Abstract
Helicobacter pylori (H. pylori) has been found in the oral cavity and stomach, and its infection is one of the most frequent worldwide. We reviewed the literature and conducted a Topic Highlight, which identified studies reporting an association between H. pylori-infection in the oral cavity and H. pylori-positive stomach bacterium. This work was designed to determine whether H. pylori is the etiologic agent in periodontal disease, recurrent aphthous stomatitis (RAS), squamous cell carcinoma, burning and halitosis. Record selection focused on the highest quality studies and meta-analyses. We selected 48 articles reporting on the association between saliva and plaque and H. pylori-infection. In order to assess periodontal disease data, we included 12 clinical trials and 1 meta-analysis. We evaluated 13 published articles that addressed the potential association with RAS, and 6 with squamous cell carcinoma. Fourteen publications focused on our questions on burning and halitosis. There is a close relation between H. pylori infection in the oral cavity and the stomach. The mouth is the first extra-gastric reservoir. Regarding the role of H. pylori in the etiology of squamous cell carcinoma, no evidence is still available.
Keywords: Helicobacter pylori, Oral pathology, Gastric infection, Burning and halitosis, Recurrent aphthous stomatitis
Core tip: Infection by Helicobacter pylori (H. pylori) is one of the most frequent worldwide, with major implications for stomach pathology over the last twenty-five years. Early diagnosis is essential for control of the infection. There has been a growing interest in H. pylori-infection in the oral cavity, since the oral-oral is one of the major transmission routes. This review describes the association between H. pylori and different oral pathologies, such as periodontal disease, canker sores, squamous cell carcinoma, burning tongue and halitosis, and their correlation with the gastric pathology.
INTRODUCTION
In 1984, Marshall and Warren[1] in the Royal Perth Hospital in Australia definitively identified the Helicobacter pylori (H. pylori). It was cultured from gastric biopsy specimens from patients with gastric inflammation and peptic ulcer. Based on these results, they proposed that H. pylori could be the etiologic agent of these conditions[1,2]. In 1994, this microorganism was recognized as a type I carcinogen, and is now considered the most common etiologic agent of infection-related cancers. As a result, in 2005 Marshall and Warren were awarded the Nobel Prize of Medicine for their seminal discovery of this bacterium and its role in peptic ulcer disease. About 10% of H. pylori individuals develop peptic ulcer disease, 1% to 3% develop gastric adenocarcinoma, and less than 0.1% mucosa associated lymphoid tissue lymphoma[3]. The global prevalence of H. pylori infection is more than 50%. This prevalence may vary significantly within and among countries, according to geography, ethnicity, age, and socioeconomic factors. Prevalence is higher in developing countries and lower in the developed world. The risk of infection increases in lower economic and socio-cultural backgrounds[4]. The main reasons for these variations involve socioeconomic differences between populations. Transmission of H. pylori is largely by the oral-oral or fecal-oral routes. Lack of proper sanitation, safe drinking water and basic hygiene, as well as poor diets and overcrowding, all play a role in the overall prevalence of infection. H. pylori infection at younger ages is markedly more prevalent in developing countries than in developed countries, and H. pylori-seropositivity rates increase progressively with age[5]. Gastric H. pylori infection is treated with systemic antibiotic therapy. In some patients, however, persistent bacterial infection is observed after treatment[6,7]. Two questions arise as to how this persistent bacterial infection is transmitted, and how the reinfection process occurs. Some researchers have suggested that oral spread would be the main route of H. pylori transmission, and both the dental plaque and the saliva could act as a reservoir and have implications in reinfection once the bacterium is eradicated from the gastric tract[8]. Zou et al[9] consider that the mouth can be a reinfection source and that eradication from the oral cavity is more difficult than gastrointestinal eradication. As mentioned above, the search for H. pylori in dental plaque, saliva, periodontal disease, canker sores, cancer, burning mouth and halitosis was rather controversial due to the different diagnostic methods and research designs used, the inclusion/exclusion criteria, and the selected controls.
DENTAL PLAQUE AND SALIVA
Frequency of H. pylori isolation in dental plaque has been variable (Table 1)[10-56]. Dental plaque was first studied in 1989 in Canada by Krajden et al[10], who performed H. pylori isolation by culture in patients with H. pylori-positive gastric pathology. H. pylori was isolated from the stomach of 29 of 71 patients examined, with only one (3%) of the 29 patients having the organism present in dental plaque. That year the same group, also in Canada, studied H. pylori strains from the stomach and plaque of this patient to determine if they were epidemiologically linked. Eight colonies cultured from the stomach and plaque specimens were isolated and resubcultured until three to five plates of each colony type (clone) were available for restriction endonuclease analysis. DNA from each isolate was digested in HindIII, HaeIII, and BgIII (Boehringer Mannheim). It was therefore evident that at least one isolate from the plaque was genetically closely related or identical to the strain from the stomach. Krajden’s team first described dental plaque as a common or rare ecological niche source of H. pylori infection[11]. Also in India, in 1991, Desai et al[13] reported that when administering the triple therapy to 24 patients with H. pylori-positive gastritis and dental plaque, stomach bacterium remitted in 100% of the patients, but H. pylori persisted in the 24 dental plaques. Therefore, they considered that the triple therapy was not sufficient for H. pylori eradication, and it should be simultaneously approached with local treatment. From 1989 to date, many researchers worldwide have identified H. pylori in plaque and saliva with varying results (Table 1). We emphasize that works such as Pustorino et al[23], in Italy, reported a low relative frequency that by dental plaque culture of 83 dyspeptic patients, and found in each patient the identical protein profile of the bacteria, both in the plate and in the stomach[23]. The persistence of bacteria in dental plaque was reported in 1996 by Pytko-Polonczyk et al[24], who after administering triple therapy, found that bacteria persisted in dental plaque in all patients. In the Kangnam Hospital of Korea, H. pylori was detected in dental plaque and saliva from 7% and 14% respectively, of patients with H. pylori-positive gastric pathology, suggesting that the oral cavity may be an important reservoir of H. pylori[31]. After triple therapy administration, Suk et al[35] in Taiwan reported an 84% resolution in stomach, but only 7% in dental plaque. Wang and colleagues conducted a comparison study of H. pylori cytotoxin genotypes in stomach and saliva. CagA, vacAm1, vacAm2, and vacAs1 genotypes were analyzed in 31 patients, and DNA sequencing in 3 subjects showed 78%, 64%, and 67% H. pylori homology from both sources, respectively. They suggested that more than one H. pylori strain could coexist in the saliva and stomach in the same patient[36]. In Venezuela, Berroteran et al[37] investigated H. pylori infection in dental plaque from 32 dyspeptic patients, and its relationship with gastric pathology. They found that 24/32 (75%) patients presented H. pylori-positive gastric pathology, and 12/32 (38%) also presented H. pylori in the dental plaque, assuming that this organism in the dental plaque could be a risk factor for gastrointestinal re-infection. On the basis of the results obtained with the rapid urease test (RUT) in the mouth, De Sousa et al[44] suggested that this methodology for H. pylori detection was not sufficiently sensitive for the determination of the microorganism in the oral cavity. The most frequent genotype in dental plaque and gastric mucosa was vacA s1bm1[51]. It was found that 47/196 (24%) patients were co-infected in both samples, 28% of whom had 2 different genotypes in saliva, and one or both in the stomach. The s1m1/s1m2 genotypes, alone or together, were found simultaneously in saliva and gastric biopsy from the same patient. Then they suggested that saliva could be the transmitting and re-infecting vector[55]. Dental plaque has been identified as the second reservoir of H. pylori, and the first extra-gastric reservoir. However, some studies revealed 100% negativity in plaque and saliva[16,18,20-22]. Microbial Culture Techniques are a useful tool for the identification of bacteria in gastric specimens. However, researchers have run into several difficulties with culture in the oral cavity. The sensitivity and specificity of H. pylori IgG antibodies in saliva (ELISA) have been estimated in 80% and 70%, respectively. On the other hand, disparate results were found regarding the effectiveness of brushing dental frequency with H. pylori presence in the dental plaque. Some investigators could not find any association[50,53].
Table 1.
