Abstract
AIM: To report our experience with long-term outcomes after multimodal management therapy.
METHODS: An observational retrospective study was performed containing seven patients with hepatoblastoma (Hbl) treated in our institution, a tertiary referral center, from 2003 to 2011. Demographic, preoperative, surgical, and outcome variables were collected. A survival analysis and a review of the current literature related to combination neoadjuvant chemotherapy and surgical resection on Hbl were performed.
RESULTS: The median age at surgery was 14.4 mo, with a male to female ratio of 4:3. Pretext staging at diagnosis was as follows: stage I, 4 cases; stage II, 2 patients; and stage III, 1 case. Mean pretreatment tumor volume was 735 cm3. Five out of seven patients received neoadjuvant chemotherapy according to SIOPEL-3 or SIOPEL-6 protocols. Tumor volume and alpha-fetoprotein levels significantly dropped after neoadjuvant therapy. Surgical procedures performed included hemihepatectomies, segmentectomies and atypical resection. All patients received chemotherapy after surgery. Median postoperative hospital stay was 8 d. All patients were alive and disease-free after a median follow-up period of 23 mo. With regards to the literature review, seventeen articles were found that were related to our search.
CONCLUSION: Our series shows how multimodal management of Hbl, exhaustive control and a meticulous surgical approach leads to almost 100% complete resection with optimal postoperative results.
Keywords: Liver tumors, Chemotherapy, Liver surgery, Multimodal management
Core tip: Complete surgical resection is the cornerstone of treatment for hepatoblastoma (Hbl), but less than 40% of patients have resectable disease at diagnosis. Our experience with long-term outcomes after multimodal management therapy and a review of the literature are reported. An observational retrospective study was performed, including seven patients with Hbl treated in our institution, a tertiary referral center, from 2003 to 2011. Our series shows how multimodal management of Hbl, exhaustive control and a meticulous surgical approach leads to almost 100% complete resection with optimal postoperative results.
INTRODUCTION
Hepatic neoplasms represent 1% of childhood malignant tumors[1]. Hepatoblastoma (Hbl) is the most frequent tumor type, accounting for almost two-thirds of primary malignant liver tumors in children, with an overall incidence of 1.5 cases per million population[2,3]. Two thirds of these tumors occur in the first 2 years of life[4].
Most children with Hbl present with an enlarging abdominal mass, and in 70% of cases are in an advanced stage at diagnosis[2]. Complete surgical resection is the cornerstone of treatment[5]; however less than 40% of patients have resectable disease at diagnosis due to local invasion, caval infiltration, or distant metastases[6]. In the early 1970s, some studies reported the response of Hbl to chemotherapy. The International Society of Pediatric Oncology (SIOP) was a pioneer in the concept of neoadjuvant chemotherapy for the management of hepatoblastoma[7,8]. Using neoadjuvant chemotherapy, 28% of patients may be down-staged, complete macroscopic resection may be achieved in 87%-91% of cases, and morbidity and mortality rates have decreased to 18% and 5%, respectively[5,9]. Chemotherapy is used to reduce tumor size in lesions that appear unresectable at diagnosis and to control residual microscopic disease after definitive resection[9]. Orthotopic liver transplantation (OLT) is an effective therapy for selected malignancies in childhood, such as multifocal Hbl without extrahepatic disease, type-2 hemangioendotheliomas, and hepatocellular carcinoma with tumors < 5 cm without vascular invasion[10].
Despite its effectiveness, isolated surgical resections may not be enough to control disease spread. Moreover, locally advanced Hbl may require extensive liver resections that may lead to increased postoperative morbidity and mortality. Few series have reported the efficacy of neoadjuvant chemotherapy and surgical resection for Hbl. We therefore report our experience with long-term outcomes of Hbl after multimodal management therapy.
