Abstract
Gastric adenosquamous carcinoma (ASC) is a rare type of gastric cancer. It is a mixed neoplasm, consisting of glandular cells and squamous cells. It is often diagnosed at an advanced stage, thus carrying a poor prognosis. We describe a case of a 73-year-old male, who presented with refractory fever and an intra-abdominal mass on imaging. He underwent a laparoscopic exploration followed by a successful totally laparoscopic total gastrectomy with D2 lymphadenectomy for gastric cancer. Postoperative pathology revealed primary gastric ASC (T4aN0M0). The patient received adjuvant radiotherapy and chemotherapy with S1 and is alive 20 mo after surgery without recurrence. This is the first case of advanced gastric ASC with fever as the initial presentation treated with totally laparoscopic total gastrectomy reported in the English literature.
Keywords: Gastric adenosquamous carcinoma, Advanced gastric cancer, Malignant tumor, Laparoscopic gastrectomy, Totally laparoscopic total gastrectomy
Core tip: Totally laparoscopic total gastrectomy for advanced primary adenosquamous carcinoma of the stomach with fever as the initial manifestation is an interesting combination of a rare lesion with an atypical presentation and an uncommon complex surgical procedure. Given the aggressive nature and advanced stage at diagnosis, there are no reports of this carcinoma in the English literature. To the best of our knowledge, this is the first case of its kind reported in the English literature.
INTRODUCTION
The first case of gastric adenosquamous carcinoma (ASC) was reported by Rolleston and Trevor[1]. ASC accounts for less than 1% of all gastric cancers worldwide[2], compared with the predominant adenocarcinoma type which has an incidence of more than 90%[3]. Since the first reported case, ASC has only been reported in case reports (Table 1), mostly from Asian countries[4-6] and very few case series (Table 2) have been reported in the English literature. It has a male to female preponderance with a 4:1 ratio, and peaks in the 6th decade of life, occurring on average earlier than sporadic adenocarcinoma[2]. It is usually aggressive in nature, diagnosed at an advanced stage and carries a poorer prognosis than adenocarcinoma[2].
Table 1.
Reported cases of gastric adenosquamous carcinoma
| Author | Year | Country | Gender | Age (yr) | Signs and symptoms | Preop diagnosis | Surgical procedure | Tumor size (cm) | Location | Metastasis | Staging | Adjuvant treatment | Prognosis |
| Rolleston and Trevor[12] | 1905 | - | M | 39 | - | - | - | - | P | - | - | - | - |
| Lubarsch[12] | 1906 | - | - | - | - | - | - | - | P | - | - | - | - |
| Herxheime[12] | 1907 | - | - | - | - | - | - | - | P | - | - | - | - |
| Boedeker[12] | 1926 | - | F | 35 | - | - | - | - | P | - | - | - | - |
| F | 65 | - | - | - | - | P | - | - | - | - | |||
| Oberling and Wolf[12] | 1927 | - | F | 67 | - | - | - | - | P | - | - | - | - |
| Pasternackv[12] | 1935 | - | M | 48 | - | - | - | - | P | - | - | - | - |
| Martin and Pollosson[12] | 1936 | - | F | 64 | - | - | - | - | B | - | - | - | - |
| Takagi[12] | 1937 | - | M | 33 | - | - | - | - | B | - | - | - | - |
| Scheffler and Falk[12] | 1940 | - | M | 74 | - | - | - | - | B | - | - | - | - |
| Wood[12] | 1943 | - | M | 51 | - | - | - | - | P | - | - | - | - |
| M | 37 | - | - | - | - | P | - | - | - | - | |||
| Strassman[12] | 1946 | - | M | 85 | - | - | - | - | B | - | - | - | - |
| O'Brien and Meehan[12] | 1950 | - | M | 40 | - | - | - | - | P | - | - | - | - |
| Milanes et al[12] | 1950 | - | M | 50 | - | - | - | - | B | - | - | - | - |
| Bellagie and Dahlin[12] | 1951 | - | M | 42 | - | - | - | - | B | - | - | - | - |
| M | 49 | - | - | - | - | B | - | - | - | - | |||
| Hirai et al[12] | 1963 | - | M | 37 | - | - | - | - | B and P | - | - | - | - |
| Straus, Heschel and Fortmann[12] | 1968 | - | F | 70 | - | - | - | - | P | - | - | - | - |
| Lee et al[1] | 1970 | SK | M | 43 | A2, EP, HP, TS | Sub-G + O | A | LN | |||||
| Taira et al[13] | 1976 | J | F | 45 | - | - | - | - | - | - | - | - | - |
| Fujitomi et al[14] | 1983 | J | - | - | - | - | - | - | - | - | Bor IV | - | - |
| Jalif et al[15] | 1984 | S1 | F | 68 | - | - | - | - | - | - | - | - | - |