Helicobacter pylori detection in dental plaque and saliva n (%)
| Ref. | Sample | Diagnostic method | Patient profile | Helicobacter pylori detection rate |
| Krajden et al[10] | Pq | MCT | Dys | 1/71 (1) |
| Shames et al[11] (Canada) | Pq/Sal | MCT, REA | Dys | Pq: 1/29 (3) |
| Sal: 0/29 (0) | ||||
| Majmudar et al[12] | Pq | MCT | Dys | 40/40 (100) |
| Desai et al[13] | Pq | RUT | Dys | Gc: 0/24 (0) |
| Tripe-H. pyloriET | Pq: 24/24 (100) | |||
| D'Alessandro et al[14] | Pq | MCT, Giemsa, RUT | Dys | 16/20 (80) |
| Nguyen et al[15] | Pq | RT-PCR (16S rRNA) | Dys | 18/25 (72) |
| Bernander et al[16] | Pq | MCT | Dys | 0/94 (0) |
| Mapstone et al[17] | Pq/Sal | PCR | Dys | Pq: 3/13 (23) |
| Sal: 2/13 (15) | ||||
| Von Recklinghausen et al[18] | Pq | MCT, RUT | Dys | 0/55 (0) |
| Li et al[19] | Sal | PCR (860-bp DNA) | Dys | 30/40 (75) |
| Hardo et al[20] | Pq | MCT, N-PCR (16S rRNA) | Dys | 1/62 (2) |
| Cammarota et al[21] | Pq | Giemsa: PCR (ureA), RUT | Dys | 0/31 (0) |
| Luman et al[22] | EGB | MCT | Dys | EGB: 52/109 (47) |
| Pq/Sal | Pq/Sal: 0/120 (0) | |||
| Pustorino et al[23] | Pq | MCT | Dys | 5/83 (6) |
| Pytko-Polonczyk et al[24] | Pq/Sal | RUT, 13-UBT | 100 Dys | Post-triple H. pyloriET |
| 50 DU | DU Gc: 1/30 (3) | |||
| DU Pq: 30/30 (100) | ||||
| Cheng et al[25] | Pq | MCT, RUT | Dys | MCT: 1/122 (1) |
| RUT: 71/122 (58) | ||||
| Oshowo et al[26] | Pq/Sal | MTC, Giemsa, RUT, REA | Dys | EGB: 116/116 (100) |
| OS | Pq: 15/116 (13) | |||
| Améndola et al[27] | Pq | MCT | Dys | 1/20 (5) |
| Mattana et al[28] | Pq | MCT | Dys | 1/62 (2) |
| Song et al[29] | Pq | N-PCR1 | Dys | 41/42 (97) |
| Doré-Davin et al[30] | Pq/Sal | N-PCR (16S rRNA-ureC), UBT | DU | 9/22 (40) |
| Kim et al[31] | Pq/Sal | MCT, Giemsa, PCR1, RUT | Dys | Gc: 29/46 (63) |
| Pq: 2/29 (7) | ||||
| Sal: 4/29 (14) | ||||
| Miyabayashi et al[32] | Pq/Sal | N-PCR (ureA) | Gtis, DU, H. pylori-pos | Diagnosis: 23/47 (49) |
| MCT | Pos-H. pyloriET: 11/23 (48) | |||
| Ozdemir et al[33] | Pq | RUT | Dys | Pq: 63/81 (78) |
| Tg | Tg: 48/81 (59) | |||
| Allaker et al[34] | EGB | PCR (ureA-cagA) | Dys, Children | EGB: 22/100 (22) |
| Pq | Pq: 15/22 (68) and 21/88 (24) | |||
| Suk et al[35] | Pq | PCR (cag-A) | Dys | Gc: 38/65 (58) |
| RUT | Pq: 28/65 (43) | |||
| Wang et al[36] | Sal | PCR-Sequence (cagA-vacA) | Gtis, DU | More than one H. pylori strain in Gc and Sal, in same patient |
| Berroteran et al[37] | EGB | PCR (urease gene cluster) | 32 Dys | Gc: 24/32 (75) |
| Pq | 20 Asym | Pq: 12/32 (38), 3/20 (15) | ||
| Gürbüz et al[38] | Pq | RUT | Dys | Gc: 65/75 (86) |
| Pq: 68/75 (90) | ||||
| Nasrolahei et al[39] | Pq | MCT, PCR1, RUT | Dys | P > 0.05 |
| Siddiq et al[40] | EGB | Giemsa, 13-UBT | Dys | Gc: 32/52 (62) |
| Pq | Pq: 48/52 (92) | |||
| Czesnikiewicz-Guzik et al[41] | Pq/Sal | MCT, 13-UBT | Dys | Gc: 51/100 (51) |
| Pq-Sal: 54/100 (54), P > 0.05 | ||||
| Kignel et al[42] | Pq/Sal | PCR | Dys | Gc: 20/49 (41) |
| Pq-Sal: 1/20 (5) | ||||
| Chitsazi et al[43] | Pq | RUT | Dys | Gc H. pylori: 16/44 (36) |
| 44 Gc H. pylori | No-Gc H. pylori: 14/44 (32) | |||
| 44 no-Gc H. pylori | P = 0.7 | |||
| De Sousa et al[44] | EGB | MCT, Giemsa, RUT | 97 Dys | Gc: 111/147 (76) |
| Pq/Sal | 50 Asym | Pq/Sal (RUT): 0/147 (0) | ||
| Sudhakar et al[45] | Pq | MCT, RUT | 50 Dys | Pq MCT: 5/50 (1) |
| 25 control | Pq RUT: 37/50 (74) | |||
| Bürgers et al[46] | SP-P | MCT, Ser (ELISA), PCR | Dys | Gc: 29/94 (31) |
| Sal | SP-P: 3/94 (3) | |||
| Sal: 7/94 (7) | ||||
| Liu et al[47] | EGB, | Giemsa, PCR, RUT | Dys | EGB: 273/443 (62) |
| Pq | Pq: 263/443 (59) | |||
| Silva et al[48] | Pq/Sal | PCR (vacA) | Dys | Gc: 30/62 (48) |
| Pq: 11/30 (37) | ||||
| Sal: 16/30 (53) | ||||
| Rasmussen et al[49] | EGB | SBlot | Dys | Gc: 66/78 (85), P < 0.0001 |
| Pq/Sal | ||||
| Medina et al[50] | EGB | PCR (ureA) | 43 Dys | 18/98 (18) |
| Pq | 55 control | EGB: 38/43 (88) | ||
| Pq/Sal: 15/43 (35) | ||||
| Assumpção et al[51] | EGB | PCR (cagA-vacA), Giemsa, RUT | Dys | Gc PCR: 95/99 (96) |
| Pq | Gc Giemsa: 39/99 (48) | |||
| Gc RUT: 47/99 (49) | ||||
| Pq PCR: 71/99 (72) | ||||
| Pq RUT: 48/99 (52) | ||||
| Navabi et al[52] | MAS | MCT, PCR, RUT | Dys | 925/1861 (49.7) |
| (95%CI: 16-83.4) | ||||
| Zou et al[9] | MAS | MCT, PCR, RUT | Dys | 490/1088 (45) |
| OR 3.61 (95%CI: 191-6.82) | ||||
| Chaudhry et al[53] | Pq | PCR (ureA-16S rRNA-860 bp DNA) | Dys | 46/89 (52) |
| TBF | ||||
| Momtaz et al[54] | EGB, Pq/Sal, Stool sample | PCR (cagA-vacA-ureC), RUT | Dys | PCR: EGB: 233/300 (78), Pq: 0/300 (0), Sal: 32/300 (11), Stool: 215/300 (72) |
| RUT: 271/300 (90) | ||||
| Román-Román et al[55] | EGB, Sal | PCR (vacA) | 162 Gtis | EGB/Sal: 47/196 (24) |
| N-PCR (cagA) | 32 GU | EGB: 103/196 (53) | ||
| Sal: 13/196 (7) | ||||
| Cai et al[56] | Pq | PCR (16S rDNA-CagA), RUT | Dys, Children | 46/235 (20) |
| Pq: 26/46 (57) |
Data not shown. Asym: Asymptomatic subject; Bs: Biopsy; DU: Duodenal ulcer; Dys: Dyspepsia; EGB: Endoscopic gastric biopsy; Gc: Gastric; Gtis: Gastritis; GU: Gastric ulcer; H. pyloriET: Helicobacter pylori eradication therapy; MAS: Meta-analysis study; MCT: Microbial culture techniques; N: Nested; OR: Odds ratio; OS: Oral swab; PCR: Polymerase chain reaction; Pq: Plaque; PS-P: Periodontal status; Ptis: Periodontitis; REA: Restriction endonuclease analysis; RT: Reverse transcription; RUT: Rapid urease test; Sal: Saliva; SBlot: Southern-blotting; SB-P: Subgingival plaque; Ser: Serology; SP-P: Supragingival plaque; TBF: Tooth brushing frequency; Tg: Tongue;
-UBT: 13C-urea breath test.
In summary, we selected 48 works that reported on the presence of H. pylori in saliva and plaque, and further considered the gastric pathology. Three of them studied only the existence of the bacteria in the saliva of patients with dyspepsia, gastritis and gastric ulcer, with positive results[19,36,55]. Bacterial detection in plaque and saliva was reported in 15 investigations, 2 of which showed negative results, both in plaque and in saliva when attempting to culture the bacteria[22,44]. In plaque, exclusively 28 clinical trials were found. The diagnostic technique most commonly used was polymerase chain reaction (PCR), together with serology; RUT and Southern-blotting were the most sensitive methods. Microbial culture was the methodology used in 18 researches to isolate the bacteria, with negative results in 5 of them, positive results between 1% and 10% in 9, and results higher than 10% in 4. Electron microscopic studies have shown that H. pylori has three stages: spiral forms, coccoid forms and degenerative forms. Spiral forms are viable, culturable, and virulent. Coccoid forms may also be viable but are nonculturable, and less virulent. Degenerative forms are pyknotic, nonculturable, coccoid forms of dead H. pylori. These forms cannot be cultured and the cell membrane has disintegrated; however, gene material can be detected by PCR. H. pylori does not seem to participate in biofilm formation in the oral cavity, despite the presence of the bacterium. In Denmark, Andersen and Rasmussen[57] conducted a mini-review and observed that in dental plaque both spiral and coccoid forms exist. Fifteen papers used 2 or more diagnostic techniques. Two meta-analyses were included, which showed a prevalence > 40%, with an OR of 3.61[9,52]. The available evidence reveals that despite some adverse results in the search for the association, dental plaque is the first extra-gastric reservoir of H. pylori. It will play a key role in the relapse of the H. pylori-positive gastric pathology. We have displayed through various research designs, the effectiveness of the eradication therapy when accompanied by adequate hygiene by , and the strong association between bacterial infection and periodontal disease with deep pockets.
PERIODONTAL DISEASE
The bacterial plaque or oral biofilm is a translucent film, mixing a biotic array (bacteria and fungi), and inter- or extracellular matrix (organic compounds and minerals), which adheres to the dental surfaces, gingival and oral epithelium, prostheses and restorations, but is not deletable with simple rinsing. It has a variable composition depending on the location and ripening time (Figure 1). When located in dental and periodontal surfaces, the biofilm is immediately responsible for both dental caries and periodontal disease. The dental plaque hosts different microbiota, and in the absence of good oral hygiene, it develops quickly and adheres to the teeth surface at the supra- and subgingival level. In periodontal lesions, the number of bacteria increases with periodontitis development, and could comprise Porphyromonas gingivalis, Fusobacterum nucleatum, and Fusobacterum periodonticum, co-aggregated with H. pylori strains.
Figure 1.
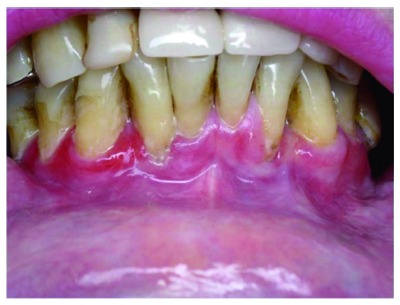
Periodontal disease. Helicobacter pylori-positive finding in subgingival plaque and stomach.
Table 2 shows a meta-analysis selection list of H. pylori detection in dental plaque between the years 1994 and 2012[58-67]. In 1994 Asikainen et al[58] carried out the first H. pylori search in the subgingival plaque of patients with periodontitis in Finland. They concluded that periodontal pockets are not a natural reservoir for H. pylori. In 1999, in Great Britain, Riggio et al[59] demonstrated the presence of H. pylori in 11/29 (38%) subgingival plaques of patients with chronic periodontitis. They suggested that, in this patient group at least, subgingival plaque may be a reservoir for H. pylori infection. But none of these researchers evaluated the gastric condition. It was determined that proper oral hygiene is required to remove H. pylori from dental plaque. They further suggested that the presence of H. pylori in dental plaque must be controlled in order to avoid its recurrence[60]. Periodontal pockets ≥ 5 mm in depth were associated with increased odds of H. pylori seropositivity (OR = 1.47, 95%CI: 1.12-1.94)[61]. The bacterium was detected in saliva, supra- and subgingival plaque, suggesting that these sites may be considered reservoirs for H. pylori in urease-positive patients. The bacterium was not found on the dorsum of the tongue of any patient[62]. Eradication of H. pylori after therapy was more effective from the stomach than from the mouth[63]. The periodontal relative frequency of H. pylori infection increased with gastric infection[65]. Bouziane et al[67] carried out a systematic review and meta-analysis which evaluated the effect of dental plaque control, periodontal therapy and bacterial eradication treatment vs eradication treatment alone in patients with gastric disease. H. pylori eradication therapy (H. pyloriET) alone would not be effective for gastric reinfection control.
