 had sufficient accuracy for detecting liver cirrhosis in NAFLD patients. The PLALA score may be an ideal scoring system for detecting cirrhosis in NAFLD patients.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is an important cause of chronic liver injury in many countries[1,2]. NAFLD represents a spectrum of conditions that are characterized histologically by macrovesicular hepatic steatosis, and the diagnosis is made after excluding a history of consumption of alcohol in amounts sufficient to be considered harmful to the liver. NAFLD range over a wide spectrum, extending from simple steatosis, which is generally benign, through to nonalcoholic steatohepatitis (NASH) to liver cirrhosis, end-stage liver disease, and even hepatocellular carcinoma despite the absence of significant alcohol consumption[3-7]. The probability of developing advanced fibrosis and hepatocellular carcinoma is significantly greater in individuals with steatohepatitis than in those with simple steatosis. Data collected from the United States have shown that the prevalence of NAFLD has increased steady in recently years, despite other diseases remaining at steady states. Natural history studies suggest that fibrosis progression occurs in 32%-37% of patients over 3-6 years[8-10], and up to 12% of patients will progress to cirrhosis over 8-10 year[11]. If these patients with NAFLD progress liver cirrhosis, they need to be kept under the surveillance data for early detection of HCC and gastroesophageal varices, similar to the case, such as hepatitis C[12-14].
Liver biopsy as a confirmation tool of NASH can reveal the histologic activity of steatosis, inflammation, and fibrosis. It is frequently used for diagnosis as the gold standard tool in patients with NASH[1,3,15]. However, it is difficult to perform liver biopsy for every patient with NAFLD to ascertain the presence of NASH and determine the stage and grade of the disease[16]. The estimated number of patients with NAFLD has reached 80-100 million in the United States, and the corresponding number of patients in Japan has been estimated at 10-20 million. The prevalence of NAFLD and nonalcoholic steatohepatitis (NASH) is increasing and is becoming a major target disease not only in Western countries, but also in Japan. Therefore, alternative diagnostic methods, noninvasive procedures such as transient elastography, have recently been developed. However, these are not appropriate for health check-ups because they cannot be used in patient with ascites, thick subcutaneous fat, narrow intercostal spaces, and hepatic atrophy.
Therefore, the aim of this study was to develop a mass screening system for general physicians, which can be used for predicting liver cirrhosis in NAFLD patients, using routine laboratory parameters.
MATERIALS AND METHODS
Patients
1048 NAFLD patients who underwent liver biopsy were enrolled between 2002 and 2011 from institutes affiliated with the Japan Study Group of NAFLD (JSG-NAFLD), represented by the following 10 hepatology centers: Yokohama City University, Asahikawa Medical College, Kurume University, Nara City Hospital, Hiroshima University, Saga Medical School, Osaka City University, Kyoto Prefectural University of Medicine, Kochi Medical School, and Saiseikai Suita Hospital. The study was conducted with the approval of the Ethics Committee of all hepatology centers. Liver biopsy was available in all NAFLD patients for the purpose of diagnosis and staging of NASH. Macrovesicular steatosis affecting at least 5% of the hepatocytes was observed in all the cases, with displacement of the nuclei to the edges of the cells[17]. The exclusion criteria included history of hepatic disease such as chronic hepatitis C or concurrent active hepatitis B (seropositive for hepatitis B surface antigen); autoimmune hepatitis; primary biliary cirrhosis; Wilson disease; hemochromatosis; α1-antitrypsin deficiency; sclerosing cholangitis; hepatic injury caused by substance abuse, or current or past consumption of > 20 g of alcohol daily. Informed consent for evaluation of liver histology was obtained from all the enrolled patients, and the present study was performed in accordance and compliance with the Ethic Principles of the 1975 Declaration of Helsinki.
