Abstract
Carfilzomib is a next-generation, selective proteasome inhibitor being evaluated for the treatment of relapsed and refractory multiple myeloma. In this open-label, single-arm phase 2 study (PX-171-003-A1), patients received single-agent carfilzomib 20 mg/m2 intravenously twice weekly for 3 of 4 weeks in cycle 1, then 27 mg/m2 for ≤ 12 cycles. The primary endpoint was overall response rate (≥ partial response). Secondary endpoints included clinical benefit response rate (≥ minimal response), duration of response, progression-free survival, overall survival, and safety. A total of 266 patients were evaluable for safety, 257 for efficacy; 95% were refractory to their last therapy; 80% were refractory or intolerant to both bortezomib and lenalidomide. Patients had median of 5 prior lines of therapy, including bortezomib, lenalidomide, and thalidomide. Overall response rate was 23.7% with median duration of response of 7.8 months. Median overall survival was 15.6 months. Adverse events (AEs) were manageable without cumulative toxicities. Common AEs were fatigue (49%), anemia (46%), nausea (45%), and thrombocytopenia (39%). Thirty-three patients (12.4%) experienced peripheral neuropathy, primarily grades 1 or 2. Thirty-three patients (12.4%) withdrew because of an AE. Durable responses and an acceptable tolerability profile in this heavily pretreated population demonstrate the potential of carfilzomib to offer meaningful clinical benefit. This trial was registered at www.clinicaltrials.gov as #NCT00511238.
Introduction
Multiple myeloma (MM) is characterized by abnormal accumulation of clonal plasma cells in bone marrow (BM). In the United States in 2012, there will be an estimated 21 700 new cases of MM and 10 710 deaths.1 The introduction of immunomodulators (thalidomide and lenalidomide) and the approval of a proteasome inhibitor (bortezomib) have significantly improved treatment options for patients with this disease.2 As a result, mortality rates have decreased3 and overall survival (OS) rates have improved over the past decade.4,5 Nevertheless, responses are temporary and the overwhelming majority of patients relapse,6,7 indicating a need for more effective therapies.8
The proteasome, present in all cells, is part of the ubiquitin-proteasome system that degrades highly regulated cellular proteins.9–11 Proteasome inhibition leads to increased rates of apoptosis induction that is particularly significant in malignant cells.12,13 The therapeutic potential of proteasome inhibitors was confirmed by the approval of bortezomib for the treatment of MM and mantle cell lymphoma.10,14–16 Carfilzomib (Onyx Pharmaceuticals) is a next-generation proteasome inhibitor that selectively and irreversibly binds to its target, resulting in sustained inhibition. Carfilzomib, alone or in combination with other agents, demonstrated antitumor activity in several in vitro and in vivo tumor models.17,18 It induced apoptosis in myeloma cell lines and in primary myeloma cells from patients whose disease was resistant to available therapies, including bortezomib.18 In vitro, compared with bortezomib, carfilzomib has greater selectivity for the chymotrypsin-like active site of the proteasome.19 In addition, carfilzomib also has fewer off-target effects, which may explain a lack of neurodegeneration in vitro, and less neurotoxicity in animal studies.20,21 As a result, carfilzomib may circumvent some of the clinical adverse events (eg, peripheral neuropathy [PN]) associated with bortezomib.20,22,23
A consecutive-day dosing regimen of carfilzomib was used in clinical studies after in vitro pharmacology experiments demonstrating sustained proteasome inhibition of up to 36 hours with this regimen.17,24 Two specific dosing regimens testing the 2- to 10-minute administration were evaluated in phase 1 studies: one using carfilzomib for 5 consecutive days on a 14-day schedule and another using carfilzomib on 2 consecutive days for 3 weeks of a 4-week schedule.24,25 Both of these dosing regimens demonstrated activity in patients with advanced MM and non-Hodgkin lymphoma, although the latter regimen (twice weekly for 3 weeks) was better tolerated and allowed the use of higher doses.24,25 These findings provided the rationale for an open-label, phase 2 pilot study (PX-171-003-A0) of carfilzomib in 46 patients with relapsed and refractory MM.26 In the pilot study, patients treated with carfilzomib 20 mg/m2 achieved an overall response rate (ORR) of 16.7% and a 23.8% clinical benefit rate (CBR) with manageable toxicities. The study was subsequently amended (PX-171-003-A1) to include an expanded dosing cohort with scheduled dose escalation from 20 to 27 mg/m2 beginning with the second dosing cycle. The investigators chose to report the results of the pilot study separately as the dose, study conduct, and analysis differ from PX-171-003-A1. The results of the amended study are reported herein.
