Abstract
AIM: To compare clinical outcomes between surgical resection (RES) and nonsurgical-RES (nRES) ablation therapies for small hepatocellular carcinoma (HCC).
METHODS: MEDLINE, Embase and Cochrane Library databases were systematically searched for studies of RES and nRES treatments for small HCC between January 2003 and October 2013. The clinical outcome measures evaluated included overall survival rate, disease-free survival rate, adverse events, and local recurrence rate. Odds ratios (ORs) with 95%CIs were calculated using either the fixed effects model or random effects model. The χ2 and I2 tests were calculated to assess the heterogeneity of the data. Funnel plots were used to assess the risk of publication bias.
RESULTS: Our analysis included 12 studies that consisted of a total of 1952 patients (RES vs nRES), five studies that consisted of 701 patients [radiofrequency ablation (RFA) vs percutaneous ethanol injection (PEI)], and five additional studies [RFA vs RFA + transcatheter arterial chemoembolization (TACE)] that all addressed the treatment of small HCC. For cases of RES vs nRES, there was no significant difference in the 1-year (OR = 0.99, 95%CI: 0.87-1.12, P = 0.85) or 3-year (OR = 0.97, 95%CI: 0.84-1.11, P = 0.98) overall survival rate; however, there was a significant increase in the RES group in the 5-year overall survival rate (OR = 0.81, 95%CI: 0.68-0.95, P = 0.01). The 1-year (OR = 0.94, 95%CI: 0.82-1.08, P = 0.37) and 5-year (OR = 0.99, 95%CI: 0.85-1.14, P = 0.85) disease-free survival rates showed no significant differences between the two groups. The 3-year disease-free survival rate (OR = 0.81, 95%CI: 0.69-0.96; P = 0.02) was higher in the RES group. For cases of RFA vs PEI, our data analysis indicated that RFA treatment was associated with significantly higher 2-year (OR = 0.76, 95%CI: 0.58-0.99, P = 0.043) and 3-year (OR = 0.73, 95%CI: 0.54-0.98, P = 0.039) overall survival rates; however, there were no significant differences in the 1-year (OR = 0.92, 95%CI: 0.72-1.17, P = 0.0502) overall survival rate or incidence of adverse events (OR = 1.84, 95%CI: 0.76-4.45, P = 0.173). For cases of RFA vs RFA+TACE, there were no significant differences in the 1-year (OR = 1.17, 95%CI: 0.88-1.56, P = 0.27) or 3-year (OR = 1.25, 95%CI: 0.90-1.73, P = 0.183) overall survival rate; however, the 5-year overall survival rate (OR = 3.19, 95%CI: 1.51-6.74, P = 0.002) in patients treated by RFA+TACE was higher than that treated by RFA alone.
CONCLUSION: Surgical resection is superior to nonsurgical ablation for the treatment of small HCC. Among the studies analyzed, RFA is the most efficacious single nonsurgical ablation treatment.
Keywords: Hepatocellular carcinoma, Meta-analysis, Surgical resection, Nonsurgical ablation, Radiofrequency ablation, Percutaneous ethanol injection, Recurrence, Survival
Core tip: The efficacy of surgical resection over nonsurgical ablation for the treatment of small hepatocellular carcinoma has long been debated. In previous studies, a meta-analysis was conducted on cases of resection (RES) vs radiofrequency ablation or RES vs other single nonsurgical ablation methods. Our meta-analysis was designed to determine the superior choice of treatment for small hepatocellular carcinoma from among surgical resection and nonsurgical ablation methods.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common malignancy, its incidence is increasing worldwide, and it is responsible for thousands of deaths every year[1]. The definition of small HCC in the Milan criteria consists of a single HCC nodule < 5 cm or up to 3 nodules and a maximum diameter of each nodule < 3 cm. As a result of technological improvements, the number of therapeutic modalities available for HCC has increased dramatically. In addition to the traditional surgical resection and liver transplantation, other surgical therapies, such as transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and percutaneous microwave coagulation therapy (MCT), have also been used[2]. Theoretically, the best treatment for small HCC is liver transplantation, but the scarcity of donor organs and high costs constrain the use of this treatment[3,4]. Therefore, the demand for novel treatment strategies for small HCC has been raised in both surgical resection (RES) and non-nonsurgical RES (nRES) cases.
