Abstract
Forty-four different animal biles obtained from both invertebrates and vertebrates (including human bile) have been used for centuries for a host of maladies in traditional Chinese medicine (TCM) beginning with dog, ox and common carp biles approximately in the Zhou dynasty (c. 1046-256 BCE). Overall, different animal biles were prescribed principally for the treatment of liver, biliary, skin (including burns), gynecological and heart diseases, as well as diseases of the eyes, ears, nose, mouth and throat. We present an informed opinion of the clinical efficacy of the medicinal uses of the different animal biles based on their presently known principal chemical components which are mostly steroidal detergent-like molecules and the membrane lipids such as unesterified cholesterol and mixed phosphatidylcholines and sometimes sphingomyelin, as well as containing lipopigments derived from heme principally bilirubin glucuronides. All of the available information on the ethnopharmacological uses of biles in TCM were collated from the rich collection of ancient Chinese books on materia medica held in libraries in China and United States and the composition of various animal biles was based on rigorous separatory and advanced chemical identification techniques published since the mid-20th century collected via library (Harvard’s Countway Library) and electronic searches (PubMed and Google Scholar). Our analysis of ethnomedical data and information on biliary chemistry shows that specific bile salts, as well as the common bile pigment bilirubin and its glucuronides plus the minor components of bile such as vitamins A, D, E, K, as well as melatonin (N-acetyl-5-methoxytryptamine) are salutary in improving liver function, dissolving gallstones, inhibiting bacterial and viral multiplication, promoting cardiac chronotropsim, as well as exhibiting anti-inflammatory, anti-pyretic, anti-oxidant, sedative, anti-convulsive, anti-allergic, anti-congestive, anti-diabetic and anti-spasmodic effects. Pig, wild boar and human biles diluted with alcohol were shown to form an artificial skin for burns and wounds one thousand years ago in the Tang dynasty (618-907 CE). Although various animal biles exhibit several generic effects in common, a number of biles appear to be advantageous for specific therapeutic indications. We attempt to understand these effects based on the pharmacology of individual components of bile as well as attempting to identify a variety of future research needs.
Keywords: Bile acids, Bile pigments, Bilirubinates, Liquid crystals, Materia medica, Mixed micelles, Bear bile, Ox gallstones, Paleo-pharmacology, Phospholipids
Core tip: We investigated during what periods of Chinese history each of the animal biles were introduced as therapeutic agents. What categories of diseases were subjected to “bile therapy” and why was a wide assortment of animal biles required for effective medicinal use? We catalogued the principal bile acids and bilirubin species in each animal bile based upon high-quality chemical analyses. We provided insights on the pharmacological mechanisms whereby different animal biles have been effective in treating a variety of acute and chronic diseases. We believe that this is the first time that this subject has been systematically collated and critically addressed.
INTRODUCTION
The usage of animal biles in China for the treatment of a wide number of disorders in human beings enjoys a three millennial history[1-4]. Documentation has been extensive as evidenced by the lineage of tomes on traditional Chinese medicine (TCM) and Chinese materia medica[5-12]. Moreover, the so-called “precious” pigment gallstone, a solid artifact found occasionally in the gallbladders of oxen, was extensively employed for multiple medicinal purposes in TCM[8].
Here, we explore critically the extensive literature on TCM and Chinese materia medica, some extending back over two millennia. We investigate the old and the latest archaeological and paleozoological evidence from China, with particular focus on the medicinal uses of animal biles and their components. We attempt to answer the following questions: (1) during what periods of Chinese history were each of the animal biles introduced as therapeutic agents? (2) which animal biles were used first for their therapeutic purposes, and which of these were most extensively employed? (3) what categories of diseases were subjected to “bile therapy” and why was a wide assortment of animal biles required for effective medicinal use? (4) how effective were diseases treated with individual biles? and (5) on the basis of published analyses of bile components, can we determine why certain animal biles were used for specific diseases and why others were not? We have also catalogued the principal bile acids and bilirubin species in each animal bile based upon high-quality rigorous chemical analyses. Finally, relying upon careful experimental studies, we provide tentative insights on the possible pharmacological mechanisms whereby different animal biles may have been effective in treating such a variety of acute and chronic diseases. We believe that this is the first time that this subject has been systematically collated and critically addressed. However, it is obvious that our understanding of these heuristic discoveries is very limited indeed and herein we will attempt to indicate future research needs.
CHEMICAL COMPOSITIONS OF ANIMAL BILES
Bile is a yellow, orange, or slightly green aqueous fluid that is the “exocrine” secretion of the liver. It forms first in bile canaliculi enclosed between parenchymal cells of the liver and flows continuously into ever enlarging ducts to exit the liver via two hepatic ducts. The network of conduits that carry bile into the duodenum is via an arborization of continually enlarging cholangiocyte-lined biliary tracts where bile is modified chemically. Animals with gallbladders, which are situated below the right lobe of the liver and are adherent to it, utilize this organ to concentrate, acidify, and store bile interdigestively. Bile is composed principally of a mixture of four dissimilar molecular lipid species: (1) in the majority of animals, bile acids are generally amidated with an amino acid, usually glycine or taurine, to form Na+ and K+ salts. This renders bile salts soluble detergent-like molecules resistant to forming insoluble salts with Ca2+. They are the principal catabolic products of cholesterol, a sterol found in the biles of all vertebrates and invertebrates; (2) bile pigments (bilirubin, biliverdin or both) the final products of heme catabolism are secreted into bile as mono-or hetero-conjugates with glucuronic acid, sometimes glucose and xylose or taurine); (3) unesterified cholesterol with traces of plant (phyto) and shellfish (choncho) sterols; and (4) phospholipids, mostly (> 96%) phosphatidylcholines in mammals but are generally absent from the bile of cartilaginous fish and reptiles. Phospholipids (and cholesterol) lower the detergent-like properties of bile salts. In addition, bile contains small amounts of proteins, especially mucin glycoproteins and a wide variety of mineral salts[13]. Bile also contains a wide variety of antioxidants, the most powerful ones being bilirubin, glutathione, vitamin E, and melatonin (N-acetyl-5-methoxytryptamine)[14].
With respect to the major biliary lipids in vertebrates including cartilaginous and bony fish, reptiles, birds and mammals, all contain conjugated steroidal bile acids as soluble salts[15]. These can be C27 bile alcohol sulfates and/or N-acyl amidates of C27 and/or C24 bile acids (Figure 1). Bile acids in bile exist invariably as a molecular mixture of congeners formed directly from cholesterol in the liver (“primary”) or bacterially modified (“secondary”). As detergent-like molecules, they solubilize as mixed micelles the insoluble lipid components of bile, namely the phospholipids and cholesterol molecules. The known naturally-occurring bile acid species run into many hundreds[16-19] and are typified by the most evolutionary advanced species in humans[20]. Whether occurring as taurine and glycine N-acyl amidates of C24 bile acids in humans, or C27 bile alcohol sulfates in reptiles, for example, all have shown historically to have similar physical-chemical and detergent-like functions[13]. Bile acids are synthesized from cholesterol only in the liver and the primary bile acids of humans are cholic acid, with three (3α,7α,12α) hydroxyl groups, and chenodeoxycholic acid (CDCA), with two hydroxyl (3α,7α) groups. Following cleavage of the amidated side chain by bacterially secreting cholylglycine or cholyltaurine amidases, secondary bile acids are produced from primary bile acids by removal of the 7-OH function by a small group of specific anaerobic intestinal bacteria (Clostridia sp.). Deoxycholic acid, with two (3α,12α) hydroxyl groups, and lithocholic acid, with a single (3α) hydroxyl group, are formed from cholic acid and chenodeoxycholic acid, respectively. “Tertiary” bile acids are the result of modification of secondary bile acids by intestinal flora or hepatocytes. These are the sulfate ester at the 3-OH group of lithocholic acids (not shown in Figure 1) and ursodeoxycholic acid (UDCA), the 7β-epimer of CDCA.
Figure 1.
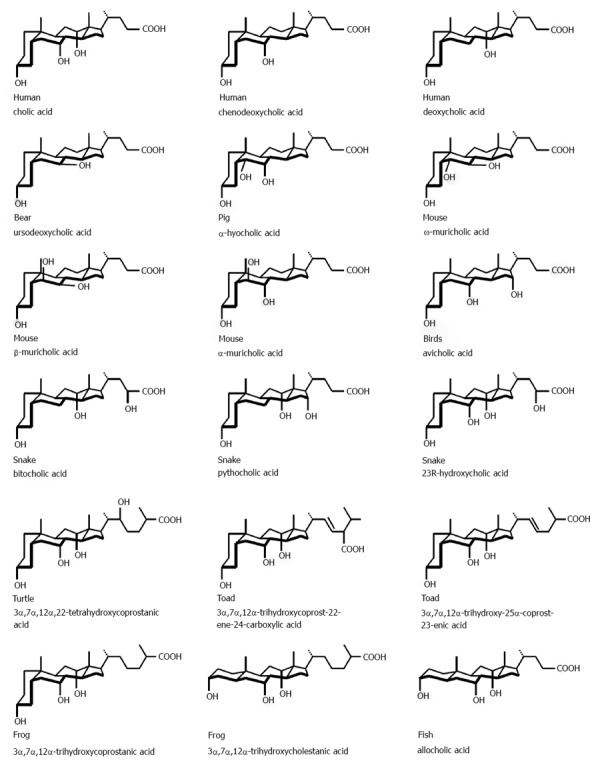
Perspective structures of major bile acids in selected animal biles according to groups of organisms: mammals (humans, bears, pigs and mice); birds;reptiles (snakes and turtles); amphibians (toads and frogs); and fish. The C24 common bile acids of higher vertebrates possess a steroid nucleus of four fused hydrocarbon rings with a cis A/B ring junction, but the biles of amphibians and fish contain C24 or C27 bile acids with trans A/B ring junctions. The polar hydroxyl functions generally are α-axial or equatorial and an aliphatic side chain is conjugated with glycine or taurine in C24 bile acids or sulfated in C27 bile acids. Because the ionized carboxylate, sulfonate or sulfate groups on the side chain render bile salts highly soluble in water, they are classified as soluble amphiphiles. The common bile acids differ in the number and orientation of the hydroxyl groups on the steroid nucleus. In humans, ursodeoxycholic acid is a secondary bile acid; whereas in a number of animals especially hystricomorpha and all ursidae, it is a primary bile acid i.e., made directly from cholesterol in the liver. Reproduced with modifications and with permission from[165].
Phospholipids are generally absent from the bile of cartilaginous fish and reptiles and present in low amounts in bony fish and most birds[15]. The phospholipid to bile acid ratio varies widely in most mammalian species and sometimes within a species. Bile with low biliary phospholipid to bile acid ratios frequently contains a high proportion of sphingomyelin. In species with a high phospholipid to bile acid ratio, the predominant biliary phospholipids are mostly phosphatidycholines and usually composed of the most hydrophilic classes in the liver[15]. Cholesterol is present in highly variable proportions in all biles including arthropods. The latter are incapable of cholesterol synthesis, but biotransform other sterols into cholesterol. The levels of cholesterol incorporated into mixed micelles depend predominantly on the amount of phospholipids present[21].
The bile of all members of the subphylum Crustacea (Phylum Arthropoda) invertebrate animals consisting of some 45000 species worldwide, contains straight chain acyl bile acids of 8-14 carbons in length amidated to a head group composed of a dipeptide. For example, in Cancer Borealis, the head group is sarcosyl (N-methyglycine)-taurine covalently linked principally to a medium chain fatty acid such as N-dodecanoyl[22].
In addition, all biles contain an acidic β-glucuronidase, an enzyme derived from hepatocellular lysosomes[23]. Human bile also contains a 92 kD alkaline sphingomyelinase[24].
BRIEF HISTORY OF THE DISCOVERY AND MEDICINAL USES OF ANIMAL BILES IN CHINA
In 1899, Chinese peasants in Henan Province of China discovered “dragon bones” inscribed with very ancient Chinese characters and apparently sold them to local pharmacists. During 1929-1933, scholars from the Historical Institute of Academia Sinica discovered tens of thousands of inscribed turtle shells, oracle bones, and other bones bearing archaic Chinese characters at Xiaotun in the Anyang district of Henan Province. Chinese archaeologists and paleo-linguists as well as European sinologists, identified that these inscriptions were most likely written during the years c. 1766 to 1154 BCE in the Shang dynasty, the first recorded Chinese dynasty for which there is both documentary and archeological evidence. This first significant corpus of recorded Chinese characters became known as the “Jia Gu Wen[25]. They provided information on the politics, culture, religion, geography, art and medicine of the period. With their help, incomplete and vague historical data accumulated representing in writing the first critical insights on early Chinese civilization. Disease terms, mostly generic, were revealed on 36 oracle bones, such as “diseased skin”, “diseased nose”, “diseased body”, “diseased foot”, “blindness”, “childhood disease”, “diseased head”, “diseased eye”, “diseased ear”, “diseased tooth”, “diseased tongue”, “diseased complaint” and “disease termination”. These inscriptions did not mention the specific names of any drugs[12,25-28]. The information from the “Jia Gu Wen” teaches us[26] that Emperor Wu Ding suffered from eye disease; his son, cranial disease; and one of his concubines, foot disease; Emperor Zhen suffered from both tooth and stomach diseases; and many royal concubines, gynecological diseases. Of interest is that Emperor Wu Ding was the 23rd Emperor of the Shang dynasty, so most of the oracle bones date from his reign, more than 3250 years ago. These simple recordings also inform us that the diseases in the time of the Shang dynasty were mainly treated with prayers, incantations and witchcraft and above all patience - “Dr. Nature” was left to cure herself.
Names of drugs and therapeutic formulae first appeared on bamboo slabs and other wood cuttings (“slips”) dated to more than 2000 years ago. These inscribed artifacts were discovered in the desert, north-west of Dunhuang of Gansu Province in 1906-1908[25], and were named “Notes Scattered in the Slippery Sand”. Before the invention of paper, ancient Chinese scholars preserved their observations and records on wooden shavings linked together with double cords of silk thread and rolled into bundles[25,29,30]. Among the hundreds of inscribed cuttings found[26], eleven contained descriptive names not only of diseases but also of herbal prescriptions for their cure. In 1930, archaeologists of the Chinese Scholars Association carried out further exploration in northwest China, and obtained over 5000 kg of wooden slivers from excavations in Juyan of Gansu Province[25]. One of the cuttings bears the name “Four Kinds of Herbs for Febrile Diseases”, and another “Formulae for Horse Injury” which contained an elaborate concoction of ginger root (Zingiber officinale; Jiang), cinnamon or cassia twig (Cinnamomum japonicum; Gui Pi), asarum herb or Chinese wild ginger root (Asarum sieboldii; Xi Xin), Chinese honey locust (Gleditsia sinensis; Zao Jia), prepared aconite root (Aconitum; Fu Zi), polygala root (Polygala; Yuan Zhi), rhubarb (Rheum officinale; Da Huang), and lepidium or descurainia seed (Lepidium apetalum; Ting Li)[26]. These valuable medical records were written at a time frame ranging from the Warring States Period to the Han dynasty[25], and encompassing Emperor Qin Shi Huang’s unification of China in 221 BCE, establishing his capital city at Xi’an.
In 1972, 92 similar cuttings inscribed with medical prescriptions and acupuncture techniques were unearthed from an ancient tomb at Wuwei County of Gansu Province and dated to c. 30 to 70 CE. In these cuttings about 100 herbs, animal and mineral products to treat different diseases were recorded[12,25,31-33]. However, the use of animal biles in therapy were not documented in any of these discoveries.
Extending from the Zhou dynasty (c. 1046-256 BCE) into the Warring States Period (c. 403-221 BCE), the names of over two hundred different plants and animals were recorded in the Book of Odes (Shi Jing) (Anon, c. the 9th century to the 5th century BCE). This work summarized husbandry methods on the planting of crops and described experience with domestication of animals in China. Furthermore, in another well-known work, the Mountain and River Classic (Shan Hai Jing)[34] more than one hundred specimens of plants, animals and minerals and parts thereof were summarized and documented for prevention and treatment of over fifty human diseases[35]. Again, we find no evidence that animal biles were used as drugs in this important work at the beginning of the Qin dynasty (c. 221 BCE).