Table 2.
Helicobacter pylori detection in periodontal disease n (%)
| Ref. | Sample | Diagnostic method | Patient profile | Helicobacter pylori detection rate |
| Asikainen et al[58] | SB-Pq (United States) | PCR (urease A) | Ptis | 0/336 (0) |
| Riggio et al[59] | SB-Pq | PCR (16S rRNA) | Chronic Ptis | 11/29 (38) |
| Avcu et al[60] | SB-Pq, SP-Pq | Camphylobacter- like organism test gels | Gastric H. pylori and B12 deficiency: (1) good oral hygiene; (2) fair oral hygiene; (3) poor oral hygiene | (1) 6/21 (28); (2) 46/51 (90); (3) 36/36 (100) post-H. pyloriET: (1) 58%; (2) 41%; (3) 5% |
| Dye et al[61] | BL | Ser (ELISA) | NHANES III; 1988-1991 | Periodontal pocket ≥ 5 mm: 493 (41) |
| Gebara et al[62] | SB-Pq, SP-Pq, Tg, Sal | PCR (16S rRNA) | GDis, RUT pos (15 gingivitis, 15 chronic Ptis) | 13/30 (43) |
| Gebara et al[63] | EGB, SB-Pq, SP-Pq, Tg, Sal | PCR (16S rRNA) | Post-H. pyloriET: GDis RUT pos (15 gingivitis, 15 Chronic Ptis) | Gastric eradication 90% H. pylori: 3/30 (10). Oral eradication: 40% H. pylori: 18/30 (60) |
| Anand et al[64] | PS | Oral Hygiene | 65 pos Ser/RUT/Giemsa; 69 control | Ptss: 30/65 (46); |
| Al Asqah et al[8] | SB-Pq | RUT | H. pylori-IgG | PDss: 37/50 (60) |
| Eskandari et al[65] | SB-Pq, SP-Pq | PCR (16S rRNA) | Chronic Ptis (23/67 Gtis) | 4/67 (6) |
| Agarwal et al[66] | SB-Pq | MCT, PCR (16S rRNA) | Chronic Ptis (30 GDss-pos; 20 GDss-neg) | 18/30 (60); 3/20 (15) |
| Bouziane et al[67] | MAS | Gtis (post-H. pyloriET) | RR 63%; [0.37 (95%CI: 0.21-0.64), P = 0.0004] |
BL: Blood; EGB: Endoscopic gastric biopsy; Gtis: Gastritis; GDss: Gastric disease; H. pyloriET: Helicobacter pylori eradication therapy; H. pylori-IgG: Presence of anti-H. pylori-IgG; MAS: Meta-analysis study; MCT: Microbial culture techniques; Neg: Negative; PDss: Negative periodontal disease; PS: Periodontal status; Ptis: Periodontitis; PCR: Polimerase chain reaction; Pos: Positive; RUT: Rapid urease test; Sal: Saliva; SB-Pq: Subgingival plaque; Ser: Serology; SP-Pq: Supragingival plaque; Tg: Tongue.
To summarize, twelve clinical trials and a meta-analysis have been included in the information assessment. In order to be able to demonstrate the association between periodontal diseases and bacterial infection, PCR was used in 7 of these trials as a diagnostic method. Only the trial by Asikainen et al[58] reported negative results. The H. pylori prevalence found with this technique was higher than 40%. The RUT was carried out in 4 clinical trials, with results similar to the PCR. Only 1 work was conducted in 4504 subjects using serology anti-H. pylori, with OR of 1.47 in patients with periodontal pocket ≥ 5 mm[61]. Emphasis is laid on the meta-analysis that evaluates the effective response to the H. pyloriET in H. pylori-positive patients presenting periodontal disease[67]. To our knowledge, there is a likelihood of super-infection of sub-gingival plaque in patients who have a poor oral hygiene and are exposed to H. pylori infection due to chronic gastric infections.
APHTHOUS STOMATITIS
Aphthous stomatitis (also termed canker sores, RAS, recurring oral aphthae and recurrent aphthous ulceration) is considered an inflammatory disease of the oral cavity characterized by the presence of erosions/ulcerations, ulcerations with necrosis, erythematous halo underlying the mucous membrane lining, which respects the keratinized mucosa (Figure 2). Its most frequent location is labial and buccal mucosa, floor of the mouth, ventral surface of the tongue, soft palate, gingiva, posterior pillars, and alveolar gum. Three variants of aphthous stomatitis exist, distinguished by the size, number and location of the lesions, the healing time of individual ulcers and whether a scar is left after healing: minor, major and herpetiform. Aphtae can recur between 1 and 6 mo later. Despite their high prevalence, etiopathogenesis remains unclear. However, canker sores are thought to have an immune pattern associated with hematological disorders, hormonal disturbances, gastric complications, food hypersensitivity, emotional states and local trauma as the most characteristic feature[68].
Figure 2.
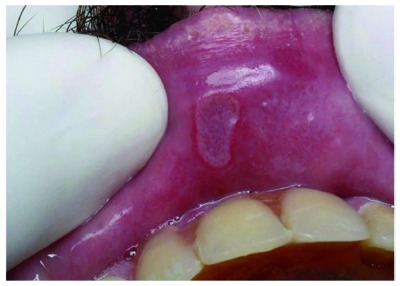
Recurrent aphthous stomatitis. Erosive necrotic lesion in lip mucosa. Helicobacter pylori-negative finding by molecular biology.
The presence of H. pylori in patients with canker sores (Table 3) has been analyzed, given the histological similarities between this condition and gastric ulcer[69-81]. Back in 1995, in Finland, Leimola-Virtanen et al[69] studied the biopsies of human immunodeficiency virus-positive patients with canker sores using Giemsa and in situ hybridization (ISH), and found positive results. Positive serology in RAS has been reported by Porter et al[70] in 1997, in London, who determined the relative frequency of anti-H. pylori IgG antibodies in minor aphthous ulceration, with no significant differences compared with control groups. The association between canker sores and H. pylori tried to be demonstrated through molecular biology, with disparate results (Table 3). In Turkey, the effect of H. pylori eradication on RAS patients was deeply studied. Thus, 23 subjects were regularly monitored for 1 year after anti-H. pylori therapy. A significant difference was observed compared with the reduction in canker sores recurrence and improvement (P < 0.05)[79]. Later, in 2013, again in Turkey, Taş et al[81] conducted a clinical trial including 46 patients with minor aphthous ulceration. Vitamin B12 serum levels were measured, gastric biopsy was done, and presence of H. pylori analyzed. Of 46 study subjects, 30 (65%) were H. pylori-positive, and followed an H. pyloriET. Three months later, they were evaluated with 13-UBT, and 18/30 (60%) were negative. New measurements of vitamin B12 were carried out, and increased levels were observed in the group that received the antibiotic therapy. It was estimated that the effects of the H. pyloriET and the increase in vitamin B12 serum levels improved the clinical course of RAS.
Table 3.
Helicobacter pylori detection in aphthous stomatitis n (%)
| Ref. | Sample (study design) | Diagnostic method | Patient profile | H. pylori detection rate |
| Leimola-Virtanen et al[69] | Bs (TS) | Giemsa, ISH | RAS | Giemsa: 14/29 (50); ISH: 6/29 (20) |
| Porter et al[70] | BL (CCS) | Ser (ELISA) | 75 RAS, 83 (15 other oral ulcerative disorders, 41 other oral mucosal lesions, 27 oral dysaesthesia), 25 control | 23/75 (31) 27/83 (33) 6/25 (24) |
| Birek et al[71] | OS (CCS) | PCR (ureC) | RAS | 23/32 (72) |
| Riggio et al[72] | Bs (CCS) | PCR (16S rRNA) | 28 RAS, 20 OLP, 13 control | 3/28 (11), 0/20 (0); 0/13 (0) |
| Shimoyama et al[73] | BL, OS (CCS) | MCT, Ser (ELISA) | RAS | 0/12 (0) |
| Victória et al[74] | OS (CCS) | N-PCR1 | 36 RAS, 48 control | 14/36 (38); 16/48 (33) |
| Iamaroon et al[75] | OS (CCS) | N-PCR (H. pyloriaA) | 22 RAU, 15 control (Tg) | 1/22 (5); 3/15 (20) |
| Elsheikh et al[76] | Bs (CCS) | PCR (16S rRNA) | 58 RAS and Lymp 88 RAS, 20 control | 39/58 (67); 9/88 (10); 0/20 (0) |
| Mansour-Ghanaei et al[77] | BL, OS (CCS) | Ser (ELISA), PCR1 | RAS | H. pylori-IgG: 26/50 (52); PCR: 1/50 (2) |
| Albanidou-Farmaki et al[78] | Sal, BL, RUT (CSS) | Ser (ELISA), 13-UBT | RAS | 34/48 (71) |
| Karaca et al[79] | Gastric Bs (CSS) | Giemsa (PTE) | RAS | 20/23 (89); P < 0.05 |
| Maleki et al[80] | RUT (CCS) | 13-UBT | 43 RAS, 44 control | 16/43 (37); 14/44 (32); P = 0.597 |
| Taş et al[81] | Gastric Bs, BL (CSS) | 13-UBT (PTE) | MAL (vitamin B12) | Pre: 30/46 (65); post: 12/30 (40) |
Data not shown. Helicobacter pylori: H. pylori; BL: Blood; Bs: Biopsy; CCS: Case-control study; CSS: Cross-sectional study; H. pylori-IgG: Presence of anti-H. pylori-IgG; ISH: In situ hybridization; Lymp: Lymphoma; MAL: Minor aphthous lesions; MCT: Microbial culture techniques; N: Nested; OLP: Oral lichen planus; OS: Oral swab; PCR: Polymerase chain reaction; PTE: Post-treatment evaluation; RAS: Recurrent aphthous stomatitis; RAU: Recurrent aphthous ulceration; RUT: Rapid urease test; Ser: Serology; Sal: Saliva; Tg: Tongue; TS: Transversal study;
-UBT: 13C-urea breath test.