Both RES and nRES treatments are recommended, and the recommendations range from an evidence-based guideline in Japan to guidelines established by the American Association for the Study of Liver Disease[5,6]. RES has been used as the standard treatment for small HCC for a long period, and in clinical studies, Takayama et al[7] reported that RES had more advantages over other treatments (i.e., survival and recurrence rate) regardless of the tumor size. However, other clinical outcomes showed that some nonsurgical ablation methods, such as RFA, could achieve a similar therapeutic effect while significantly avoiding postoperative complications[8]. Thus, alternative therapies for small HCC are controversial. In previous studies, a meta-analysis was conducted on cases of RES vs RFA or RES vs other single nonsurgical ablation methods. Our meta-analysis was designed to determine the superior choice for treatment of small HCC out of surgical resection and nonsurgical ablation methods.
MATERIALS AND METHODS
Study selection
A search was performed using MEDLINE, Embase, and Cochrane Library databases for publications dated from January 2003 to October 2013 by two investigators separately. The corresponding author was consulted when the criteria for inclusion or exclusion of a study were controversial. The following MeSH search headings in English were used: hepatocellular carcinoma, HCC, liver cancer, hepatic tumor, liver resection, surgical resection, hepatectomy, radiofrequency ablation, microwave, high-intensity focused ultrasound, cryoablation, ethanol, and acetic acid.
Criteria for inclusion
To be eligible for the meta-analysis, a study had to fulfill the following criteria: (1) treatment of HCC by RES vs nRES or nRES vs nRES; (2) tumor size meeting the Milan criteria; (3) no antitumor treatment before the intervention; (4) no previous or simultaneous malignancies; and (5) description of the details of overall survival rate, recurrence-free survival rate, tumor progression rate, and major complications.
Criteria for exclusion
The study was excluded if it (1) dealt with recurrent HCC or metastatic carcinoma; (2) did not have appropriate data or could not allow to extract available data from the published results; or (3) was an abstract or review without original data. If two or more similar studies were reported by the same author in one institution, the one with higher quality was included or the largest one was included if they were of same quality.
Statistical analysis
The meta-analysis was performed using State Software (State 12). The odds ratios (OR) with 95%CI were calculated using either the fixed-effect or random-effect model depending on the absence or presence of significant heterogeneity. The χ2 and I2 tests were calculated to assess heterogeneity. We considered an I2 > 50% as significant heterogeneity, and a P-value < 0.05 was considered statistically significant. If P < 0.05 and I2 > 50%, the random-effect model was used; otherwise, the fixed effects model was used. Funnel plots were used to assess the risk of publication bias.
RESULTS
Included studies
MEDLINE, Embase, and Cochrane Library databases were systematically searched for studies on RES vs nRES or nRES vs nRES treatments for small HCC published between January 2003 and October 2013. The identification of studies for inclusion was shown in Figure 1. Twelve studies of RES vs RES[9-20] (2 RCTs, Huang et al[9] and Chen et al[10]), five studies[21-25] of RFA vs PEI (5 RCTs), and five studies[26-30] of RFA vs RFA + TACE (1 nRCT, Zhao et al[27]) were included.
Figure 1.
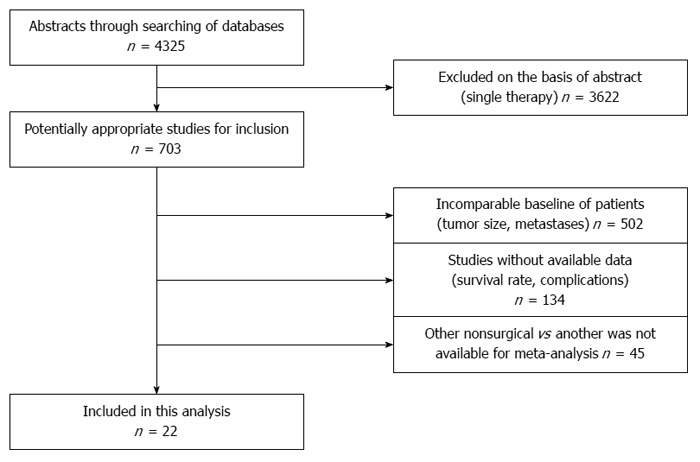
Identification of studies for inclusion in the meta-analysis.