In the Winter of 1973, medical writings were unearthed from an ancient tomb at Mawangdui in Hunan Province. In this excavation most of the medical inscriptions were written on silk fragments, and a minority on bamboo cuttings[1,2,12,25,36-40]. In some cases, the Chinese script resembles Qin bronze inscriptions rather than those of the Han dynasty, so the manuscripts may well date to as early as 300 BCE, although certain linguistic considerations suggest an even earlier date, perhaps in the Warring States period (c. 400 BCE)[12,41]. The texts of the manuscripts contain no titles, but the transcribing paleographers[1] accorded them the following names: (1) Moxa manual of the eleven tracts on the upper and lower limbs; (2) Moxa manual of the eleven tracts according to the Yin and Yang; (3) Method of taking the pulse; (4) Fatal prognoses determined by the Yin and Yang; and (5) Prescriptions for fifty-two types of diseases (Wu Shi Er Bing Fang). The latter probably represented the earliest extant writings of Chinese medical prescriptions (c. 475 to 221 BCE) and included 291 prescriptions for the treatment of the 52 categories of disease. Short illustrated works on dietetics and calisthenics were also included.
Most notable is that in the Prescriptions for Fifty-two Types of Diseases, dog and ox biles were recorded for the first time as being efficacious in treating health disorders[1,3,40]. Not only is this the earliest time that animal biles (Figure 2) were recorded in an ancient Chinese text, but it is also significant that they were mentioned as being useful as drugs for therapeutic purposes! Additionally, the earliest recorded monograph on materia medica in China, Shen Nong’s Herbal Classic, which appeared in the years c. 475 to 206 BCE, recorded the use of common carp bile as a drug in addition to dog and ox biles. Therefore, these paleo-archaeological discoveries provide irrefutable evidence that animal biles have been employed therapeutically for more than 2500 years in China, with dog and ox biles being the first to be used, followed closely by common carp bile (Figure 2).
Figure 2.

Chronology for the introduction of different animal biles used therapeutically in traditional Chinese medicine. The main Chinese dynasties are listed to the left of year in 500-year increments to indicate the approximate beginning of the dynasty. BCE: Before the common era; CE: Common era which corresponds to the common Gregorian calendar.
The Chinese historical record[8] mentions an anecdotal report of Hua Tuo (c. 110 to 207 CE), an outstanding surgeon who treated a patient with dog bile. The Annals of the Wei Kingdom (Wei Guo Zhi)[26] recorded that during the Eastern Han period (25 to 220 CE), the daughter of Mayor Liu Xun of Henei City suffered from an abscess on her left knee. Hua Tuo was summoned to take care of her, and having examined the patient, he incised and drained the abscess. After evacuating the pus, he filled the empty space with fresh gallbladder bile of a domestic dog. This apparently caused the inflammation to abate, the girl’s pain disappeared and healing was induced. This medical record (c. 290 CE) was the first to document the use of an animal bile to heal a drained abscess cavity[8], and it is also the first example of the antiseptic and anti-inflammatory properties of gallbladder bile.
CONTINUING DISCOVERY AND MEDICINAL USES OF A TOTAL OF FORTY-FOUR DIFFERENT ANIMAL BILES (INCLUDING HUMAN BILE)
Table 1 provides a chronological listing of the animal biles used therapeutically in China from the earliest times together with their origins as documented in Chinese materia medica (Figure 3). We also provide the principal chemical (i.e., bile salt/alcohol and bile pigment) compositions. These will now be discussed individually with particular attention to their chronological introduction into therapy, their apparent clinical usefulness (Table 2) and we make a reasonable conjecture as to why they might have been beneficial for certain diseases. Table 3 lists animal biles according to groups of organisms that were therapeutically used in China.
Table 1.
A chronological list of animal biles used therapeutically in China from the earliest times
| Chinese name (Pin Yin) | English name | Latin nomenclature | Earliest recorded book, year and author | Bile salt and alcohol composition1 | Bile pigment composition1 |
| Gou Dan | Dog | Canis familiaris | Prescriptions for Fifty-two Types of diseases (c. 475 to 221 BCE); author(s) unknown | TC, TCDC, TDC[87,102,166] | BDG, BDGC, BMX[167,168] |
| Niu Dan | Ox | Bos taurus domesticus; Bubalus bulalis | TC, TDC, TCDC, TLC, GC, GDC, GCDC, GLC, AC[91,100,107,166] | BMG, BMGC, BDG, UCB, BV[167,169,170] | |
| Li Yu Dan | Common carp | Cyprinus carpio | Shen Nong’s Herbal Classic (c. 475 to 206 BCE); author(s) unknown | TCDC, TC, TDC, AC, α-cyprinol-26 SO4, α-latimerol-SO4[87,91,107] | BV, BMG, PB, UCB[169-171] |
| Shu Dan | Mouse | Mus musculus | Variorum of Shen Nong’s Herbal Classic (c. 492 CE); Tao Hong-Jing (c. 452 to 536 CE) | TC, TCDC, TDC, T-β-MC, T-ω-MC, TUDC[172] | BMG, BDG, UCB[173] |
| Ran She Dan | Python | Python molurus bivittatus | TPC, TC, TDC[87,106,107] | BV, BMG, UCB[174] | |
| Yang Dan | Goat and sheep | Capra hircus | TC, TCDC, TDC, GC, GDC[87,100,107,166] | BMG[167,168] | |
| Zhu Dan | Pig | Sus scrofa domestica | Records of Famous Physicians (c. 510 CE); Tao Hong-Jing (c. 452 to 536 CE) | GHC, GHDC, GHOC, GC, GCDC, GDC, THC, THDC, TC, TCDC, TDC[89,94,166] | BMG, BV[167,168,170] |
| Fu She Dan | Pallas pit viper | Agkistrodon halys | TC, TAC, TCDC, TDC[107] | BV, BMG, UCB[174] | |
| Ji Dan | Chicken | Gallus gallus domesticus | TAC, TC, TCDC[89,166] | BV, BMG, BMGC, BDG, BDGC, UCB[167,175] | |
| Xiang Dan | Elephant | Elephas maximus | Lei’s Treatise on Preparation of Drugs (c. the 5th century); Lei Xiao (c. the 5th century) | 3α,7α,25,26-tetrahydroxy-5β-cholestane SO4[107] | ND |
| Li Yu Dan | Murrel | Ophicephalus argus | Ri Hua-Zi’s Collected Materia Medica (c. the 6th century); Ri Hua-Zi (c. the 6th century) | TCDC, TC, TDC[107] | BV, BMG, PB, UCB[168,170,171] |
| Xiong Dan | Bear | Selenarctos thibetanus; Ursus arctos | Treatise on Properties of Drugs (c. 643 CE or earlier); Zhen Quan (c. 540 to 643 CE) | TUDC, TC, TCDC, TDC[66] | BR, BFL, BFU[70] |
| Ye Zhu Dan | Wild boar | Sus scrofa | A Dietetic Materia Medica (c. 710 CE); Meng Xian (c. 621 to 714 CE) | GHC[87] | BMG, BV[167,168,170] |
| Jiao Yu Dan | Shark | Mustelus manazo | 5β-scymnol SO4[107] | BV, BMG, PB, UCB[168,170,171] | |
| Qing Yu Dan | Black carp | Mylopharyngodon piceus | 5α-cyprinol-26 SO4[107] | BV, BMG, PB, UCB [168,170,171] | |
| Ling Yang Dan | Antelope | Saiga tatarica | Medical Secrets of an Official (c. 752 CE); Wang Tao (c. 702 to 772 CE) | TC, TCDC, TDC[87,107] | ND |
| Huan Yu Dan | Grass carp | Ctenopharyngodon idellus | A Supplement to Materia Medica (c. 758 CE); Chen Zang-Qi (c. the 8th century) | TCDC, TC, TDC, 5α-cyprinol-26 SO4[107] | BV, BMG, PB, UCB[168,170,171] |
| Hu Dan | Tiger | Panthera tigris | TC, TCDC, TDC[107] | ND | |
| Ren Dan | Human | Homo sapiens | GC, GCDC, GDC, GUDC, GLC, TC, TCDC, TDC, TUDC, TLC, SGLC, STLC[13,176] | BDG, BMG, BMGG1, BDGC, BMGC, BMX, UCB[173] | |
| Ta Dan | Otter | Lutra lutra | Illustrated Pharmacopoeia (c. 1061 CE); Su Song (c. 1019 to 1101 CE) | TC, TCDC, TDC, GC, GDC[87,107] | ND |
| Hu Dan | Fox | Vulpes vulpes | TC, TCDC, TDC[87,107] | ND | |
| Wei Dan | Hedgehog | Erinaceus europaeus; Hemiechinus dauuricus | Amplified Materia Medica (c. 1116 CE); Kou Zong-Shi (c. the 12th century) | TCDC, TC, TDC[107] | ND |
| E Dan | Goose | Anser domestica | Pharmacopoeia in Southwestern China (c. in the middle of the 15th century); Lan Mao (c. 1397 to 1496 CE) | TCDC, TACDC, TPHC, TBC[107] | BV, BMG, BMGC, UCB[168] |
| Ya Dan | Duck | Anas domestica | Compendium of Materia Medica (1596 CE); Li Shi-Zhen (1518 to 1593 CE) | TCDC, TACDC, TPHC, TBC, 3α7α11β-trihydroxy-5β-cholan-24-oic acid[107] | BV, BDG, BDGC[177] |
| Lu Dan | Deer | Cervus nippon; Cervus elaphus | GC, GCDC, GDC[107] | ND | |
| Hou Dan | Horseshoe crab | Tachypleus tridentatus | Medium-chain fatty acids conjugated with sarcosyl taurine[22,107] | ND | |
| Ji Yu Dan | Crucian carp | Carassius auratus | α-cyprinol-26 SO4[107] | BV, BMG, PB, UCB[168,170,171] | |
| Bie Dan | Turtle | Amyda sinensis | 3α7α22-trihydroxy-5β-cholan-27-oic acid, sterocholanic acid, 3α12α22-trihydroxy-5β-cholan-27-oic acid[107] | BV, BMG, UCB[168,174] | |
| Gui Yu Dan | Mandarin fish | Siniperca chuatsi | TCDC, TC, TDC[107] | BV, BMG, PB, UCB[168,170,171] | |
| Wu Ya Dan | Crow | Corvus macrorhynchus | TCDC, TC, TDC[107] | BV[168] | |
| Yuan Dan | Soft-shelled turtle | Pelochelys bibroni | TsteroC, TsteroCDC, TsteroDC[107] | BV, BMG, UCB[168,174] | |
| Ha Ma Dan | Chinese forest frog | Rana limnocharis | Pentahydroxy C27 and C26 bile alcohol SO4, taurine conjugated C27 trihydroxy acids[107] | BV, UCB[168,170,178,179] | |
| Wu She Dan | Black snake | Zaocys dhumnades | TC, TAC, TCDC, TDC[107] | BV, BMG, UCB[174] | |
| Gui Dan | Tortoise | Chinemys reevesii | TsteroC, TsteroCDC, TsteroDC[107] | ND | |
| Bian Fu Dan | Bat | Vespertilio superans; Pipistrellus abramus; Plecotus auritus; Rhinolophus ferrum-equnum | TC[107] | ND | |
| Ci Wu Dan | Raven | Corvus monedula dauuricus | A Supplement to the Compendium of Materia Medica (c. 1765 CE); Zhao Xue-Min (1719 to 1805 CE) | TCDC, TC[107] | BV[168] |
| Mao Niu Dan | Yak | Bos grunniens | Jing Zhu Materia Medica (c. 1840 CE); Dan-Zeng Peng-Cuo (c. the 19th century) | TC, TCDC, TDC, GC, GCDC, GDC[107] | BMG, BMGC, BDG, UCB, BV[167,169,170] |
| Ye Niu Dan | Gaur | Bos gaurus | TC, TCDC, TDC, GC, GCDC, GUDC[107] | BMG, BMGC, BDG, UCB, BV[167,169,170] | |
| Shi Long Zi Dan | Skink | Eumeces chinensis Gray; Eumeces elegans; Sphenomorphus indicus | TC, TCDC, GHDC, Tvaranic acid, T3α,7α,12α-trihydroxy-5β-cholestan-27-oic acid[90,107] | BV, BMG, UCB[170] | |
| Ying Wu Dan | Parrot | Psittacula alexandri fasciata | TC, TCDC[107] | BV[168] | |
| Ma Dan | Horse | Equus caballus | TC[87,107] | BDG, BDGC, BMG, BMGC[175] | |
| Tu Jiu Dan | Vulture | Gypaetus barbatus aureus | TC, TCDC, TalloC[107] | ND | |
| Yuan Dan | Kite | Milvus korschun lineatus | Great Dictionary of Chinese Materia Medica (1977 CE); Jiangsu New Medical College | TC, TCDC, TalloC[107] | ND |
| Chan Chu Dan | Toad | Bufo bufo gargarizans; Bufo melanostictus | Bufol SO4, Ranol SO4, hexahydroxy-27-nor-C26-bile alcohol SO4[107] | BV[168,170,178,179] |
Bile salt and bilirubin compositions are shown according to the rank order of species from high to low, respectively. AC: Allocholate; C: Cholate; DC: Deoxycholate; GC: Glycocholate; GCDC: Glycochenodeoxycholate; GDC: Glycodeoxycholate; GHC: Glycohyocholate; GHDC: Glycohyodeoxycholate; GHOC: Glyco-3α-hydroxy-6-oxo-5β-cholate; GLC: Glycolithocholate; GUDC: Glycoursodeoxycholate; TAC: Tauroallocholate; TBC: Taurobitocholate; TC: Taurocholate; TCDC: Taurochenodeoxycholate; TDC: Taurodeoxycholate; THC: Taurohyocholate; THDC: Taurohyodeoxycholate; TLC: Taurolithocholate; T-β-MC: Tauro-β-muricholate; T-ω-MC: Tauro-ω-muricholate; TPC: Tauropythocholate; TPHC: Taurophocacholate; TsteroC: Taurosterocholate; TsteroCDC: Taurosterochenodeoxycholate; TsteroDC: Taurosterodeoxycholate; TUDC: Tauroursodeoxycholate; SGLC: Sulfoglycolithocholate; STLC: Sulfotaurolithocholate; BDG: Bilirubin diglucuronide; BDGC: Bilirubin diglucoside; BFL: Biliflavin; BFU: Bilifulvin; BMG: Bilirubin monoglucuronide; BMGC: Bilirubin monoglucoside; BMGG1: Bilirubin monoglucuronide monoglucoside; BMX: Bilirubin monoxyloside; BR: Bilirubin; BV: Biliverdin; PB: Phycobilin; UCB: Unconjugated bilirubin; ND: Not determined.
Figure 3.

Four pages from Li Shi-Zhen’s Compendium of Materia Medica (Ben Cao Gang Mu) published in 1596 CE in the Ming Dynasty. A: Goat (top left), pig (top right), Procapra przewalskii (Przewalskii’s gazelle) (bottom left) and dog (bottom right); B: Crow (top left) and raven (bottom right); C: Python (top right) and black snake (bottom left); D: Common carp (top right). The biles of these animals were introduced into traditional Chinese medicine as important drugs to treat various diseases as discussed in the text.
Table 2.