MATERIALS AND METHODS
All patients diagnosed with Hbl and treated at our institution, a tertiary referral center, between 2003 and 2011 were included in this observational retrospective analysis. According to imaging techniques, such as computerized tomography (CT) scan or magnetic resonance imaging (MRI), all patients were assigned a PRETEXT (pre-treatment extent of disease) stage, with four groups of patients identified as PRETEXT I-IV, both at diagnosis and after preoperative chemotherapy, according to the classification proposed by the Liver Tumors Strategy Group (SIOPEL) for their SIOPEL-1 study[11]. Standard follow-up was based on serial AFP levels every three months for the first year and every six months for the next ten years. MRI every six months was our protocoled imaging technique for the immediate 5 years after surgery.
Demographic (age, gender, and weight); preoperative [initial presentation, alpha-fetoprotein (αFP) levels, location and volume of the tumor, method of diagnosis, tumor spread at diagnosis, and preoperative chemotherapy]; surgical (procedure performed, tumor characteristics, histology, margins, and vascular invasions); and outcome (complications of treatment, length of hospital stay, recurrence, and overall and disease-free survival) variables were collected.
A review of the literature was carried out to identify all series that reported hepatoblastomas treated with a combination of neoadjuvant chemotherapy and surgical resection. The Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and MEDLINE databases were searched using the keywords (preoperative chemotherapy OR neoadjuvant treatment OR locally advanced) AND (hepatoblastoma) to identify studies published up to September 2012. Free text words were used instead of MeSH terms to avoid missing recent articles that had not yet been given a MeSH label. Two investigators independently performed the literature search. Electronic links to related articles and references of selected articles were hand-searched as well. The search was not restricted to any language, but only studies published in English were taken into account.
Statistical analysis
Data were expressed as median and range. Independent and paired non-parametric tests were used for baseline comparisons. SPSS software 14.0 was used for statistical analysis.
RESULTS
Descriptive results
Seven children with Hbl were referred to our hospital between 2003 and 2012. The male to female ratio was 4:3. The median age at surgery was 14.4 mo (range, 3-31 mo). Golabi-Behmel syndrome (congenital syndrome X-linked with an increased risk of embryonal cancer)[12] was associated in a male patient, but none of the patients were preterm. Median weight of the patients prior to the surgery was 9.26 kg (range, 4.8-13.5 kg). The most common symptom found was a palpable abdominal mass (85%). The median αFP level at diagnosis was 141.7 ng/mL (range, 379-483756 ng/mL). Thrombocytosis was found in 71.4% of cases. PRETEXT staging at diagnosis was as follows: Stage I, 4 patients; Stage II, 2 cases; and Stage III, 1 patient. There were no metastases at diagnosis. An additional criterion of PRETEXT staging, P1 (the involvement of either the left or right branch of the portal vein), was suspected in three cases (two right branches and one left branch)[13]. Mean tumor volume at diagnosis was 735 cm3 (range, 150-1950 cm3) (Table 1).
Table 1.
Patients features and outcomes
| Age (yr) | PRE-TEXT pre-QT | Segments involved at diagnosis | AFP at diagnosis (ng/mL) | Histology | Chemotherapy (No. of cycles) | POST-TEXT at surgery (localization) | Surgery | Postoperative events | Follow-up (mo) | Current status |
| 3 | III, P1 (right branch) | I, V, VI, VII, VIII | 18597.37 | Embryonal | Neoadjuvant PLA (4 cycles) + adjuvant (2c) | II (V, VI, VII, VIII) | Right hepatectomy | Subphrenic abscess (drainage) | 18 | CR |
| 4 | II, P1 (left branch) | IVb, V, VIII | 551.21 | Epithelial mesenchymal mixed | Neoadjuvant PLA (4c) + adjuvant (2c) | I (IVb) | Segmentectomy IVb | Uneventful | 19 | CR |
| 8 | I | II, III | 473856.00 | Epithelial fetal | Neoadjuvant PLADO (4c) + adjuv (2c) | I (II, III) | Left hepatectomy | Uneventful | 56 | CR |
| 13 | I | VIII | 14277.00 | Epithelial fetal | Neoadjuvant PLA (4c) + adjuv (2c) | I (VIII) | Right hepatectomy | Uneventful | 28 | CR |
| 15 | I | VI | 401800.00 | Epithelial mesenchymal mixed | Adjuvant PLADO (4c) | I (VI) | Tumorectomy | Uneventful | 106 | CR |
| 25 | II, P1 (right branch) | V, VI, VII, VIII | 83100.50 | Embryonal fetal epithelial | Neoadjuvant PLADO (4c) + adjuv (2c) | II, P0 (VII, VIII) | Bisegmentectomy VII, VIII | Uneventful | 13 | Lung metastasis |
| 31 | I | VI | 379.00 | Epithelial | Adjuvant PLADO (4c) | I (VI) | Segmentectomy VI | Uneventful | 50 | CR |
PLA: Cisplatin; DO: Doxorubicin; P1: Involvement of either the left or the right branch of the portal vein; CR: Complete remission.