Anthropometric and biochemical measurements
Body mass index (BMI) was calculated as body weight (kg) divided by height (m2). Fasted Human blood was collected from all biopsy-proven patients in the morning after overnight for 12 h. In patients with NAFLD, the blood cell counts and the serum levels of aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase, albumin, ferritin, cholinesterase (ChE), fasting plasma glucose, fasting immunoreactive insulin, hyaluronan, and collagen IV were measured consecutively in the each hospital’s laboratory. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated for NAFLD patients as using the following formula: fasting insulin (μU/mL) × fasting plasma glucose (mg/dL)/405.
Histological evaluation
All patients had undergone percutaneous liver biopsy under ultrasound guidance. Liver biopsies were obtained with 16 or 18 gauge needle biopsy apparatus. The number of biopsy specimen fragments was one or two. Liver tissue specimens were fixed in formalin, embedded in paraffin and stained, and analyzed independently by an expert pathologist who was blinded to the clinical data. Fatty liver was defined as the presence of > 5% steatosis. In addition to steatosis, the diagnosis of NASH was histologically confirmed as the presence of lobular inflammation, hepatocyte injures including hepatocyte ballooning cells or perisinusoidal/pericellular fibrosis in zone 3 of the hepatic acini[4,18,19]. The grading and staging of NASH was assessed by Brunt’s modified semi-quantitative system, which classifies inflammatory activity into 3 grades [grade 1, mild; grade 2, moderate; grade 3, severe, and the stage of fibrosis into a 4 stages (stage 1, zone3 perivenular and/or perisinusoidal fibrosis; stage 2, stage 1 with periportal fibrosis; stage 3, bridging fibrosis; and stage 4, cirrhosis)]. The individual parameters of fibrosis were scored independently according to the NASH Clinical Research Network (CRN) scoring system developed by the NASH CRN[20].
Statistical analysis
Statistical analysis was performed by using SPSS software version 12.0 for windows (SPSS, Chicago, IL, United States). Continuous variables were expressed as mean ± SD. Qualitative data were represented as numbers, with the percentages indicated within parentheses. The statistical significance of differences between the two groups in the quantitative data were assessed using the t-test or the χ2 test. Data sets involving more than two independent groups were assessed by Kruskal-Wallis test, because the variables were often not normally distributed. The diagnostic performance was assessed by analysis of receiver-operating characteristic (ROC) curves, and the ROC curve was a plot of sensitivity versus (1-specificity) for all possible cutoff values. The probabilities of a true-positive (sensitivity) and true-negative (specificity) were determined for selected cutoff values, and the area under the ROC curve (AUROC) was calculated for each index. Statistical significance was defined as a P < 0.05.
RESULTS
Patient and laboratory characteristics of enrolled subjects
Using a multicenter database, 1048 biopsy-proven cases of NAFLD were investigated (fibrosis stage 0, 216; stage 1, 334; stage 2, 270; stage 3, 190; stage 4, 38). The clinical laboratory data and liver biopsy specimens with characteristics of individuals with fibrosis stages 0-3 and stage 4 (cirrhosis) are shown in Table 1. The age, AST/ALT ratio (AAR), ALP, hyaluronan, and collagen IV were significantly higher, and ChE, albumin, hemoglobin, and platelet were significantly decreased in NAFLD patients with liver cirrhosis (fibrosis stage 4), compared with those with no cirrhosis (fibrosis stages 0-3).
Table 1.