| Sato et al[16] | 1984 | J | F | 76 | - | - | TG + S | - | - | - | Bor III | - | - |
| Matsumoto et al[17] | 1984 | J | M | 56 | UAD | - | TG | 5.0 × 4.0 | A and B | LN | Bor III | - | - |
| Masuda et al[18] | 1985 | J | - | 53 | UAP | ASC | Sub-G | - | B | - | Bor III | - | - |
| 3Horikawa et al[19] | 1987 | J | F | 56 | - | - | - | - | - | - | EGC | - | - |
| 3Johzaki et al[20] | 1988 | J | F | 78 | - | - | Par-G | - | - | - | EGC | - | - |
| 3Butov et al[21] | 1989 | R | - | - | - | - | - | - | - | - | EGC | - | - |
| Shigematsu et al[22] | 1989 | J | F | 74 | EP + AL | - | - | - | - | LN | Bor III | - | - |
| 4Yamamoto et al[23] | 1989 | J | M | 61 | - | Sub-G | - | - | L, L1 LN | - | - | - | |
| Honda et al[24] | 1990 | J | M | 57 | UAP | - | - | - | Angle region | - | Grade II ASC | - | - |
| Tsukino et al[25] | 1990 | J | M | 61 | - | - | - | - | - | LN | Bor IV | - | - |
| Kawabe et al[26] | 1990 | J | - | - | - | - | - | - | Fornix and B | - | - | - | - |
| Coard et al[27] | 1991 | J and C | - | - | - | - | - | - | - | - | - | - | - |
| Tenma et al[28] | 1993 | J | M | 51 | - | - | TG post chemo | - | - | -ve | Bor III | - | - |
| Ito et al[29] | 1993 | J | - | - | - | - | - | - | - | - | - | - | - |
| Cabello Rodríguez et al[30] | 1994 | S1 | F | 84 | - | - | - | - | A | - | - | - | - |
| Toyota et al[6] | 1996 | J | F | 72 | RHE | - | P | - | - | - | Bor 2 + EGC | - | - |
| 3Yoshida et al[5] | 1996 | J | M | 66 | EP | SCC | Par-DG | 2.1 × 2.0 | A | L, LN | - | - | Died 2 yr post op2 |
| Manna et al[31] | 1998 | P | M | 55 | - | - | - | - | - | - | - | - | - |
| Mori et al[32] | 2000 | J | M | 59 | - | - | TG + H | - | RS | L, LN | - | - | Died 2 mo post H1 |
| Blázquez et al[33] | 2005 | S1 | F | 56 | CA | - | - | - | A | - | - | - | Died few days post op |
| Endo et al[7] | 2005 | J | M | 55 | F and A1 | ASC | Par-G + D3 | 7.0 × 8.0 | A | LN, L2 | T2N2M0 | - | - |
| Nomura et al[34] | 2006 | J | F | 62 | A1 | - | DG | B and A | - | LN | T3N3M0 | Neoadjuvant chemo | - |
| Terada[35] | 2009 | J | F | 87 | N and V | SCC | TG + C1 + S | 10 × 8 × 7 | - | SM, LV | Bor IV | - | Died 5 mo post op |
| F | 77 | EP | SCC | G | 6 × 5 × 7 | - | SM, LV | Bor III | - | Died 8 mo post op | |||
| Faria et al[2] | 2010 | P | F | 84 | EP | -ve | Sub-G | 8 × 5 × 1.1 | A | LN, L | T3N1M1 | - | - |
| Fukuda et al[36] | 2012 | J | M | 70 | - | - | TG + D2 + H | - | - | L | - | - | - |
| Ebi et al[8] | 2012 | J | M | 74 | AP and M | SCC | Par-G | - | B | LN | Advanced type 3 | S1 monotherapy | Alive post op 2 yr 10 mo |
| Saito et al[37] | 2013 | J | M | 60 | UBP | ASC | No surgery | - | B-A | LN | - | DC-S1 | Pt died 3 mo post chemo |
| 3Kimura et al[41] | 2013 | J | M | 77 | - | - | LADG | - | - | LN; Recurrence in liver | ECG | S-1 and cisplatin (CDDP) | Alive 14 mo post op |
| Harsha Ajoodhea et al (present case) | 2013 | C | M | 73 | Fever | ADC | TLTG+D2 | 8 × 7.5 | B-A | -ve | T4a N0 M0 | Rand S1 | Alive at 20 mo post op |
Died of lymphangitis carcinomatosa of the lung 2 mo after right hepatectomy;
Died of liver failure due to multiple metastases 2 yr after operation;
Early gastric cancer;
Hypesthesia and pain in both legs, and progressive difficulty in walking. A1: Anemia; A2: Anorexia; AL: Appetite loss; CA: Chronic anemia; EP: Epigastric pain; HP: Hunger pain; F: Fatigue; V: Vomiting; N: Nausea; RHE: Routine health examination; TS: Tarry stool; M: Melena; UAP: Upper abdominal pain; UAD: Upper abdominal discomfort; J: Japan; S1: Spain; P: Portugal; M: Maroc; C: China; R: Russia; SK: South Korea; JC: Jamaica and Commonwealth Caribbean; S: Splenectomy; Sub-G: Subtotal gastrectomy; Par-G: Partial gastrectomy; P: Pylorogastrectomy; H: Hepatectomy; DG: Distal gastrectomy; C1: Cholecystectomy; ASC: Adenosquamous carcinoma; Par-DG: Partial distal gastrectomy; SSC: Squamous cell carcinoma; ADC: Adenocarcinoma; A: Antrum; B: Body; F: Fornix; C: Cardia; P: Pylorus; O: Omentectomy; RS: Remnant stomach; Borr: Borrmann type; L: liver; L1: Lung; SM: Systemic metastases; LN: Lymph node metastases; LV: Lymphovascular metastases; L2: Lymphatics; R: Radiotherapy; S1: S1 chemotherapy; DC-S1: Docetaxel, cisplatin and S-1 (DCS); EGC: Early gastric cancer; LADG: Laparoscopic-assisted distal gastrectomy; TLTG: Totally laparoscopic total gastrectomy.
Table 2.