In summary, we evaluated 13 articles reporting the potential association between recurrent canker sores and the action of H. pylori in their etiology. The designs were 9 case-control studies, 1 cross-sectional, 2 clinical trials of the effectiveness of eradication therapy and 1 meta-analysis. The latter evaluated nine of the works included in our analysis, whose data and conclusions are, in our opinion, correct. The samples used in the research were 3 tissue (lesion biopsy), 4 swabs, 3 sera, 2 expired air and 1 saliva. In 3 papers, 2 or more samples were used. The diagnostic methods were PCR in 6 of the publications, ELISA in 3, 13-UBT in 2. Other techniques were Giemsa, ISH and culture. Four reports used 2 diagnostic methods. Results showed no association in 6 researches, 4 of them using PCR as a diagnostic method. Clinical trials of therapeutic effectiveness showed reduction in episodes of relapse of canker sores after the eradication therapy in patients with gastric H. pylori-positive and recurrent canker sores. These patients were anemic, and the presence of the bacterium had not been evaluated in canker sores injury[79,81]. We have investigated the presence of the bacterium in canker sores from patients with dyspepsia. Samples were taken by biopsy, and the following diagnostic techniques were used: HE, Periodic acid-Schiff (PAS), Giemsa and PCR. Negative results were obtained with all the methods. All the patients underwent video-esofagogastricoduodenoscopy with biopsy of the greater and lesser curvature of the gastric antrum up to 5 cm from the pylorus. Erosive and chronic active H. pylori-positive gastritis was found in 63% of the patients, who had an iron deficiency anemia[82]. We believe that the action of the bacteria in the RAS is due to H. pylori-positive gastric disease and deficiency conditions. The presence of bacterial infection in the occurrence and recurrence of canker sores would be associated with anemia, produced by H. pylori-positive stomach diseases. Antibiotic therapy and the return to normal hematological values lead to a decrease in the number of canker sores, their size and relapse.
ORAL CANCER
In 2011, the American Cancer Society estimated 39400 new cases of oral and pharynx cancer, and 7900 deaths from this cause. The incidence varies according to gender, racial or ethnic group, and geographic location. The latter is directly related to particular habits and the differential exposure to environmental carcinogens[83]. The most frequent cancer in the oral cavity is squamous cell carcinoma of the mucosa, which represents 90% of all malignant neoplasms in this location (Figure 3). The remaining 10% includes salivary gland tumors, sarcomas of the soft tissues and jaw bones, non-Hodgkin’s lymphoma, metastasis of extra oral primary tumors and melanomas. Oral squamous cell carcinoma is the sixth most common cancer in the world, with approximately 350000 deaths and 650000 new diagnoses per year[84-86]. It was estimated that biological carcinogens cause 18% of all cases of cancer[87]. In 1994, H. pylori was considered a carcinogenic agent type 1 by the Organización Mundial de la Salud/International Agency for Research on Cancer, OMS/IARC (http://www.iarc.fr).
Figure 3.
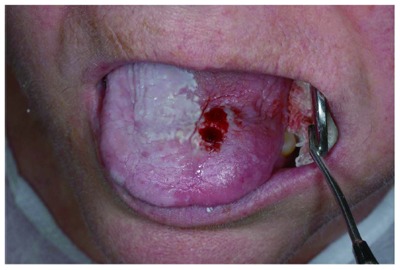
Oral squamous cell carcinoma grade 2 in lingual dorsum. Helicobacter pylori-negative finding by molecular biology. Biopsy area.
Few studies have addressed the relationship between oral cancer and H. pylori action (Table 4)[88-93]. The first study was carried out by Grandis et al[88] who studied the serology of 21 patients with oral cancer and 21 controls, and observed a similar seroprevalence in both patients and controls, and they could not establish a significant association. Serologic studies showed a similar relative frequency in patients with cancer and controls[92]. A group of Indian researchers evaluated the presence of H. pylori in serum and tissue samples from 20 patients with oral cancer and 20 controls, using culture and PCR. While this study showed no significant differences, the OR of H. pylori-positive patients was 3.0 (95%CI: 0.34-26.4), while it was lower for only PCR-positive subjects (OR = 1.5, 95%CI: 0.28-8.0)[93].
Table 4.
Helicobacter pylori detection in oral cancer n (%)
| Ref. | Sample (study design) | Diagnostic method | Patient profile | H. pylori detection rate |
| Grandis et al[88] | BL (CCS) | Ser (ELISA) | 21 HNSCC 21 controls | 12/21 (57) 13/21 (62), P > 0.05 |
| Singh et al[89] | Bs (CCS) | MCT, Giemsa, RUT | 26 OC 26 controls | MTC: 0/26 (0); 0/26 (0) Giemsa: 4/26 (15); 0/26 (0) RUT: 3/26 (11); 0/26 (0) |
| Okuda et al[90] | Bs, OS (PRS) | MTC, RT PCR1 | GDss (58 OC) | Bs: 54/116 (46); OS: 14/116 (12); OC: 11/58 (19) |
| Kanda et al[91] | Bs, urine (PRS) | MTC, Ser (ELISA), PCR1, IHC | HNSCC | Bs: 0/31 (0) MTC, IHC, PCR. Urine: 21/31 (68) Ser |
| Fernando et al[92] | BL (CCS) | Ser (ELISA) | 53 cases 60 controls | 14/53 (23) 10/60 (17) |
| Dayama et al[93] | Bs (CCS) | MTC; PCR (16S rRNA) | 20 OC 20 controls | OC: 3/20 (15) MCT, PCR Controls: 1/20 (5) MTC and 2/20 (10) PCR |
Data not shown. BL: Blood; Bs: Biopsy; CCS: Case-control study; GDss: Gastric disease; HNSCC: Head and neck squamous cell carcinomas; ISH: In situ hybridization; MCT: Microbial culture techniques; OC: Oral cancer; OS: Oral swab; PCR: Polymerase chain reaction; PRS: Prospective study; RUT: Rapid urease test; Ser: Serology.
In summary, only a few researchers have looked for the bacteria in oral cancer. All of them have included patients with squamous cell carcinoma. We have analyzed 6 works, 4 of which are case- control studies, and 2 prospective studies. The samples used to search the bacteria were biopsy, swabs and serum, and the diagnostic methods were culture, serology, histopathology, 13-UBT, ISH, RUT and PCR. No relationship was found with the bacteria. These results are in line with those found by our group in the study of 8 oral squamous cell carcinomas in patients with dyspepsia. We studied biopsy, and used HE, PAS, Giemsa and PCR as diagnostic techniques, with negative results for all these methods. The relationship between H. pylori and the pathogenesis of gastric cancer has been well described, due to the ability of H. pylori to modify the host’s immune response. It might behave similarly in the progression of oral carcinoma; however, this association has not been demonstrated yet. In the future, a prospective cohort design will be required to be able to determine a potential association between H. pylori and oral cancer.
BURNING, HALITOSIS, AND LINGUAL DORSUM HYPERPLASIA
The oral burning sensation can be a symptom of an underlying disease or a syndrome of unknown etiology. If the local or systemic factors involved are identified, these are referred to as the cause of the burning[94,95].
Halitosis means abnormal odour of exhaled air regardless of its origin. Halitosis can be oral, nasal, gastric or systemic (diabetes, nephropathies, trimethylaminuria). Miyazaki et al[96] have classified.
Genuine halitosis
It was subdivided into: (1) Physiological-halitosis, in which there is no disease; and (2) Pathologic-halitosis, which may be oral or extra-oral. Oral pathologic-halitosis occurs as a result of a pathological process in the mouth, either dental or mucosal (caries, periodontal disease, canker sores, cancer, etc.). Non-oral pathological-halitosis can originate from the upper respiratory tract and from other sources that are carried by blood and exhaled in the lung[96-98].
Pseudo-halitosis
It is characterized by absence of halitosis. However, the patient believes that he has oral malodour.
Halitophobia
It occurs when the patient perceives his bad breath after treatment of a true halitosis or pseudohalitosis, with physical evidence or a negative effect in his social life, which indicates the persistence of the bad smell. However, the patients are convinced that their “Halitosis” is socially offensive[96,99,100].
Bad breath or oral malodour is the term used for halitosis of oral origin[101]. Oral halitosis is caused by volatile sulfur compounds (VSC), such as methyl mercaptan, hydrogen sulfide, methyl disulfide, which are generated by the action of bacterial metabolism that degrades the sulfur containing amino acids present in the oral cavity. Anaerobic and gram-negative bacteria are the agents most frequently involved[102-104]. These bacteria are found in gingival grooves, in periodontal pockets, and in posterior lingual dorsum[105]. De Boever et al[106] considered that the anaerobic flora of the tongue plays an essential role in halitosis origin. There are different research lines that postulate lingual anaerobic flora action as one of the causes of halitosis appearance. These researches argue that the tongue acts as a reservoir that allows the accumulation and stagnation of bacteria and food waste[107-110]. The three main methods of diagnosing halitosis are gas chromatography, organoleptic measurement, and sulphide monitoring[99]. Loesche et al[110] reported that 74% of the bacteria cultured from the lingual dorsum were Veillonella parvula, Actinomyces odontolyticus, Streptococcus intermedius and Clostridium innocuum.
Table 5 shows the different research groups that attempted to relate halitosis to H. pylori[111-123]. The first were Tiomny et al[111], who in 1992 in Israel studied 6 patients with halitosis, 5 of whom were H. pylori-positive. They found that halitosis had disappeared after H. pyloriET, and highlighted the possible connection between halitosis and H. pylori infection. At the University of Bari (Italy), Ierardi and partners associated halitosis with H. pylori infection and correlated H. pylori-eradication in dyspeptic patients. They established the levels of VSCs at diagnosis and in subsequent controls after H. pyloriET was established[112]. In 2001 we reported a patient with severe BHH of lingual dorsum, who underwent biopsy of the affected tongue area. H. pylori was observed by Giemsa and PCR[113]. In Croatia a group of researchers, using PCR, detected H. pylori in the lingual dorsum of 43/268 (16%) patients with burning mouth syndrome (BMS), migratory glossitis and atrophic glossitis, with controls being H. pylori-negative[114]. Serin et al[115] administered triple H. pyloriET for 2 wk (omeprazole 20mg, clarithromycin 500mg, amoxicillin 1000mg), to subjects with halitosis and H. pylori-positive gastric disease. They reported that in patients with confirmed H. pyloriET, halitosis was the most successfully resolved symptom (62% to 3%). Therefore, they considered that halitosis is a frequent and treatable symptom of H. pylori-positive non-ulcer dyspepsia, and may be a valid indication for H. pyloriET. In the year 2005 in Argentina, we designed a case-control study to determine whether H. pylori was a risk factor in subjects with burning, halitosis, and lingual dorsum hyperplasia or BHH (Figure 4). A total of 124 subjects with different gastric diseases were studied: 46 patients with BHH and 78 patients with other oral diseases. H. pylori detection in the oral cavity by histopathologic diagnosis and molecular biology was confirmed in 40/46 (87%) patients with BHH, and in 2/78 (2.6%) subjects with other diseases (RR 13.01, 95%CI: 6-28.20). Gastric relative risk was RR 8.02, 95%CI 4.28-15.01. This trial showed, for the first time, an association between H. pylori and BHH, and further considered this bacterium a risk factor for gastric infection[82]. In Greece, Katsinelos et al[117] carried out a cohort-study with 18 patients with dyspepsia. All patients underwent gastric biopsy, and H. pylori status was determined histopathologically by hematoxylin and eosin, and Giemsa staining. A biopsy specimen was also taken from the antrum for RUT (CLOtest, Kimberly-Clark/Ballard Medical Products). All H. pylori-positive patients were prescribed a 10-d course of triple drug for H. pyloriET (20 mg omeprazole, 500 mg clarithromycin and 1000 mg amoxicillin, all twice daily). In case of eradication failure, a 10-d course of quadruple drug for H. pyloriET (20 mg omeprazole twice daily, 600 mg bismuth subcitrate twice daily, 500 mg metronidazole twice daily, and 500 mg tetracycline, 4 times daily) was administered. Four to six weeks after completion of H. pyloriET, the symptom of halitosis was re-evaluated, and repeated endoscopy or 13-UBT was performed to assess H. pylori-status in the gastric mucosa. Triple H. pyloriET was sufficient to eradicate H. pylori in 14/18 (78%) patients. In the 4/18 (22%) patients who remained H. pylori-positive after triple drug H. pyloriET, quadruple drug H. pyloriET successfully eradicated the bacterium. Halitosis did not recur in 16/18 (89%) patients. The results obtained revealed that H. pyloriET should be indicated to H. pylori-positive patients with halitosis[117]. Suzuki et al[119] studied the relationship between halitosis and H. pylori-presence in saliva. A total of 326 subjects were recruited, 251 with halitosis and 75 without halitosis. The clinical symptoms associated with oral malodour and periodontal symptoms were significantly greater in the H. pylori-positive subjects. In South Korea, Yoo et al[120] evaluated halitosis in 72 H. pylori-positive patients. Patients were divided into two groups, 24 with erosive gastric lesions and 48 with non-erosive gastric lesions. Researchers considered that halitosis could be an effective biomarker predicting that the gastric mucosa of affected patients might show erosive change beyond inflamed condition.