Out of a total of 1952 patients, 953 were allocated to the RES group and 999 to the nRES group to evaluate the treatment options used for HCC. The age, mean tumor size, and mean AFP were well controlled by the study authors when patients were enrolled. Dynamic CT was performed in follow-up, and the follow-up time was considered to be sufficient. Main serious complications included liver failure, biliary fistula, abdominal bleeding, wound infection or dehiscence in the surgical group and pleural effusion, liver abscess, and abdominal bleeding in the RFA group. The demographic and clinical characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of cases using surgical resection vs nonsurgical ablations
| Ref. | Treatment | M/F | Mean tumor size (cm) | Mean age (yr) | Mean AFP (ng/mL) | Child-Pugh A/B | Mean follow-up (mo) |
| Huang et al[9] | PEI | 19/19 | ≤ 2.0 (n = 21) | 63 ± 10.9 | > 200 (n = 7) | 29/3 | 37.7 ± 14.5 |
| RES | 27/11 | ≤ 2.0 (n = 24) | 59 ± 11.4 | > 200 (n = 8) | 28/0 | 38.4 ± 16.4 | |
| Chen et al[10] | RFA | 56/15 | ≤ 3.0 (n = 37) | 51.9 ± 11.2 | > 200 (n = 31) | 71/0 | 27.9 ± 10.6 |
| RES | 75/15 | ≤ 3.0 (n = 42) | 49.4 ± 10.9 | > 200 (n = 30) | 90/0 | 29.2 ± 11.9 | |
| Huang et al[11] | RFA | 85/30 | ≤ 3.0 (n = 45) | 55.91 ± 12.68 | > 400 (n = 32) | 106/9 | 37.2 (6.0-60) |
| RES | 79/36 | ≤ 3.0 (n = 57) | 56.57 ± 14.30 | > 400 (n = 21) | 110/5 | 46.4 (1.2-60) | |
| Feng et al[12] | RFA | 79/5 | ≤ 2.0 (n = 31) | 51 (24-83) | 215.5 (0.5-8530) | 39/45 | 36 |
| RES | 75/9 | ≤ 2.0 (n = 25) | 47 (18-76) | 262.8 (1.7-10220) | 43/41 | 36 | |
| Cho et al[13] | PEI | 86/30 | ≤ 2.0 (n = 43) | 58.0 ± 9.7 | > 20 (n = 47) | 92/24 | 68 |
| RES | 91/25 | ≤ 2.0 (n = 43) | 56.0 ± 8.9 | > 20 (n = 47) | 92/24 | 68 | |
| Abu-Hilal et al[14] | RFA | 27/7 | 3.8 (1.3-5) | 65 | - | 27/7 | 30 (0-60) |
| RES | 26/8 | 3.0 (2-5) | 67 | - | 25/9 | 43 (2-129) | |
| Ueno et al[15] | RFA | 100/55 | 2.7 ± 0.1 | 66 (40-79) | 131 ± 33 | - | 36.8 ± 1.5 |
| RES | 82/41 | 2.0 ± 0.1 | 67 (28-85) | 382 ± 108 | - | 35.0 ± 1.7 | |
| Kagawa et al[16] | RFA + TACE | 39/23 | ≤ 2.0 (n = 19) | 67.5 ± 8.4 | > 400 (n = 5) | - | 49 (1-102) |
| RES | 40/15 | ≤ 2.0 (n = 9) | 66.1 ± 8.4 | > 400 (n = 10) | - | 50 (9-95) | |
| Nishikawa et al[17] | RFA | 95/67 | 1.99 ± 0.62 | 68.4 ± 8.7 | 74.7 ± 181.1 | 102/22 | 37.2 (2.4-84) |
| RES | 50/19 | 2.68 ± 0.49 | 67.4 ± 9.7 | 376 ± 1989.8 | 45/5 | 39.6 (8.4-84) | |
| Guo et al[18] | RFA | 78/16 | ≤ 3.0 (n = 62) | 56 (19-75) | > 200 (n = 34) | 63/31 | 28 (7-75) |
| RES | 94/8 | ≤ 3.0 (n = 75) | 51.5 (18-75) | > 200 (n = 34) | 95/7 | 32 (6-86) | |
| Kim et al[19] | RFA + TACE | 31/6 | 3.46 ± 0.75 | 61.7 ± 11.1 | ≥ 100 (n = 7) | 37/0 | 29.9 ± 7.8 |
| RES | 36/11 | 3.66 ± 0.76 | 58.8 ± 10.7 | ≥ 100 (n = 14) | 45/2 | 31.7 ± 10 | |
| Lai et al[20] | RFA | 19/12 | 1.8 ± 0.6 | 63.1 ± 12.8 | 201.3 (2-2221.9) | - | 35.1 ± 17.4 |
| RES | 55/25 | 2.9 ± 1.1 | 60.8 ± 9.9 | 256.5 (91.5-5193) | - | 29.7 ± 19.9 |
RES: Resection; RFA: Radiofrequency ablation; PEI: Percutaneous ethanol injection; TACE: Transcatheter arterial chemoembolization; M: Male; F: Female; AFP: Alpha-fetoprotein.