Reported case series of gastric adenosquamous carcinoma
| Author | Year | Country | No. of cases | Mean age (yr) | Location |
| Boswell and Helwig[12] | 1965 | - | 111 | 49.1 | P: 9 pts; LC: 1 pt; F: 1 pt |
| Urban et al[12] | 1966 | - | 102 | 54.7 | P and B: 1 pt; C and B: 1 pt |
| P: 6 pts; C: 2 pts | |||||
| Aoki et al[38] | 1978 | Japan | 113 | 61.2 | A: 5 pts; B: 2 pts; F: 4 pts |
| Mori et al[4] | 1986 | Japan | 28 | - | - |
| Namatame et al[39] | 1986 | Japan | 54 | 62.8 | Lower body along lesser curvature |
| Rottenberg[40] | 1987 | Russia | 5 | - | B and P |
10 males and 1 female;
6 males and 1 female;
10 males and 1 female;
2 males and 3 females. Pt: Patient; A: Antrum of stomach; P: Pylorus; LC: Lesser curvature of stomach; F: Fundus of stomach; B: Body of stomach; C: Cardia of stomach.
The application of laparoscopic approaches for distal gastric lesions, benign or early gastric cancers, has been accepted worldwide. The advantages and long-term benefits in these patients are also very well known and appreciated. Despite the advances in minimally invasive techniques and their advantages, several issues have been raised in the setting of a totally laparoscopic total gastrectomy. Moreover, in patients with advanced cancer, a minimally invasive approach is not commonly used. The case presented here is that of a patient with an advanced primary ASC extending to the serosal layer. This is the first case of ASC treated completely laparoscopically reported in the English literature.
CASE REPORT
We report a case of a 73-year-old male, who was referred to the surgical department after refractory medical treatment for an irregular high fever. The patient was previously treated for pneumonia 2 mo before admitted to the Infectious Disease Department because of irregular high fever. Except for mild anemia (hemoglobin 8 g/L), white blood cell (WBC) count of 15.8 × 109/L and C-reactive protein (CRP) level of 72.4 mg/L, all other laboratory tests, physical examination and past/family history were insignificant. Despite aggressive treatment, his fever (Figure 1A) persisted and the WBC count (Figure 1B) and CRP level (Figure 1C) increased. He was then referred to the surgical department due to the presence of an intra-abdominal mass found on imaging. An abdominal ultrasound revealed a hypoechoic mass in the upper abdomen suggesting gastric cancer with surrounding lymph node involvement. Abdominal computed tomography (CT) showed a mass in the upper abdomen between the stomach and the left lobe of the liver (Figure 2). Gastroscopy showed a raised lesion on the gastric antrum surface, with ulceration, and involving the lower part of the body of the stomach (Figure 3). The preoperative biopsy pathology showed an adenocarcinoma, and the patient was found to be Helicobacter pylori positive. He underwent a totally laparoscopic total gastrectomy accompanied by D2 lymphadenectomy, and an esophagojejunal Roux-en-Y anastomosis was performed. The patient was placed in the supine position with the head slightly elevated under general anesthesia. The surgeon and the second assistant holding the laparoscope stood on the right side of the patient and the first assistant stood on the left. Carbon dioxide pneumoperitoneum was established (CO2 at 15 mmHg) using a Veress needle. The first 10-mm trocar was placed at the umbilicus for the laparoscope. A 30-degree telescope was inserted to examine the peritoneal cavity to rule out metastatic disease and assess the feasibility of the procedure. After general examination, an additional four trocars (one of 12 mm, three of 5 mm) were inserted and the five trocars were arranged in a V-shape. The operation was started by retracting the greater omentum superiorly using the grasper and bluntly dissecting along the transverse colon border to enter the lesser sac (Figure 4A). The stomach together with the greater omentum was then retracted superiorly using the grasper to allow better visualization of anatomical landmarks. The right gastroepiploic vessels were identified, clipped using Hem-o-lock and excised (Figure 4B and C). Next, mobilization began at the superior edge of the pancreas, thus revealing the celiac trunk. The common hepatic artery, right gastric artery, left gastric artery and splenic artery and their respective lymph nodes were identified and excised. The right gastric artery, left gastric artery and vein were then clipped using the Hem-o-lock and excised (Figure 4D). The hepatoduodenal and hepatogastric ligaments were then dissected. The esophageal wall was detached from the surrounding tissue and completely mobilized (Figure 4E). The cardia vagus nerve was cut 3 cm above the cardia. Once the structures were completely mobilized, the duodenum was then transected using an endoscopic linear stapler 3 cm from the pylorus (Figure 4F). The jejunum was stapled using an endoscopic linear stapler 20 cm from the ligament of Treitz. A small opening was made 10 cm from the stump on the distal jejunum and the latter was then pulled up to the esophagus, in which a small side opening was also made. A side-to-side antiperistaltic esophagojejunostomy was then performed using linear staplers (Figure 4G) and the entry hole was then closed using staplers (Figure 4H). The esophageal wall was sutured to the diaphragm wall to avoid tension, thus preventing anastomotic failure. The specimen was placed in a retrieval bag and pulled out through an enlarged umbilical incision. Pneumoperitoneum was re-established. A routine Roux-en-Y anastomosis was performed laparoscopically between the distal jejunum (40 cm from the esophagojejunostomy) and the proximal jejunum. Any defects in the mesentery were closed. The postoperative pathology result was an 8 cm × 7.5 cm sized gastric ASC (Figure 5) located in the body and antrum, with a 3 cm × 2 cm central ulcer invading the serosal layer. Pathological findings showed an adenocarcinoma component with glandular formation, and a squamous cell component with keratinization (Figure 6A and B). There was no obvious border between the two components. The adenocarcinoma cells were well to moderately differentiated with a proportion of 40% and were found in the upper one-third, and the squamous cells were well differentiated with a proportion of 60% and were found in the lower two-thirds of the stomach. Additional immunohistochemical staining for CK Low (Figure 6C) showed strong positive staining for adenocarcinoma, and staining for P63 (Figure 6D) showed nuclear positive staining for squamous cell carcinoma. No lymph node metastasis (0/35), lymphatic or vascular invasion were found and a negative margin was achieved (TNM staging was Stage IV-T4aN0M0 and Bormann type 4).