A list of diseases treated by animal biles in traditional Chinese medicine
| Disease | Animal bile or gallstones |
| Digestive system | |
| Jaundice | Bear, ox |
| Biliary colic | Bear, python, yak |
| Epigastric pain | Fox, gaur, kite |
| Gastric regurgitation | Bear, dog, pig |
| Infantile malnutrition | Bear, dog, pig, python, tiger, wild boar |
| Hemorrhoids | Bear, duck, goose, hedgehog, ox, python, turtle |
| Anal fistula | Bear, turtle |
| Dysentery | Bear, dog, python, tiger |
| Diarrhea | Bear, dog, pig |
| Constipation | Pig |
| Intestinal parasites | Bear, ox, dog, pallas pit viper, crucian carp, horseshoe crab |
| Stuck fishbone | Black carp, crucian carp, grass carp, mandarin fish |
| Acute alcoholism | Fox |
| Alcoholic cirrhosis | Fox |
| Food poisoning | Parrot |
| Skin | |
| Infectious skin diseases | Bear, chicken, common carp, crucian carp, dog, elephant, goat and sheep, horse, ox, pallas pit viper, pig, python, vulture, wild boar |
| Burns | Pig, human, wild boar |
| Traumatic injury | Deer, dog, human, mouse |
| Darkish complexion | Antelope, goat and sheep |
| Tinea versicolor | Antelope, goat and sheep |
| Chloasma, freckle, ephelis | Antelope, goat and sheep |
| Shampoo for hair | Pig |
| Leprosy | Black snack, horseshoe crab |
| Eyes | |
| Infectious eye diseases | Black carp, chicken, common carp, crow, dog, duck, goat and sheep, hedgehog, otter, pig, python, skink, tortoise |
| Improving visual acuity | Bat, bear, common carp, crow, mouse, ox, pig, python, skink, tortoise |
| Optic atrophy | Common carp, elephant, goat and sheep, mouse, python, raven |
| Night blindness | Bat, common carp, mouse, pig, raven |
| Glaucoma and cataract | Black carp, elephant |
| Eye injury | Chicken, goat and sheep |
| Ears | |
| Suppurative otitis media | Dog, mouse |
| Deafness | Crucian carp |
| Nose | |
| Rhinitis | Dog |
| Nasal sinusitis | Bear |
| Rhinorrhea | Bear |
| Nasal polyp | Dog |
| Mouth | |
| Tonsillitis | Black carp, ox gallstones, shark |
| Gingivitis | Python |
| Gingival atrophy | Python |
| Dental caries | Python |
| Halitosis | Elephant |
| Throat | |
| Laryngitis | Bear, black carp, common, grass carp, pig, murrel |
| Pharyngitis | Shark, soft-shelled turtle |
| Infantile hoarseness and aphonia | Chinese forest frog |
| Respiratory system | |
| Bronchitis | Ox gallstones, toad |
| Pneumonia | Ox gallstones |
| Cough | Ox gallstones, pig |
| High fever in children | |
| Infantile convulsions | Bear, ox (bile), ox gallstones, tiger |
| High fever in children | Human, ox gallstones, pig, python |
| Cardiovascular system | |
| Apoplexy | Ox gallstones |
| Angina pectoris | Bear |
| Neuropsychiatric disorders | |
| Epilepsy | Fox, Ox gallstones, wild boar |
| Coma | Ox gallstones, fox |
| Gynecological disorders | |
| Irregular or scanty menstruation | Otter |
| Amenorrhea or amenia Menostasis | Otter |
| Chancre | Mouse |
| Endocrine system | |
| Diabetes mellitus | Crucian carp, pig |
| Urinary system | |
| Diuresis | Chicken |
| Cystitis | Chicken |
| Hematuria | Bear |
| Strangury | Bear, chicken |
Table 3.
A list of animal biles according to organism groupings used therapeutically in China
| Class | Animal bile |
| Mammals | Antelope, bat, bear, deer, dog, elephant, fox, gaur, goat and sheep, hedgehog, horse, human, mouse, otter, ox, pig, tiger, wild boar, yak |
| Birds | Chicken, crow, duck, goose, kite, parrot, raven, vulture |
| Reptiles | Black snake, pallas pit viper, python, skink, soft-shelled turtle, tortoise, turtle |
| Amphibians | Chinese forest frog, toad |
| Fish | Black carp, common carp, crucian carp, grass carp, mandarin fish, murrel, shark |
| Arthropods | Horseshoe crab |
Dog bile
Dog bile[1,3,5,40] was believed to be effective for external use principally, to treat infectious skin diseases including carbuncle, furuncle, multiple abscesses, ulcerated scrofula, excess granulation tissue, and “malignant boil” (most likely, “rodent ulcer,” i.e., basal cell carcinoma). Other applications included skin lacerations from trauma of all kinds, including knife stab, spear, and arrow wounds. Domestic dog bile was also used as nasal drops to treat a “stuffy” nose caused by chronic rhinitis or nasal polyps, and epistaxis; it was employed as eye drops for conjunctival congestion, itchy dry eyes, and suppurative opthalmopathy; and it was prescribed as ear drops for acute suppurative and chronic otitis media. Dog bile was also ingested orally to treat heartburn from gastric regurgitation and diarrhea especially with visible blood (hematochezia) and mucus (probably dysentery or chronic inflammatory bowel disease). It was also believed applicable for patients with symptoms of distention and a full sensation in both chest and upper abdomen, or “blood stasis” (Xue Yu). Blood stasis was considered to be eliminated after half the volume of a dog’s gallbladder bile was ingested with hot wine. Dog bile was also believed effective in reversing traumatic injury to abdominal viscera. Because the more hydrophobic bile acids of bile (Table 1) increase intestinal peristalsis, dog bile was frequently used to expel intestinal worms. Hence it had a central role in treating infantile malnutrition with digestive disturbances secondary to intestinal nematodes and trematodes. Based on paleopathological evidence, these infestations were most likely due to roundworms (nematodes). Dog bile was also prescribed for patients with polydypsia, polyphagia and polyuria, mostly likely symptomatic of diabetes mellitus.
Ox bile
Ox bile[1,3,5,8,40] was introduced to treat jaundice and also to expel intestinal parasites. Ox bile was always available in plentiful supply and was also compounded into pills with flavescent sophora root (Sophora flavescens; Ku Shen), rough gentian root (Gentiana scabra; Long Dan Cao), and honey. These pills were prescribed for patients, with or without malnutrition, who had contracted jaundice (Gu Dan) believed to be due to immoderate eating and drinking. Gu Dan is one of the five types of jaundice in TCM. The yellow discoloration of the skin was believed to be caused by improper diet, and was manifested in dizziness, anxiety, gastric discomfort, abdominal fullness, diarrhea, and oliguria. It is likely that it represents one of several infectious hepatitides, such as the acute stages of viral hepatitis A, B or C. It was believed that ox bile was effective topically for healing hemorrhoids especially those that had become infected. We know now that bile acids possess strong astringent properties. For eternal use, ox bile was also used in treating furuncle, carbuncle, and all kinds of scabs from trauma and sores of the skin. Furthermore, ox bile was believed to improve visual acuity when taken internally mixed with sophora fruit (Styphnolobium japonicum; Huai Zi). The prescriptions provide for two to seven grains of ox bile following 100 d fermentation with black beans to be taken every evening. In addition, a fermented mixture of ox bile with powdered arisaema tuber (Arisaema; Nan Xing Mo) was thought to be highly effective in treating febrile infantile convulsions. To cure male impotence and increase libido, a mixture of the fruit of ailanthus-like prickly ash (Zanthoxylum ailanthoides; Shi Zhu Yu) fermented with ox bile for 100 d was prescribed.
Ox gallstones
Ox gallstones[5,8,9] also known as ox bezoars or “calculus bovis” in the literature, were first recorded in Shen Nong’s Herbal Classic[5], during the Qin (221-206 BCE) and the Western Han Dynasties (206 BCE-25 CE). These gallstones were considered a superior (i.e., top grade) therapeutic drug, and have been employed for over two thousand years in TCM, mainly as a tranquilizer and sedative. Ox gallstones were believed to be successful in curing a number of childhood diseases. This was especially the case with febrile infantile convulsions, which in the ancient literature were characterized quite accurately by sudden onset, upward gazing of the eyes, lockjaw, rigidity of the neck, limb convulsions, frothy salivation, “rattling” of pharyngeal phlegm and eventually coma. Ox gallstones were also prescribed for children as a remedy for polydipsia, diarrhea, and vomiting “caused by fright”, and nocturnal crying “induced by fear” (most-likely febrile nightmares). Furthermore, in TCM, ox gallstones were documented extensively to treat (1) Re Ji Sheng Feng, a morbid condition caused epidemic febrile diseases, characterized by high fever with convulsions, muscular rigidity and even opisthotonos followed by coma; (2) Re Ru Xin Bao, a morbid condition occurring in the Ying-Xue system caused by epidemic febrile diseases in which patients have high fever, delirium, cold limbs or convulsions ending in coma; (3) Re Ru Xue Fen, an epidemic febrile disease with invasion of the Xue system by “pathogenic heat”, characterized by high nocturnal fever, impairment of consciousness, restlessness or convulsions, eruptions, hemorrhage with a red raw tongue; (4) Re Sheng Fa Jing, a convulsive seizure caused by impaired nourishment of the “channels” owing to accumulation of “pathogenic heat” or intense heat impairment of the Yin fluid, usually associated with high fever, stiff neck, trismus, rigidity or spasms, abdominal distension, constipation, and even opisthotonos and loss of consciousness; and (5) Re Ru Xue Shi, in which the uterus during menstruation or after childbirth is affected by “exopathic” heat. This is characterized by recurrent fever and chills, tenderness and fullness in the lower abdomen, and nocturnal delirium, most likely puerperal sepsis.
Ox gallstones were also used to treat apoplexy, with aphasia, and coma. In addition, ox gallstones were mixed and prescribed together with other herbal remedies. For example, “Bezoar Bolus for Resurrection” (An Gong Niu Huang Wan) was, and still is used as a common drug in China. It contains powdered ox gallstones, curcuma root (Curcuma zedoaria; Yu Jin), rhinoceros horn (Rhinocerotidae; Xi Jiao), baikal skullcap root (Scutellaria baicalensis; Huang Qin), coptis root (Coptis chinensis; Huang Lian), realgar (Arsenic sulfide; Xiong Huang), cape jasmine fruit (Gardenia jasminoides; Zhi Zi), cinnabar (Mercury sulfide; Zhu Sha), musk (Moschus berezovskii; She Xiang) and pearl. According to the therapeutic theories of TCM, the preparation could eliminate “evil” heat and toxic materials from the body, and arouse patients from unconsciousness apparently by eliminating “phlegm”. Therefore, it was considered effective in the treatment of all febrile diseases, with “evil” heat involving the pericardium and the “evil” heat of phlegm stagnating the heart. This condition was characterized by high fever, irritability, coma, delirium and a yellow-coated tongue. Besides Bezoar Bolus for Resurrection for mild cases of each of these syndromes, Bezoar Bolus for Purging the Heart-Fire (Niu Huang Qing Xin Wan) most likely angina pectoris was also recommended. It contained powdered ox gallstones, cinnabar (Mercury sulfide; Zhu Sha), coptis root (Coptis chinensis; Huang Lian), baikal skullcap root (Scutellaria baicalensis; Huang Qin), cape jasmine fruit (Gardenia jasminoides; Zhi Zi), and curcuma root (Curcuma zedoaria; Yu Jin).
Common carp bile
Common carp bile[5,8,9] was prescribed as drops to treat eye diseases such as acute conjunctivitis, phlyctenular and lagophthalmic keratitis (probably trachoma), suppurative blepharitis, and vesicular dermatitis of the eyelids. Common carp bile was also promoted as a remedy for improving visual acuity and for treating optic atrophy and night blindness (nyctalopia). It was also believed to be effective in treating inflammation of the throat and infectious skin diseases such as chancre (possibly impetigo) in children.
Mouse bile
Mouse bile[6,8,9] was used topically to improve visual acuity as well as to ameliorate impaired hearing. As eye drops it was considered effective in treating optic atrophy and night blindness; as ear drops for suppurative otitis media; and as a dressing for external skin trauma such as incisional wounds. When mixed together with bear bile, aconitus root (Aconitum Carmichaeli; Chuan Wu Tou), asarum herb or Chinese wild ginger root (Asarum sieboldii; Xi Xin), chalcanthite (Copper sulfate pentahydrate; Dan Fan) and musk (Moschus berezovskii; She Xiang), it was believed to be salutary for chronic use in older individuals who were “hard of hearing”.
Python bile
Python bile[6,8,9] was prescribed mainly for patients with intermittent colicky abdominal pain apparently due to intestinal parasites, which based on the paleopathological evidence most likely were Ascaris lumbricoides and schistosomes. It was also employed for patients with hemorrhoids and bloody dysentery. To treat high fever in children, python bile was diluted with water and dropped into the nose; it was also given as an enema to treat infantile dysentery complicated by malnutrition. Python bile was employed externally to treat ulcers (chancres) of the external female genitalia (most likely venereal) since some secondary bile acids are potent topical microbicidal agents[42], and chancre complicated by fistulae in children, as well as leprosy. Together with musk, python bile was considered effective in the treatment of gingival atrophy, gingivitis, and dental caries. Python bile was also advocated as a remedy to improve visual acuity, remove nebulae, diminish eye swelling, and abate ophthalmalgia.
Goat and sheep biles
Goat and sheep biles[6,8,9]. In TCM, goat and sheep biles were considered to have similar therapeutic effects and therefore are not distinguished by nomenclature; so accordingly they are combined in this document. They were believed to be effective in treating optic atrophy, including acute hemorrhagic conjunctivitis, marginal suppurative blepharitis, and epiphora (non-emotional tearing). Goat bile was also used to treat temporary blindness following a life-threatening illness (which was most likely smallpox), and eye injury from foreign bodies. These biles were believed effective in ameliorating various infectious skin diseases, chancre in children (most likely impetigo), and constipation. When compounded with pig pancreas and asarum herb, these biles were also prescribed as a facial lotion for minimizing chloasma in pregnant women and to dermabrase freckles. Also, when goat bile was decocted thrice with ox bile and wine the resulting liquor was believed effective in reversing any olive discoloration of the skin from itchy dermatitis, which was most likely secondary to tinea versicolor.
Pig bile
Pig bile[7-9] was one of the most common domestic animal biles used medicinally in ancient China; moreover it was deployed often as an inexpensive substitute for rarer animal biles such as that of black bears, as it is to this day[43,44]. The Longmen Grottoes in Luoyang of Henan Province, which were built in 386 to 534 CE, were found to contain 110 prescriptions for Chinese medical herbs, and 19 acupuncture modalities for 41 disease categories, hereafter called the Longmen Prescriptions (Long Men Chu Fang)[10]. They embodied 122 types of the most commonly used medical “herbs”, including domestic pig bile. To publicize these prescriptions, they were initially carved on stones and therefore preceded wood block printing which, though invented earlier (c. 220 CE), only became practiced widely from the time of the Eastern Jin dynasty (317-420 CE).
Domestic pig bile was used to treat (1) febrile diseases and thirst caused by high fever or “pathogenic” heat, as well as in patients suffering from (2) Shao Yin diseases complicated by dysentery; (3) dysentery due to “damp-heat” pathogens; (4) intermittent diarrhea, and dysentery with bloody, mucoid stools; (5) five types of infantile malnutrition as well as their cold equivalents complicated by cough; (6) diabetes mellitus characterized by polydipsia, polyphagia and polyuria; and (7) puerperal fever and bed sores in “lying-in” women. Furthermore, pig bile following dilution with wine was painted on skin to treat burns caused by boiling water and fire, as well as a shampoo to clean greasy hair. It was also believed that pig bile was effective in curing various infectious infantile skin diseases, nasal eczema, allergies and favus of the scalp.