Characteristics of patients in the estimation and validation groups
| Variables | Fibrosis stages 0-3 (non-cirrhosis)1 | Fibrosis stage 4 (cirrhosis)1 | P value2 |
| Age (yr) | 51.1 ± 15.0 | 63.9 ± 9.6 | < 0.0010 |
| n | 1010 | 38 | - |
| BMI (kg/m2) | 27.8 ± 4.9 | 28.6 ± 3.9 | 0.3648 |
| AST (IU/L) | 58.3 ± 38.2 | 70.2 ± 74.7 | 0.0721 |
| ALT (IU/L) | 92.2 ± 64.4 | 67.5 ± 65.6 | 0.0208 |
| AAR | 0.71 ± 0.29 | 1.15 ± 0.41 | < 0.0010 |
| ALP (IU/L) | 258.8 ± 94.9 | 312.2 ± 155.6 | 0.0012 |
| GGT (IU/L) | 88.2 ± 93.8 | 95.4 ± 73.0 | 0.6408 |
| ChE (IU/L) | 383.0 ± 95.3 | 298.7 ± 127.0 | < 0.0010 |
| Albumin (g/dL) | 4.42 ± 0.41 | 3.69 ± 0.47 | < 0.0010 |
| Ferritin (ng/mL) | 255.3 ± 249.8 | 227.5 ± 198.0 | 0.5677 |
| Fasting glucose (mg/dL) | 113.3 ± 38.9 | 124.0 ± 57.2 | 0.1116 |
| Fasting insulin (μU/mL) | 14.8 ± 13.8 | 18.4 ± 10.5 | 0.1938 |
| HOMA-IR | 4.31 ± 5.10 | 6.05 ± 5.64 | 0.1015 |
| Hemoglobin (g/dL) | 14.5 ± 1.6 | 13.2 ± 1.5 | < 0.0010 |
| Platelet (× 104/μL) | 22.6 ± 6.53 | 12.08 ± 4.40 | < 0.0010 |
| Hyaluronan (ng/mL) | 54.5 ± 81.0 | 250.7 ± 191.0 | < 0.0010 |
| Collagen IV (ng/mL) | 4.70 ± 3.60 | 8.14 ± 1.80 | < 0.0010 |
Results are presented as numbers for qualitative data or as mean ± SD for quantitative data;
P values were calculated by the t test or the χ2 test. ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AAR: AST/ALT ratio; BMI: Body mass index; ChE: Cholinesterase; GGT: γ-glutamyl transpeptidase; HOMA-IR: Homeostasis model assessment-insulin resistance.
Multiple logistic regression analysis of factors related to fibrosis: Fibrosis stage 0-3 vs fibrosis stage 4 (cirrhosis)
Multiple logistic regression analysis is performed by using age, AAR, serum ChE, albumin, hemoglobin, platelet, hyaluronan, and collagen IV, which were significantly increased or decreased in NAFLD patients with liver cirrhosis (fibrosis stage 4) compared with NAFLD patients without cirrhosis (fibrosis stages 0-3), by univariate analysis (P < 0.0001) (Table 2). On the factors using multiple logistic regression analysis associated with fibrosis stage 0-3 compared with stage 4, AAR (P = 0.0427), serum albumin level (P < 0.001), and platelet (P < 0.001) were the factors related to progression to cirrhosis.
Table 2.
Multiple logistic regression analysis of factors associated with stage 0-3 compared with stage 4 (cirrhosis)
| Variables | OR | 95%CI | P value |
| Age (yr) | 0.976 | 0.935-1.018 | 0.2610 |
| AAR | 0.122 | 0.054-0.279 | 0.0427 |
| Cholinesterase (IU/L ) | 1.000 | 0.995-1.005 | 0.9200 |
| Albumin (g/dL) | 5.977 | 2.430-14.703 | < 0.0001 |
| Hemoglobin (g/dL) | 0.988 | 0.761-1.283 | 0.9263 |
| Platelet (× 104/μL) | 1.282 | 1.169-1.405 | < 0.0001 |
| Hyaluronan (ng/mL) | 1.000 | 0.997-1.003 | 0.8879 |
| Type IV collagen 7s | 0.941 | 0.883-1.004 | 0.0671 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AAR: AST/ALT ratio.