Therapeutic approach to locally advanced hepatoblastoma
Chemotherapy was given to all patients. Five out of the seven patients received four cycles of pre-operative neoadjuvant chemotherapy, with additional two post-surgery. The remaining two patients underwent primary surgery and received four cycles of adjuvant chemotherapy. The pathological diagnosis of Hbl was confirmed by percutaneous biopsy previous to neoadjuvant chemotherapy in all cases. Chemotherapy regimens included the SIOPEL study protocols. PLADO regimen or SIOPEL-3 protocols (platinum on day 1 at a dose of 2.7 mg/kg per day and doxorubicin at a dose of 1 mg/kg per day on day 2 and 3 mg/kg per day every 20 d) was used in four patients, and cisplatin alone or SIOPEL-6 (at a dose of 2.7 mg/kg per day) was used in three patients. Neutropenia cases were treated with granulocyte stimulating grow factor, and trimethoprim/sulfamethoxazole was used for prophylaxis against pneumocystis pneumonia. Neither mortality nor long-term toxicity related with chemotherapy was reported. The patients who underwent neoadjuvant chemotherapy were reassessed every two months and all of them received four cycles before surgery.
Tumor volume significantly dropped after neoadjuvant chemotherapy (from an initial median of 735-287 cm3; P = 0.02). The same happened to αFP levels, although statistical significance was not reached (from pretreatment median of 141-7.9 ng/mL; P = 0.10) (Figure 1).
Figure 1.
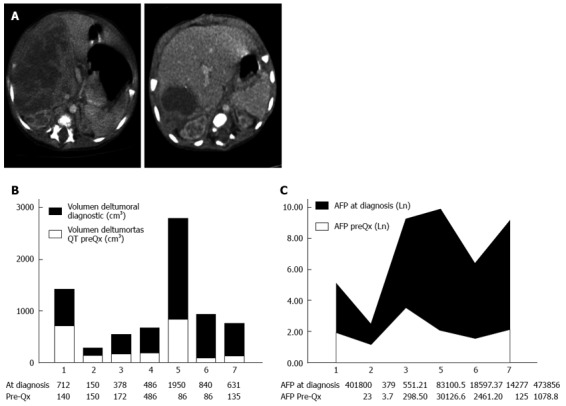
Changes after neoadjuvant chemotherapy. A: Computerized tomography: Down-staging effect of neoadjuvant chemotherapy; B: Tumor volume at diagnosis and before surgery; C: AFP level at diagnosis and before surgery.
Surgical procedures performed included right hepatectomy in two patients, left hepatectomy in one, bisegmentectomy VII-VII in one, segmentectomy VI and IVb in two, and an atypical resection of a pediculated tumor arising from segment VI in one patient. The only postoperative complication was a subphrenic abscess that required percutaneous drainage. The median postoperative hospital stay was 8 d (range, 5-26 d).
The histological types encountered were as follows: embryonal and mixed embryonal/fetal subtype in three patients, mixed epithelial and mesenchymal type in two patients, and purely fetal type in two patients. All specimens had tumor-free margins.