In the contemporary medical literature[45,46], it has been documented that pig bile possesses anti-allergic properties against delayed-type hypersensitivity, a feature shared with bear bile but not with ox or chicken biles. Pig bile was also believed to improve eyesight so that it was used to treat acute conjunctivitis, cloudy vision, and to remove opacities in the cornea (nebulae) - most likely from trachoma. In addition, pig bile was used to treat tonsilitis and acute sore throat (Hou Feng). In TCM, Hou Feng is a general term for a serious condition such as sudden swelling and pain in the throat, difficult breathing, discomfort upon swallowing, and excessive salivation (most likely a peri-tonsillar abscess or “quinsy”). It could also promote peristalsis and hence bowel movement, thereby a cathartic effect relieving constipation (especially due to “pathogenic heat”). In this case, the bile was administered by enema, dripped into the large bowel employing a reed as a guide. Pig bile was also used effectively to treat whitlow or paronychial inflammation with accumulation of pus. In this regard it is known today, and verified by experiment, that pig bile (as well as bear bile) share anti-inflammatory as well as analgesic properties[47].
Bile of Pallas’s pit viper
Bile of Pallas’s pit viper[7-9] was used to treat ulcers of the female external genitals (most likely venereal, see python bile above), and various fistulae and sinuses such as those occurring in the perianal region, most likely secondary to chronic inflammatory diseases of the colon and rectum. It was also indicated for perforated dacryocystitis and scrofula (i.e., tuberculosis) of the neck. This viper bile could also destroy and eliminate intestinal worms and as well as cure continuous uterine hemorrhage (metrorrhagia).
Chicken bile
Chicken bile[7-9] was believed to be efficacious in treating various infectious skin diseases, including eczema and intertrigo on the pinna of the ear. As eye drops, it was used in newborns to treat acute conjunctivitis, epiphora, xerophthalmia, and sensation of a foreign body in the eye. When chicken bile was diluted with water, the solution was used topically for hemorrhoids and for the treatment of strangury (slow and painful discharge of urine). For an infected urinary tract, it was employed to induce a diuresis thereby ameliorating “secondary” urolithiasis and as well as cystitis.
Elephant bile
Elephant bile[8,9,48] was introduced to improve visual acuity. Upon drying, it was compounded into pills with gallbladder biles of the common carp, bear, ox and musk, plus abalone shell (Haliotis asinina; Shi Jue Ming). These pills were ingested with green tea to treat eye diseases including blindness from optic atrophy, mature cataract, and mild nebulae (corneal opacities), as well as glaucoma. Elephant bile was believed efficacious in treating a large number of infectious skin diseases. Furthermore, when diluted with water, it was employed as a gargle to alleviate halitosis!
Murrel bile
Murrel bile[8,9,49]: Upon dilution with water, murrel bile was employed to treat inflammatory diseases of the throat, and hence it was believed especially effective in healing acute pharyngitis. As a liniment, it was employed for the treatment of tinea capitis and for clarifying corneas with nebulae which were most likely secondary to trachoma.
Bear bile
Bear bile[8,9,50] is considered the “king” of animal biles both in ancient times and currently in the Orient and it has a highly respected history being used widely in therapy throughout ancient China. In Asia generally and TCM in particular, top-grade bear bile has been valued “more than gold” principally for its preventative as well as therapeutic efficacy. Bear bile was widely employed in treating patients who suffered from (1) jaundice caused by summer heat; (2) summer diarrhea (Shu Xie) caused by summer heat, which is marked by passage of watery or sticky stools and dark-colored urine, restlessness, thirst, and sweating; (3) abdominal pain due to hepatobiliary diseases and gastric malfunction; (4) epigastric colic due most likely to biliary ascariasis; (5) five types of infantile malnutrition; (6) infantile convulsions; (7) blood retention syndrome (Xu Xue Zheng), which in TCM shows that stagnated blood accumulated in a channel or an organ, e.g., in the uterus, manifested by distension and pain in the lower abdomen, chills and fever, delirium or other nocturnal mental disorders; or in the middle-Jiao, marked by pain, tenderness and guarding over the epigastrium; (8) strangury complicated by hematuria (Xue Lin) that is one of the five types of strangury in TCM, manifested by dribbling of bloody urine, accompanied by urethral pain, distension and pain/discomfort in the lower abdomen; (9) chancre, pyogenic nasal infection and nasal eczema, as well as various kinds of infectious skin diseases; and (10) hemorrhoids and anal fistulae. Bear’s bile was believed to be highly effective in treating rhinorrhea with purulent nasal discharge as in (9) above. Bear bile apparently could be used to destroy intestinal worms such as ascariasis and oxyuriasis in children, and together with the fruit of Rangoon creeper, it was beneficial in treating emaciation in children due to these intestinal parasites. It was also believed effective in treating inflammation of the throat. Lastly, bear’s bile was believed to improve visual acuity by “dissolving” nebulae.
Wild boar bile
Wild boar bile[8,9,51] upon dilution with wine was advocated for treating various infectious skin diseases including paronychia (whitlow) as well as burns caused by boiling water and/or fire. It was decocted with jujube juice and ingested to treat the various kinds of infantile malnutrition, as well as epilepsy.
Shark bile
Shark bile[8,9,51] obtained from their fresh gallbladders was made into pills with alum powder and physically placed in the throat to terminate acute tonsillitis and pharyngitis.
Black carp bile
Black carp bile[8,9,51] was employed to treat acute conjunctivitis and ophthalmalgia. Pills of Fish Biles were compounded with biles of black carp, common carp, goat, ox and bear, musk (Moschus berezovskii; She Xiang), and abalone shell (Haliotis asinina; Shi Jue Ming). The pills were swallowed with tea to treat various kinds of ophthalmopathies such as optic atrophy, cataract, nebulae, and glaucoma. Black carp bile was believed to be effective in treating herpetic ulcers (cold-sores), malignant boil (“rodent ulcers”), tonsillitis, pharyngitis, as well as fishbones struck in the throat.
Antelope bile
Antelope bile[8,9,52] was used as a lotion for the face after being decocted (concentrated, or boiled down) thrice with ox bile and vinegar. Furthermore, this decoction was used as a tincture to lighten the dark complexion of chloasma and freckles during pregnancy.
Grass carp bile
Grass carp bile[8,9,53]: Following dilution with water, grass carp bile was used to treat diseases of the pharynx, especially acute pharyngitis. In a clinical emergency, grass carp bile was mixed with wine and gargled by patients with a fishbone or other foreign body stuck in the throat or esophagus. The bile diluted with wine apparently softened bones rapidly and allowed them to slip down into the stomach or alternatively they were regurgitated sometimes with induction of vomiting.
Tiger bile
Tiger bile[8,9,53] was used to treat infantile dysentery complicated by malnutrition, and febrile convulsions accompanied by restlessness in infants.
Human bile
Human bile[8,53]: When diluted with wine, human bile (obtained from fresh cadavers, often after battles) was used by soldiers to paint-on sword wounds in northern battlefields of ancient China. It was also believed to be highly effective in treating patients with malarial-type diseases characterized by intermittent high fevers, rigors and sweating, as well as languor and dysphagia. To compound a preparation, human gallbladder bile (obtained at necropsy) was placed in a bowl that was filled with polished glutinous rice and a little musk. The bowl was then placed in a shady place to dry in air. To treat chronic ‘malarial’ disease, half of the polished glutinous rice (Oryza sativa; Nuo Mi) and bile, that had now become green in color (from oxidation of bilirubin to biliverdin), was ingested with a decoction of dried tangerine or orange peel (Citrus reticulata or Citrus sinensis; Chen Pi). To treat dysphagia, the other half that had become black in color with passage of time, was ingested with a decoction of the pith stem of the rich-paper plant (Tetrapanax papyriferus; Tong Cao). After drying, human bile was ground into powder with cinnabar (mercury sulfide; Zhu Sha), realgar (Arsenic sulfide; Xiong Huang) and musk (Moschus berezovskii; She Xiang), and pills were made the size of broad beans. These were believed effective in treating patients with intermittent rigor, high fever and severe chills, most likely from malaria.
Otter bile
Otter bile[8,9,54] was believed effective in treating patients with opthalmopathy such as vertigo, motes, blurred vision, and hypopsia (impaired vision). Pills of Otter Bile were made from otter plus dog gallbladder bile, sal ammoniac (Ammonium chloride; Nao Sha), Chinese prickly ash (Zanthoxylum bungeanum; Chuan Jiao), and fried leech (Hirudinea; Shui Zhi). When the pills were ingested three times a day with liquor of Chinese angelica root (Angelica sinensis; Dang Gui), they restored normal menstrual function in patients with irregular or scanty menstruation, as well as amenorrhea.
Fox bile
Fox bile[8,9,54]: When diluted with warm water, fox bile was used to resuscitate patients who became unconscious suddenly, most likely from a syncopal attack or epilepsy. This bile was also believed to be potent in reversing acute alcoholism, and treating alcoholic liver disease (Jiu Zheng). Jiu Zheng is a chronic disease with formation of a firm “mass” in the hepatic region of the upper abdomen due to long-term habitual drinking. Most likely this was micronodular or Laennec’s (alcoholic) cirrhosis possibly complicated by a hepatoma. Pills were made from fox bile, cinnabar (Mercury sulfide; Zhu Sha), white arsenic (Arsenic trioxide; Pi Shuang), asafetida (Ferula assafoetida; A Wei), musk (Moschus berezovskii; She Xiang), and mung beans (Vigna radiata; Lu Dou). The pills were also ingested with cold vinegar to treat malarial symptoms, and were believed effective in treating epigastric pain and stomach aches most likely secondary to peptic ulcer disease.
Hedgehog bile
Hedgehog bile[8,9,55] was employed as astringent eye drops to cure epiphora most likely from ectropion. After diluted with water, the bile was used also as a liniment for hemorrhoids. It was apparently effective in treating acute conjunctivitis and conjunctival congestion from smallpox.
Goose bile
Goose bile[8,9,56] was used solely for treating hemorrhoids. A mixture of goose (and sometimes bear) biles mixed together with honey was believed effective in treating internal and external hemorrhoids with or without bleeding, infection or prolapse.
Duck bile
Duck bile[8,9] was used primarily as eye drops in the treatment of acute conjunctivitis, as well as a liniment for treating hemorrhoids.
Deer bile
Deer bile[8,9] was utilized chronically to remove “toxic” substances from the body and promote subsidence of inflammatory swelling; therefore, it was employed topically to treat various infectious skin diseases and apparently through “trans-dermal absorption” some internal diseases.
Horseshoe crab bile
Horseshoe crab bile[8,9] was used to treat tongue swelling with numbness (most likely from leprosy) and to expel intestinal parasites. In the treatment of leprosy, horseshoe crab bile was mixed together with alum (hydrated aluminium potassium sulfate; Bai Fan), green vitriol (Iron sulfate; Lu Fan), mercury and musk (Moschus berezovskii; She Xiang), and was ingested daily with spring water.
Crucian carp bile
Crucian carp bile[8,9] was employed externally to treat chancres, vulval erosions, and pruritus vulvae. Because bile of the crucian carp could also expel intestinal worms, it was effective in the treatment of abdominal pain caused by intestinal parasites, especially in children. In addition, bile of crucian carp was capable of softening fishbones and pieces of wood including bamboo, stuck in the throat or esophagus, thereby allowing them to slide into the stomach. Crucian carp bile was also used in treating carbuncles of the head in malnourished infants. Together with pumice stone (Volcanic rocks that are composed of 65%-75% Silicon dioxide and 9%-20% Aluminium oxide; Fu Shi), ground gecko or red-spotted house lizard (Gekko gecko; Ge Jie) and cicada slough (Cicadidae; Chan tui), crucian carp bile was employed to treat diabetes mellitus at least as inferred by the combined complaints of polydipsia, polyphagia and polyuria. Furthermore, in the treatment of deafness, crucian carp bile was placed in the cavity of a scallion (large green onion) together with black donkey fat (Equus asinus; Wu Lu Zhi) and sesame oil (Sesamum indicum; Ma You) for seven days, and then used as ear drops.
Turtle bile
Turtle bile[8,9] was prescribed to treat hemorrhoids complicated by perianal fistulae.
Mandarin fish bile
Mandarin fish bile[8,9] was applicable to cases where a fishbone or other bone, or foreign-body including wood or bamboo was stuck in the throat. The bile salts acted as a calcium “chelator”[57] when gargled with powder of Chinese honey locust seed (Gleditsia sinensis; Zao Jia Zi) and wine which performed possibly as an organic solvent.
Crow bile
Crow bile[8,9] was believed effective in treating acute conjunctivits, blepharitis, vesiculo-dermatitis of the eyelids, and keratitis.
Soft-shelled turtle bile
Soft-shelled turtle bile[8,9] also appeared to have a unique role in therapy, in that it was believed to be effective in treating acute and chronic pharyngitis when ingested with ginger juice and peppermint.
Chinese forest frog bile
Chinese forest frog bile[8,9] was used to treat hoarseness and aphonia most likely secondary to laryngitis in infants and young children.
Black snake bile
Black snake bile[8,9] was employed to treat leprosy, as inferred from the association with paralysis, numbness, and swelling of the tongue. Long Dan Extract for Leprosy was prepared as follows: after its seeds were removed, a wax gourd was placed in a hole dug in the ground one meter deep. After both black snake bile and a pear were added to the gourd, the hole was covered with earth. The wax gourd was checked every three days for about 50 d until the black snake bile, plus the other ingredients were intimately co-dissolved. To treat leprosy, the juice was ingested daily with wine.
Tortoise bile
Tortoise bile[8,9] was widely used for treating patients with persistent eye swelling following an attack of smallpox.
Bat bile
Bat bile[8,9] was believed to improve visual acuity since bats fly at night and hence was used in treating night blindness (nyctalopia). Most likely its beneficial effect was because of this bile’s high vitamin A content.
Raven bile
Raven bile[9,58] was prescribed to improve visual acuity and in treating optic atrophy and night-blindness. The extraordinary visual acuity of this raptor must also have played a role in this heuristic discovery! Raven bile was also believed effective in removing toxic substances from the intestine with gamboge (a yellow pigment extracted from specific evergreen trees) (Clusiaceae; Teng Huang).
Yak bile
Yak bile[59] was used only to treat epigastric colic in infants, children and adults, caused by various digestive diseases.
Gaur bile
Gaur bile[59] was used to treat epigastric pain.
Skink bile
Skink bile[59], the bile of this family of lizards was believed effective in treating several eye diseases such as acute conjunctivitis, blepharitis, and keratitis.
Parrot bile
Parrot bile[59] was used solely to treat food poisoning when ingested immediately following the onset of the acute symptoms.
Horse bile
Horse bile[59]: Despite being without a gallbladder, equine hepatic bile was aspirated by fistulation of the bile ducts and used in treating infectious skin diseases.
Vulture bile
Vulture bile[59], again as a raptor with particularly good visual acuity, it was believed to improve vision and hence was used to treat opthalmopathy. It was also effective in treating various kinds of infectious skin diseases such as carbuncle, furuncle, multiple abscesses, scrofula (tuberculosis of lymph glands in the neck), and rodent ulcers (basal cell carcinomata).
Kite bile
Kite bile[9], the bile of this raptor, was believed effective in treating epigastric pain most likely caused by hepatobiliary, gastric or esophagus diseases.
Toad bile
Toad bile[9], when ingested orally, was considered highly effective in treating acute and chronic bronchitis. This was the only bile in addition to that of the pig used for treating pulmonary diseases.
In summary, forty-four different animal biles, including human bile, were used as drugs in TCM to treat patients with a great variety of different diseases (Table 2) and recorded in books of Chinese materia medica for over 2500 years.
CRITERIA FOR COLLECTING ANIMAL BILES AND OX GALLSTONES
The most prized of all animal biles in treating disease was that of the Chinese black bear introduced after the Han dynasty and prior to that of the Sui dynasty (Figure 2). In addition, ox gallstones were used therapeutically during the Han dynasty[5]. Therefore, it is not surprising that Chinese patients and physicians generally considered that both bear bile, which contains high UDCA levels in conjugated form[60-62], and ox gallstones largely consisting of non-polymerized calcium bilirubinates were “more precious than gold” with respect to safeguarding their health and well-being and reversing health disorders[4,63]. Also, ancient Chinese physicians and pharmacists had established a series of novel methods for collecting biles from animals and gallstones from oxen.