Figure 1.
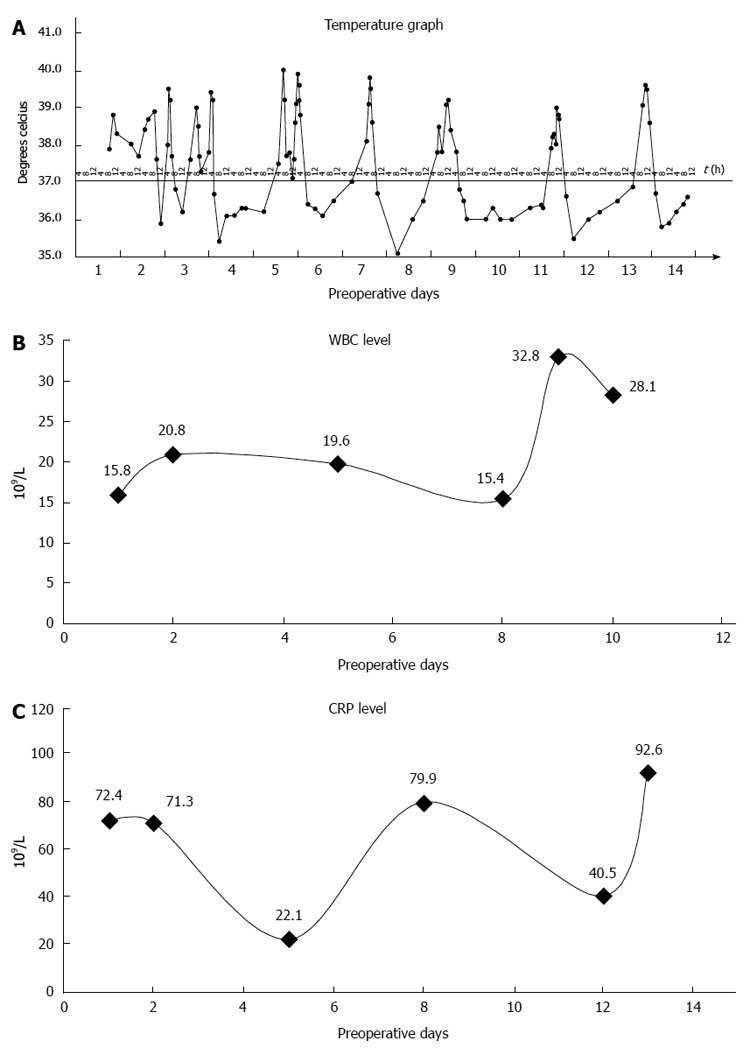
Preoperative graphs. A: Preoperative temperature; B: Preoperative white blood cell (WBC) count; C: C-reactive protein (CRP) level.
Figure 2.
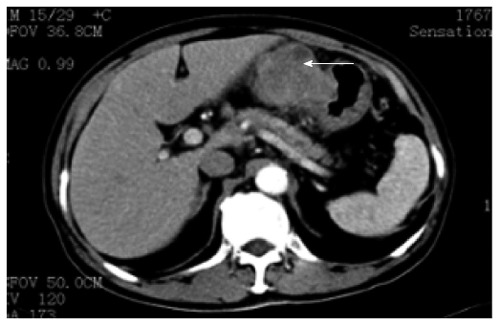
Abdominal computed tomography showing an upper abdominal mass between left lateral hepatic lobe and stomach, as shown by arrow.
Figure 3.
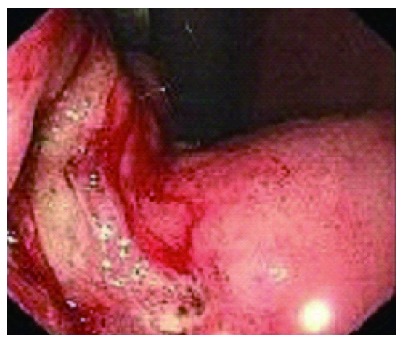
Gastroscopy. Gastric antrum raised lesion, with ulceration, easy to bleed and involving the lower part of the body of the stomach.
Figure 4.
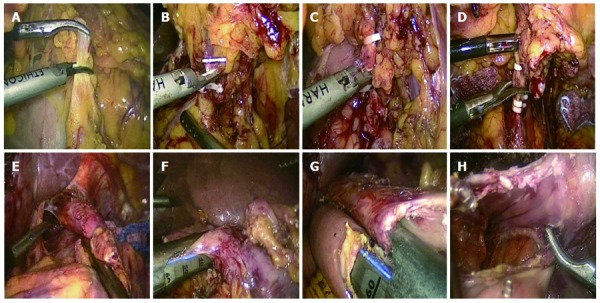
Surgical procedure. A: Dissection of the greater omentum; B, C: Clipping and cutting of right gastroepiploic vein and artery, respectively; D: Clipping and cutting of left gastric artery; E: Mobilization of esophagus; F: Transection of duodenum; G: Antiperistaltic reconstruction of esophagus and jejunum using linear stapler; H: Entry hole as seen after stapling.
Figure 5.