Methods
This was a multicenter, open-label, single-arm, phase 2 study (www.clinicaltrials.gov; #NCT00511238) involving 30 centers in the United States and Canada. The study was conducted in accordance with US Food and Drug Administration and International Conference on Harmonisation Guidelines for Good Clinical Practice, Declaration of Helsinki, Health Canada, and applicable local health authority and Institutional Review Board requirements. All patients provided written informed consent in accordance with federal, local, and institutional guidelines. The investigators and representatives from Onyx Pharmaceuticals (formerly Proteolix, South San Francisco, CA) designed the study. Data were collected and analyzed by medical and biometrics representatives from Onyx in conjunction with the investigators and an Independent Review Committee (IRC). All participating institutions received financial support for conducting the study.
Patients
Eligible patients were ≥ 18 years old, with measurable progressive MM, responsive to at least 1 prior regimen by the International Myeloma Working Group (IMWG) Uniform Response Criteria and European Blood and Marrow Transplantation Group criteria (ie, minimal response [MR] or better)27 and refractory to their most recent therapy (ie, ≤ 25% response or progression either during therapy or ≤ 60 days after completion of therapy). Patients must have received ≥ 2 prior regimens for relapsed disease, including bortezomib, thalidomide, or lenalidomide, an alkylating agent, or an anthracycline alone or in combination. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0-2. Laboratory entry criteria included platelet count ≥ 50 000/mm3, hemoglobin concentration ≥ 8.0 g/dL, and absolute neutrophil count ≥ 1000/mm3, serum aspartate aminotransferase or alanine aminotransferase concentrations < 3 times the upper limit of normal, serum total bilirubin concentration < 2 times the upper limit of normal, and measured or calculated creatinine clearance > 30 mL/min.
Treatment
Intravenous carfilzomib was given over 2-10 minutes on days 1, 2, 8, 9, 15, and 16 of each 28-day cycle, for up to 12 cycles. The dose for cycle 1, determined based on the findings from the 2 phase 1 studies, was 20 mg/m2, which was escalated to 27 mg/m2 for all cycles thereafter provided that patients tolerated the initial dose level of 20 mg/m2. Oral or intravenous dexamethasone (4 mg) was administered before each dose of carfilzomib in cycle 1 and as needed thereafter as premedication to help prevent potential infusion reactions. To avoid a therapeutic effect, higher doses of dexamethasone were not allowed. All patients were to receive oral and intravenous fluids before dosing to assure adequate hydration. Those patients with a high tumor burden, thus considered to be at increased risk for tumor lysis syndrome, could receive allopurinol for tumor lysis syndrome prophylaxis. Antiviral prophylaxis, bisphosphonates, and erythropoietic agents were permitted during the study. Carfilzomib was withheld for grade 3 or 4 hematologic or nonhematologic toxicities and resumed at reduced doses of 15 mg/m2 in cycle 1 or 20 mg/m2 in cycle 2 and above on resolution. Up to 2 dose level reductions were permitted per patient; if toxicity continued, the patient was withdrawn. The maximum treatment duration was 12 cycles in patients with stable disease or better. Patients who completed this study and wished to continue to receive carfilzomib were eligible to participate in an extension study (PX-171-010; #NCT00884312), allowing for prolonged therapy. Patients were followed for disease progression and OS for up to 3 years after discontinuation or completion of therapy.
Assessment of efficacy
Response assessments were conducted on day 15 of cycle 1, on day 1 of cycles 2-12, and at the end of the study. The primary efficacy endpoint was ORR (proportion of patients with stringent complete response [sCR], complete response [CR], very good partial response [VGPR], and partial response [PR]) based on responses as assessed by study investigators and reviewed and adjudicated by an IRC consisting of 3 expert clinicians not affiliated with this study, using IMWG Uniform Response Criteria.28 sCR, CR, VGPR, PR, and MR required 2 consecutive assessments made at any time before progression or initiation of any new therapy. Secondary endpoints included CBR rate (proportion of patients with MR or better) according to IMWG criteria and European Blood and Marrow Transplantation Group criteria27 (for MR), duration of response (time from first confirmed response of PR or better [for ORR] or MR or better [for CBR] to confirmed disease progression [PD] or death), progression-free survival (PFS; time from start of treatment to IRC-confirmed PD or death from any cause, irrespective of disease status), and OS (time from start of treatment to death from any cause). In contrast to the pilot study PX-171-003-A0, the IRC for PX-171-003-A1 also reviewed and adjudicated all of the secondary endpoints.
Disease progression required any one of the following criteria: (1) increase in serum monoclonal protein by 25% or higher above the lowest response level and an absolute increase of ≥ 5 g/L; (2) increase in urine monoclonal protein by ≥ 25% above the lowest remission value and an absolute increase in excretion by ≥ 200 mg/24 hours; (3) increase in size of soft-tissue plasmacytoma by ≥ 50% or appearance of a new plasmacytoma; (4) appearance of new bone lesions or increase in size of existing bone lesions by ≥ 50%; or (5) unexplained hypercalcemia (> 2.875mM or > 11.5 g/dL). Progression also had to be confirmed by 2 consecutive assessments.