For example, Su Song (c. 1019 to 1101 CE) in his Illustrated Pharmacopoeia (c. 1061 CE) described a detailed method for collecting gallbladder bile from the python. In the southern part of China, the python was often raised purposefully for collecting gallbladder bile, and usually bile was harvested from the python in the early summer. An adult python was immobilized in a wooden “sandwich” cage and its abdomen was exposed. During a mini-laparotomy, liver and gallbladder were carefully examined. If the python’s gallbladder was as large as a duck’s head in size, a careful surgical cholecystectomy was performed. After the liver was returned to the abdominal cavity, the abdominal incision was closed with silk sutures. Postoperatively, this python continued to be raised because its muscle meat, subcutaneous fat and skin could be used to treat other diseases. The fresh gallbladder bile was often dried in a shadow place away from direct sunlight. If the gallbladder contained dark green colored bile with a very thin wall and possessed a pleasant sweet and bitter taste, it was categorized as “high grade”.
Collecting gallbladder biles from fish such as common carp, grass carp, black carp, murrel, crucian carp and shark was often carried out in wintertime[8]. These fresh gallbladder biles were dried in a cool place, and stored for later medicinal use. In addition, human bile was obtained from fresh cadavers, often in the ‘field’ after battles. When diluted with wine, a phase transition to multilayered phospholipid-cholesterol vesicles took place (“artificial membranes”)[64], human bile was used as an artificial “skin” by soldiers to paint-on sword wounds in northern battlefields of ancient China[8].
Ancient Chinese medical works also described how to differentiate authentic bear bile (treasured above all others) from other animal biles[8]. When a gallbladder bile sample was dropped upon the surface of water, “high quality” bear biles spread on the surface quickly, whereas “low quality” bile spread slowly. Additionally, when a sample of high quality bear’s bile sank to the bottom of a vessel containing pure water, it drew out a “yellow tail”. When a layer of dust was placed on the surface of the water it could be dispelled by placing a drop of high quality bear’s bile on the water surface[8]. This was a serendipitous example of the differential surface activity of the bile acids in bile, especially between the major hydrophilic (e.g., UDCA conjugates) and hydrophobic (e.g., CDCA conjugates) species[65] that occur in variable proportions in bear bile[66], as well as the high specific gravity (d = 1.3) of bilirubin[67]. Obviously, bilirubin does not undergo oxidation to biliverdin when a bile contains a high proportion of UDCA conjugates because of the latter’s potent anti-oxidant effects[68].
In bear’s bile, the notable component is the high level of the taurine conjugate of ursodeoxycholic acid (TUDCA) (Figure 1). In bears, UDCA is a primary bile acid that is synthesized directly from cholesterol in the liver[66]. Clearly gallbladder bile from bears is highly valued in TCM. Since UDCA in bears is a primary bile acid[66], this allows hepatic bile, highly enriched in TUDCA, to be harvested indefinitely from bears with chronic biliary fistulae. Frequently such bears were confined individually to iron cages or were kept free-living on bear farms (now illegal in China). After a successful cholecystostomy or choledocotomy under anesthesia, a bear was placed in a specific, custom-fabricated “iron-cage” filled together with a sterile drainage tube, one end of which was sutured into the common bile duct and the other leading to a glass-receptacle firmly holstered externally to the abdominal wall. Usually with the aid of a stop-cock, 20-100 mL of bear bile (approximately one third of the daily bile secretion) was collected daily, and dried at 65 °C for 3-4 d. By this means up to more than 36 kg of dilute fistula bile or 1.5 kg of dried bear bile could be harvested each year from one “free-living” bear[69]. By contrast, only 50 g or so of the fresh bile could be obtained from the gallbladder of a sacrificed bear. Studies by the same authors[69] showed that bears secreted the highest amount of bile between July and September, followed by decreasing flows from October to December, from April to June, and then from January to March[69]. Also, chemical compositions and pharmacological effects of the drained bear’s bile were believed identical to fresh bear bile in terms of the quality and quantity of bile acids (including increased TUDCA levels) and bilirubin conjugates, biliflavin and bilifulvin. Moreover, the minor components of bile such as, amino acids and trace elements, displayed no change in bile of bears who were drained for two years[70-73]. Of note is that pig bile is often substituted for bear bile in therapy in today’s China because the Asiatic black bear is an endangered species. This bear is listed in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Appendix I (viz. http://www.cites.org/eng/app/appendices.shtml).
Tao Hong-Jing (c. 452 to 536 CE) in his Records of Famous Physicians (c. 510 CE) wrote a fanciful description of the pathobiology of ox gallstones (i.e., principally calcium bilirubinate): “Ox gallstones are mainly obtained from oxen raised in the central regions of China, and are one of the most valued of drugs. An ox suffering from gallstones roars constantly and its body is ‘luminous’ at night. If a basin of water is placed before the ox, a frightening noise will cause the animal to vomit the gallstones into the basin. These gallstones are called Sheng Huang and are the most costly.” Based on stone size and texture, ox gallstones were divided into three kinds. The San Huang was composed of small granules like green beans. The Man Huang was described as being soft “like the yolk of an hen’s egg”, and was always found in the intrahepatic bile ducts. The Tuan Huang was an agglomeration of small stones that varied in size. According to the methods of collecting stones, gallstones were divided into four kinds. The Sheng Huang has been described above. The Zhong Huang was obtained in abattoirs during victualling an ox. The Xin Huang was obtained at necropsy after a sick ox had died. This stone which was as soft as an egg yolk at first, became hard once it was placed in water. The stones obtained from the intrahepatic bile ducts were called Gan Huang. Li Shi-Zhen (1518 to 1593 CE) in his Compendium of Materia Medica (1596 CE) wrote: “For the ox to form gallstones, it must be diseased. Only a sick ox suffers from the formation of gallstones and dies easily of their complications. Based on one of the therapeutic theories of TCM, i.e., combating poison with poison (Yi Du Gong Du), ox gallstones were also advocated for treating hepatobiliary diseases associated with jaundice in humans”.
Although ancient Chinese scholars did not understand how gallstones formed in gallbladder and intrahepatic ducts of oxen, they knew how to identify “true” from “false” ox gallstones. After a fresh ox gallstone was smeared on a finger nail, if the yellow color could not be easily washed off with water, it was considered a “true” ox gallstone[7]. Furthermore, if the yellow color spread and painted the entire finger nail and remained there for a long time, it was also a “true” ox gallstone[54]. Kou Zong-Shi in his Amplified Materia Medica (c. 1116 CE) stated that ox gallstones were light in weight and soft, as well as sweet-smelling, most likely the pure bile pigment salt, i.e., calcium bilirubinate stones. On the other hand, yak gallstones obtained from the western part of China were very hard, and without a sweet smell; we believe that these were most likely cholesterol or even calcium carbonate stones. Moreover, it was very easy to pass off gallstones obtained from camels as “ox” gallstones, and these could be obtained easily. This medical scholar went on to state that more attention should be paid to this because the effects of “false” ox gallstones were completely different medicinally from those of “true” ox gallstones[54].
As in ancient times, ox gallstones are still a very popular drug in China. There are more than 154 traditional Chinese herbal preparations containing this form of calcium bilirubinate as an ingredient[4]. However, it is very difficult to obtain natural ox gallstones in sufficient quantities by collecting them from abattoirs. Since 1955, Chinese scientists introduced a method for making artificial ox gallstones ex vivo employing fresh ox gallbladder bile. Moreover, since 1980, Chinese scientists developed a facile technique to produce “natural” ox gallstones in situ, akin to the culturing of pearls[4]. A mini-laparotomy is performed on a restrained cow and a tiny hole is made in the fundus of the gallbladder, which is grasped with a forceps. A pinhead-sized natural ox gallstone is then implanted in the gallbladder. The fundus of the gallbladder is then sutured and the abdominal incision is closed. This natural ox gallstone performs as a nucleus and develops into a large pigment gallstone within 1-3 years. During this period, 98% of oxen so “implanted” form gallstones and on average, a 4 g pigment gallstone can be obtained at slaughter[4]. A number of pharmacological and clinical studies have confirmed that the chemical compositions and therapeutic effects of both the artificial ox gallstones and the “man-made” natural ox gallstones are identical to those of spontaneously forming ox gallstones[4,63]. Furthermore, artificial ox gallstones or the prepared synthetic calcium bilirubinate salt have been used to treat many thousands of patients with high fever, pneumonia, pyogenic tonsillitis, and bronchitis in China[4].
ANIMAL BILES USED THERAPEUTICALLY IN JAPAN
Chronologically, as might be anticipated the first country to be influenced by the Asiatic spread of TCM was Japan. Traditional Chinese medicine entered Japan during the Qin (221-206 BCE) and the Han (206 BCE to 25 CE) Dynasties[74-76]. Chinese herbs were readily collected, transplanted and cultivated. Many herbaria were constructed in Japan, and books based on TCM were translated into Japanese and published in Japan. An impetus to further progress was made by the dispersion of Buddhism in Japan during the Sui (581-618 CE) and the Tang (618-907 CE) dynasties. These Chinese dynasties saw long uninterrupted periods of peace with marked economical, political and cultural advancements. For example, Jian Zhen (688 to 763 CE) an illustrious Buddhist monk and physician, was officially invited at age 55 at the request of Japanese Monks to visit Japan to preach Buddhism. In 753 CE, he brought hundreds of Chinese medical books with him[77], established a charity clinic in Kobe, a large city on the southern side of the main island of Honshú, and taught TCM to Japanese physicians. After his death, he was subsequently venerated as a “saint” and worshiped in Japanese temples. He was the author of the Eminent Monk Jian Zhen’s Secret Remedies, which was treasured in Japan as a highly innovative medical, especially therapeutic, work. Seventy-three years after the Newly-revised Materia Medica[78] was published in China, this book was also introduced into Japan[74,76,79], and became an important reference book for Japanese scholars researching Chinese materia medica. In fact, the Japanese Government proclaimed that every scholar wishing to take the governmental examination to become a practicing physician needed to study this book.
For example, the most detailed lore we have from Japan concerning an animal bile was that of the Asiatic black bear (Ursus Thibetanus) also known as the moonbear or white-chested bear. Its range is wide in Asia, including in the Honshú and Shikoku islands of Japan. The species can be very aggressive towards humans and Rudyard Kipling described it as “the most bizarre of the Ursine species.” In Japan, black bear’s bile, Fel Ursi[80], was classified into two groups, bile from summer-bears and bile from winter-bears, according to the season of capturing the bear (Figure 4). Summer-bear bile was described as “high grade” being obtained from a gallbladder with a thick wall that could be either transparent or opaque. The amount of gallbladder bile obtained during the summer from bears was not that plentiful, but was invariably clear and always yellow-orange in color. It was also called “amber” gall and its quality was believed superior to that of winter-bear bile, since it was scarce. The bile from winter-bears obtained after August was found in a gallbladders that contained a thin wall. The amount of this bile was plentiful presumably because the animals were either hibernating or in a pre-hibernating stage, hence there was minimal hormonal stimulus to gallbladder contraction. Its color was brown-black with a glossy sheen. Additionally, bear bile was further divided into two groups according to the place of collection, bile from “hillock-bears” and bile from “island-bears”. Hillock-bear bile was generally from the Ishikawa district in northern Japan. It possessed a pleasant sweet smell, and was labeled “high grade”. Island-bear bile from Matumeezo district in the east of Japan was “low-grade”, had a pronounced smell of raw fish, mostly salmon, and therefore unpleasant to drink.
Figure 4.

Three woodcuts from Pictures of the Famous Products of Mountain and Sea (Sankai Meizan Zue) illustrated by Shitomi Kangetsu (1747 to 1797 CE) and published in 1799 CE, showing how to catch a bear and harvest bear bile. Capturing a bear in a cave (top panel). Seizing a bear by killing with a heavy crossbow (middle panel). Killing a bear with an axe (bottom panel).
MODERN CHEMICAL RESEARCH ON THE PRINCIPAL COMPONENTS OF ANIMAL BILES WITH POTENTIAL MECHANISMS UNDERLYING THEIR MEDICINAL ACTIONS
Since 1900, modern medical research on animal biles entered a new era. In 1900-1901, Danish and Swedish explorers led by Amdrup and Kolthoff respectively set out to explore Greenland[81]. During the expeditions, teams obtained gallbladders from polar bears (Thalarctos maritimus) and brought them back to Hammarsten’s laboratory in Uppsala, Sweden. Hammarsten isolated an unknown bile acid from polar bear bile, and named it ursocholeinic acid (Ursocholeinsäure)[82,83]. Twenty-five years later, Shoda in Japan crystallized a “new” dihydroxy bile acid from bile of presumably an Asiatic black bear (origin and species unspecified)[84], and renamed it ursodeoxycholic acid (UDCA). In 1954, Kanazawa et al[85] succeeded in synthesizing UDCA from cholic acid. Shortly thereafter, UDCA was introduced into the Japanese pharmacopoeia for the therapy of a variety of hepatobiliary diseases. Over subsequent years, the compositions of bile acids in vertebrates were investigated extensively[60,61,66,86-107]. In 1972, American investigators at the Mayo Clinic reported that long-term ingestion of chenodeoxycholic acid (CDCA) by humans could desaturate bile with cholesterol and lead to the dissolution of cholesterol gallstones[108]. Somewhat later, Japanese investigators also published similar findings in gallstone patients receiving daily doses of UDCA[109,110]. As a result, two purified bile acids, CDCA and UDCA (Figure 1), originally isolated as extracts of bird (goose) and mammalian (bear) biles, respectively[111], were approved for treatment of cholesterol gallstones, and in the case of UDCA for the early stages of several chronic hepatic diseases[108-110,112-118]. These bile acids are now prepared semi-synthetically from cholic acid by removal of the 12α OH group (CDCA) and epimerization of the 7-OH group (UDCA).
Specifically, long-term administration of UDCA to humans may or may not reduce biliary cholesterol secretion, but it induces a phase transition in gallbladder bile[119], and promotes physical-chemical (liquid-crystalline) dissolution of small cholesterol gallstones[109]. It was then discovered that UDCA might be efficacious in treating primary biliary cirrhosis[120] and a number of other chronic cholestatic liver diseases[118]. Since these earlier studies, well-controlled clinical studies have confirmed the modest beneficial effects of UDCA in a broad variety of cholestatic liver diseases, provided therapy is initiated in their early stages, including primary biliary cirrhosis, primary sclerosing cholangitis, pediatric cholestatic disorders, perhaps cystic fibrosis and drug-induced cholestasis[118,121-126]. It is believed that UDCA reduces biliary “cytotoxicity” by replacing the more detergent and potent endogenous dihydroxy bile acids such as deoxycholate (DC) and chenodeoxycholate (CDC) conjugates within the bile acid pool, principally by inhibiting their active sodium-coupled absorption from the distal small intestine. UDCA therapy also promotes membrane insertion of biliary lipid transporters at the level of the hepatocyte canaliculus[127,128].
Furthermore, black bear bile per se has been advocated for centuries to improve liver function and when compounded together with Chinese herbs, it was believed highly effective in treating acute and chronic hepatitis, toxic hepatitis, hepatic coma, acute and chronic gastritis, and peptic ulcer, as well as in relieving the pain of gastric and duodenal ulcers. In addition, there was experience of its use for treating patients with coronary heart disease, angina pectoris, glaucoma, hemorrhoids, and epilepsy[129]. Over the past twenty-five years, clinical, pharmacological, toxicological, and metabolic studies on both CDCA and UDCA have appeared in increasing numbers[13,101,115,117,130,131]. Although today CDCA (Chenodiol) has fallen into disuse because of its hepatotoxicity in therapeutic doses, UDCA (Ursodiol) has enjoyed increasing applications for a number of chronic liver diseases especially in their early stages and is considered safe in pregnancy[118,121-126].