A total of 701 patients in five RCT studies were analyzed to compare PEI and RFA methods for the treatment of small HCC. Dynamic CT was performed in follow-up, and the clinical characteristics of the patients are shown in Table 2.
Table 2.
Characteristics of cases involving percutaneous ethanol injection vs radiofrequency ablation
| Ref. | Treatment | M/F | Mean tumor size (cm) | Mean age (yr) | Mean AFP (ng/mL) | Child-Pugh A/B | Mean follow-up (mo) |
| Brunello et al[21] | PEI | 49/30 | 2.25 ± 0.54 | 70.3 ± 8.1 | 16.5 (MD) | 39/30 | 25.3 (MD) |
| RFA | 43/27 | 2.42 ± 0.49 | 69.0 ± 7.7 | 22.0 (MD) | 39/31 | 26.1 (MD) | |
| Lin et al[22] | PEI | 34/18 | 2.8 ± 0.9 | 67 ± 6.0 | > 400 (n = 8) | 39/12 | 23.8 ± 10.4 |
| RFA | 35/17 | 2.9 ± 0.8 | 59 ± 10 | > 400 (n = 7) | 41/11 | 24.5 ± 11.3 | |
| Shiina et al[23] | PEI | 87/27 | ≤ 2.0 (n = 57) | ≤ 65 (n = 41) | > 400 (n = 7) | 85/29 | 2.9 (MD) |
| RFA | 79/39 | ≤ 2.0 (n = 45) | ≤ 65 (n = 45) | > 400 (n = 6) | 85/33 | 3.1 (MD) | |
| Lin et al[24] | PEI | 39/23 | 2.3 ± 0.8 | 60 ± 8 | 400 (n = 9) | 47/15 | 26 ± 12 |
| RES | 30/22 | 2.5 ± 1.0 | 61 ± 10 | 400 (n = 10) | 46/46 | 28 ± 12 | |
| Lencioni et al[25] | PEI | 30/20 | 2.8 ± 0.8 | 69 ± 7.4 | 54 (MD) | 35/15 | 22.4 ± 8.6 |
| RFA | 36/16 | 2.8 ± 0.6 | 67 ± 6.0 | 57 (MD) | 45/7 | 22.9 ± 9.4 |
PEI: Percutaneous ethanol injection; RFA: Radiofrequency ablation; M: Male; F: Female; AFP: Alpha-fetoprotein; MD: Median; RES: Resection.
Five studies were used to compare TACE and radiofrequency ablation with radiofrequency ablation alone for small HCC; however, only Shibata et al[26] and Zhao et al[27] were specialized in the treatment of small HCC. We extracted data from Peng et al[28], Morimoto et al[29], and Cheng et al[30] because the tumor sizes met the Milan criteria; however, there were limited patient descriptions.
RES vs nRES
Overall survival rate: The meta-analysis shows that there was no significant difference between the two groups in the 1-year (all trials reported these data, OR = 0.99, 95%CI: 0.87-1.12, P = 0.85) or 3-year (11 trials reported these data, OR = 0.97, 95%CI: 0.84-1.11, P = 0.98) overall survival rate. However, there was a significant improvement in the 5-year overall survival rate in the RES group (nine trials reported these data, OR = 0.81, 95%CI: 0.68-0.95, P = 0.01) (Figure 2A).
Figure 2.
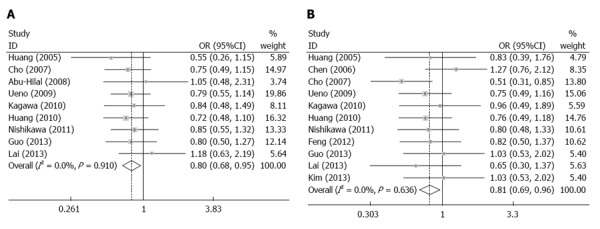
Comparison of methods for surgical resection and nonsurgical ablation for small hepatocellular carcinoma based on the overall survival rate and disease-free survival rate. A: 5-year overall survival rate; B: 3-year disease-free survival rate.