As noted, an IRC was used for primary determination of response and progression to reduce the bias inherent in this open-label, uncontrolled design. Because comparisons of survival inherently favor patients who respond, every attempt was made to minimize this effect. Thus, the survival data for patients with responses ≥ PR versus the survival data for all other patients were analyzed according to the Simon-Makuch landmark transient-state method,29 as was previously applied in a study of patients with relapsed, refractory MM receiving bortezomib.30
Assessment of safety
Adverse events (AEs) were assessed at each visit, graded according to National Cancer Institute Common Terminology Criteria (NCI-CTC) for Adverse Events (Version 3.0), and tabulated by system organ class, frequency, severity grade, and relationship to study treatment. Results of laboratory assessments were assessed as change from baseline and shift in toxicity grade. Patients underwent complete neurologic evaluation during initial screening, on day 1 of odd-numbered cycles, and at the end of treatment, using the neurotoxicity subscale of the Gynecologic Oncology Group's Functional Assessment of Cancer Therapy.31 A Safety Oversight Group reviewed all reports of serious AEs and Common Terminology Criteria for Adverse Events grade 3 or 4 treatment-related AEs and provided recommendations about stopping or continuing the trial.
Statistical analysis
The primary efficacy population comprised all patients who received ≥ 1 dose of study drug, had a baseline disease assessment, and had ≥ 1 post-baseline disease assessment, or patients who discontinued treatment before day 1 of cycle 2 because of an AE that was considered to be possibly or probably related to carfilzomib, irrespective of availability of baseline and post-baseline assessment. The safety population comprised all patients who received ≥ 1 dose of study drug.
A target sample size of 250 patients was calculated based on a 1-stage, single-arm, phase 2 design such that a meaningful increase in the CBR rate (defined per protocol as ORR > 10%), if present, could be detected with an ∼ 80% probability while maintaining an upper limit on the type 1 error rate at conventional levels. The ORR was estimated based on the crude proportion of evaluable patients who achieved a sCR, CR, VGPR, or PR. This estimate was to be accompanied by a 2-sided 95% Clopper-Pearson exact CI. A 1-sample χ2 test with a 1-sided significance level of 2.5% was used to test the null hypothesis proportion of 10% versus the alternative hypothesis proportion of 16%. The study was deemed successful if the lower boundary of the 2-sided 95% CI about the ORR was > 10%. The data for CBR were assessed in the same manner.
For time-to-event endpoints, Kaplan-Meier32 plots were prepared, along with the estimates of median and 95% CI. The time to response was from the date of the first administration of carfilzomib to first evidence of a confirmed response. Median follow-up for OS was estimated according to the Kaplan-Meier estimate of potential follow-up, also termed the “reverse Kaplan-Meier” method.33 PFS was censored at the last disease assessment visit for patients who met one of the following conditions: (1) nonprotocol anticancer treatment started before documentation of PD or death, (2) lost to follow-up, or (3) alive and did not have documentation of PD before data analysis cutoff date. OS for patients who were alive or lost to follow-up at the data analysis cutoff date was censored on the date the subject was last known to be alive.
Exploratory subgroup analyses evaluated the potential impact(s) of the following factors on response to carfilzomib: baseline patient characteristics of age, sex, and race; prognostic factors of ECOG status, hemoglobin, plasma cell involvement, cytogenetics or FISH, serum β2-microglobulin, and International Staging System (ISS) stage; prior antimyeloma treatment history; and history of PN or active grade 1 or 2 PN at study entry. Specific efficacy endpoints considered in these analyses were ORR, CBR, duration of response (DOR), PFS, and OS. All statistical analyses were performed using SAS Version 9.1 or later.
Results
Disposition of patients
From July 2008 until October 2009, 266 patients were enrolled at 30 sites in the United States and Canada. The data cutoff date for this analysis was February 11, 2011. All patients enrolled received ≥ 1 dose of study drug, so were evaluable for safety. The efficacy population comprised 257 patients; 9 patients were excluded because of missing baseline and/or post-baseline disease assessment.