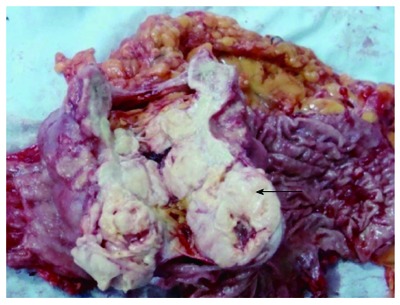
Gross Operative specimen. An 8 cm × 7.5cm mass with a 3 cm × 2 cm ulcer, invading the serosal layer (mass cut open), as shown by arrow.
Figure 6.
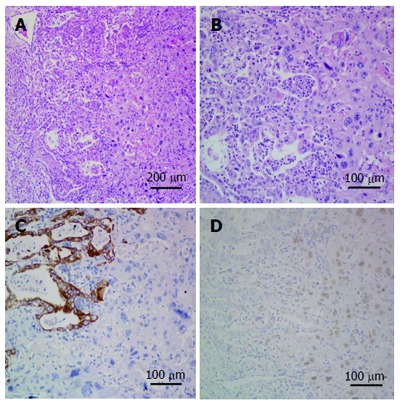
Microscopic specimen. A and B: Both adenocarcinoma and squamous cell carcinoma components. Adenocarcinoma component shows gland formation and squamous carcinoma component shows keratinization (hematoxylin and eosin staining, A: × 10 magnification; B: × 20 magnification); C: Immunohistochemical (IHC) staining for cytokeratin (low), on the left side adenocarcinoma is strongly positive and on the right side squamous cell carcinoma is negative (IHC × 20 magnification); D: IHC staining for P63, on the left side adenocarcinoma is negative and on the right side squamous cell carcinoma is nuclear positive (IHC × 20 magnification).
After surgery, there was an immediate decrease in temperature (Figure 7A), WBC count (Figure 7B) and CRP level (Figure 7C). The patient was started on liquid food 3 d after surgery, followed by semi-liquid food on the 8th day. The length of postoperative hospital stay was 14 d. No postoperative morbidity was noted. After operation, the patient received adjuvant radiotherapy and chemotherapy with S1. He was followed for nearly 20 mo and was in good health without recurrence as confirmed by CT scan at follow-up. His nutritional status was reported to be better compared to preoperative status.
Figure 7.
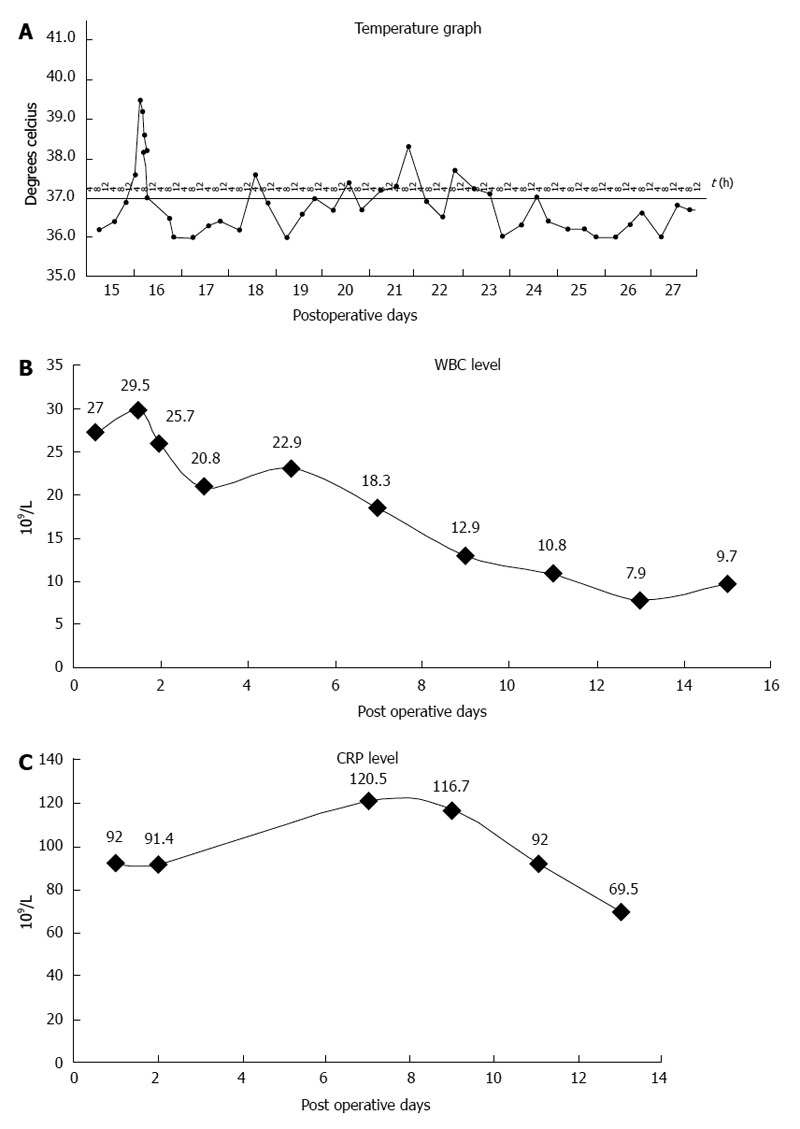
Postoperative graphs. A: Postoperative temperature; B: Postoperative white blood cell (WBC) count; C: Postoperative C-reactive protein (CRP) level.
DISCUSSION
ASC is a special type of gastric cancer. It occurs mainly in the distal and the body of the stomach (Table 1). Macroscopically, most tumors are Bormann type 2 or 3 advanced gastric cancer[7]. Very few reports are available in the English literature on early gastric ASC[41]. The most common site of metastasis is the liver[7] and lymphovascular metastases are common[8]. For the diagnosis of true ASC, collision tumors should be excluded. True ASC is a combination of adenocarcinoma and squamous cell carcinoma, with transitions between the two components[9]. Although the lesion is a combination of the two different histologic types, the biologic behavior is decided by the adenocarcinoma component.