ROC curve for differentiating stage 4 fibrosis based on albumin, platelet, and AAR
We performed ROC curve analysis for differentiating fibrosis stage 4 (cirrhosis) based on albumin, platelet, and AAR (Figure 1). For detecting cirrhosis (fibrosis stage 4) compared with non-cirrhosis (fibrosis stage 0-3), the AUROC for AAR, albumin, and platelet was 0.843, 0.898, and 0.918, respectively. Based on the ROC curve, the cutoff level for the diagnosis of AAR, albumin, and platelet was set at ≥ 0.9, ≤ 4.0 g/dL, and ≤ 15.3 × 104/mL, respectively. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of AAR, albumin, and platelet were 76.3%/84.2%/81.6% (sensitivity), 82.9%/84.6%/88.6% (specificity), 98.9%/99.3%/99.2% (NPV), and 13.9%/17.0%/21.2% (PPV), respectively.
Figure 1.
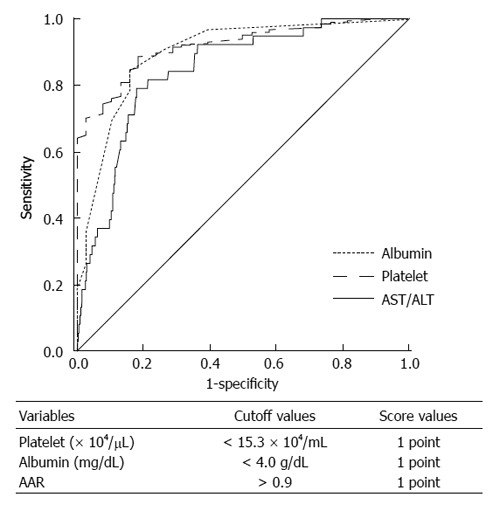
Receiver operating characteristic curve. Receiver operating characteristic curve for differentiating fibrosis stage 4 based on albumin, platelet, and AAR; aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio. PLALA score (platelet, albumin, AAR).
PLALA score (platelet, albumin, AAR)
By multiple logistic regression analysis, three variables remained significant, including platelet, albumin, and AAR. Thus, these three variables, platelet < 15.3 × 104/μL, albumin < 4.0 g/dL, and AAR > 0.9, were combined to form an easily calculated composite score for predicting NAFLD with cirrhosis, called the PLALA score. The three variables were given a score of 1 point each (Figure 1), and a score of 0-3 was calculated. Figure 2 shows the percentage of patients with cirrhosis (fibrosis stage 4) with a platelet < 15.3 × 104/μL, albumin < 4.0 g/dL, and AAR > 0.9.
Figure 2.

The percentage of patients with cirrhosis. The percentage of patients with cirrhosis (stage 4) with a platelet < 15.3 ×104/μL, albumin < 4.0 g/dL, and alanine aminotransferase ratio; aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio (AAR) > 0.9.
The diagnostic accuracy of the scoring system in distinguishing patients with and without cirrhosis was confirmed in 1048 patients. The percentage of patients with cirrhosis (fibrosis stage 4) with a PLALA score of 0, 1, 2, and 3 was 0%, 13%, 29%, and 58%, respectively (Figure 3). When using a PLALA score of 2 as a cutoff, the sensitivity, specificity, NPV, and PPV were 86.8%, 90.8%, 99.5%, and 26.2%, respectively. All of these data were superior to those of platelet, albumin, and AAR.
Figure 3.

PLALA score and fibrosis stage of 1048 biopsy-proven nonalcoholic fatty liver disease patients.
DISCUSSION
We developed a simple scoring system to differentiate cirrhosis from non-cirrhosis in NAFLD patients. The three variables platelet, albumin, and AAR were combined in an unweighted sum (platelet < 15.3 × 104/μL; 1 point, serum albumin < 4.0 g/dL; 1 point, and AAR > 0.9; 1 point) and formed an easily calculated composite score for predicting cirrhosis in NAFLD patients, called the PLALA score. A PLALA score (2 and 3) was useful for detecting liver cirrhosis in NAFLD patients (sensitivity, 86.8%; specificity, 90.8%; NPV, 99.5%; PPV, 26.2%).