Table 5.
Helicobacter pylori detection in burning, halitosis, and lingual dorsum hyperplasia n (%)
| Ref. | Diagnostic method | Patient profile | H. pylori detection rate | Hal detection rate |
| Tiomny et al[111] | SHal | 6 Hal | 5/6 (83) | Post-H. pyloriET: 0/11(0) |
| Ierardi et al[112] | OHal | Dys [Hal: 52 (90)] | Diagnosis: 30/52 (58) | Diagnosis: 52/52 (100) |
| Double H. pyloriET: 11/30 (64) | Double H. pyloriET: 11/30 (64) | |||
| Triple H. pyloriET: 2/11 (18) | Triple H. pyloriET: 2/11 (18) | |||
| Adler et al[113] | EGB, SHal, OHal, Giemsa, PCR (SSA) | Dys, BHH | Tg: 1/1; Gc: 1/1 | Case report |
| Gall-Troselj et al[114] | N-PCR (ureA) | AG: 87 (32) | Tg: 43/268 (16) | 17/87 (20), |
| BMG: 37 (14) | 1/37 (3), | |||
| BMS: 144 (54) | 12/54 (22), | |||
| (54 Tg, 90 no-Tg) | 13/90 (14) | |||
| Serin et al[115] | EGB, Giemsa | Dys, Hal, EGB: H. pylori-pos, no lesion and no atrophy | EGB pos-H. pyloriET: 39/148 (26) | Hal post-H. pyloriET: 40/148 (3) |
| Adler et al[113] | EGB, SHal, OHal, Giemsa, PCR (SSA) y (16SrRNA) | GDSS [BHH: 46 (37), no-BHH: 78 (63)] | 40/46 (87); 2/78 (3) | RR 13.01 CI 6-28.20 |
| Brailo et al[116] | Ser (ELISA) | 150 BMS | 29/150 (19) | 19/150 (13) |
| Katsinelos et al[117] | EGB, Giemsa, RUT | Dys, Hal, | Diagnosis: 18/18 (100) | Diagnosis: 18/18 (100) |
| Triple H. pyloriET: 4/18 (22) | Triple H. pyloriET: 2/18 (11) | |||
| Quad H. pyloriET: 0/18 (0) | Quad H. pyloriET: 2/18 (11) | |||
| Moshkowitz et al[118] | EGB, RUT | GDSS [GERD: 72 (55)] | H. pylori vs Hal: P > 0.05 | Hal vs GERD: P < 0.05 |
| Suzuki et al[119] | OLT, GC, PCR (16S rRNA) | Non-Dys (251 Hal, 75 no-Hal) | Sal: 21/326 (6) | H. pylori-pos: Higher VSC and OLT Score |
| Yoo et al[120] | OHal, GC, 13-UBT | H. pylori-pos [EGL: 24 (33), NEGL: 48 (675)] | 72/72 (100) | VSC: EGL vs NEGL: P < 0.01 |
| Lee et al[121] | EGB, OHal | Dys, Hal | Gc: 68/88 (77) | Korea Red Ginseng Supplementation |
| Kinberg et al[122] | EGB | Hal [GDSS: 36 (38)] | 14/94 (15) | OHal: 74/94: (78) |
| Tangerman et al[123] | EGB, OLT, GC, RUT | GDSS, Hal | 11/49 (22) | VSC: 9/49 (18); H. pylori: 1/9 (11) |
AG: Atrophic glossitis; BHH: Burning, halitosis, and lingual dorsum hyperplasia; BMS: Burning mouth syndrome; BMG: Benign migratory glossitis; Dys: Dyspepsia; EGB: Endoscopic gastric biopsy; EGL: Erosive gastric lesions; GC: Gas chromatography; Gc: Gastric; GERD: Gastroesophageal reflux disease; GDSS: Gastric disease; Hal: Halitosis; H. pyloriET: Helicobacter pylori eradication therapy; N: Nested; NEGL: Non-erosive gastric lesions; OHal: Objective halimeter levels > 100 ppb; OLT: Organoleptic test; PCR: Polymerase chain reaction; Pos: Positive; Quad: Quadruple; RR: Relative risk; RUT: Rapid urease test; Sal: Saliva; Ser: Serology; SHal: Subjective halitosis; SSA: 26-kDa species-specific antigen gene; Tg: Tongue; VSC: Volatil sulfur compounds; 13-UBT: 13C-urea breath test.
Figure 4.
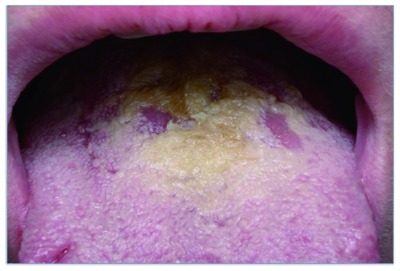
Burning, halitosis, and lingual dorsum hyperplasia. Negative for mycological microscopy and culture. Helicobacter pylori-positive finding in lingual dorsum and stomach.
In summary, in this chapter we have analyzed 10/14 studies on the possible association between halitosis and H. pylori infection. Nine of them included patients with H. pylori-positive gastric disease, and 5/10 were based on H. pyloriET results. In these researches, halitosis had been resolved in 90% to 100% of the cases. Regarding burning sensation, 2/14 articles reported H. pylori-positivity in 10% to 20% of the cases. A burning tongue was reported in one of them. In 2/14 we have described a particular clinical syndrome (BHH)[82,113]. We estimated a high risk of H. pylori-infection in patients with this syndrome. Our data suggest that halitosis is not a consequence of gastric pathology; however the work shows that in lingual dorsum, the action of the bacterium is the etiologic agent of BHH syndrome. H. pylori is one of the risk factors of halitosis, with an oral or gastric origin. Therefore, the search for the bacterium must be oral and gastric. Oral and/or gastric infection by H. pylori may occur with intense burning. In those cases, it is necessary to determine whether the patient experiences gastric discomfort. If so, it would be necessary to look for the bacteria. We have described a clinical syndrome characterized by “burning, halitosis and lingual dorsum hyperplasia”. Discrepancies in the diagnosis of those patients who consulted due to these symptoms and further referred chronic gastric discomfort made us hypothesize about the association between H. pylori both in tongue and stomach. In our opinion, and given the biological implications of the bacteria, it is necessary to focus on patients with BHH. Then, we must objectively diagnose halitosis, ask the patient if he/she has experienced discomfort in the gastric tract, evaluate burning and properly inspect his/her mouth. Lab tests must include anti-H. pylori antibodies. Cytobrush on tongue surface must also be performed to reach an H. pylori diagnosis by molecular biology. Before a positive result in mouth might be obtained and prior to any therapy, the patient must be referred to the gastroenterology unit for his/her examination, given the biological behavior of H. pylori.
CONCLUSION
Infection by H. pylori is one of the most frequent infections in the world, and has revolutionized the gastric pathology in the last twenty-five years. Early diagnosis is essential for infection control. The interest in H. pylori-infection in the oral cavity has progressively increased, since the presence of this bacterium in the mouth determines an oral-oral way of transmission. This review describes the relation between H. pylori and different oral pathologies, such as periodontal disease, canker sores, squamous cell carcinoma, burning tongue and halitosis, and its association with gastric pathology.
Designs for prospective cohorts are required to demonstrate the bacterial action in the mouth using sensitive and specific diagnostic methodologies. In terms of H. pylori identification methods, culture is considered the gold standard. However, though sensitive, the method is not specific, since additional testing must be performed on the isolates. H. pylori PCR amplification is the method of choice[124]. However, several H. pylori genes have extensive polymorphism and particular genes are absent in some strains, as cagA[125]. Among the genes that have been tested, ureA and ureC (also named glmM) appear sensitive, but lack specificity. Therefore, the concurrent H. pylori detection of multiple genes and the use of different sets of primers are required to reach a specific and sensitive diagnosis of the infection[82]. Another alternative has been the use of H. pylori ribosomal gene 16SrRNA, which is present in all bacteria and is specific to a given bacterial genus[126,127]. Reviewing the literature herein cited, we found that the additional post-culture methods or the chosen molecular targets are not always described. In this sense, a methodological consensus would be required to validate the real location of the bacteria in the mouth, since this methodological diversity hinders the proper interpretation of the results.
ACKNOWLEDGMENTS
We thank Marcela Rossi, Jorge Sucar, and Valeria Melia for language revision.