The highlights of this case report are as follows: (1) atypical presentation in the patient; (2) the presence of ASC at final postoperative diagnosis; and (3) totally laparoscopic total gastrectomy for advanced gastric cancer. The chief complaints of patients with gastric ASC are similar to those of any gastric adenocarcinoma patient, and include epigastric pain, nausea and vomiting[8]. This is the first case of advanced gastric ASC reported in the English literature with fever as the initial presentation. Preoperatively, it was difficult to rule out lung infection as the cause of fever, given the patient’s previous history of pneumonia. As a result of non-responsiveness to medical treatment, the patient underwent surgery for suspected gastric cancer. The preoperative fever in this patient is believed to be associated with the necrotic tissues found in the lesion. There was a decrease in temperature immediately after the operation, along with a reduction in the WBC count and CRP level, which confirmed that the gastric mass was the cause of the fever.
Of interest was the presence of squamous cells in a glandular cellular area not contiguous with squamous epithelium. The final pathological diagnosis was gastric ASC. Since the first case reported more than one century ago, no confirmed evidence has been available on the pathogenesis of this lesion.
A few hypotheses[2] have been proposed regarding its origin, such as: (1) metaplastic transformation of an adenocarcinoma; (2) cancerization of metaplastic squamous cells; (3) cancerization of ectopic squamous epithelium; (4) collision of an adenocarcinoma and a squamous cell carcinoma; and (5) stem cell differentiation towards both cellular lines. Furthermore, another noteworthy point in the present report is the mismatch of preoperative and postoperative pathological results. This may be explained by the fact that despite gastroscopy, which is the gold standard for gastric cancer, the tissue sample taken during the biopsy showed that differentiation into adenosquamous cells had not occurred or had not yet occurred. Therefore, our preoperative biopsy result showed only gastric adenocarcinoma. Hence, ASC cannot be ruled out preoperatively and should be considered in the differential diagnosis, especially in patients with atypical presentations.
To the best of our knowledge, this is the first case of primary advanced gastric ASC with fever as the initial manifestation treated by totally laparoscopic total gastrectomy reported in the English literature. Laparoscopic surgery has been adopted since 1991 in the treatment of gastric cancer. In 1994, laparoscopy-assisted distal gastrectomy (LADG) with lymph node dissection was first performed by Kitano et al[10]. As the safety and surgical quality of LADG have been proved to be equivalent to open gastrectomy, LADG has been accepted as a less invasive treatment for early gastric cancer[11]. Advances in laparoscopic surgical techniques have now made it possible to perform all necessary surgical procedures, including intra-abdominal reconstruction[11]. This has led to the introduction of totally laparoscopic distal gastrectomy. Unfortunately, totally laparoscopic total gastrectomy with D2 lymphadenectomy is not yet popular due to the complexity of the procedure. Our case underwent a totally laparoscopic total gastrectomy with D2 lymphadenectomy and esophagojejunostomy Roux-en-Y anastomosis (antiperistaltic side-to-side anastomosis). The patient had an uneventful and quick recovery after operation without morbidity. He received adjuvant radiotherapy and S1 chemotherapy and is still alive 20 mo after operation, without recurrence. Therefore, laparoscopic surgery should be considered for advanced gastric cancer patients in selected high volume centers and by highly specialized laparoscopic surgeons.
In conclusion, primary advanced gastric ASC can present with atypical manifestations and should be considered in the differential diagnosis in atypical gastric cancer patients. Surgery should be attempted in such patients.
COMMENTS
Case characteristics
A case of advanced gastric adenosquamous carcinoma (ASC) in a 73-year-old male who presented with an irregular high fever refractory to medical treatment.
Clinical diagnosis
He was diagnosed initially with a lung infection due to the presence of an irregular high fever at presentation.
Differential diagnosis
In the differential diagnosis, lung infection, fever of unknown origin, and cancer-related fever were considered and therefore, blood and sputum cultures, sputum acid fast staining, PPD test, TSPOT test, bone marrow aspiration, abdominal ultrasound, computed tomography and bone scintigraphy were ordered.
Imaging diagnosis
Abdominal ultrasound revealed a hypoechoic mass in the upper abdomen suggesting gastric cancer with surrounding lymph node involvement. Abdominal computed tomography showed a mass in the upper abdomen between the stomach and the left lobe of the liver.
Pathological diagnosis
The preoperative pathological diagnosis was adenocarcinoma, and the patient was found to be Helicobacter pylori positive.
Treatment
A totally laparoscopic total gastrectomy was performed and the patient received postoperative radiotherapy and chemotherapy with S1.
Experiences and lessons
Primary gastric ASC can present with atypical manifestations and should be considered in the differential diagnosis in atypical gastric cancer patients, even if the preoperative pathological results state otherwise. Surgery should be attempted in such patients.
Peer review
Adenosquamous carcinoma of the stomach is very rare, and fever is an unusual presentation of gastric cancer. Furthermore, totally laparoscopic surgery is not usually performed for advanced gastric cancer. Thus, the present case is interesting and worth reporting.