Disease-free survival rate: The meta-analysis shows that there was no significant difference in the 1-year (all trials reported these data, OR = 0.94, 95%CI: 0.82-1.08, P = 0.37) or 5-year (nine trials reported these data, OR = 0.99, 95%CI: 0.85-1.14, P = 0.85) disease-free survival rate between the two groups. The 3-year disease-free survival rate (11 trials reported these data OR = 0.81, 95%CI: 0.69-0.96, P = 0.02) was better in the RES group (Figure 2B).
Adverse events: The meta-analysis (six trials reported these data) showed that there was a significant decrease in the incidence of adverse events in the RES group (OR = 0.22, 95%CI: 0.15-0.34, P < 0.01).
Local recurrence rate: The local recurrence rate until the end of the follow-up period (four trials reported these data) was significantly higher in the nRES group when compared with the RES group (OR = 1.83, 95%CI: 1.07-3.13, P = 0.03).
RFA vs PEI
Survival rate: Our meta-analysis indicated that there was no significant difference in the 1-year (OR = 0.92, 95%CI: 0.72-1.17, P = 0.0502) overall survival rate between the two groups; however, RFA treatment resulted in significantly higher 2-year (OR = 0.76, 95%CI: 0.58-0.99, P = 0.043) and 3-year (OR = 0.73, 95%CI: 0.54-0.98, P = 0.039) overall survival rates (Figure 3A-C).
Figure 3.
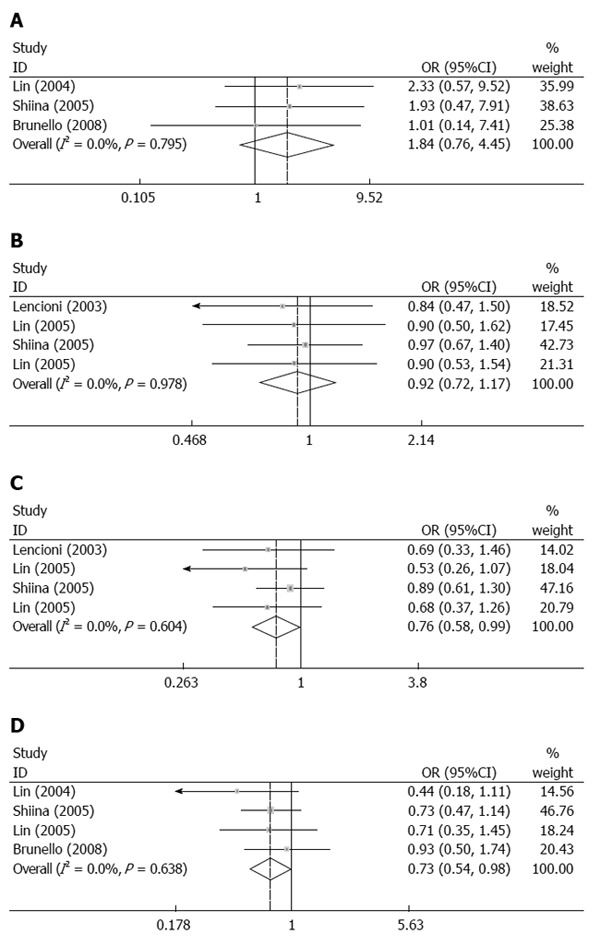
Comparison of adverse events and 1-, 2-, and 3-year overall survival rates between percutaneous ethanol injection and radiofrequency ablation for treatment of small hepatocellular carcinoma. A: Adverse events; B: 1-year overall survival rate; C: 2-year overall survival rate; D: 3-year overall survival rate. All analyses used the Mantel-Haenszel method.
Adverse events: No significant differences in adverse events were found between the two groups (OR = 1.84, 95%CI: 0.76-4.45, P = 0.173) (Figure 3D).
RFA vs RFA + TACE
Survival rate: There was no significant difference in the 1-year (OR = 1.17, 95%CI: 0.88-1.56, P = 0.27) and 3-year (OR = 1.25, 95%CI: 0.90-1.73, P = 0.183) survival rates. The 5-year survival rate (OR = 3.19, 95%CI: 1.51-6.74, P = 0.002) in patients treated by RFA+TACE was higher than that by RFA alone (Figure 4A-C).
Figure 4.