Footnotes
P- Reviewer: Codoner-Franch P, Du YQ, Mansour-Ghanaei F S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 3.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabwera HM, Logan RP. Epidemiology of Helicobacter pylori: transmission, translocation and extragastric reservoirs. J Physiol Pharmacol. 1999;50:711–722. [PubMed] [Google Scholar]
- 5.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, Vaz Coelho LG, Fock M, Fedail S, Cohen H, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guidelines. 2010 [PubMed] [Google Scholar]
- 6.Niv Y. H pylori recurrence after successful eradication. World J Gastroenterol. 2008;14:1477–1478. doi: 10.3748/wjg.14.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 8.Al Asqah M, Al Hamoudi N, Anil S, Al Jebreen A, Al-Hamoudi WK. Is the presence of Helicobacter pylori in dental plaque of patients with chronic periodontitis a risk factor for gastric infection? Can J Gastroenterol. 2009;23:177–179. doi: 10.1155/2009/950527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou QH, Li RQ. Helicobacter pylori in the oral cavity and gastric mucosa: a meta-analysis. J Oral Pathol Med. 2011;40:317–324. doi: 10.1111/j.1600-0714.2011.01006.x. [DOI] [PubMed] [Google Scholar]
- 10.Krajden S, Fuksa M, Anderson J, Kempston J, Boccia A, Petrea C, Babida C, Karmali M, Penner JL. Examination of human stomach biopsies, saliva, and dental plaque for Campylobacter pylori. J Clin Microbiol. 1989;27:1397–1398. doi: 10.1128/jcm.27.6.1397-1398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shames B, Krajden S, Fuksa M, Babida C, Penner JL. Evidence for the occurrence of the same strain of Campylobacter pylori in the stomach and dental plaque. J Clin Microbiol. 1989;27:2849–2850. doi: 10.1128/jcm.27.12.2849-2850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majmudar P, Shah SM, Dhunjibhoy KR, Desai HG. Isolation of Helicobacter pylori from dental plaques in healthy volunteers. Indian J Gastroenterol. 1990;9:271–272. [PubMed] [Google Scholar]
- 13.Desai HG, Gill HH, Shankaran K, Mehta PR, Prabhu SR. Dental plaque: a permanent reservoir of Helicobacter pylori? Scand J Gastroenterol. 1991;26:1205–1208. doi: 10.3109/00365529108998615. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Seri S. Comparison of three different methods for evaluation of Helicobacter pylori (H.P.) in human dental plaque. Boll Soc Ital Biol Sper. 1992;68:769–773. [PubMed] [Google Scholar]
- 15.Nguyen AM, Engstrand L, Genta RM, Graham DY, el-Zaatari FA. Detection of Helicobacter pylori in dental plaque by reverse transcription-polymerase chain reaction. J Clin Microbiol. 1993;31:783–787. doi: 10.1128/jcm.31.4.783-787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernander S, Dalén J, Gästrin B, Hedenborg L, Lamke LO, Ohrn R. Absence of Helicobacter pylori in dental plaques in Helicobacter pylori positive dyspeptic patients. Eur J Clin Microbiol Infect Dis. 1993;12:282–285. doi: 10.1007/BF01967259. [DOI] [PubMed] [Google Scholar]
- 17.Mapstone NP, Lynch DA, Lewis FA, Axon AT, Tompkins DS, Dixon MF, Quirke P. Identification of Helicobacter pylori DNA in the mouths and stomachs of patients with gastritis using PCR. J Clin Pathol. 1993;46:540–543. doi: 10.1136/jcp.46.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Recklinghausen G, Weischer T, Ansorg R, Mohr C. No cultural detection of Helicobacter pylori in dental plaque. Zentralbl Bakteriol. 1994;281:102–106. doi: 10.1016/s0934-8840(11)80643-2. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Musich PR, Ha T, Ferguson DA, Patel NR, Chi DS, Thomas E. High prevalence of Helicobacter pylori in saliva demonstrated by a novel PCR assay. J Clin Pathol. 1995;48:662–666. doi: 10.1136/jcp.48.7.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardo PG, Tugnait A, Hassan F, Lynch DA, West AP, Mapstone NP, Quirke P, Chalmers DM, Kowolik MJ, Axon AT. Helicobacter pylori infection and dental care. Gut. 1995;37:44–46. doi: 10.1136/gut.37.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cammarota G, Tursi A, Montalto M, Papa A, Veneto G, Bernardi S, Boari A, Colizzi V, Fedeli G, Gasbarrini G. Role of dental plaque in the transmission of Helicobacter pylori infection. J Clin Gastroenterol. 1996;22:174–177. doi: 10.1097/00004836-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Luman W, Alkout AM, Blackwell CC, Weir DM, Plamer KR. Helicobacter pylori in the mouth--negative isolation from dental plaque and saliva. Eur J Gastroenterol Hepatol. 1996;8:11–14. doi: 10.1097/00042737-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Pustorino R, Nicosia R, D’Ambra G, Di Paola M, Brugnoletti O, Grippaudo G, Paparo BS. The mouth-stomach crossing of Helicobacter pylori. Riv Eur Sci Med Farmacol. 1996;18:183–186. [PubMed] [Google Scholar]
- 24.Pytko-Polonczyk J, Konturek SJ, Karczewska E, Bielański W, Kaczmarczyk-Stachowska A. Oral cavity as permanent reservoir of Helicobacter pylori and potential source of reinfection. J Physiol Pharmacol. 1996;47:121–129. [PubMed] [Google Scholar]
- 25.Cheng LH, Webberley M, Evans M, Hanson N, Brown R. Helicobacter pylori in dental plaque and gastric mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:421–423. doi: 10.1016/s1079-2104(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 26.Oshowo A, Tunio M, Gillam D, Botha AJ, Holton J, Boulos P, Hobsley M. Oral colonization is unlikely to play an important role in Helicobacter pylori infection. Br J Surg. 1998;85:850–852. doi: 10.1046/j.1365-2168.1998.00724.x. [DOI] [PubMed] [Google Scholar]
- 27.Améndola R, Roldán CD, Morgade L, Solagna A, Lineado A, Musi AO, Valero J, Zerbo O, Kogan Z, Ferro FE, et al. [Is dental plaque a normal Helicobacter pylori reservoir?] Acta Gastroenterol Latinoam. 1998;28:199–201. [PubMed] [Google Scholar]
- 28.Mattana CM, Vega AE, Flores G, de Domeniconi AG, de Centorbi ON. [Isolation of Helicobacter pylori from dental plaque] Rev Argent Microbiol. 1998;30:93–95. [PubMed] [Google Scholar]
- 29.Song Q, Lange T, Spahr A, Adler G, Bode G. Characteristic distribution pattern of Helicobacter pylori in dental plaque and saliva detected with nested PCR. J Med Microbiol. 2000;49:349–353. doi: 10.1099/0022-1317-49-4-349. [DOI] [PubMed] [Google Scholar]
- 30.Doré-Davin C, Heitz M, Yang H, Herranz M, Blum AL, Corthésy-Theulaz I. Helicobacter pylori in the oral cavity reflects handling of contaminants but not gastric infection. Digestion. 1999;60:196–202. doi: 10.1159/000007659. [DOI] [PubMed] [Google Scholar]
- 31.Kim N, Lim SH, Lee KH, You JY, Kim JM, Lee NR, Jung HC, Song IS, Kim CY. Helicobacter pylori in dental plaque and saliva. Korean J Intern Med. 2000;15:187–194. doi: 10.3904/kjim.2000.15.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter. 2000;5:30–37. doi: 10.1046/j.1523-5378.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozdemir A, Mas MR, Sahin S, Sağlamkaya U, Ateşkan U. Detection of Helicobacter pylori colonization in dental plaques and tongue scrapings of patients with chronic gastritis. Quintessence Int. 2001;32:131–134. [PubMed] [Google Scholar]
- 34.Allaker RP, Young KA, Hardie JM, Domizio P, Meadows NJ. Prevalence of helicobacter pylori at oral and gastrointestinal sites in children: evidence for possible oral-to-oral transmission. J Med Microbiol. 2002;51:312–317. doi: 10.1099/0022-1317-51-4-312. [DOI] [PubMed] [Google Scholar]
- 35.Suk FM, Chen SH, Ho YS, Pan S, Lou HY, Chang CC, Hsieh CR, Cheng YS, Lien GS. It is difficult to eradicate Helicobacter pylori from dental plaque by triple therapy. Zhonghua Yixue Zazhi (Taipei) 2002;65:468–473. [PubMed] [Google Scholar]
- 36.Wang J, Chi DS, Laffan JJ, Li C, Ferguson DA, Litchfield P, Thomas E. Comparison of cytotoxin genotypes of Helicobacter pylori in stomach and saliva. Dig Dis Sci. 2002;47:1850–1856. doi: 10.1023/a:1016417200611. [DOI] [PubMed] [Google Scholar]
- 37.Berroteran A, Perrone M, Correnti M, Cavazza ME, Tombazzi C, Goncalvez R, Lecuna V. Detection of Helicobacter pylori DNA in the oral cavity and gastroduodenal system of a Venezuelan population. J Med Microbiol. 2002;51:764–770. doi: 10.1099/0022-1317-51-9-764. [DOI] [PubMed] [Google Scholar]
- 38.Gürbüz AK, Ozel AM, Yazgan Y, Celik M, Yildirim S. Oral colonization of Helicobacter pylori: risk factors and response to eradication therapy. South Med J. 2003;96:244–247. doi: 10.1097/01.SMJ.0000051069.50950.2B. [DOI] [PubMed] [Google Scholar]
- 39.Nasrolahei M, Maleki I, Emadian O. Helicobacter pylori colonization in dental plaque and gastric infection. Rom J Gastroenterol. 2003;12:293–296. [PubMed] [Google Scholar]
- 40.Siddiq M, Haseeb-ur-Rehman A. Evidence of Helicobacter pylori infection in dental plaque and gastric mucosa. J Coll Physicians Surg Pak. 2004;14:205–207. [PubMed] [Google Scholar]
- 41.Czesnikiewicz-Guzik M, Bielanski W, Guzik TJ, Loster B, Konturek SJ. Helicobacter pylori in the oral cavity and its implications for gastric infection, periodontal health, immunology and dyspepsia. J Physiol Pharmacol. 2005;56 Suppl 6:77–89. [PubMed] [Google Scholar]
- 42.Kignel S, de Almeida Pina F, André EA, Alves Mayer MP, Birman EG. Occurrence of Helicobacter pylori in dental plaque and saliva of dyspeptic patients. Oral Dis. 2005;11:17–21. doi: 10.1111/j.1601-0825.2004.01043.x. [DOI] [PubMed] [Google Scholar]
- 43.Chitsazi MT, Fattahi E, Farahani RM, Fattahi S. Helicobacter pylori in the dental plaque: is it of diagnostic value for gastric infection? Med Oral Patol Oral Cir Bucal. 2006;11:E325–E328. [PubMed] [Google Scholar]
- 44.De Sousa L, Vásquez L, Velasco J, Parlapiano D. [Isolation of Helicobacter pylori in gastric mucosa, dental plaque and saliva in a population from the Venezuelan Andes] Invest Clin. 2006;47:109–116. [PubMed] [Google Scholar]