Footnotes
Supported by Natural Science Foundation of Zhejiang Province, China, grant No. LQ13H160007
P- Reviewer: Takahashi Y, Zhu YL S- Editor: Gou SX L- Editor: Ma JY E- Editor: Wang CH
References
- 1.Lee YB, Choi IJ. Adenoacanthoma of pyloric antrum of the stomach. Report of a case. Yonsei Med J. 1970;11:60–66. doi: 10.3349/ymj.1970.11.1.60. [DOI] [PubMed] [Google Scholar]
- 2.Faria GR, Eloy C, Preto JR, Costa EL, Almeida T, Barbosa J, Paiva ME, Sousa-Rodrigues J, Pimenta A. Primary gastric adenosquamous carcinoma in a Caucasian woman: a case report. J Med Case Rep. 2010;4:351. doi: 10.1186/1752-1947-4-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori M, Iwashita A, Enjoji M. Adenosquamous carcinoma of the stomach. A clinicopathologic analysis of 28 cases. Cancer. 1986;57:333–339. doi: 10.1002/1097-0142(19860115)57:2<333::aid-cncr2820570224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida K, Manabe T, Tsunoda T, Kimoto M, Tadaoka Y, Shimizu M. Early gastric cancer of adenosquamous carcinoma type: report of a case and review of literature. Jpn J Clin Oncol. 1996;26:252–257. doi: 10.1093/oxfordjournals.jjco.a023224. [DOI] [PubMed] [Google Scholar]
- 6.Toyota N, Minagi S, Takeuchi T, Sadamitsu N. Adenosquamous carcinoma of the stomach associated with separate early gastric cancer (type IIc) J Gastroenterol. 1996;31:105–108. doi: 10.1007/BF01211195. [DOI] [PubMed] [Google Scholar]
- 7.Endo K, Kohnoe S, Okamura T, Haraguchi M, Adachi E, Toh Y, Baba H, Maehara Y. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer. 2005;8:173–177. doi: 10.1007/s10120-005-0330-y. [DOI] [PubMed] [Google Scholar]
- 8.Ebi M, Shimura T, Yamada S, Hirata Y, Tsukamoto H, Okamoto Y, Mizoshita T, Tanida S, Kataoka H, Kamiya T, et al. A patient with gastric adenosquamous carcinoma with intraperitoneal free cancer cells who remained recurrence-free with postoperative S-1 chemotherapy. Intern Med. 2012;51:3125–3129. doi: 10.2169/internalmedicine.51.8402. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Classification of Tumours. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- 10.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 11.Sakaguchi Y, Ikeda O, Ohgaki K, Oki E, Chinen Y, Sakamoto Y, Minami K, Toh Y, Okamura T. Totally Laparoscopic Gastrectomy for Gastric Cancer Associated with Recklinghausen’s Disease. Diagn Ther Endosc. 2010;2010:682401. doi: 10.1155/2010/682401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::aid-cncr2820240518>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Taira Y, Ogata H, Tsuchiyama H. Two autopsy cases of primary adenoacanthoma of the stomach. Acta Pathol Jpn. 1976;26:223–228. doi: 10.1111/j.1440-1827.1976.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujitomi Y, Uchida Y, Shibata O, Baba K, Shigemitsu O, Hadama T, Shirabe J, Nakayama I, Kai T. [Case of adenosquamous carcinoma of the stomach showing Borrmann 4 type and elevated serum CEA] Nihon Shokakibyo Gakkai Zasshi. 1983;80:1327–1330. [PubMed] [Google Scholar]
- 15.Jalif AR, López OJ, Díaz de Battaglini SM, Bur EG. [Gastric adenoacanthoma. Report of a case and review of the literature] Acta Gastroenterol Latinoam. 1984;14:79–84. [PubMed] [Google Scholar]
- 16.Sato N, Wada K, Kobayashi K, Hirai H, Yagi M. [A case of primary adenosquamous carcinoma of the stomach associated with gastric polyposis] Gan No Rinsho. 1984;30:292–295. [PubMed] [Google Scholar]
- 17.Matsumoto K, Sugiyama Y, Miyagishima K, Murakami T, Yamagata N, Tsuchida H, Sasamura M. [Primary adenosquamous carcinoma of the stomach--a case report] Gan No Rinsho. 1984;30:1726–1731. [PubMed] [Google Scholar]
- 18.Masuda T, Kizaki M, Akaiwa Y, Kamiya T, Suzuki O, Okawa H, Kiryu Y, Kikuchi K, Kakumoto Y, Nakagawa Y. [A case of primary adenosquamous carcinoma of the stomach preoperatively diagnosed by endoscopic biopsy] Gan No Rinsho. 1985;31:212–216. [PubMed] [Google Scholar]
- 19.Horikawa M, Miyagi N, Nakatani K, Shiratori T, Tsutsumi M, Takahashi S, Konishi Y. [A case of early primary adenosquamous carcinoma of the stomach] Gan No Rinsho. 1987;33:305–310. [PubMed] [Google Scholar]
- 20.Johzaki H, Murayama H, Maekawa T, Kikuchi M, Satoh M. [A case of small squamous cell carcinoma with a grading of IIa + IIc, an early gastric cancer type] Gan No Rinsho. 1988;34:1025–1030. [PubMed] [Google Scholar]
- 21.Butov IuL, Sadchikov VD. [Adenosquamous early gastric carcinoma] Arkh Patol. 1989;51:76–77. [PubMed] [Google Scholar]