The median age was 63 years, 58% were male, and 71% were white (Table 1). Patients had been diagnosed with MM a median of 5.4 years before study entry and had received a median of 5 (range, 1-20) lines of therapy for MM; the majority (82%) had received ≥ 4 lines of therapy and 95% were refractory to their last line of therapy. Patients may have been exposed to a given drug in more than one line of therapy. All but 1 patient had received bortezomib; 73% were refractory to bortezomib in any prior line of therapy, and 45% were refractory to bortezomib in their most recent line of therapy. All patients had received an immunomodulator, and most had received high-dose corticosteroid therapy (98%) and therapy with an alkylating agent (92%). Seventy-four percent of patients had received at least 1 stem cell transplant. Eighty percent of patients (n = 214) were refractory or intolerant to both bortezomib and lenalidomide where intolerance to a previous therapy was defined as having had that therapy discontinued because of toxicity. Most patients had either IgG (73%) or IgA (17%) myeloma and 69% had ISS stage II or III disease at diagnosis. Of the 234 patients with cytogenetic or FISH data, 75 (28% overall) had markers indicating poor prognosis, including either t(4;14), t(14;16), deletion (17p;13) by cytogenetics/FISH, or deletion (13q;14) by cytogenetics. The majority of patients (77%) had PN of grade 1 or 2 at baseline.
Table 1.
Baseline demographics and clinical characteristics (N = 266)
| Characteristic | Value |
|---|---|
| Median age, y (range) | 63 (37-87) |
| Male, n (%) | 155 (58) |
| Ethnicity, n (%) | |
| Black | 53 (20) |
| Asian/Pacific Islander | 6 (2) |
| White | 190 (71) |
| Hispanic | 10 (4) |
| Other | 7 (3) |
| Median time from diagnosis, y (range) | 5.4 (0.5-22.3) |
| Ig class heavy chain, n (%) | |
| IgG | 193 (73) |
| IgA | 45 (17) |
| IgD | 2 (1) |
| Light chain only or not specified | 26 (10) |
| International Staging System stage, n (%) | |
| I | 76 (29) |
| II | 102 (38) |
| III | 81 (31) |
| ECOG performance status, n (%) | |
| 0 | 69 (26) |
| 1 | 162 (61) |
| 2 | 35 (13) |
| Cytogenetic/FISH prognostic markers, n (%) | |
| Normal/favorable | 159 (60) |
| Unfavorable | 75 (28) |
| Unknown/not done | 32 (12) |
| PN, n (%)* | |
| 0 | 60 (23) |
| 1 | 178 (67) |
| 2 | 28 (11) |
| Median creatinine clearance, mL/min (range) | 70 (16-203) |
| Median serum β2-microglobulin, mg/L (range)† | 4.3 (0.4-20.5) |
| Prior lines of therapy, median (range) | 5 (1-20) |
| ≥ 4, n (%) | 217 (82) |
| Refractory to last regimen | |
| Progressive disease on therapy | 198 (74) |
| Progressive disease within 60 d | 38 (14) |
| ≤ 25% response | 16 (6) |
| Prior agents, median (range) | 13 (3-45) |
| Bortezomib, n (%)‡ | 265 (99.6) |
| In most recent prior regimen, n (%) | 132 (50) |
| Immunomodulatory agent, n (%) | 266 (100) |
| Lenalidomide, n (%) | 249 (94) |
| Thalidomide, n (%) | 199 (75) |
| Pomalidomide, n (%) | 9 (3) |
| Corticosteroid, n (%) | 261 (98) |
| Alkylating agent, n (%) | 246 (92) |
| Stem cell transplant, n (%) | 198 (74) |
| Anthracycline, n (%) | 171 (64) |
Based on physical assessment at screening (NCI-CTC scale).
n = 259.
One patient who had not received prior bortezomib was enrolled. The deviation from the inclusion criteria was discovered after the patient had initiated treatment.
The median duration of carfilzomib treatment was 3.0 months (range, 0.03-16.9 months). Approximately one-third of patients (31%) completed more than 6 cycles; 40 patients (15%) completed 12 cycles, and 31 patients (12%) continued on to extension protocol PX-171-010. Patients who discontinued therapy did so primarily because of PD (59%) or AEs (12%). Ninety-seven patients (36.5%) discontinued during the first 2 cycles of treatment, including 51 patients (19%) who discontinued during cycle 1. Of the 97 patients, 61 discontinued within the first 2 cycles because of progressive disease. The remaining 36 patients discontinued for reasons of AEs (n = 14), withdrawn consent (n = 10), death (n = 6), or MD discretion/lack of efficacy/seeking alternative treatment (n = 6). A total of 69 patients (26%) did not escalate to the 27 mg/m2 dose of carfilzomib, primarily because of early discontinuation.