Review of current literature
Using the aforementioned criteria, seventeen articles were found that included case reports. We selected articles in which most of the study patients had been treated with neoadjuvant chemotherapy and surgical resection, as well as those reporting locally-advanced hepatoblastomas (stage post-TEST III or IV). Seven studies were included (Table 2).
Table 2.
Review of current literature
| Study | NACH/N (n) | Gender (M/F) | Age (mo) | CH regimen | CH morbidity | PR CH | Complete resection | Postoperative events | OS | DFS |
| Pritchard et al[8], 2000 SIOPEL-1 | 138/154 | 97/ 57 | 16.5 | PLADO (cis+dox) | 2% death 6% myelotoxicity < 2% others | 82% | 92% | 5 deaths 8% infections 3% bleeding 9% others | 75% | 66% |
| Katzenstein et al[18], 2002 | 33/33 | 21/12 | 22 | Car-vin-5FU | 60% myelotoxicity | 82% | 58% | - | 57% | 48% |
| Perilongo et al[14], 2004 SIOPEL-2 | 135/135 | 81/54 | 16-25 | Cis Car-dox-cis | Neutropenia 43%-81% Infections 40%-76% Transfusion 19%-76% | 90% (SR HB) 78% (HR HB) 90% (SR-HR) | 97% (SR HB) 67% (HR HB) 100% (SR-HR) | - | 91% (SR HB) 53% (HR HB) 86% (SR-HR) | 89% (SR HB) 48% (HR HB) 89% (SR-HR) |
| Towu et al[16], 2004 | 54/56 | 34/22 | 12 | 22 PLADO 14 SIOPEL-2 17 SIOPEL-3 | - | 92% | 74% | 1 death 22% (bile leakage, collections, others) | 75% | - |
| Zsíros et al[15], 2010 SIOPEL-3 | 150/151 | 90/61 | 21 | Cis/car-dox | 1 death 76% neutropenia 51% infections 33% renal toxicity | 78.70% | 76.20% | 4 deaths | 69%1 | 65% |
| Lautz et al[19], 2010 | 14/14 | 7/7 | 8 | Cis-vin-5FU Others | - | 61% | 85% | 1 Iscq cholangio 1 portal thrombosis | 88% | 77% |
| Hishiki et al[17], 2011 | 185/212 | 132/80 | 17 | Cis-pir Iof-car-pir-eto | 90% neutropenia 10% infections < 10% others | 65% | 63% | - | 81% | 62.4% |
3-year overall survival. NACH: Number of patients with neoadjuvant chemotherapy; N: Total number of patients; CH: Chemotherapy; PR: Partial response; OS: 5-year overall survival; DFS: Disease-free survival; cis: Cisplatin; dox: Doxorubicin; car: Carboplatin; pir: Pirarubicin; iof: Ifosfamide; eto: Etoposide; vin: Vincristine; 5FU: 5-fluorouracil; SR HB: Standard risk hepatoblastoma; HR HB: High risk hepatoblastoma; SR-HR: Standard risk hepatoblastoma treated as high risk hepatoblastoma.
Pritchard et al[8] published in 2000 the first international study (SIOPEL-1), applying preoperative chemotherapy (PLADO: cisplatin plus doxorubicin) and delayed surgery. However, the prognosis with advanced stages remained unsatisfactory. To improve the survival of these patients, the SIOPEL group intensified the chemotherapy in their subsequent studies. Thus, in the SIOPEL-2 study the patients were classified in two groups: one for patients with Hbl confined to the liver and involving no more than three hepatic sectors (standard-risk Hbl) treated with cisplatin alone every 14 d; and one for those with Hbl extending into all four sectors and/or with lung metastases or intra-abdominal extra hepatic spread (high-risk Hbl) treated with cisplatin alternating every 14 d with carboplatin and doxorubicin. In 2004, Perilongo et al[14] published their results in which, despite chemotherapy intensification, only half of the high-risk Hbl patients were long-term survivors. Later, the SIOPEL-3 study[15] showed an improved survival in this group of patients. This study was designed to test the efficacy of this treatment strategy including only high-risk Hbl patients: tumor in all liver sections, vascular invasion, extrahepatic extension, metastatic disease or αFP less than 100 ng/mL at diagnosis.