- 22.Shigematsu T, Nakamura K, Fukui K, Morita H, Samejima Y, Goto T, Inagaki J, Kimura K, Kasugai T, Nishikawa K. [A primary gastric adenosquamous carcinoma with remarkable lymphatic metastasis diagnosed by the stomach and lymph node biopsy] Gan No Rinsho. 1989;35:421–426. [PubMed] [Google Scholar]
- 23.Yamamoto K, Ohnishi A, Noda S, Umezaki H, Yamamoto T. [An autopsy case of carcinomatous sensory neuropathy associated with gastric adenosquamous carcinoma] Rinsho Shinkeigaku. 1989;29:493–496. [PubMed] [Google Scholar]
- 24.Honda H, Satomi T, Fujita T, Hamagaki H, Hayashi M, Ichimiya M, Numoto S. [A primary adenosquamous carcinoma of the stomach difficult to differentiate from gastric ulcer] Gan No Rinsho. 1990;36:193–197. [PubMed] [Google Scholar]
- 25.Tsukino H, Kusumoto S, Nagamachi S, Watanabe K, Koga Y, Kataoka H. [A case of primary adenosquamous carcinoma of the stomach showing Borrmann 4 type] Rinsho Hoshasen. 1990;35:649–652. [PubMed] [Google Scholar]
- 26.Kawabe K, Nakanuma Y, Terada T, Nakamura Y. Adenosquamous carcinoma of the stomach presenting “giant gastric folds”. Gastroenterol Jpn. 1990;25:739–745. [PubMed] [Google Scholar]
- 27.Coard KC, Titus IP. Adenosquamous carcinoma of the stomach. With a note on pathogenesis. Trop Geogr Med. 1991;43:234–237. [PubMed] [Google Scholar]
- 28.Tenma K, Nakamura T, Kano T, Suzuki T, Ohashi Y, Masaoka T, Sato K. [A case report of adenosquamous cell carcinoma of the stomach responding well to combination chemotherapy] Gan To Kagaku Ryoho. 1993;20:647–650. [PubMed] [Google Scholar]
- 29.Ito T, Nishida H, Kawada T, Senjyu S, Nanbu K, Nishikawa J, Onuki M, Mitamura K, Ito Y, Koike T. [A case of gastric adenosquamous carcinoma with cystic hepatic metastasis] Nihon Shokakibyo Gakkai Zasshi. 1993;90:1445–1449. [PubMed] [Google Scholar]
- 30.Cabello Rodríguez M, Somoza de Saint-Palais M, Rueda Pérez JM, Montero Vázquez JM, Merino Royo E, Carabot Rodríguez-Rubio A, Barrera Alvarez F, Cabezudo San José R. [Adenosquamous carcinoma of the stomach] Rev Esp Enferm Dig. 1994;86:757–760. [PubMed] [Google Scholar]
- 31.Manna ED, Seixas AA, de Araújo RP, Ferro MC. [Primary adenosquamous carcinoma of the stomach] Rev Assoc Med Bras. 1998;44:152–154. doi: 10.1590/s0104-42301998000200016. [DOI] [PubMed] [Google Scholar]
- 32.Mori E, Watanabe A, Maekawa S, Itasaka H, Maeda T, Yao T. Adenosquamous carcinoma of the remnant stomach: report of a case. Surg Today. 2000;30:643–646. doi: 10.1007/s005950070105. [DOI] [PubMed] [Google Scholar]
- 33.Blázquez S, Raventós A, Díaz ML, García-Fontgivell JF, Martínez S, Sirvent JJ. Adenosquamous gastric carcinoma in Caucasian patient. Rev Esp Enferm Dig. 2005;97:211–212. doi: 10.4321/s1130-01082005000300009. [DOI] [PubMed] [Google Scholar]
- 34.Nomura M, Inoue Y, Fujita S, Sakao J, Hirota M, Souda S. [A case of gastric adenosquamous carcinoma with abdominal paraaortic lymph node metastases successfully treated by TS-1 plus CDDP neoadjuvant chemotherapy] Gan To Kagaku Ryoho. 2006;33:99–103. [PubMed] [Google Scholar]
- 35.Terada T. Adenosquamous Carcinoma of the Stomach: Report of Two Cases. Gastroenterol Res. 2009;2:54–56. doi: 10.4021/gr2009.01.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda Y, Takeshima F, Ogihara K, Shoji H, Akazawa Y, Tsutsumi T, Abe K, Mizuta Y, Isomoto H, Nakao K. [Successful management of liver metastasis from gastric adenosquamous carcinoma with adjuvant chemotherapy and radiofrequency ablation] Nihon Shokakibyo Gakkai Zasshi. 2012;109:606–614. [PubMed] [Google Scholar]
- 37.Saito H, Oyama K, Fushida S, Tsukada T, Okamoto K, Nakanuma N, Kinoshita J, Makino I, Nakamura K, Hayashi H, et al. [A case of gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor] Gan To Kagaku Ryoho. 2013;40:799–802. [PubMed] [Google Scholar]
- 38.Aoki Y, Tabuse K, Wada M, Katsumi M, Uda H. Primary adenosquamous carcinoma of the stomach: experience of 11 cases and its clinical analysis. Gastroenterol Jpn. 1978;13:140–145. doi: 10.1007/BF02773859. [DOI] [PubMed] [Google Scholar]
- 39.Namatame K, Ookubo M, Suzuki K, Sagawa H, Nagashima T, Kataba Y, Maruyama U, Watanabe H. [A clinicopathological study of five cases of adenosquamous carcinoma of the stomach] Gan No Rinsho. 1986;32:170–175. [PubMed] [Google Scholar]
- 40.Rottenberg VI. [Glandular-squamous cell stomach cancer] Arkh Patol. 1987;49:19–24. [PubMed] [Google Scholar]
- 41.Kimura Y, Matsuda H, Saeki H, Oki E, Morita M, Sugimachi K, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, et al. Case of early adenosquamous carcinoma of the stomach. Fukuoka Igaku Zasshi. 2013;104:315–320. [PubMed] [Google Scholar]