Efficacy
In 257 response-evaluable patients, the ORR was 23.7% by IRC assessment and the CBR rate was 37.0% (Table 2). There was 92.1% concordance between the IRC-assessed and investigator-assessed ORR, and an 81.3% concordance between IRC- and investigator-assessed response grades overall. The ORR in patients whose disease was refractory to their last prior therapy was similar (23.5%; 57 of 243 patients) to the ORR of 23.7% for response-evaluable patients and to the ORR of 22.9% in the 266 patients in the safety population. The ORR and CBR rate in patients with grade 1 or 2 PN were 23.8% and 36.1%, respectively. The ORR was somewhat lower in patients with ≥ 2 prior lines of bortezomib-based therapy compared with those with < 2 (18.5% vs 29.5%) and in patients refractory to bortezomib in their last line of therapy compared with those who did not receive bortezomib in their last line of therapy (18.6% vs 28.3%). Patients (n = 214 of 266) refractory or intolerant to both bortezomib and lenalidomide had an ORR of 20.1% (95% CI, 14.9%-26.1%), whereas patients refractory to both bortezomib and lenalidomide (n = 169 of 266) had an ORR of 15.4% (95% CI, 10.3%-21.7%).
Table 2.
Best overall responses (n = 257*)
| All patients (n = 257) | Patients with unfavorable cytogenetic/FISH markers (n = 71) | |
|---|---|---|
| Response category, n (%) | ||
| Complete response | 1 (0.4) | 0 (0) |
| Very good partial response | 13 (5.1) | 3 (4.2) |
| Partial response | 47 (18.3) | 18 (25.4) |
| Minimal response | 34 (13.2) | 3 (4.2) |
| Stable disease | 81 (31.5) | 28 (39.4) |
| Progressive disease | 69 (26.8) | 15 (21.1) |
| Not evaluable | 12 (4.7) | 4 (5.6) |
| Overall response, n (%) | 61 (23.7) | 21 (29.6) |
| 95% CI | 18.7-29.4 | 19.3-41.6 |
| Clinical benefit rate, n (%) | 95 (37.0) | 24 (33.8) |
| 95% CI | 31.1-43.2 | 23.0-46.0 |
| PFS, median (95% CI), mo | 3.7 (2.8-4.6) | 3.6 (2.3-4.6) |
| Median duration of response, mo (95% CI)† | 7.8 (5.6-9.2) | 6.9 (3.7-8.5) |
| Mean treatment duration, mo (range)‡ | 3.0 (0.03-16.9) | 3.6 (0-11.1) |
Response-evaluable population.
Calculated for patients with partial response or better.
Before the opening of the extension study (PX-171-010), individual protocol exceptions were granted for 7 patients to continue receiving treatment beyond 12 cycles.
The median DOR in the 61 patients who achieved PR or better was 7.8 months (95% CI, 5.6-9.2 months; Table 2). In the 95 patients with MR or better, the corresponding DOR was 8.3 months (95% CI, 6.5-9.7 months). The median DOR for patients refractory or intolerant to both bortezomib and lenalidomide was 7.4 months (95% CI, 5.6-8.4 months), and the DOR for patients refractory to both bortezomib and lenalidomide was 7.8 months (95% CI, 6.7-10.1 months). The median time to best overall response (≥ PR) was 1.9 months.