In addition, a unique property of all biles with high phospholipid to bile acid ratios is that when diluted below the critical micellar concentrations of the bile acids, biliary mixed micelles undergo a phase transition into multilamellar liquid-crystals[132,133] composed principally of biliary phospholipids and cholesterol with bilirubin conjugates attached via π-orbital OH interactions[21]. These can serve as an “artificial skin” with antioxidant properties[64,133,134] for burns and wounds, a discovery made in China during the Tang dynasty (618-907 CE). For example, human, wild boar and domestic pig biles were used topically to treat burn patients for more than one thousand years in China. Recent clinical observations[135] showed that human hepatic bile displays even better effects than pig bile, and dilute hepatic bile better than concentrated gallbladder bile. Presumably, the reason for this is when bile is diluted with an alcohol-H2O mixtures, the dilute bile phase separates as lamellar liquid crystals, in other words multilayered artificial membranes[133] composed principally of bile phospholipid/cholesterol and bilirubin. When these are painted on burns and wounds, it provides an “artificial skin” with antioxidant properties. This occurs only when the bile salts present in the bile are diluted below their critical micellar concentrations, forming liquid crystals, e.g., lamellar liposomes or artificial lipid membranes composed of biliary phospholipid and cholesterol that phase separate from the dilute bile (Figure 5). Concomitantly, the minor components of bile, including melatonin, bilirubin, vitamin E, zinc and selenium, together with alcohol, for external use, have anti-inflammatory, anti-immune and antioxidant effects, all salutary in the control of bacterial infection with respect to burned skin surfaces[136-139].
Figure 5.
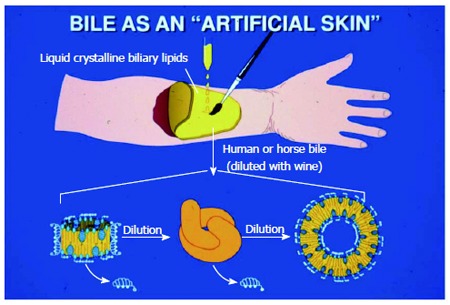
When human, domestic pig, or wild boar gallbladder biles, all with high phospholipid to bile acid ratios, are diluted progressively with low concentrations of alcohol such as with wine, the bile acid concentration decreases below its critical micellar concentration in the bile, and the specimen becomes cloudy from phase separation of previously micellar solubilized phospholipids plus cholesterol. This occurs because liquid crystals (lamellar liposomes) composed of phospholipids and cholesterol form spontaneously in the dilute non-micellar bile and also have potent antioxidant properties from bound bilirubin conjugate molecules. Such preparations were employed as an artificial skin and were used widely in China to dress and cover burns and battle wounds. See text for details.
Furthermore, calcium, a small hydrated divalent cation, moves freely from blood to bile via osmotic, solvent drag and Gibbs-Donnan forces[140,141]. Bile acids are the major calcium binding agents in bile and calcium binding is augmented by biliary phosphatidylcholine[57], with consequent decreases in unbound calcium concentrations. This diminishes the thermodynamic driving force for precipitation of several calcium-sensitive anions especially those of CO32+, PO43+ and unconjugated bilirubin as insoluble salts in bile[142-146]. Possibly for this reason, it is notable that animal biles were a first line of “attack” in TCM to dislodge fishbones stuck in the throat and esophagus. This salutary effect was most likely by calcium chelation and detergent softening of bones since the prescribed therapy was prolonged and frequently successful[8]. Other beneficial roles of biliary lipids in general and whole bile in particular, include inhibition of bacterial and microbial growth and by exhibiting anti-inflammatory[42], antipyretic, sedative, hypnotic, anticonvulsant, antispasmodic, analgesic, inotropic and antiallergic effects[16,46,47,69,73,129,131,135].
Gallbladder bile with concentrated levels of hydrophobic bile acids have powerful detergent-like actions and can kill pathogenic organisms via cell membrane and cell wall disruption[42]. Furthermore, gallbladder biles with concentrated levels of hydrophilic bile acids such as mouse and hibernating bear biles act as emollients with softening, soothing, cleansing and mild antiseptic actions. All biles have high levels of fat-soluble vitamins A, D, E and K[147-149], whose dietary deficiencies in ancient times lead to night blindness (nystalopia), infertility, childhood rickets, hemorrhagic diathesis and osteopenia in adults. As highlighted throughout our work, all biles that were used in TCM possess small but potent levels of bilirubin and infrequently biliverdin or varying combinations of each. These agents are now known to have antipyretic, antioxidant, anti-inflammatory, anti-immune, anti-atherogenic[134], and anti-oncogenic properties[150]. We have discussed in this article that when diluted below the critical micellar concentration, the phospholipid (mostly phosphatidycholines and to a lesser extent sphingomyelins) plus cholesterol of bile phase separate forming artificial biomembranes[132,133] applicable for multiple purposes, such as covering wounds, burns, and ulcers.
In addition, bile is an excretory conduit for multiple rare metals which act as prosthetic groups or cofactors for a host of metabolic processes[151]. They include magnesium, zinc, copper, selenium, calcium, and iron. And finally, bile is the main excretory route for pineal and extrapineal melatonin (N-acetyl-5-methoxytryptamine), a potent free-radical scavenger, sedative and hypnotic[152]. Hence, not only did Chinese physicians know that the best natural hypnotic was animal bile, but because of the multifaceted free radical scavenging and antioxidant properties of melatonin[153], bile is capable of detoxifying a wide variety of free radicals and reactive oxygen intermediates. Melatonin also protects membrane lipids, lipid-derived intermediates, nuclear DNA and many other molecules from oxidative damage in metabolism and hence abates organ dysfunction[153]. Bile acids are now known to have multiple effects on the activation of nuclear receptors and multiple signaling pathways in cells of the liver and gastrointestinal tract. So whether expression levels of numerous genes controlling fatty acid, glucose, lipoprotein and energy metabolism are behind many of their heuristic actions is indeed possible. In like manner linear tetrapyrroles in bile, besides their potent effect in scavenging reactant oxygen species, may also generate reactive oxygen species through the action of NADPH oxidase which is critical in signal transduction and defense against pathogenic attack.
Besides bile acids and phospholipids, other components of bile were also employed as drugs in TCM. For example, bilirubin (the principal lipopigment in bile) was isolated in rural areas by adding lime (CaCO3) to ox bile to induce its precipitation. The calcium salt was employed in treatment of many diseases, particularly hyperpyrexia-induced infantile convulsions[8,9,12,70,112,154-156]. Even though the concentration of bilirubins in bile is low, the gallstones that form in ox gallbladders are pigment stones composed of very pure calcium bilirubinate. When acidified and extracted into an organic solvent, these provided pure unconjugated bilirubin. Accordingly, ox gallstones have been employed for more than 2500 years in China for procuring pure unconjugated bilirubin for multiple therapeutic purposes[5]. Clinical studies and animal experiments have confirmed that the pharmacological effects of bilirubin from natural or artificially produced ox gallstones[4] are similar and include antipyretic, anticonvulsive, antihypertensive, bacterial growth inhibition, and expectorant, as well as inotropic effects on cardiac muscle[4,157]. As first introduced over 100 years ago in the West, ox bile components, principally bile acids precipitated with iron sulfate to form ferrous salts of bile acids knows as “Bilron” have also been shown to be effective in treating patients with steatorrhea due to bile acid deficiency especially following ileectomy for Crohn’s disease[158,159].
CONCLUSION
Even though forty-four animal biles (including human bile) have been used as highly popular drugs for more than 2500 years in TCM, only pig, ox and bear biles are extensively employed in China today. Modern physiological, physical-chemical, molecular biological and nuclear receptor regulation, as well as homeostatic research on bile acid and bilirubin metabolism in animal and model biles have provided important insights on the possible pharmacological mechanisms involved the mode of action of various animal biles. These underscore the success of the heuristic methods used for millennia in TCM[134,160-162]. Furthermore, over the past two decades it has been discovered that bile acids are potent regulatory molecules and do so via activation of specific nuclear receptors, a G-coupled protein receptor and several cell signaling pathways within the gastrointestinal tract and liver[20,162-165]. The activation and repression of genes encoding enzymes, other proteins and transporters by bile acids all show that they are involved in the regulation of bile acid, glucose, fatty acid and cholesterol synthesis, transport and metabolism, as well as triglyceride homeostasis and nutrient signaling that controls the utilization of energy[162]. Therefore, this review may stimulate systematic chemical and medicinal investigations with respect to the common and uncommon components of animal biles. It is our hope that the physiological functions and therapeutic mechanisms discovered in China heuristically over thousands of years may be further extended by modern chemical and pharmacological insights and methodologies.
ACKNOWLEDGMENTS
We are greatly indebted to Helen H Wang (Beth Israel Deaconess Medical Center) for collecting information of animal biles from books on traditional Chinese medicine and Chinese materia medica, and to Dr. Lee Hagey (University of California at San Diego) for providing data on bile acid compositions from his unpublished observations on rare animal biles. The authors thank Helen H. Wang (Beth Israel Deaconess Medical Center), Drs. Timothy T. Kuo (Harvard University) and Mei J. Xu (University of Massachusetts), and Mr. Kevin F. McGeeney (University College Dublin) for their critical reading of the manuscript.
Footnotes
Supported by Grants DK54012, DK73917, to Wang DQ-H; DK36588, DK34854, and DK73687, to Carey MC; all from the National Institutes of Health (US Public Health Service)
P- Reviewer: Assfalg M, Makishima M S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
References
- 1.Research Group for Collating Mawangdui Medical Books. A transcription of some of the medical texts contained in the silk manuscripts and unearthed at the No. 3 Han tomb at Mawangdui. Wen Wu. 1975;6:1–5 and 9: 35-48. [Google Scholar]
- 2.Research Group of the History of Medicine, the Academy of Traditional Chinese Medicine. A brief study of four lost ancient medical texts contained in the silk manuscripts recovered from No. 3 tomb at Mawangdui. Wen Wu. 1975;6:16–19. [Google Scholar]
- 3.Ma JX. Achievements of materia medica in medical books unearthed at the No. 3 Han tomb at Mawangdui, Part I, Part II, Part III, and Part IV. Zhongyi Zazhi. 1986;5:57–60; 6: 59-61; 7: 57-59; and 8: 57-59. [Google Scholar]
- 4.Chen SP. [Research on Calculus bovis] Zhongyao Tongbao. 1987;12:59–61. [PubMed] [Google Scholar]
- 5.Chen SP Anon. Shen Nong’s Herbal Classic (Shen Nong Ben Cao Jing) Shanghai: Shanghai Zhonghua Publishing House, c. 475 to 206 BCE, reprinted; 1941. [Google Scholar]
- 6.Tao HJ. Variorum of Shen Nung’s Herbal Classic (Ben Cao Jing Ji Zhu) Shanghai: Shanghai Qunlian Publishing House, c. 492 CE, reprinted; 1955. [Google Scholar]
- 7.Tao HJ. Records of Famous Physicians (Ming Yi Bie Lu) Beijing: People’s Publishing House, c. 510 CE, reprinted; 1986. [Google Scholar]
- 8.Li SZ. Compendium of Materia Medica (Ben Cao Gang Mu) Beijing: People’s Health Publishing House, 1596, reprinted; 1957. [Google Scholar]
- 9.Jiangsu New Medical College. Great Dictionary of Chinese Materia Medica. Shanghai: People’s Publishing House of Shanghai; 1977. [Google Scholar]
- 10.Li YQ. The Longmen prescriptions are the oldest carved ones on stone in China. Zhonghua Yishi Zazhi. 1986;16:222–223. [Google Scholar]
- 11.Unschuld PU. Medicine in China: A History of Pharmaceutics. Berkeley: University of California Press; 1986. [Google Scholar]
- 12.Shi DB. The Great Chinese Encyclopedia: Traditional Chinese Medicine. Beijing, Shanghai: The Great Chinese Encyclopedia Publishing House; 1992. [Google Scholar]
- 13.Carey MC, Duane WC. Enterohepatic circulation. In: Arias IM BJ, Fausto N, Jakoby WB, Schachter DA, Shafritz DA, et al., editors. The liver: Biology and Pathobiology. New York: Raven Press, Ltd; 1994. [Google Scholar]
- 14.Tan D, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999;65:2523–2529. doi: 10.1016/s0024-3205(99)00519-6. [DOI] [PubMed] [Google Scholar]
- 15.Moschetta A, Xu F, Hagey LR, van Berge-Henegouwen GP, van Erpecum KJ, Brouwers JF, Cohen JC, Bierman M, Hobbs HH, Steinbach JH, et al. A phylogenetic survey of biliary lipids in vertebrates. J Lipid Res. 2005;46:2221–2232. doi: 10.1194/jlr.M500178-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Sobotka H. Physiological chemistry of the bile. Baltimore: Williams and Wilkins Co; 1937. [Google Scholar]
- 17.Haslewood GAD. Bile Salts. London: Methuen and Co; 1967. [Google Scholar]
- 18.Haslewood GAD. The Biological Importance of Bile Salts. Amsterdam, North Holland: Elsevier; 1978. [Google Scholar]
- 19.Une M, Hoshita T. Natural occurrence and chemical synthesis of bile alcohols, higher bile acids, and short side chain bile acids. Hiroshima J Med Sci. 1994;43:37–67. [PubMed] [Google Scholar]
- 20.Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res. 2010;51:226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50 Suppl:S406–S411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lester R, Carey MC, Little JM, Cooperatein LA, Dowd SR. Crustacean intestinal detergent promotes sterol solubilization. Science. 1975;189:1098–1100. doi: 10.1126/science.1162360. [DOI] [PubMed] [Google Scholar]