Bhala et al[21] reported that 2.4% of patients with NAFLD followed up for approximately 85.6 ± 54.5 mo developed HCC, and 66.7% of the patients with NAFLD-associated HCC had cirrhosis (fibrosis stage 4). Hashimoto et al[22] reported that 88% of patients with NASH-associated HCC had advanced fibrosis (stage 3 or 4). Therefore, advanced fibrosis was recognized as an important risk factor for HCC. Furthermore, HCC was the major cause of mortality in NASH patients with advanced fibrosis[22]. It is important to closely follow cirrhosis patients with NASH. In the field of NAFLD, various scoring systems have been reported, for example NAFIC[23], FIB4 index[24], HAIR[25], BAAT[26], BARD[13], NAFLD fibrosis score[27], and N score (Nippon)[28]. These scoring systems can differentiate NASH from NAFLD or differentiate advanced fibrosis (stages 3 or 4) from mild fibrosis (stage 0-2). However, the PLALA score developed in our study, with the three variables platelet, albumin, and AAR, differentiates cirrhosis (stage 4) from non-cirrhosis in the NAFLD patients (stages 0-3), and it is easy to calculate.
The platelet is one of the most commonly reported parameters associated with clinically significant portal hypertension in compensated cirrhosis patients[29]. The levels of serum albumin reflect the protein-synthesizing capacity of the liver. Patients with advanced cirrhosis almost always have hypoalbuminemia caused by decreased protein synthesis in the hepatocytes. AAR reflects fibrosis of the liver[30,31]. When only one of three values is positive, factors that may cause false-positive low cutoff values for platelet are older age (decreased platelet production), idiopathic thrombocytopenic purpura, idiopathic portal hypertension, and drugs. Factors that may cause false-positive low levels of serum albumin are loss of urinary albumin due to renal dysfunction (e.g., nephritic syndrome and diabetic nephropathy), severe burns, and inadequate protein intake. The possibility of false-positive values for AAR > 0.9 is within the normal range of AST and ALT.
Liver cirrhosis simultaneously induces liver fibrosis, portal hypertension, and decreased production of albumin. Thus, PLALA score includes: platelet, which reflects portal hypertension; albumin, which reflects protein production; and AAR, which reflects liver fibrosis. A PLALA score of 2 or 3 points is highly diagnostic for liver cirrhosis in patients with false-positive results for NAFLD. If these NAFLD patients with liver cirrhosis have early detection of HCC and portal hypertension, such as gastroesophageal varices, it is important for them to need to be kept under surveillance.
This study had several limitations. The study had a largely retrospective design. The proportion of patients with advanced fibrosis was small. Therefore, in contrast to the NPVs, the PPVs did not have sufficient accuracy for the diagnosis of advanced fibrosis. Therefore, it would seem appropriate to consider liver biopsy in all patients with values above the cutoff of the selected index, PLALA (2 and 3). We previously reported, possibly for the first time, that transient elastography and acoustic radiation force impulse elastography can be used to measure the severity of fibrosis in patients with NAFLD[32,33]. It is possible that a combination of transient elastography and the aforementioned scoring systems may provide better performance than each of them used alone, although this needs to be verified. The patients were recruited from multiple hepatology centers in Japan with a particular interest in the study of NAFLD; therefore, the possibility of some referral bias cannot be ruled out. Patient selection bias could also have existed, because liver biopsy might have been considered for NAFLD patients who were likely to have NASH and progression of fibrosis. Thus, the findings may not represent NAFLD patients in the community at large. We also acknowledge that the pathological diagnosis was mainly determined using liver tissues derived from percutaneous liver biopsies, which are prone to sampling errors and/or inter-observer variability[34,35]. There is a possibility that our results might not be adaptable for NAFLD patients of other races, because all participants were Japanese. Because of these limitations, the present results need to be validated in independent populations by other investigators.
In conclusions, the PLALA score may be an ideal scoring system for detecting cirrhosis in NAFLD patients, because it is easy to use, cost effective, and accurate. Therefore, we consider that this scoring system is useful for mass screening by general physicians, using routine laboratory parameters.