In addition, the results of an English institution with 54 patients of all stages were reported by Towu et al[16] in 2004. Hishiki et al[17] published the results of 185 patients in a Japan centre in 2011, as well. Additionally, we have considered two studies that regard only patients with advanced stages: Katzenstein et al[18] include thirty three patients with stage III (unresectable or nodal involvement) and IV Hbl (metastatic disease), and Lautz et al[19] who include fourteen patients underwent resection for POST-TEXT IV or centrally located POST-TEXT III after neoadjuvant chemotherapy.
Statistical analysis
All patients are alive after a median follow-up period of 23 mo (range, 18-111 mo). The median disease-free survival is 23 mo (range, 6-111 mo). One patient developed distant metastases in the middle right lobe lung six months later that required a pulmonary atypical resection; after 18-mo follow-up the patient is free of disease. The remaining 6 cases have no evidence of recurrence or a rise in AFP levels.
DISCUSSION
Management of Hbl has evolved from unresectable or extensive surgical resections with high rates of morbidity and mortality to the current standard of care, namely neoadjuvant chemotherapy followed by surgery[20]. Complete tumor resection is essential for cure; therefore, any strategy that may reduce tumor volume, and thus lead to an increased resection rate, would provide a survival benefit[21]. An initial surgical approach may be acceptable for resectable disease, but a combined approach may be preferable in advanced stages[18]. Our series shows how multimodal management, exhaustive control, and a meticulous surgical approach led to almost 100% complete resection with optimal postoperative results. However, we are aware that our study is limited by its small sample size.
The prognosis for children with Hbl has improved over the last few decades; survival in the 1970s with surgical resection alone was about 10%-20%[22]. After the routine use of preoperative cisplatin and doxorubicin (PLADO regimen), surgical resection was soon achieved in 87% of cases, whereas historically only 30% of cases were operable upfront[23]. Liver transplantation later proved to be an effective treatment for certain children with Hbl. Criteria established by SIOPEL recommend that it should therefore be considered in patients with neoplasm in all 4 liver sections, tumor extension into the vena cava or all 3 hepatic veins, invasion of the main and/or left and right portal veins, or recurrent disease after resection (rescue transplant)[24]. In our cohort, liver transplantation was not performed in any patient.
Unfortunately, although results have improved, complete resection rates are still between 60%-75% and free-of-disease survival rates between 65%-80%. In our series, surgical outcomes and hospital stay are in accordance with the literature. The experience of an active pediatric liver transplant program would surely be helpful for this multidisciplinary approach and for getting optimal surgical resections.
Our results support the key role of neoadjuvant chemotherapy when the tumor appears in advanced stages and a complete resection at initial diagnosis is unlikely to occur. In addition, preoperative chemotherapy has led to an increase in surgical resection rates, allowing more limited hepatectomies and decreasing the rate of postoperative complications. Postoperative chemotherapy shows also good results, thereby avoiding reoperation for positive resection margins. Multidisciplinary management of Hbl is mandatory, as the childhood population is especially susceptible for complications during surgical procedure. The combination of chemotherapeutic regimes and surgical techniques has shown to be the best treatment option, and has led to improved free-of-disease rates and long-term survival.
COMMENTS
Background
Hepatoblastoma (Hbl) is the most frequent malignant liver tumor in childhood. Complete surgical resection is the most important treatment, but a limited percentage have resectable disease at diagnosis. The International Society of Pediatric Oncology (SIOP) was a pioneer in the concept of neoadjuvant chemotherapy for the management of these neoplasms. Multimodal management is currently the best treatment option for Hbl.
Research frontiers
Neoadjuvant chemotherapy is used in many tumors in order to reduce the tumor size, allow complete resection, and to control residual microscopic disease. In the area of Hbl management, the current research hotspot is how a multimodal therapy with several regimes of preoperative chemotherapy may increase the rate of feasible surgical resection, improve postoperative morbidity, and consequently get better long-term survival outcomes, especially in advanced Hbl at diagnosis.