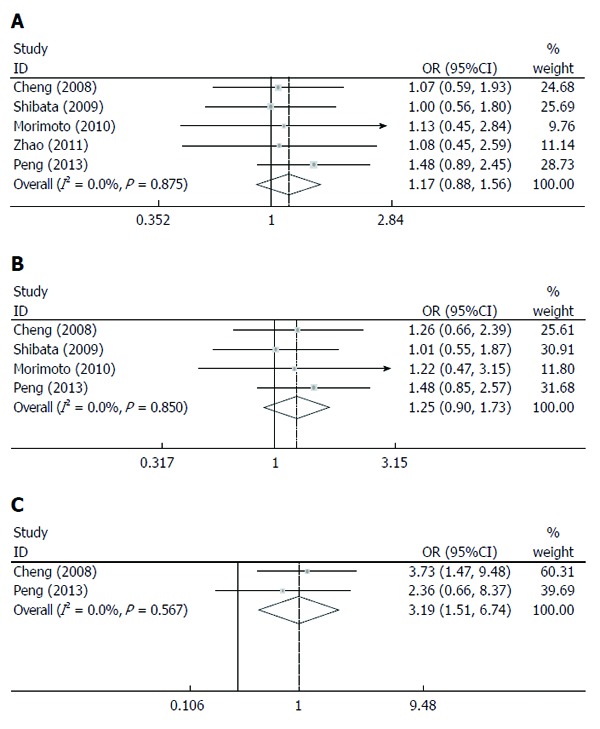
Comparison between transcatheter arterial chemoembolization plus radiofrequency ablation and radiofrequency ablation alone as a treatment for small hepatocellular carcinoma based on overall survival rate. A: 1-year overall survival rate; B: 3-year overall survival rate; C: 5-year overall survival rate. All analyses used the Mantel-Haenszel method.
Sensitivity analysis
The test for heterogeneity showed that there was no significant heterogeneity by incorporating multiple studies. The fixed-effect model was used to calculate the survival rate and recurrence rate. The results were similar, and the combined results were highly reliable.
Publication bias
The publication bias in this study was detected using a funnel plot of 1-year overall survival rate and 5-year overall survival rate data (Figure 5). The basic symmetry of the funnel plot suggested that there was no publication bias in these studies.
Figure 5.
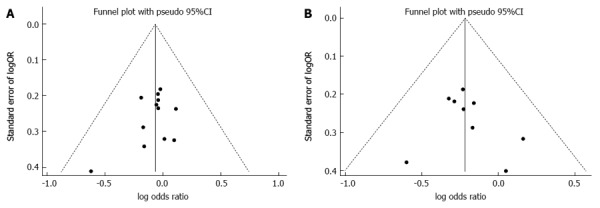
Funnel plot of surgical resection vs nonsurgical ablations. A: 1-year overall survival rate; B: 5-year disease-free survival rate.
DISCUSSION
Small HCC is a common malignant disease, especially in Asian regions[31]. Because of the limitations of donor scarcity and high costs, surgical resection and certain nonsurgical ablation methods were frequently used for the treatment of small HCC. According to a search of the literature, radiofrequency ablation was the most common method used among various nonsurgical ablation techniques. Few clinical trials have emphasized the therapeutic effects of surgical resection vs other nonsurgical methods, and a very limited number of high quality RCTs were available.
Our meta-analysis suggested that the incidence of adverse events after nRES for HCC was lower than that in the RES group, which might be explained by the specific characteristics of these therapies. The main nonsurgical therapies included were RFA, PEI and TACE. The principle of RFA is that heat generated by high radiofrequency waves inactivates local tumor cells quickly and effectively. In PEI, anhydrous alcohol dehydrates cancer cells, which degenerates and necrotizes them directly, and thus promotes tumor intravascular thrombosis. For TACE, a designated amount of embolization agents is injected into the target artery to produce ischemic necrosis of the tumor tissue. When compared with RES, these methods significantly reduce the physical injury and liver dysfunction that may lead to increased hospitalization.
In our meta-analysis, we showed that the local recurrence rate was higher after RFA than after RES, which can be potentially explained in two aspects. First, the safety margin of RFA was narrower than that of RES[32]. Second, the nRES methods were not able to find the potential sites of microscopic diseases; therefore, the clearance of tumors is more complete in RES because the method usually involves the removal of the entire tumor-containing segment[33].