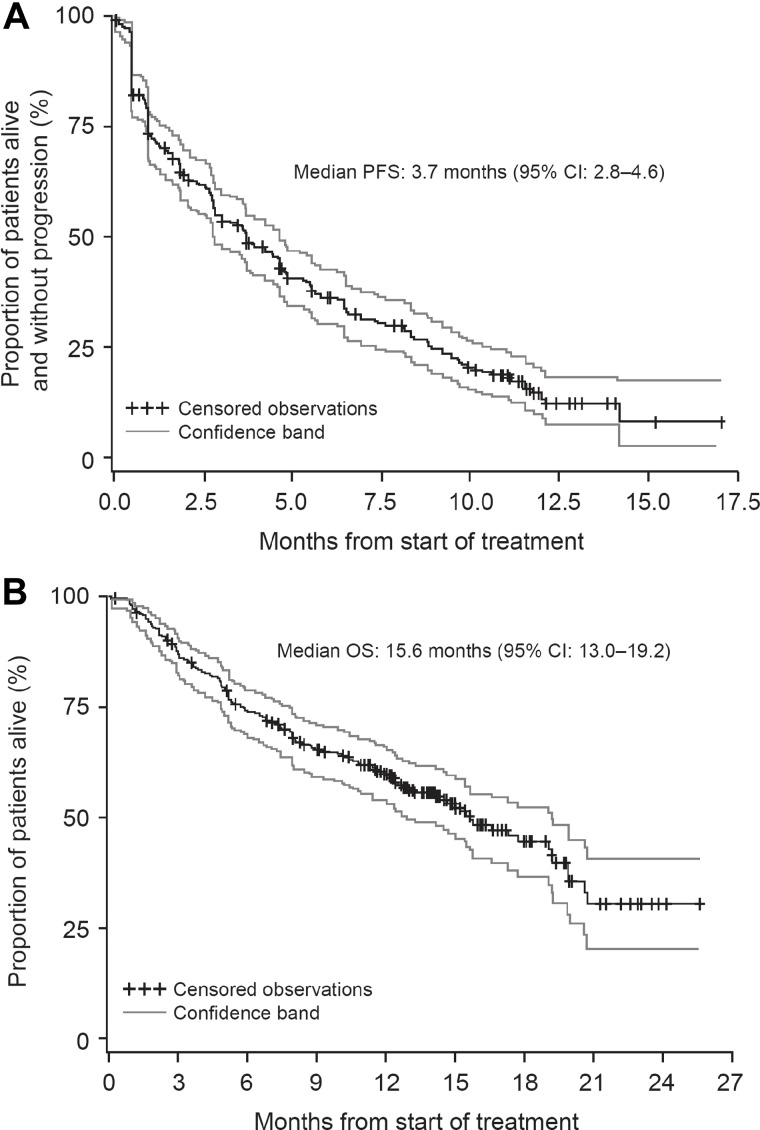
Other secondary endpoints of the study included a median PFS for all response-evaluable patients (n = 257) of 3.7 months (95% CI, 2.8-4.6 months; Figure 1A), and a median OS of 15.6 months (95% CI, 13.0-19.2 months; Figure 1B). The median OS for all 266 patients was 15.4 months (95% CI, 12.5-19.0 months). The median OS in patients refractory or intolerant to both bortezomib and lenalidomide (n = 214) was 13.2 months (95% CI, 10.6-16.6 months), whereas patients refractory to both bortezomib and lenalidomide (n = 169) had a median OS of 11.9 months (95% CI, 8.4-14.7 months). Subgroup analyses of PFS and OS by depth of response showed trends of improved outcomes as the level of response improved (supplemental Figure A1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and a landmark analysis at 2 months from the start of treatment showed that patients who achieved at least a MR lived longer than those who did not achieve a response (χ2 = 45.7, P < .0001; supplemental Figure 2A)
Figure 1.
Survival. PFS (A) and OS (B) in response evaluable patients (n = 257) treated with single-agent carfilzomib.
Prognostic factors
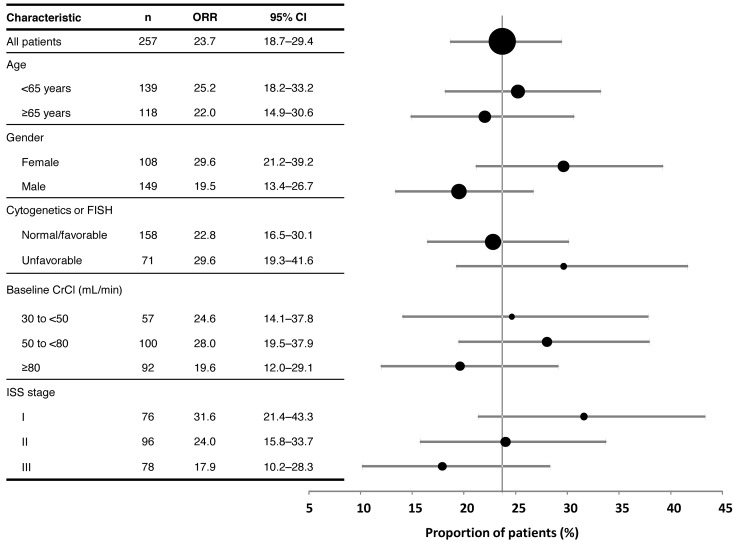
A multivariate analysis evaluated the impact of factors including sex, the extent of plasma cell involvement in bone marrow, adverse cytogenetics, renal impairment, ISS stage, and ECOG performance status on responses to carfilzomib. This analysis demonstrated that responses were not unfavorably influenced by the majority of these factors, most notably, unfavorable cytogenetic/FISH markers and renal impairment (Figure 2). Furthermore, the durations of CBR were similar for patients with and without unfavorable cytogenetic/FISH markers, at ∼ 8 months for each subgroup. The ORRs in the subpopulations of patients with ISS stage III (n = 78) and those aged ≥ 65 years (n = 118) were 17.9% and 22.0%, respectively. Responses were slightly higher in patients with baseline ISS stage I and female sex (Figure 2), although these differences were determined to be statistically nonsignificant.
Figure 2.
Responses according to demographic and baseline disease characteristics.
Safety
The mean dose per patient was 23.1 mg/m2, and the median dose per patient was 23.5 mg/m2, which resulted in a median relative dose intensity of 92%. The majority of patients received ≥ 80% of their planned dose and more than half of the patients received ≥ 90% of their planned dose. The median time on study was 3.0 months (range, 0.03-16.9 months) and the median cumulative dose of carfilzomib was 470 mg/m2 (range, 20-2647 mg/m2). Doses of dexamethasone administered during the study ranged from 4 to 26 mg/28-day cycle.