Innovations and breakthroughs
Although there are several studies in the literature that report the benefit of a multidisciplinary treatment for Hbl in the last few decades, there are fewer studies concerning Hbl in its advanced stages and the prognosis for such cases remained unsatisfactory until recently. Three international studies conducted by the SIOP published their results applying preoperative chemotherapy in progressive stages. Their survival outcomes showed an improvement along the sequential studies, especially with regards to high-risk Hbl. Unfortunately, despite improving results, complete resection rates cannot be always achieved. The current report contains a series of cases, including advanced stages of Hbl, with very good surgical and survival outcomes.
Applications
The results support the key role of multidisciplinary therapy, such as neoadjuvant chemotherapy in unresectable tumors upfront, complete surgical resection, and postoperative chemotherapy.
Terminology
Cisplatin is a chemotherapy drug. It was the first member of a class of platinum-containing anti-cancer drugs, which now also includes carboplatin and oxaliplatin. Doxorubicin is an antineoplastic chemotherapy drug that is a standard component in treating many types of tumors.
Peer review
This is an interesting study in which the authors reported the experience of a single center in the management of Hbl over ten years with a multimodal management therapy. The results are in accordance with the literature and suggest that it is the best option for improving the prognosis of Hbl.
Footnotes
P- Reviewer: Dziri C, Wang JG S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Ishak KG, Glunz PR. Hepatoblastoma and hepatocarcinoma in infancy and childhood. Report of 47 cases. Cancer. 1967;20:396–422. doi: 10.1002/1097-0142(1967)20:3<396::aid-cncr2820200308>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Herzog CE, Andrassy RJ, Eftekhari F. Childhood cancers: hepatoblastoma. Oncologist. 2000;5:445–453. doi: 10.1634/theoncologist.5-6-445. [DOI] [PubMed] [Google Scholar]
- 3.Reyes JD, Carr B, Dvorchik I, Kocoshis S, Jaffe R, Gerber D, Mazariegos GV, Bueno J, Selby R. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr. 2000;136:795–804. [PubMed] [Google Scholar]
- 4.Ross JA. Hepatoblastoma and birth weight: too little, too big, or just right? J Pediatr. 1997;130:516–517. [PubMed] [Google Scholar]
- 5.Schnater JM, Aronson DC, Plaschkes J, Perilongo G, Brown J, Otte JB, Brugieres L, Czauderna P, MacKinlay G, Vos A. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer. 2002;94:1111–1120. [PubMed] [Google Scholar]
- 6.Shukla PJ, Barreto SG, Qureshi SS, Hawaldar R, Shrikhande SV, Ramadwar MR, Banavali S. Hepatoblastoma: a single institutional experience of 18 cases. Pediatr Surg Int. 2008;24:799–802. doi: 10.1007/s00383-008-2169-x. [DOI] [PubMed] [Google Scholar]
- 7.von Schweinitz D, Byrd DJ, Hecker H, Weinel P, Bode U, Bürger D, Erttmann R, Harms D, Mildenberger H. Efficiency and toxicity of ifosfamide, cisplatin and doxorubicin in the treatment of childhood hepatoblastoma. Study Committee of the Cooperative Paediatric Liver Tumour Study HB89 of the German Society for Paediatric Oncology and Haematology. Eur J Cancer. 1997;33:1243–1249. doi: 10.1016/s0959-8049(97)00095-6. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks-Mireaux C, Keeling J, Phillips A, Vos A, Plaschkes J. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–3828. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 9.Tsay PK, Lai JY, Yang CP, Hung IJ, Hsueh C, Tsai MH, Jaing TH. Treatment outcomes for hepatoblastoma: experience of 35 cases at a single institution. J Formos Med Assoc. 2011;110:322–325. doi: 10.1016/S0929-6646(11)60048-X. [DOI] [PubMed] [Google Scholar]