- 45.Sudhakar U, Anusuya CN, Ramakrishnan T, Vijayalakshmi R. Isolation of Helicobacter pylori from dental plaque: A microbiological study. J Indian Soc Periodontol. 2008;12:67–72. doi: 10.4103/0972-124X.44098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bürgers R, Schneider-Brachert W, Reischl U, Behr A, Hiller KA, Lehn N, Schmalz G, Ruhl S. Helicobacter pylori in human oral cavity and stomach. Eur J Oral Sci. 2008;116:297–304. doi: 10.1111/j.1600-0722.2008.00543.x. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Yue H, Li A, Wang J, Jiang B, Zhang Y, Bai Y. An epidemiologic study on the correlation between oral Helicobacter pylori and gastric H. pylori. Curr Microbiol. 2009;58:449–453. doi: 10.1007/s00284-008-9341-3. [DOI] [PubMed] [Google Scholar]
- 48.Silva DG, Stevens RH, Macedo JM, Albano RM, Falabella ME, Veerman EC, Tinoco EM. Detection of cytotoxin genotypes of Helicobacter pylori in stomach, saliva and dental plaque. Arch Oral Biol. 2009;54:684–688. doi: 10.1016/j.archoralbio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Rasmussen LT, Labio RW, Gatti LL, Silva LC, Queiroz VF, Smith Mde A, Payão SL. Helicobacter pylori detection in gastric biopsies, saliva and dental plaque of Brazilian dyspeptic patients. Mem Inst Oswaldo Cruz. 2010;105:326–330. doi: 10.1590/s0074-02762010000300015. [DOI] [PubMed] [Google Scholar]
- 50.Medina ML, Medina MG, Martín GT, Picón SO, Bancalari A, Merino LA. Molecular detection of Helicobacter pylori in oral samples from patients suffering digestive pathologies. Med Oral Patol Oral Cir Bucal. 2010;15:e38–e42. [PubMed] [Google Scholar]
- 51.Assumpção MB, Martins LC, Melo Barbosa HP, Barile KA, de Almeida SS, Assumpção PP, Corvelo TC. Helicobacter pylori in dental plaque and stomach of patients from Northern Brazil. World J Gastroenterol. 2010;16:3033–3039. doi: 10.3748/wjg.v16.i24.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navabi N, Aramon M, Mirzazadeh A. Does the presence of the Helicobacter pylori in the dental plaque associate with its gastric infection? A meta-analysis and systematic review. Dent Res J (Isfahan) 2011;8:178–182. doi: 10.4103/1735-3327.86033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhry S, Idrees M, Izhar M, Butt AK, Khan AA. Simultaneous amplification of two bacterial genes: more reliable method of Helicobacter pylori detection in microbial rich dental plaque samples. Curr Microbiol. 2011;62:78–83. doi: 10.1007/s00284-010-9662-x. [DOI] [PubMed] [Google Scholar]
- 54.Momtaz H, Souod N, Dabiri H, Sarshar M. Study of Helicobacter pylori genotype status in saliva, dental plaques, stool and gastric biopsy samples. World J Gastroenterol. 2012;18:2105–2111. doi: 10.3748/wjg.v18.i17.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Román-Román A, Giono-Cerezo S, Camorlinga-Ponce M, Martínez-Carrillo DN, Loaiza-Loeza S, Fernández-Tilapa G. vacA genotypes of Helicobacter pylori in the oral cavity and stomach of patients with chronic gastritis and gastric ulcer. Enferm Infecc Microbiol Clin. 2013;31:130–135. doi: 10.1016/j.eimc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Cai H, Li W, Shu X, Peng K, Zhang Y, Jiang M. Genetic variation of Helicobacter pylori in the oral cavity and stomach detected using thymine adenine cloning in children with chronic gastritis. Pediatr Infect Dis J. 2014;33:e1–e6. doi: 10.1097/INF.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 57.Andersen LP, Rasmussen L. Helicobacter pylori-coccoid forms and biofilm formation. FEMS Immunol Med Microbiol. 2009;56:112–115. doi: 10.1111/j.1574-695X.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 58.Asikainen S, Chen C, Slots J. Absence of Helicobacter pylori in subgingival samples determined by polymerase chain reaction. Oral Microbiol Immunol. 1994;9:318–320. doi: 10.1111/j.1399-302x.1994.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 59.Riggio MP, Lennon A. Identification by PCR of Helicobacter pylori in subgingival plaque of adult periodontitis patients. J Med Microbiol. 1999;48:317–322. doi: 10.1099/00222615-48-3-317. [DOI] [PubMed] [Google Scholar]
- 60.Avcu N, Avcu F, Beyan C, Ural AU, Kaptan K, Ozyurt M, Nevruz O, Yalçin A. The relationship between gastric-oral Helicobacter pylori and oral hygiene in patients with vitamin B12-deficiency anemia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:166–169. doi: 10.1067/moe.2001.113589. [DOI] [PubMed] [Google Scholar]
- 61.Dye BA, Kruszon-Moran D, McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am J Public Health. 2002;92:1809–1815. doi: 10.2105/ajph.92.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gebara EC, Pannuti C, Faria CM, Chehter L, Mayer MP, Lima LA. Prevalence of Helicobacter pylori detected by polymerase chain reaction in the oral cavity of periodontitis patients. Oral Microbiol Immunol. 2004;19:277–280. doi: 10.1111/j.1399-302X.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 63.Gebara EC, Faria CM, Pannuti C, Chehter L, Mayer MP, Lima LA. Persistence of Helicobacter pylori in the oral cavity after systemic eradication therapy. J Clin Periodontol. 2006;33:329–333. doi: 10.1111/j.1600-051X.2006.00915.x. [DOI] [PubMed] [Google Scholar]
- 64.Anand PS, Nandakumar K, Shenoy KT. Are dental plaque, poor oral hygiene, and periodontal disease associated with Helicobacter pylori infection? J Periodontol. 2006;77:692–698. doi: 10.1902/jop.2006.050163. [DOI] [PubMed] [Google Scholar]
- 65.Eskandari A, Mahmoudpour A, Abolfazli N, Lafzi A. Detection of Helicobacter pylori using PCR in dental plaque of patients with and without gastritis. Med Oral Patol Oral Cir Bucal. 2010;15:e28–e31. doi: 10.4317/medoral.15.e28. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal S, Jithendra KD. Presence of Helicobacter pylori in subgingival plaque of periodontitis patients with and without dyspepsia, detected by polymerase chain reaction and culture. J Indian Soc Periodontol. 2012;16:398–403. doi: 10.4103/0972-124X.100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouziane A, Ahid S, Abouqal R, Ennibi O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: a systematic review and meta-analysis. J Clin Periodontol. 2012;39:1166–1173. doi: 10.1111/jcpe.12015. [DOI] [PubMed] [Google Scholar]
- 68.Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Häyrinen-Immonen R. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221–234. doi: 10.1006/ijom.2002.0446. [DOI] [PubMed] [Google Scholar]
- 69.Leimola-Virtanen R, Happonen RP, Syrjänen S. Cytomegalovirus (CMV) and Helicobacter pylori (HP) found in oral mucosal ulcers. J Oral Pathol Med. 1995;24:14–17. doi: 10.1111/j.1600-0714.1995.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 70.Porter SR, Barker GR, Scully C, Macfarlane G, Bain L. Serum IgG antibodies to Helicobacter pylori in patients with recurrent aphthous stomatitis and other oral disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:325–328. doi: 10.1016/s1079-2104(97)90237-7. [DOI] [PubMed] [Google Scholar]
- 71.Birek C, Grandhi R, McNeill K, Singer D, Ficarra G, Bowden G. Detection of Helicobacter pylori in oral aphthous ulcers. J Oral Pathol Med. 1999;28:197–203. doi: 10.1111/j.1600-0714.1999.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 72.Riggio MP, Lennon A, Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med. 2000;29:507–513. doi: 10.1034/j.1600-0714.2000.291005.x. [DOI] [PubMed] [Google Scholar]
- 73.Shimoyama T, Horie N, Kato T, Kaneko T, Komiyama K. Helicobacter pylori in oral ulcerations. J Oral Sci. 2000;42:225–229. doi: 10.2334/josnusd.42.225. [DOI] [PubMed] [Google Scholar]
- 74.Victória JM, Kalapothakis E, Silva Jde F, Gomez RS. Helicobacter pylori DNA in recurrent aphthous stomatitis. J Oral Pathol Med. 2003;32:219–223. doi: 10.1034/j.1600-0714.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 75.Iamaroon A, Chaimano S, Linpisarn S, Pongsiriwet S, Phornphutkul K. Detection of Helicobacter pylori in recurrent aphthous ulceration by nested PCR. J Oral Sci. 2003;45:107–110. doi: 10.2334/josnusd.45.107. [DOI] [PubMed] [Google Scholar]
- 76.Elsheikh MN, Mahfouz ME. Prevalence of Helicobacter pylori DNA in recurrent aphthous ulcerations in mucosa-associated lymphoid tissues of the pharynx. Arch Otolaryngol Head Neck Surg. 2005;131:804–808. doi: 10.1001/archotol.131.9.804. [DOI] [PubMed] [Google Scholar]
- 77.Mansour-Ghanaei F, Asmar M, Bagherzadeh AH, Ekbataninezhad S. Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Med Sci Monit. 2005;11:CR576–CR579. [PubMed] [Google Scholar]
- 78.Albanidou-Farmaki E, Giannoulis L, Markopoulos A, Fotiades S, Aggouridaki X, Farmakis K, Papanayotou P. Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis. 2005;11:22–26. doi: 10.1111/j.1601-0825.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 79.Karaca S, Seyhan M, Senol M, Harputluoglu MM, Ozcan A. The effect of gastric Helicobacter pylori eradication on recurrent aphthous stomatitis. Int J Dermatol. 2008;47:615–617. doi: 10.1111/j.1365-4632.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- 80.Maleki Z, Sayyari AA, Alavi K, Sayyari L, Baharvand M. A study of the relationship between Helicobacter pylori and recurrent aphthous stomatitis using a urea breath test. J Contemp Dent Pract. 2009;10:9–16. [PubMed] [Google Scholar]
- 81.Taş DA, Yakar T, Sakalli H, Serin E. Impact of Helicobacter pylori on the clinical course of recurrent aphthous stomatitis. J Oral Pathol Med. 2013;42:89–94. doi: 10.1111/j.1600-0714.2012.01197.x. [DOI] [PubMed] [Google Scholar]