AEs most frequently associated with treatment discontinuation were hypercalcemia (n = 6) associated with progressive disease (in 5 of 6 patients), congestive heart failure, cardiac arrest, dyspnea, pneumonia, and spinal cord compression (n = 4 each), and increased serum creatinine (n = 3). However, there was no evidence of cumulative toxicity with successive cycles of treatment throughout the course of this study or in those patients who continued to receive treatment under the extension protocol (PX-171-010).34,35 AEs led to at least one dose level reduction in 17.7% of patients, 2 dose level reductions in 5.6% of patients, and dose delays in 21.4%. Twenty-four patients (9%) died on study or within 30 days after their last dose; this includes the 6 patients who died within the first 2 dosing cycles mentioned in “Disposition of patients”; 12 (4.5%) died from causes related to PD and 11 (4.1%) as a result of an AE (cardiac arrest [n = 3], hepatic failure [n = 2], acute coronary syndrome secondary to PD [n = 1], dyspnea [n = 1], intracranial hemorrhage [n = 1], pneumonia [n = 1], sepsis [n = 1], and sepsis secondary to PD [n = 1]). In 5 patients (2%), the cause of death (cardiac arrest [n = 2], dyspnea, hepatic failure, and unknown cause [n = 1 each]) was deemed possibly or probably related to carfilzomib treatment.
The most common treatment-emergent AEs were fatigue (49%) and anemia (46%), and the most common grade 3 or 4 AEs were thrombocytopenia (29%) and anemia (24%). The most common AEs of any grade possibly related to carfilzomib were fatigue (37%) and nausea (34%; Table 3). Gastrointestinal symptoms, although common, were mild and manageable with routine supportive care. Fatigue and hematologic AEs were not dose-limiting or common reasons for discontinuation. Dyspnea was also seen in 34% of patients, although only half of these events (17%) were considered to be related to carfilzomib and only 3% were of grades 3 or 4. In general, dyspnea tended to be transient, did not increase with cumulative exposure, and was not associated with progressive lung injury. No particular cardiac AE occurred at an incidence of > 5%; 10 patients (3.8%) receiving carfilzomib experienced congestive heart failure, 4 (1.5%) had cardiac arrest, and 2 (0.8%) had myocardial infarction during the study. Febrile neutropenia, another AE often of major concern in the setting of relapsed/refractory MM, had very low incidence (< 1% for events of any grade).
Table 3.
Incidence and severity of treatment-emergent AEs (≥ 25%) and carfilzomib-related AEs (N = 266)
| AE | All grades,* n (%) | Grades 3 or 4,* n (%) | All grades carfilzomib-related,† n (%) |
|---|---|---|---|
| Hematologic | |||
| Anemia | 122 (46) | 63 (24) | 59 (22) |
| Thrombocytopenia | 103 (39) | 77 (29) | 77 (29) |
| Lymphopenia | 62 (23) | 52 (20) | 44 (17) |
| Neutropenia | 48 (18) | 29 (11) | 40 (15) |
| Leukopenia | 37 (14) | 18 (6.8) | 31 (12) |
| Nonhematologic | |||
| Fatigue | 130 (49) | 20 (7.5) | 98 (37) |
| Nausea | 119 (45) | 5 (1.9) | 90 (34) |
| Dyspnea | 90 (34) | 9 (3.4) | 45 (17) |
| Diarrhea | 86 (32) | 2 (0.8) | 64 (24) |
| Pyrexia | 83 (31) | 4 (1.5) | 40 (15) |
| Headache | 74 (28) | 5 (1.9) | 46 (17) |
| Upper respiratory tract infection | 71 (27) | 12 (4.5) | 15 (5.6) |
| Increased serum creatinine | 67 (25) | 7 (2.6) | 44 (17) |
| Other events of clinical interest | |||
| Vomiting | 59 (22.2) | 2 (0.8) | 44 (16.5) |
| PN‡ | 33 (12.4) | 3 (1.1) | 22 (8.3) |
| Hypophosphatemia | 32 (12.0) | 16 (6.0) | 19 (7.1) |
| Pneumonia | 32 (12.0) | 25 (9.4) | 13 (4.9) |
| Hyponatremia | 31 (11.7) | 22 (8.3) | 13 (4.9) |
| Renal failure (acute) | 13 (4.9) | 9 (3.4) | 4 (1.5) |
| Febrile neutropenia | 2 (0.8) | 2 (0.8) | 2 (0.8) |
| Tumor lysis syndrome | 1 (0.4) | 0 (0) | 0 (0) |
Irrespective of cause.