- 10.Malek MM, Shah SR, Atri P, Paredes JL, DiCicco LA, Sindhi R, Soltys KA, Mazariegos GV, Kane TD. Review of outcomes of primary liver cancers in children: our institutional experience with resection and transplantation. Surgery. 2010;148:778–782; discussion 782-784. doi: 10.1016/j.surg.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Perilongo G, Shafford E, Plaschkes J. SIOPEL trials using preoperative chemotherapy in hepatoblastoma. Lancet Oncol. 2000;1:94–100. doi: 10.1016/s1470-2045(00)00018-8. [DOI] [PubMed] [Google Scholar]
- 12.DeBaun MR, Ess J, Saunders S. Simpson Golabi Behmel syndrome: progress toward understanding the molecular basis for overgrowth, malformation, and cancer predisposition. Mol Genet Metab. 2001;72:279–286. doi: 10.1006/mgme.2001.3150. [DOI] [PubMed] [Google Scholar]
- 13.Otte JB. Progress in the surgical treatment of malignant liver tumors in children. Cancer Treat Rev. 2010;36:360–371. doi: 10.1016/j.ctrv.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Perilongo G, Shafford E, Maibach R, Aronson D, Brugières L, Brock P, Childs M, Czauderna P, MacKinlay G, Otte JB, et al. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer. 2004;40:411–421. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Zsíros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A, Laithier V, et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28:2584–2590. doi: 10.1200/JCO.2009.22.4857. [DOI] [PubMed] [Google Scholar]
- 16.Towu E, Kiely E, Pierro A, Spitz L. Outcome and complications after resection of hepatoblastoma. J Pediatr Surg. 2004;39:199–202; discussion 199-202. doi: 10.1016/j.jpedsurg.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Hishiki T, Matsunaga T, Sasaki F, Yano M, Ida K, Horie H, Kondo S, Watanabe K, Oue T, Tajiri T, et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2: report from the JPLT. Pediatr Surg Int. 2011;27:1–8. doi: 10.1007/s00383-010-2708-0. [DOI] [PubMed] [Google Scholar]
- 18.Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu-Mares W, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Newman K, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. J Clin Oncol. 2002;20:2789–2797. doi: 10.1200/JCO.2002.06.155. [DOI] [PubMed] [Google Scholar]
- 19.Lautz TB, Ben-Ami T, Tantemsapya N, Gosiengfiao Y, Superina RA. Successful nontransplant resection of POST-TEXT III and IV hepatoblastoma. Cancer. 2011;117:1976–1983. doi: 10.1002/cncr.25722. [DOI] [PubMed] [Google Scholar]
- 20.Cyriac S, Seshadri RA, Warrier A, Sagar TG. Hepatoblastoma: Analysis of treatment outcome from a tertiary care center. J Indian Assoc Pediatr Surg. 2011;16:11–14. doi: 10.4103/0971-9261.74514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana AN, Qidwai A, Pritchard J, Ashraf MS. Successful treatment of multifocal unresectable hepatoblastoma with chemotherapy only. Pediatr Hematol Oncol. 2006;23:153–158. doi: 10.1080/08880010500457566. [DOI] [PubMed] [Google Scholar]
- 22.Hermann RE, Lonsdale D. Chemotherapy, radiotherapy, and hepatic lobectomy for hepatoblastoma in an infant: report of a survival. Surgery. 1970;68:383–388. [PubMed] [Google Scholar]
- 23.Ninane J, Perilongo G, Stalens JP, Guglielmi M, Otte JB, Mancini A. Effectiveness and toxicity of cisplatin and doxorubicin (PLADO) in childhood hepatoblastoma and hepatocellular carcinoma: a SIOP pilot study. Med Pediatr Oncol. 1991;19:199–203. doi: 10.1002/mpo.2950190310. [DOI] [PubMed] [Google Scholar]
- 24.Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J, Brock P, Dicks-Mireaux C, Phillips A, Vos A, Plaschkes J. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418–1425. doi: 10.1016/s0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]