- 82.Adler I, Denninghoff VC, Alvarez MI, Avagnina A, Yoshida R, Elsner B. Helicobacter pylori associated with glossitis and halitosis. Helicobacter. 2005;10:312–317. doi: 10.1111/j.1523-5378.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 83.Murugan AK, Munirajan AK, Tsuchida N. Ras oncogenes in oral cancer: the past 20 years. Oral Oncol. 2012;48:383–392. doi: 10.1016/j.oraloncology.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol. 2007;43:523–534. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 85.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45:324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 87.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 88.Grandis JR, Perez-Perez GI, Yu VL, Johnson JT, Blaser MJ. Lack of serologic evidence for Helicobacter pylori infection in head and neck cancer. Head Neck. 1997;19:216–218. doi: 10.1002/(sici)1097-0347(199705)19:3<216::aid-hed9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 89.Singh K, Kumar S, Jaiswal MS, Chandra M, Singh M. Absence of Helicobacter pylori in oral mucosal lesions. J Indian Med Assoc. 1998;96:177–178. [PubMed] [Google Scholar]
- 90.Okuda K, Ishihara K, Miura T, Katakura A, Noma H, Ebihara Y. Helicobacter pylori may have only a transient presence in the oral cavity and on the surface of oral cancer. Microbiol Immunol. 2000;44:385–388. doi: 10.1111/j.1348-0421.2000.tb02510.x. [DOI] [PubMed] [Google Scholar]
- 91.Kanda T. Investigation of Helicobacter pylori in tumor tissue specimens from patients of head and neck tumor. Practica Oto-Rhino-Laryngologica. 2005;98:571–575. [Google Scholar]
- 92.Fernando N, Perera N, Vaira D, Holton J. Helicobacter pylori in school children from the Western province of Sri Lanka. Helicobacter. 2001;6:169–174. doi: 10.1046/j.1523-5378.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 93.Dayama A, Srivastava V, Shukla M, Singh R, Pandey M. Helicobacter pylori and oral cancer: possible association in a preliminary case control study. Asian Pac J Cancer Prev. 2011;12:1333–1336. [PubMed] [Google Scholar]
- 94.Zakrzewska JM, Hamlyn PJ. Facial pain. In: Crombie IK, Croft PR, Linton SJ, Le Resche L, Von Korff M, et al., editors. Epidemiology of pain. Seattle: International Association for the Study of Pain; 1999. pp. 177–202. [Google Scholar]
- 95.Zakrzewska JM, Forssell H, Glenny AM. Intervenciones para el tratamiento del syndrome de ardor bucal (Cochrane Review). La Biblioteca Cochrane Plus. Oxford: Update Software; 2006. [Google Scholar]
- 96.Miyazaki H, Arao M, Okamura K, Kawaguchi Y, Toyofuku A, Hoshi K. Tentative classification of halitosis and its treatment needs. Niigata Dent J. 1999;29:11–15. [Google Scholar]
- 97.Delanghe G, Ghyselen J, van Steenberghe D, Feenstra L. Multidisciplinary breath-odour clinic. Lancet. 1997;350:187. doi: 10.1016/S0140-6736(05)62354-9. [DOI] [PubMed] [Google Scholar]
- 98.Tangerman A, Winkel EG. Intra- and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol. 2007;34:748–755. doi: 10.1111/j.1600-051X.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 99.Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc. 2000;66:257–261. [PubMed] [Google Scholar]
- 100.Scully C, Greenman J. Halitology (breath odour: aetiopathogenesis and management) Oral Dis. 2012;18:333–345. doi: 10.1111/j.1601-0825.2011.01890.x. [DOI] [PubMed] [Google Scholar]
- 101.Tangerman A. Halitosis in medicine: a review. Int Dent J. 2002;52 Suppl 3:201–206. doi: 10.1002/j.1875-595x.2002.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 102.Tonzetich J, McBride BC. Characterization of volatile sulphur production by pathogenic and non-pathogenic strains of oral Bacteroides. Arch Oral Biol. 1981;26:963–969. doi: 10.1016/0003-9969(81)90104-7. [DOI] [PubMed] [Google Scholar]
- 103.Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5:195–201. doi: 10.1111/j.1399-302x.1990.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 104.Rosenberg M, Septon I, Eli I, Bar-Ness R, Gelernter I, Brenner S, Gabbay J. Halitosis measurement by an industrial sulphide monitor. J Periodontol. 1991;62:487–489. doi: 10.1902/jop.1991.62.8.487. [DOI] [PubMed] [Google Scholar]
- 105.Bosy A, Kulkarni GV, Rosenberg M, McCulloch CA. Relationship of oral malodor to periodontitis: evidence of independence in discrete subpopulations. J Periodontol. 1994;65:37–46. doi: 10.1902/jop.1994.65.1.37. [DOI] [PubMed] [Google Scholar]
- 106.De Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodor. J Am Dent Assoc. 1995;126:1384–1393. doi: 10.14219/jada.archive.1995.0049. [DOI] [PubMed] [Google Scholar]
- 107.Messadi DV. Oral and nonoral sources of halitosis. J Calif Dent Assoc. 1997;25:127–131. [PubMed] [Google Scholar]
- 108.Scully C, el-Maaytah M, Porter SR, Greenman J. Breath odor: etiopathogenesis, assessment and management. Eur J Oral Sci. 1997;105:287–293. doi: 10.1111/j.1600-0722.1997.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 109.Messadi DV, Younai FS. Halitosis. Dermatol Clin. 2003;21:147–155, viii. doi: 10.1016/s0733-8635(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 110.Loesche WJ, Kazor C. Microbiology and treatment of halitosis. Periodontol 2000. 2002;28:256–279. doi: 10.1034/j.1600-0757.2002.280111.x. [DOI] [PubMed] [Google Scholar]
- 111.Tiomny E, Arber N, Moshkowitz M, Peled Y, Gilat T. Halitosis and Helicobacter pylori. A possible link? J Clin Gastroenterol. 1992;15:236–237. doi: 10.1097/00004836-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 112.Ierardi E, Amoruso A, La Notte T, Francavilla R, Castellaneta S, Marrazza E, Monno RA, Francavilla A. Halitosis and Helicobacter pylori: a possible relationship. Dig Dis Sci. 1998;43:2733–2737. doi: 10.1023/a:1026619831442. [DOI] [PubMed] [Google Scholar]
- 113.Adler I, Denninghoff V, Alvarez MI, Neffen E, Avagnina A, Elsner B. Evidencia observada en el paciente. Infección intrabucal por Helicobacter en paciente de sexo masculino. Caso clínico. Rev del Ateneo Argentino de Odontología. 2001;40:10–15. [Google Scholar]
- 114.Gall-Troselj K, Mravak-Stipetić M, Jurak I, Ragland WL, Pavelić J. Helicobacter pylori colonization of tongue mucosa--increased incidence in atrophic glossitis and burning mouth syndrome (BMS) J Oral Pathol Med. 2001;30:560–563. doi: 10.1034/j.1600-0714.2001.300909.x. [DOI] [PubMed] [Google Scholar]
- 115.Serin E, Gumurdulu Y, Kayaselcuk F, Ozer B, Yilmaz U, Boyacioglu S. Halitosis in patients with Helicobacter pylori-positive non-ulcer dyspepsia: an indication for eradication therapy? Eur J Intern Med. 2003;14:45–48. doi: 10.1016/s0953-6205(02)00206-6. [DOI] [PubMed] [Google Scholar]
- 116.Brailo V, Vuéiaeeviae-Boras V, Alajbeg IZ, Alajbeg I, Lukenda J, Aeurkoviae M. Oral burning symptoms and burning mouth syndrome-significance of different variables in 150 patients. Med Oral Patol Oral Cir Bucal. 2006;11:E252–E255. [PubMed] [Google Scholar]
- 117.Katsinelos P, Tziomalos K, Chatzimavroudis G, Vasiliadis T, Katsinelos T, Pilpilidis I, Triantafillidis I, Paroutoglou G, Papaziogas B. Eradication therapy in Helicobacter pylori-positive patients with halitosis: long-term outcome. Med Princ Pract. 2007;16:119–123. doi: 10.1159/000098364. [DOI] [PubMed] [Google Scholar]
- 118.Moshkowitz M, Horowitz N, Leshno M, Halpern Z. Halitosis and gastroesophageal reflux disease: a possible association. Oral Dis. 2007;13:581–585. doi: 10.1111/j.1601-0825.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki N, Yoneda M, Naito T, Iwamoto T, Masuo Y, Yamada K, Hisama K, Okada I, Hirofuji T. Detection of Helicobacter pylori DNA in the saliva of patients complaining of halitosis. J Med Microbiol. 2008;57:1553–1559. doi: 10.1099/jmm.0.2008/003715-0. [DOI] [PubMed] [Google Scholar]
- 120.Yoo SH, Jung HS, Sohn WS, Kim BH, Ku BH, Kim YS, Park SW, Hahm KB. Volatile sulfur compounds as a predictor for esophagogastroduodenal mucosal injury. Gut Liver. 2008;2:113–118. doi: 10.5009/gnl.2008.2.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee JS, Kwon KA, Jung HS, Kim JH, Hahm KB. Korea red ginseng on Helicobacter pylori-induced halitosis: newer therapeutic strategy and a plausible mechanism. Digestion. 2009;80:192–199. doi: 10.1159/000229997. [DOI] [PubMed] [Google Scholar]
- 122.Kinberg S, Stein M, Zion N, Shaoul R. The gastrointestinal aspects of halitosis. Can J Gastroenterol. 2010;24:552–556. doi: 10.1155/2010/639704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tangerman A, Winkel EG, de Laat L, van Oijen AH, de Boer WA. Halitosis and Helicobacter pylori infection. J Breath Res. 2012;6:017102. doi: 10.1088/1752-7155/6/1/017102. [DOI] [PubMed] [Google Scholar]
- 124.Liu H, Rahman A, Semino-Mora C, Doi SQ, Dubois A. Specific and sensitive detection of H. pylori in biological specimens by real-time RT-PCR and in situ hybridization. PLoS One. 2008;3:e2689. doi: 10.1371/journal.pone.0002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Camorlinga-Ponce M, Romo C, González-Valencia G, Muñoz O, Torres J. Topographical localisation of cagA positive and cagA negative Helicobacter pylori strains in the gastric mucosa; an in situ hybridisation study. J Clin Pathol. 2004;57:822–828. doi: 10.1136/jcp.2004.017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kolbert CP, Persing DH. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr Opin Microbiol. 1999;2:299–305. doi: 10.1016/S1369-5274(99)80052-6. [DOI] [PubMed] [Google Scholar]
- 127.Smith SI, Oyedeji KS, Arigbabu AO, Cantet F, Megraud F, Ojo OO, Uwaifo AO, Otegbayo JA, Ola SO, Coker AO. Comparison of three PCR methods for detection of Helicobacter pylori DNA and detection of cagA gene in gastric biopsy specimens. World J Gastroenterol. 2004;10:1958–1960. doi: 10.3748/wjg.v10.i13.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]