COMMENTS
Background
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver damage in many countries around the world. Although liver biopsy is useful as the gold standard method for diagnosis of nonalcoholic steatohepatitis (NASH) with cirrhosis, it is difficult to perform liver biopsy for every patient with NAFLD. However, noninvasive markers for predicting cirrhosis in NAFLD patients have not yet been well established.
Research frontiers
In the field of NAFLD, various scoring systems related liver fibrosis have been reported, for example FIB4 index, NAFLD fibrosis score. These scoring systems can differentiate advanced fibrosis (stage 3 or 4) from mild fibrosis (stage 0-2). However, NASH-related cirrhosis (stage 4) is cause of most complication, especially hepatocellular carcinoma and portal hypertension. Therefore, the research hotspot is how to find noninvasive scoring systems for detecting with NASH-associated cirrhosis (stage 4).
Innovations and breakthroughs
The PLALA score developed with the three variables, differentiates cirrhosis (stage 4) from non-cirrhosis in the NAFLD patients (stages 0-3). When using a PLALA score of 2 as a cutoff, the sensitivity, specificity, negative predictive value, and positive predictive value were 86.8%, 90.8%, 99.5%, and 26.2%, respectively.
Applications
The study results suggest that PLALA score is to develop a mass screening system for general physicians, which can be used for predicting liver cirrhosis in NAFLD patients, using routine laboratory parameters.
Terminology
NAFLD is mainly represents a spectrum of liver disease from simple steatosis to nonalcoholic fatty steatohepatitis, which can progress to cirrhosis and hepatocellular carcinoma, despite the absence of significant alcohol consumption. PLALA score is constructed from platelet, Alb, AAR. These three variables were combined to form an easily calculated composite score for predicting NAFLD with cirrhosis.
Peer review
The manuscript aimed to develop a simple noninvasive scoring system for predicting liver cirrhosis in nonalcoholic fatty liver disease patients by using early available clinical and biochemical variables. This article is interesting, original and well written, and gives good clues to the readers.
Footnotes
P- Reviewer: Borg BB, Jin S, Morales-Gonzalez JA S- Editor: Gou SX L- Editor: A E- Editor: Ma S
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–138. doi: 10.1046/j.1440-1746.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 4.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 5.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Saibara T. Nonalcoholic steatohepatitis in Asia-Oceania. Hepatol Res. 2005;33:64–67. doi: 10.1016/j.hepres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 9.Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–378. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Yoneda M, Imajo K, Eguchi Y, Fujii H, Sumida Y, Hyogo H, Ono M, Suzuki Y, Kawaguchi T, Aoki N, et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J Gastroenterol. 2013;48:1051–1060. doi: 10.1007/s00535-012-0704-y. [DOI] [PubMed] [Google Scholar]
- 13.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 16.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 18.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286–5296. doi: 10.3748/wjg.v16.i42.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 23.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 24.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 26.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 27.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 28.Miyaaki H, Ichikawa T, Nakao K, Yatsuhashi H, Furukawa R, Ohba K, Omagari K, Kusumoto Y, Yanagi K, Inoue O, et al. Clinicopathological study of nonalcoholic fatty liver disease in Japan: the risk factors for fibrosis. Liver Int. 2008;28:519–524. doi: 10.1111/j.1478-3231.2007.01614.x. [DOI] [PubMed] [Google Scholar]
- 29.Schepis F, Cammà C, Niceforo D, Magnano A, Pallio S, Cinquegrani M, D’amico G, Pasta L, Craxì A, Saitta A, et al. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology. 2001;33:333–338. doi: 10.1053/jhep.2001.21410. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi M, Maggioli C, Zaccherini G. Human albumin in the management of complications of liver cirrhosis. Crit Care. 2012;16:211. doi: 10.1186/cc11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95–106. doi: 10.1038/ncpgasthep1025. [DOI] [PubMed] [Google Scholar]
- 33.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, Munteanu M, Thabut D, Cadranel JF, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 35.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]