Although RFA has been suggested to cause more complications than nRES, patients treated by RFA had longer overall survival rate and disease-free survival rate. This may be due to low local recurrences and improvements in surgical techniques, including surgical maneuvers and technical innovations. Many studies have suggested that anatomic resection seems to be superior to nonanatomic resection because of poorer liver function reserve in the nonanatomic resection group[34]. Additionally, the use of a laparoscope and the da Vinci Surgical System may also reduce prognostic risk factors, leading to longer survival[35].
Our analysis of RFA and PEI treatments for small HCC indicated that RFA was associated with a significantly longer overall survival rate. This may be due to a lower recurrence rate in the RFA group[22]. The reported major complications vary from study to study. Lencioni et al[25] and Livraghi et al[36] concluded that the risk of adverse events was significantly higher after PEI treatments. These results were similar to those reported by Shiina et al[23]. However, there was no significant difference between the two groups in our meta-analysis. A few studies have assessed RFA vs RFA plus TACE treatments for small HCC; however, there were limited useful data that could be extracted. Our study showed that the overall survival rate of patients who were treated by RFA plus TACE was higher than that treated by RFA alone. The efficacy of RFA can be enhanced by occlusion of the hepatic artery because the blood supply to a classical HCC is primarily provided by the hepatic artery. In the TACE plus RFA group, a larger area that included regions of the surrounding nontumorous liver parenchyma was coagulated[29].
For our meta-analysis, we searched through the relatively recent literature for multiple nRES trials to complete a comprehensive analysis of various indexes. RCT and nRCT trials were both included in this study because very few high quality RCT trials were available. We also acknowledge that the number of trials on nonsurgical ablation was insufficient, which might be due to the primary selection of RFA among nRES methods in clinical work. Thus, additional data are needed to obtain a more accurate conclusion.
In conclusion, surgical resection was superior to nonsurgical ablation methods for the treatment of small HCC in terms of a longer survival rate. However, the incidence of adverse events after RES was higher than that after nRES. We acknowledge that the number of cases undergoing PEI and TACE was insufficient, but the studies that we assessed suggest that RFA is the best single nonsurgical ablation method. However, RFA plus TACE was better than RFA alone for the treatment of small HCC.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a common malignancy, its incidence is increasing worldwide, and it is responsible for thousands of deaths every year. Theoretically, the best treatment for small HCC is liver transplantation, but the scarcity of donor organs and high costs constrain the use of this treatment. Therefore, the demand for novel treatment strategies for small HCC has been raised for both surgical resection (RES) and nonsurgical-RES (nRES) cases. However, alternative therapies for small HCC are controversial. Hence, it is necessary to compare all the treatment options for small HCC, including surgical resection and nonsurgical ablation methods.
Research frontiers
Meta-analysis, a quantitative technique for therapeutic evaluation, may be used when controversy persists after several studies. The meta-analysis was designed to determine the superior treatment for small HCC from among surgical resection and common nonsurgical ablation methods.
Innovations and breakthroughs
The current analysis comprehensively compared the effectiveness and safety of surgical resection and common nonsurgical ablation methods for the treatment of HCC. Meanwhile, it also provided evidence for the superior choice among nonsurgical ablation methods for treatment of small HCC. The analysis indicated that the overall and recurrence-free survival rates of patients in the resection group were significantly higher than those in patients who underwent radiofrequency ablation (RFA).
Applications
Surgical resection was superior to nonsurgical ablation methods in the treatment of small HCC in terms of longer survival; however, the incidence of adverse events after RES was higher than that after nRES. Among the studies analyzed, RFA is the best nonsurgical ablation method, and the combination of transcatheter arterial chemoembolization (TACE) and RFA was more efficacious than RFA alone.
Terminology
The principle of RFA is that heat generated by high radio frequency waves inactivates the local tumor cells quickly and effectively. Anhydrous alcohol dehydrates cancer cells, which degenerates and necrotizes the cells directly and promotes tumor intravascular thrombosis. For TACE, a designated amount of embolization agents are injected into the target artery to produce ischemic necrosis of the tumor tissue. All of these methods are common ablation methods for small HCC.
Peer review
This is an important subject. Meta-analysis, a quantitative technique for therapeutic evaluation, may be used when controversy persists after several studies.