- 23.Cahalane MJ, Neubrand MW, Carey MC. Physical-chemical pathogenesis of pigment gallstones. Semin Liver Dis. 1988;8:317–328. doi: 10.1055/s-2008-1040553. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson A, Duan RD. Alkaline sphingomyelinases and ceramidases of the gastrointestinal tract. Chem Phys Lipids. 1999;102:97–105. doi: 10.1016/s0009-3084(99)00078-x. [DOI] [PubMed] [Google Scholar]
- 25.Hu QL. The Great Chinese Encyclopedia: Archaeology. Beijing, Shanghai: The Great Chinese Encyclopedia Publishing House; 1986. [Google Scholar]
- 26.Chen T. The annals of the three kingdoms (San Guo Zhi) Shanghai: Shanghai Zhonghua Publishing House, c. 290 CE, reprinted; 1959. [Google Scholar]
- 27.Gwei-Djen L, Needham J. Records of diseases in ancient China. Am J Chin Med (Gard City N Y) 1976;4:3–16. doi: 10.1142/s0192415x76000032. [DOI] [PubMed] [Google Scholar]
- 28.Zhan Y. [Textual research on medicine in oracle inscriptions of the Shang (Yin) period (c 16th-11th century B.C.)] Zhonghua Yishi Zazhi. 1986;16:15–23. [PubMed] [Google Scholar]
- 29.Beijing University. Chronology of Science and Technology in the Ancient China. Beijing: People’s Education Publishing House; 1977. [Google Scholar]
- 30.Research Institute of the History of Natural Sciences. Achievements of Ancient Chinese Science and Technology. Beijing: Chinese Young’s Publishing House; 1978. [Google Scholar]
- 31.The Gansu Provincial Museum. Excavation of the Eastern Han tomb at Han-tan-po in Wuwei county of Gansu Province and the discovery of numerous wooden slips inscribed with medical prescriptions. Wen Wu. 1973;12:18–21. [Google Scholar]
- 32.Luo FY. Some notes on the Eastern Han Dynasty wooden slips inscribed with medical prescriptions unearthed at Wuwei county of Gansu Province. Wen Wu. 1973;12:30–31. [Google Scholar]
- 33.Research Group of the History of Medicine, the Academy of Traditional Chinese Medicine. The Eastern Han Dynasty wooden slips inscribed with medical prescriptions unearthed at Wuwei county of Gansu Province and its significance in Chinese medical history. Wen Wu. 1973;12:23–29. [Google Scholar]
- 34.Luo FY Anon. The Mountain and River Classic (Shan Hai Jing) Shanghai: Shanghai Zhonghua Publishing House, c. 770 to 221 BCE, reprinted; 1959. [Google Scholar]
- 35.Wang YX. Medical records in the Mountain and River Classic. Zhonghua Yishi Zazhi. 1985;15:192–193. [Google Scholar]
- 36.Editorial Correspondent. Some problems concerning the researches into the woman’s corpse unearthed from the No. 1 Han tomb at Mawangdui of Changsha City. Wen Wu. 1973;7:74–80. [Google Scholar]
- 37.Xin Hua News Agent. Anatomical studies of the woman corpse unearthed from the No. 1 Han tomb at Mawangdui in Changsha made a contribution to scientific researches. Wen Wu. 1975;7:73. [Google Scholar]
- 38.Research Group of the History of Medicine, the Academy of Traditional Chinese Medicine. Preliminary investigations of the text and paintings of medical gymnastics, etc. contained in the silk manuscripts recovered from No. 3 tomb at Mawangdui. Wen Wu. 1975;6:6–13. [Google Scholar]
- 39.Zhong YY, Li X. The oldest medical prescriptions; prescriptions for fifty-two types of diseases discovered in China. Wen Wu. 1975;9:49–60. [Google Scholar]
- 40.Research Group on the Prescriptions for Fifty-two Types of Diseases. Prescriptions for Fifty-two Types of Diseases Unearthed from the No. 3 Han Tomb at Mawangdui. Beijing: Historical Relics Publishing House; 1979. [Google Scholar]
- 41.Sun Q. [Infering the date of writing of the Recipes for 52 kinds of disease by the archaic linguistics of the Book of Song] (Chi) Zhonghua Yi Shi Za Zhi. 1986;16:243–246. [PubMed] [Google Scholar]
- 42.Herold BC, Kirkpatrick R, Marcellino D, Travelstead A, Pilipenko V, Krasa H, Bremer J, Dong LJ, Cooper MD. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob Agents Chemother. 1999;43:745–751. doi: 10.1128/aac.43.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin DL, Chang HC, Chang CP, Chen CY. Identification and differentiation of bear bile used in medicinal products in Taiwan. J Forensic Sci. 1997;42:817–823. [PubMed] [Google Scholar]
- 44.Espinoza EO, Shafer JA, Hagey LR. International trade in bear gall bladders: forensic source inference. J Forensic Sci. 1993;38:1363–1371. [PubMed] [Google Scholar]
- 45.Kubo M, Nakagami T, Yamasaki N, Ito M, Nogami M, Taji S. Studies on medicinal resources from livestock. I. Anti-allergic effects of pig bile. (1) Chem Pharm Bull (Tokyo) 1989;37:3409–3411. doi: 10.1248/cpb.37.3409. [DOI] [PubMed] [Google Scholar]
- 46.Nakagami T, Yamasaki N, Taji S. Studies on medicinal resources from livestock. II. Anti-allergic effects of pig bile. (2) Chem Pharm Bull (Tokyo) 1990;38:706–708. doi: 10.1248/cpb.38.706. [DOI] [PubMed] [Google Scholar]
- 47.Li YW, Zhu XY, But PP, Yeung HW. Ethnopharmacology of bear gall bladder: I. J Ethnopharmacol. 1995;47:27–31. doi: 10.1016/0378-8741(95)01249-d. [DOI] [PubMed] [Google Scholar]
- 48.Lei X. Lei’s Treatise on Preparation of Drugs. Leigong Paozhi Lun. c. 420 CE-479 CE [Google Scholar]
- 49.Ri HZ. Ri Hua-Zi’s Collected Materia Medica. Ri Hua Zi Zhu Jia Ben Cao. c. the 6th century [Google Scholar]
- 50.Zhen Q. Treatise on Property of Drugs. Yao Xing Lun. c. 643 CE or earlier [Google Scholar]
- 51.Meng X. A Dietetic Materia Medica (Shi Liao Ben Cao) Shanghai: Shanghai Dadong Publishing House, c. 710 CE, reprinted; 1934. [Google Scholar]
- 52.Wang T. The Medical Secrets of an Official (Wai Tai Mi Yao) Beijing: People’s Health Publishing House, c. 752 CE, reprinted; 1955. [Google Scholar]
- 53.Chen ZQ. A supplement to materia medica. Compendium of Materia Medica. Ben Cao Shi Yi. c. 758 CE [Google Scholar]
- 54.Su S. Illustrated Pharmacopoeia. Tujing Bencao. 1061 CE [Google Scholar]
- 55.Kou ZS. Amplified Materia Medica (Ben Cao Yan Yi) Shanghai: Shanghai Commercial Press, c. 1116 CE, reprinted; 1957. [Google Scholar]
- 56.Lan M. Pharmacopoeia of Southwestern China (Dian Nan Ben Cao) Kunming: People’s Publishing House of Yunnan, c. middle of the 15th century, reprinted; 1973. [Google Scholar]
- 57.Donovan JM, Leonard MR, Batta AK, Carey MC. Calcium affinity for biliary lipid aggregates in model biles: complementary importance of bile salts and lecithin. Gastroenterology. 1994;107:831–846. doi: 10.1016/0016-5085(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 58.Zhao XM. A Supplement to the Compendium of Materia Medica (Ben Cao Gang Mu Shi Yi) Beijing: People’s Health Publishing House, c. 1765 CE, reprinted; 1957. [Google Scholar]
- 59.Dan-Zeng PC. Jing Zhu materia medica (Jing Zhu Ben Cao) Xining: People’s Publishing House of Qinghai, 1840, reprinted; 1984. [Google Scholar]
- 60.MacDonald IA, Williams CN, Sutherland JD, MacDonald AC. Estimation of ursodeoxycholic acid in human and bear biles using Clostridium absonum 7 beta-hydroxysteroid dehydrogenase. Anal Biochem. 1983;135:349–354. doi: 10.1016/0003-2697(83)90695-4. [DOI] [PubMed] [Google Scholar]
- 61.MacDonald AC, Williams CN. Studies of bile lipids and bile acids of wild North American black bears in Nova Scotia, showing a high content of ursodeoxycholic acid. J Surg Res. 1985;38:173–179. doi: 10.1016/0022-4804(85)90024-1. [DOI] [PubMed] [Google Scholar]
- 62.Hagey LR. Personal communications to Wang DQ-H and Carey MC. 1997 [Google Scholar]
- 63.Yuan H. [Pharmacological action of cultured calculus bovis] Zhongguo Zhongyao Zazhi. 1991;16:105–108, 128. [PubMed] [Google Scholar]
- 64.Carey MC, Small DM. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978;61:998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carey MC, Montet JC, Phillips MC, Armstrong MJ, Mazer NA. Thermodynamic and molecular basis for dissimilar cholesterol-solubilizing capacities by micellar solutions of bile salts: cases of sodium chenodeoxycholate and sodium ursodeoxycholate and their glycine and taurine conjugates. Biochemistry. 1981;20:3637–3648. doi: 10.1021/bi00515a052. [DOI] [PubMed] [Google Scholar]
- 66.Hagey LR, Crombie DL, Espinosa E, Carey MC, Igimi H, Hofmann AF. Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. J Lipid Res. 1993;34:1911–1917. [PubMed] [Google Scholar]
- 67.Bonnett R, Davies JE, Hursthouse MB, Sheldrick GM. The structure of bilirubin. Proc R Soc Lond B Biol Sci. 1978;202:249–268. doi: 10.1098/rspb.1978.0066. [DOI] [PubMed] [Google Scholar]
- 68.Lapenna D, Ciofani G, Festi D, Neri M, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol. 2002;64:1661–1667. doi: 10.1016/s0006-2952(02)01391-6. [DOI] [PubMed] [Google Scholar]
- 69.Wang YL, Li XH. Progress in the studies of the drained bear bile. Zhongguo Zhongyao Zazhi. 1991;16:592–594. [Google Scholar]
- 70.Zhang NR. [Bile pigments in bear gall] Zhongyao Tongbao. 1987;12:11–13, 62. [PubMed] [Google Scholar]
- 71.Wang FS, Xu LX, Zhao YJ, Liu AR, Jin LZ, Zhang XQ. [Determination of bile acids in bear gall drainage by thin layer chromatographic scanning] Yaoxue Xuebao. 1989;24:397–400. [PubMed] [Google Scholar]
- 72.Zhao DY, Wei ZK, Tian H. [Studies on calculus bovis by FTIR-PAS] Zhongguo Zhongyao Zazhi. 1989;14:34–36, 63. [PubMed] [Google Scholar]
- 73.Li J, Wu Z, Zhang J, Xiong C, Liu Y, Yuan H, Xu H. [Pharmacological studies on the comparison between the drainage and certified Fel Ursi] Zhongguo Zhongyao Zazhi. 1991;16:749–752, 763. [PubMed] [Google Scholar]
- 74.Nakayama T. Researches on the History of Traditional Chinese Medicine in Japan. Tokyo: Tokyo Publishing House; 1931. [Google Scholar]
- 75.Beijing College of Traditional Chinese Medicine. A History of Chinese Medicine. Shanghai: Shanghai Science and Technology Publishing House; 1978. [Google Scholar]
- 76.Otruka Y, Kimura S, Mana J. Encyclopedia of Traditional Medicine in East-Asia. Japan: Kodansha; 1988. [Google Scholar]
- 77.Zhang HB. An outstanding Buddhist monk and physician, Jian Zhen, of the Tang Dynasty missionary to Japan and the exchange of medicine between China and Japan. Zhongguo Zhongyao Zazhi. 1989;14:57–59. [Google Scholar]
- 78.Su J. The Newly-revised Materia Medica (Xin Xiu Ben Cao) Shanghai: Shanghai Science and Technology Publishing House, c. 659 CE, reprinted; 1959. [Google Scholar]
- 79.Taki M. Comprehensive Annotated Bibliography of Chinese Medical Literature: Lost or Still Existing. Tokyo: Ise Ki Ko, c. 1825, reprinted; 1933. [Google Scholar]
- 80.Ono R. An Instruction to Compendium of Materica Medica. Tokyo: Honzo Komoku Keimo; c. 1803-1806. [Google Scholar]
- 81.Vahl M, Amdrup GC, Bobé L, Jensen ADS. Greenland. Copenhagen, London: Commission for the Direction of the Geological and Geographical Investigations in Greenland; 1928. [Google Scholar]
- 82.Hammarsten O. Untersuchungen über die Gallen einiger Polarthiere. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1902;36:525–555. [Google Scholar]
- 83.Hammarsten O. Untersuchungen über die Gallen einiger Polarthiere. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1901;32:435–466. [Google Scholar]
- 84.Shoda H. Über die Ursodesoxycholsäure aus bärengallen und ihre physiologische Wirkung. J Biochem. 1927;7:505–517. [Google Scholar]
- 85.Kanazawa T, Shimazaki A, Sato T, Hoshino T. Synthesis of ursodeoxycholic acid and its conjugated bile acid. Proc Jpn Acad. 1954;30:391–441. [Google Scholar]
- 86.Takuma T. The bile components of the polar bear (Ursus maritimus, phipps) and the Japanese bear (Ursus thebetanus japonicus, schleger) Biochemistry. 1949;21:26–27. [Google Scholar]
- 87.Haslewood GA, Wootton V. Comparative studies of ‘bile salts’; preliminary survey. Biochem J. 1950;47:584–597. doi: 10.1042/bj0470584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimura M, Osada E, Hashiba Y, Yoshizaki M, Shiho D. [Fundamental research for the pharmacological activity of oriental drugs. V. On the relationship between content and spasmolytic activity of components of the several kinds of bear fel] Yakugaku Zasshi. 1967;87:801–806. doi: 10.1248/yakushi1947.87.7_801. [DOI] [PubMed] [Google Scholar]
- 89.Haslewood GA. Bile salts of germ-free domestic fowl and pigs. Biochem J. 1971;123:15–18. doi: 10.1042/bj1230015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kothari RM, Godbole NN, Vaidya VG. Separation and identification of bile acids in some reptiles using thin layer chromatography. Biochem Biophys Res Commun. 1972;49:736–739. doi: 10.1016/0006-291x(72)90472-x. [DOI] [PubMed] [Google Scholar]
- 91.Yamasaki K, Ikawa S, Ayaki Y, Yamamoto Y. Natural occurrence of allocholic acid in ox and carp bile. J Biochem. 1972;72:769–772. doi: 10.1093/oxfordjournals.jbchem.a129957. [DOI] [PubMed] [Google Scholar]
- 92.Kurozumi K, Harano T, Yamasaki K, Ayaki Y. Studies on bile acids in bear bile. J Biochem. 1973;74:489–495. doi: 10.1093/oxfordjournals.jbchem.a130268. [DOI] [PubMed] [Google Scholar]
- 93.Yamasaki K. Chemistry and pharmacological action of bear bile. Taisha. 1973;10:757–761. [Google Scholar]
- 94.Egger G, Kutz K, Strebel H, Bircher J, Weber M, Scholl E, Preisig R. Bile formation in the intact pig. Am J Vet Res. 1974;35:1203–1208. [PubMed] [Google Scholar]
- 95.Uji A. [Studies on bear gall. II. Determination of conjugated bile acids in bear gall by TLC-autodetector (author’s transl)] Yakugaku Zasshi. 1975;95:214–218. doi: 10.1248/yakushi1947.95.2_214. [DOI] [PubMed] [Google Scholar]
- 96.Uji A. Studies on bear bile. III. On the bile acids in bear bile and the related crude drugs. Syoyakugaku Zasshi. 1975;29:22–30. [Google Scholar]
- 97.Uji A. [Studies on bear gall. IV. On the free amino acids in bear gall and the related crude drugs (author’s transl)] Yakugaku Zasshi. 1975;95:460–464. doi: 10.1248/yakushi1947.95.4_460. [DOI] [PubMed] [Google Scholar]
- 98.Uji A, Takiura K. [Studies on bear gall. I. Determination of bile acids in bear gall by gas chromatography (author’s transl)] Yakugaku Zasshi. 1975;95:114–119. doi: 10.1248/yakushi1947.95.1_114. [DOI] [PubMed] [Google Scholar]
- 99.Ikawa S, Tammar AR. Bile acids of snakes of the subfamily Viperinae and the biosynthesis of C-23-hydroxylated bile acids in liver homogenate fractions from the adder, Vipera berus (Linn.) Biochem J. 1976;153:343–350. doi: 10.1042/bj1530343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khatim MS, Gumaa KA. Biliary lipids in ox and sheep. Comp Biochem Physiol A Comp Physiol. 1982;73:193–196. doi: 10.1016/0300-9629(82)90054-8. [DOI] [PubMed] [Google Scholar]
- 101.Tracy JD, Jensen AH, Allhands RV. Bile acid metabolism in the pig. Cornell Vet. 1986;76:128–138. [PubMed] [Google Scholar]
- 102.Wildgrube HJ, Stockhausen H, Petri J, Füssel U, Lauer H. Naturally occurring conjugated bile acids, measured by high-performance liquid chromatography, in human, dog, and rabbit bile. J Chromatogr. 1986;353:207–213. doi: 10.1016/s0021-9673(01)87090-4. [DOI] [PubMed] [Google Scholar]
- 103.Yang YM. [Differentiation and identification of snake bile and the bile of some small animals] Zhong Yao Tong Bao. 1987;12:7–9. [PubMed] [Google Scholar]
- 104.Zhang S. Studies on the bile acid compositions of ten species of snakes by thin layer chromatography. Zhongchengyao. 1989;11:35–36. [Google Scholar]
- 105.Legrand-Defretin V, Juste C, Henry R, Corring T. Ion-pair high-performance liquid chromatography of bile salt conjugates: application to pig bile. Lipids. 1991;26:578–583. doi: 10.1007/BF02536421. [DOI] [PubMed] [Google Scholar]
- 106.You YJ, Lin JG, Ji LF. [Studies on chemical constituents of the gall of Python molurus bivittatus Schlegel] Yaoxue Xuebao. 1992;27:674–678. [PubMed] [Google Scholar]
- 107.Hagey LR. Bile acid biodiversity in vertebrates: chemistry and evolutionary implications. [Ph.D. thesis] San Diego, CA: University of California; 1992. [Google Scholar]
- 108.Danzinger RG, Hofmann AF, Schoenfield LJ, Thistle JL. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972;286:1–8. doi: 10.1056/NEJM197201062860101. [DOI] [PubMed] [Google Scholar]
- 109.Nakagawa S, Makino I, Ishizaki T, Dohi I. Dissolution of cholesterol gallstones by ursodeoxycholic acid. Lancet. 1977;2:367–369. doi: 10.1016/s0140-6736(77)90301-4. [DOI] [PubMed] [Google Scholar]
- 110.Nakayama F. Oral cholelitholysis--cheno versus urso: Japanese experience. Dig Dis Sci. 1980;25:129–134. doi: 10.1007/BF01308311. [DOI] [PubMed] [Google Scholar]
- 111.Carey MC. Editorial: Cheno and urso: what the goose and the bear have in common. N Engl J Med. 1975;293:1255–1257. doi: 10.1056/NEJM197512112932412. [DOI] [PubMed] [Google Scholar]
- 112.Hsu HY. A Study of Chinese Animal Drugs. Taipei: National Research Institute of Chinese Medicine Press; 1977. [Google Scholar]
- 113.Okumura M, Tanikawa K, Chúman Y, Kóji T, Nakagawa S, Nakamura Y, Iino H, Yamasaki S, Hisatsugu T. Clinical studies on dissolution of gallstones using ursodeoxycholic acid. Gastroenterol Jpn. 1977;12:469–475. doi: 10.1007/BF02781340. [DOI] [PubMed] [Google Scholar]
- 114.Schoenfield LJ, Lachin JM. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann Intern Med. 1981;95:257–282. doi: 10.7326/0003-4819-95-3-257. [DOI] [PubMed] [Google Scholar]
- 115.Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. part I. Dig Dis Sci. 1982;27:737–761. doi: 10.1007/BF01393771. [DOI] [PubMed] [Google Scholar]
- 116.Makino I, Takebe K. Bear bile and ursodeoxycholic acid. Sougou Rinsho. 1982;31:2385–2387. [Google Scholar]
- 117.Ward A, Brogden RN, Heel RC, Speight TM, Avery GS. Ursodeoxycholic acid: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1984;27:95–131. doi: 10.2165/00003495-198427020-00001. [DOI] [PubMed] [Google Scholar]
- 118.Festi D, Montagnani M, Azzaroli F, Lodato F, Mazzella G, Roda A, Di Biase AR, Roda E, Simoni P, Colecchia A. Clinical efficacy and effectiveness of ursodeoxycholic acid in cholestatic liver diseases. Curr Clin Pharmacol. 2007;2:155–177. doi: 10.2174/157488407780598171. [DOI] [PubMed] [Google Scholar]
- 119.Salvioli G, Igimi H, Carey MC. Cholesterol gallstone dissolution in bile. Dissolution kinetics of crystalline cholesterol monohydrate by conjugated chenodeoxycholate-lecithin and conjugated ursodeoxycholate-lecithin mixtures: dissimilar phase equilibria and dissolution mechanisms. J Lipid Res. 1983;24:701–720. [PubMed] [Google Scholar]
- 120.Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 121.James OF. Ursodeoxycholic acid treatment for chronic cholestatic liver disease. J Hepatol. 1990;11:5–8. doi: 10.1016/0168-8278(90)90263-q. [DOI] [PubMed] [Google Scholar]
- 122.de Caestecker JS, Jazrawi RP, Petroni ML, Northfield TC. Ursodeoxycholic acid in chronic liver disease. Gut. 1991;32:1061–1065. doi: 10.1136/gut.32.9.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 124.Rust C, Beuers U. Medical treatment of primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2005;28:135–145. doi: 10.1385/CRIAI:28:2:135. [DOI] [PubMed] [Google Scholar]
- 125.Pusl T, Beuers U. Ursodeoxycholic acid treatment of vanishing bile duct syndromes. World J Gastroenterol. 2006;12:3487–3495. doi: 10.3748/wjg.v12.i22.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48:792–800. doi: 10.1016/j.jhep.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 127.Heuman DM. Hepatoprotective properties of ursodeoxycholic acid. Gastroenterology. 1993;104:1865–1870. doi: 10.1016/0016-5085(93)90672-y. [DOI] [PubMed] [Google Scholar]
- 128.Ikegami T, Matsuzaki Y. Ursodeoxycholic acid: Mechanism of action and novel clinical applications. Hepatol Res. 2008;38:123–131. doi: 10.1111/j.1872-034X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 129.Ma CS, Wang YS. Dr. Wang Yao-Yu’s experience on the use of bear bile. Liaoning Zhongyi Zazhi. 1981;9:9. [Google Scholar]
- 130.Reynier MO, Montet JC, Gerolami A, Marteau C, Crotte C, Montet AM, Mathieu S. Comparative effects of cholic, chenodeoxycholic, and ursodeoxycholic acids on micellar solubilization and intestinal absorption of cholesterol. J Lipid Res. 1981;22:467–473. [PubMed] [Google Scholar]
- 131.Ye D, Han DY, Wang JR. [Effects of ursodeoxycholic acid on mechanical and electric activities in isolated guinea pig heart muscles] Zhongxi Yijie He Zazhi. 1991;11:289–290, 262. [PubMed] [Google Scholar]
- 132.Hay DW, Cahalane MJ, Timofeyeva N, Carey MC. Molecular species of lecithins in human gallbladder bile. J Lipid Res. 1993;34:759–768. [PubMed] [Google Scholar]
- 133.Cohen DE, Thurston GM, Chamberlin RA, Benedek GB, Carey MC. Laser light scattering evidence for a common wormlike growth structure of mixed micelles in bile salt- and straight-chain detergent-phosphatidylcholine aqueous systems: relevance to the micellar structure of bile. Biochemistry. 1998;37:14798–14814. doi: 10.1021/bi980182y. [DOI] [PubMed] [Google Scholar]
- 134.Vítek L, Ostrow JD. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15:2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- 135.Shang WZ, Li YL. Forty-five cases of burn patients treated with human bile. Zhongxiyi Jiehe Zazhi. 1990;10:599. [Google Scholar]
- 136.Montilla P, Cruz A, Padillo FJ, Túnez I, Gascon F, Muñoz MC, Gómez M, Pera C. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J Pineal Res. 2001;31:138–144. doi: 10.1034/j.1600-079x.2001.310207.x. [DOI] [PubMed] [Google Scholar]
- 137.Ferencík M, Ebringer L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol (Praha) 2003;48:417–426. doi: 10.1007/BF02931378. [DOI] [PubMed] [Google Scholar]
- 138.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 139.Vítek L. [Cytoprotective effects of bilirubin] Cas Lek Cesk. 2005;144 Suppl 1:58–62. [PubMed] [Google Scholar]
- 140.Moore EW. Biliary calcium and gallstone formation. Hepatology. 1990;12:206S–214S; discussion 214S-218S. [PubMed] [Google Scholar]
- 141.Rege RV, Dawes LG, Moore EW. Biliary calcium secretion in the dog occurs primarily by passive convection and diffusion and is linked to bile flow. J Lab Clin Med. 1990;115:593–602. [PubMed] [Google Scholar]
- 142.Williamson BW, Percy-Robb IW. Contribution of biliary lipids to calcium binding in bile. Gastroenterology. 1980;78:696–702. [PubMed] [Google Scholar]
- 143.Moore EW, Celic L, Ostrow JD. Interactions between ionized calcium and sodium taurocholate: bile salts are important buffers for prevention of calcium-containing gallstones. Gastroenterology. 1982;83:1079–1089. [PubMed] [Google Scholar]
- 144.Rajagopalan N, Lindenbaum S. The binding of Ca2+ to taurine and glycine-conjugated bile salt micelles. Biochim Biophys Acta. 1982;711:66–74. doi: 10.1016/0005-2760(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 145.Baruch E, Lichtenberg D, Barak P, Nir S. Calcium binding to bile salts. Chem Phys Lipids. 1997;57:17–27. doi: 10.1016/0009-3084(91)90045-d. [DOI] [PubMed] [Google Scholar]
- 146.Stevens RD, Lack L, Killenberg PG. Calcium binding by monosulfate esters of taurochenodeoxycholate. J Lipid Res. 1991;32:621–627. [PubMed] [Google Scholar]
- 147.Ullrey DE. Biological availability of fat-soluble vitamins: vitamin A and carotene. J Anim Sci. 1972;35:648–657. doi: 10.2527/jas1972.353648x. [DOI] [PubMed] [Google Scholar]
- 148.Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev. 1984;64:478–504. doi: 10.1152/physrev.1984.64.2.478. [DOI] [PubMed] [Google Scholar]
- 149.Eggermont E. Recent advances in vitamin E metabolism and deficiency. Eur J Pediatr. 2006;165:429–434. doi: 10.1007/s00431-006-0084-5. [DOI] [PubMed] [Google Scholar]
- 150.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19:1216–1219. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 151.Carey MC, Spivak W. Physical chemistry of bile pigments and porphyrins with particular reference to bile. In: Ostrow JD, et al., editors. Bile pigments and jaundice: molecular, metabolic and medical aspects. New York: Marcel Dekker; 1986. pp. 81–132. [Google Scholar]
- 152.Reiter RJ, Tan DX, Jou MJ, Korkmaz A, Manchester LC, Paredes SD. Biogenic amines in the reduction of oxidative stress: melatonin and its metabolites. Neuro Endocrinol Lett. 2008;29:391–398. [PubMed] [Google Scholar]
- 153.Reiter RJ, Tan DX, Cabrera J, D’Arpa D, Sainz RM, Mayo JC, Ramos S. The oxidant/antioxidant network: role of melatonin. Biol Signals Recept. 1999;8:56–63. doi: 10.1159/000014569. [DOI] [PubMed] [Google Scholar]
- 154.Liu SS. An Anthology and Bibliography of Works on Chinese Medical Drugs from 1820-1961. Vol. I. Beijing: Science Publishing House; 1975. [Google Scholar]
- 155.Liu SS Anon. A Dictionary of Chinese Pharmacology. Shanghai: The World Book Company Press; 1935. [Google Scholar]
- 156.Liu SS. An Anthology and Bibliography of Works on Chinese Medical Drugs from 1962-1974. Vol. II. Beijing: Science Publishing House; 1979. [Google Scholar]
- 157.Fordtran JS, Bunch F, Davis GR. Ox bile treatment of severe steatorrhea in an ileectomy-ileostomy patient. Gastroenterology. 1982;82:564–568. [PubMed] [Google Scholar]
- 158.Little KH, Schiller LR, Bilhartz LE, Fordtran JS. Treatment of severe steatorrhea with ox bile in an ileectomy patient with residual colon. Dig Dis Sci. 1992;37:929–933. doi: 10.1007/BF01300393. [DOI] [PubMed] [Google Scholar]
- 159.Nair PP, Kritchevsky D. The Bile Acids: Chemistry, physiology and metabolism. Vol. 1, Chemistry, Physiology and Metabolism Vol. 2, Pathophysiology Vol. 3. New York: Plenum, 1971, 1973; 1976. [Google Scholar]
- 160.Setchell KDR, Kritchevsky D, Nair PP. The Bile Acids: Chemistry, Physiology, and Metabolism. Vol 4 (Methods and Applications) New York: Plenum; 1988. [Google Scholar]
- 161.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 163.Chiang JY. Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G349–G356. doi: 10.1152/ajpgi.00417.2002. [DOI] [PubMed] [Google Scholar]
- 164.Kuipers F, Stroeve JH, Caron S, Staels B. Bile acids, farnesoid X receptor, atherosclerosis and metabolic control. Curr Opin Lipidol. 2007;18:289–297. doi: 10.1097/MOL.0b013e3281338d08. [DOI] [PubMed] [Google Scholar]
- 165.Mukhopadhyay S, Maitra U. Chemistry and biology of bile acids. Curr Sci. 2004;87:1666–1683. [Google Scholar]
- 166.Alvaro D, Cantafora A, Attili AF, Ginanni Corradini S, De Luca C, Minervini G, Di Biase A, Angelico M. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp Biochem Physiol B. 1986;83:551–554. doi: 10.1016/0305-0491(86)90295-6. [DOI] [PubMed] [Google Scholar]
- 167.Fevery J, Van de Vijver M, Michiels R, Heirwegh KP. Comparison in different species of biliary bilirubin-IX alpha conjugates with the activities of hepatic and renal bilirubin-IX alpha-uridine diphosphate glycosyltransferases. Biochem J. 1977;164:737–746. doi: 10.1042/bj1640737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cornelius CE. Comparative Bile Pigment Metabolism in Vertebrates. In: Ostrow JD E, ed , et al., editors. Bile Pigments and Jaundice: Molecular, Metabolism, and Medical Aspects. New York: Marcel Dekker; 1986. pp. 601–647. [Google Scholar]
- 169.Garner RJ. Bile pigment metabolism in cattle; bile pigments in ox bile, intestinal contents, and faeces. J Comp Pathol. 1955;65:271–277. doi: 10.1016/s0368-1742(55)80027-6. [DOI] [PubMed] [Google Scholar]
- 170.Colleran E, O’Carra PD. Chemistry and Physiology of Bile Pigments. In: Berk PD, Berlin NI, et al., editors. Bethesda, Maryland: Fogarty International Center, DHEW Publ. No. (NIH) 77-1100; 1977. pp. 35, 69–80. [Google Scholar]
- 171.Low PS, Bada JL. Bile pigments in the blood serum of fish from the family Cottidae. Comp Biochem Physiol A Comp Physiol. 1974;47:411–418. doi: 10.1016/0300-9629(74)90003-6. [DOI] [PubMed] [Google Scholar]
- 172.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 173.Spivak W, Carey MC. Reverse-phase h.p.l.c. separation, quantification and preparation of bilirubin and its conjugates from native bile. Quantitative analysis of the intact tetrapyrroles based on h.p.l.c. of their ethyl anthranilate azo derivatives. Biochem J. 1985;225:787–805. doi: 10.1042/bj2250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Noonan NE, Olsen GA, Cornelius CE. A new animal model with hyperbilirubinemia: the indigo snake. Dig Dis Sci. 1979;24:521–524. doi: 10.1007/BF01489319. [DOI] [PubMed] [Google Scholar]
- 175.Cornelius CE, Kelley KC, Himes JA. Heterogeneity of bilirubin conjugates in several animal species. Cornell Vet. 1975;65:90–99. [PubMed] [Google Scholar]
- 176.Wang DQ, Carey MC. Characterization of crystallization pathways during cholesterol precipitation from human gallbladder biles: identical pathways to corresponding model biles with three predominating sequences. J Lipid Res. 1996;37:2539–2549. [PubMed] [Google Scholar]
- 177.Israels LG, Novak W, Foerster J, Zipursky A. The early-appearing bilirubin in ducks. Can J Physiol Pharmacol. 1966;44:864–866. doi: 10.1139/y66-105. [DOI] [PubMed] [Google Scholar]
- 178.Cole KD, Little GH. UDP-glucuronosyltransferase activity and bilirubin conjugation in the bullfrog. Biochem J. 1983;212:265–269. doi: 10.1042/bj2120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cole KD, Little GH. Bile pigments and bilirubin UDP-glucuronyl transferase during the metamorphosis of Rana catesbeiana tadpoles. Comp Biochem Physiol B. 1983;76:503–506. doi: 10.1016/0305-0491(83)90283-3. [DOI] [PubMed] [Google Scholar]