Footnotes
Supported by National Key Basic Research Program of China - 973 Program, No. 2012CB526706; International Science and Technology Cooperation Program of the Ministry of Science and Technology, No. 2011DFA32980; The Innovation Program of Shanghai Municipal Education Commission, No. 2013ZZ060; and the National Natural Science Foundation of China, No. NSFC81271694
P- Reviewer: Panduro A, Tandon RK, Torres MI, Shehata MMM S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51–58. doi: 10.1111/j.1440-1746.2011.06947.x. [DOI] [PubMed] [Google Scholar]
- 3.Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013;11:190. doi: 10.1186/1477-7819-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merchant N, David CS, Cunningham SC. Early Hepatocellular Carcinoma: Transplantation versus Resection: The Case for Liver Resection. Int J Hepatol. 2011;2011:142085. doi: 10.4061/2011/142085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 7.Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:422–424. doi: 10.1007/s00534-009-0239-7. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 9.Huang GT, Lee PH, Tsang YM, Lai MY, Yang PM, Hu RH, Chen PJ, Kao JH, Sheu JC, Lee CZ, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann Surg. 2005;242:36–42. doi: 10.1097/01.sla.0000167925.90380.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 12.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Cho YB, Lee KU, Suh KS, Kim YJ, Yoon JH, Lee HS, Hahn S, Park BJ. Hepatic resection compared to percutaneous ethanol injection for small hepatocellular carcinoma using propensity score matching. J Gastroenterol Hepatol. 2007;22:1643–1649. doi: 10.1111/j.1440-1746.2007.04902.x. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg. 2008;12:1521–1526. doi: 10.1007/s11605-008-0553-4. [DOI] [PubMed] [Google Scholar]
- 15.Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, Hasegawa S, Tsubouchi H. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16:359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 16.Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, Watanabe T, Mine T. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010;116:3638–3644. doi: 10.1002/cncr.25142. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S, et al. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol. 2011;11:143. doi: 10.1186/1471-230X-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo WX, Sun JX, Cheng YQ, Shi J, Li N, Xue J, Wu MC, Chen Y, Cheng SQ. Percutaneous radiofrequency ablation versus partial hepatectomy for small centrally located hepatocellular carcinoma. World J Surg. 2013;37:602–607. doi: 10.1007/s00268-012-1870-z. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Shin SS, Kim JK, Choi SK, Heo SH, Lim HS, Hur YH, Cho CK, Jeong YY, Kang HK. Radiofrequency ablation combined with transcatheter arterial chemoembolization for the treatment of single hepatocellular carcinoma of 2 to 5 cm in diameter: comparison with surgical resection. Korean J Radiol. 2013;14:626–635. doi: 10.3348/kjr.2013.14.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai EC, Tang CN. Radiofrequency ablation versus hepatic resection for hepatocellular carcinoma within the Milan criteria--a comparative study. Int J Surg. 2013;11:77–80. doi: 10.1016/j.ijsu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 22.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 26.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 27.Zhao M, Wang JP, Li W, Huang ZL, Zhang FJ, Fan WJ, Zhang L, Li XS, Pan CC, Wu PH. [Comparison of safety and efficacy for transcatheter arterial chemoembolization alone and plus radiofrequency ablation in the treatment of single branch portal vein tumor thrombus of hepatocellular carcinoma and their prognosis factors] Zhonghua Yixue Zazhi. 2011;91:1167–1172. [PubMed] [Google Scholar]
- 28.Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 30.Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669–1677. doi: 10.1001/jama.299.14.1669. [DOI] [PubMed] [Google Scholar]
- 31.Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. Dig Dis. 2001;19:263–268. doi: 10.1159/000050692. [DOI] [PubMed] [Google Scholar]
- 32.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346–350. [PubMed] [Google Scholar]
- 33.Makuuchi M, Hasegawa H, Yamazaki S, Takayasu K, Moriyama N. The use of operative ultrasound as an aid to liver resection in patients with hepatocellular carcinoma. World J Surg. 1987;11:615–621. doi: 10.1007/BF01655837. [DOI] [PubMed] [Google Scholar]
- 34.Cucchetti A, Cescon M, Ercolani G, Bigonzi E, Torzilli G, Pinna AD. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol. 2012;19:3697–3705. doi: 10.1245/s10434-012-2450-z. [DOI] [PubMed] [Google Scholar]
- 35.Bentas W, Wolfram M, Jones J, Bräutigam R, Kramer W, Binder J. Robotic technology and the translation of open radical prostatectomy to laparoscopy: the early Frankfurt experience with robotic radical prostatectomy and one year follow-up. Eur Urol. 2003;44:175–181. doi: 10.1016/s0302-2838(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 36.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]