≥ 10% incidence; considered to be probably or possibly related to study drug.
Grouped PN included all events of neuropathy, peripheral neuropathy, peripheral sensory neuropathy, and peripheral motor neuropathy.
In this study, the most frequently reported renal AE was increased serum creatinine (25%). The majority of these events were grade 1 or 2 in severity, and none led to discontinuation of carfilzomib. Acute renal failure was reported in 13 patients (5%), 9 of whom experienced serious grade 3 acute renal failure. These acute renal failure events either resolved or stabilized with study drug interruption (n = 4), dose reduction (n = 1), discontinuation (n = 2), or no change in study drug dosing (n = 3). Chronic renal failure was reported in 10 patients (3.8%), with 3 of these events considered serious and 2 resulting in discontinuation of carfilzomib.
At baseline, 77% of patients had grade 1 or 2 neuropathy, but only 33 patients (12.4%) experienced new-onset or worsening of preexisting PN (defined as any event of neuropathy, peripheral neuropathy, peripheral sensory neuropathy, and peripheral motor neuropathy). Grade 3 PN occurred in 3 patients (1.1%), all of whom had grade 1 neuropathy at baseline. In 2 of these patients, the neuropathy resolved with study drug interruption, and the third patient completed the study with no changes to the study medication. PN (all grades) was considered related to treatment in 22 patients (8.3%) and related to disease progression in the remaining 11. No patients experienced grade 4 PN or were discontinued from study.
Discussion
With an ORR of 23.7%, a CBR rate of 37.0%, a median DOR of 7.8 months, and an OS of 15.6 months, responses to single-agent carfilzomib in this group of heavily pretreated patients (most of whom had received prior bortezomib, an immunomodulatory agent, corticosteroids, and an alkylating agent) are notable. The OS is particularly impressive as 73% of patients had disease refractory to bortezomib and 80% were refractory or intolerant to both bortezomib and lenalidomide. A median OS of 9 months is typically seen for similar patients in late-line settings.36 In addition, there was a high rate of concordance between IRC and investigator assessment of responses. Although the rate of CRs achieved in this study (0.4%) appears low, complete responses are rare in populations of patients with refractory myeloma.30 This may probably be the result of the exposure of the majority of the present study population to nearly all available approved anti-MM agents, especially the most effective agents, bortezomib and lenalidomide.30,37,38 Notably, very high CR rates have been achieved in trials conducted with carfilzomib-based regimens in the frontline disease setting, lending further support to this hypothesis.39–41
Both the ORR and the overall PFS were heavily impacted by the significant proportion (36.5%) of patients discontinuing therapy during the first 2 treatment cycles, including 22.9% because of PD. Interestingly, this was also observed in the phase 2 pilot study PX-171-003-A0.26 In the case of PX-171-003-A1, this was probably in part the result of the application of the IMWG response criteria and a study design calling for 2 assessments of response as early as day 15 of cycle 1 and day 1 of cycle 2 (day 29 on study). The early response assessments were intended to provide a measure for evaluating the time to achieve a first response, but in a study requiring PD at study entry, this may have led to premature determinations of disease progression in cycles 1 and 2. Almost all patients were experiencing rapid disease progression when they entered the study, and this progression could likely not be arrested by carfilzomib at 20 mg/m2. All patients who discontinued from the study during cycles 1 and 2 because of progressive disease were permitted to seek other treatment. In subsequent studies, the dosing regimen has been amended to escalate the dose of carfilzomib on day 8 of cycle 1 rather than waiting until day 1 of cycle 2 to provide the patient with the higher dose of 27 mg/m2. The doses of dexamethasone administered were similar between responders and nonresponders. Furthermore, no pattern of increased dexamethasone use was observed in responding patients, nor was there evidence of increasing dexamethasone use as the number of carfilzomib doses increased.
In the pilot study PX-171-003-A0, carfilzomib at 20 mg/m2 achieved an ORR of 16.7% and CBR of 23.8% in a similar patient population.26 A subsequent multivariate analysis based on this and other studies demonstrated an apparent dose-response to carfilzomib in patients with MM, with the 20-mg/m2 dose at the lower end of the effective range.42 The ORR of 23.7% and 37.0% CBR achieved in this study with carfilzomib at 27 mg/m2 support this observation. Ongoing and future studies evaluating earlier escalation to 27 mg/m2 on day 8 rather than at cycle 2, or higher doses of carfilzomib administered using adjusted dosing schedules, may have an impact on these parameters of response in similar patient populations.43
The rates and durations of response to carfilzomib were not significantly affected by most high-risk disease factors, including unfavorable cytogenetic status, age, and ISS staging where rapid relapses and aggressive disease are hallmarks.44 Although there were trends toward slightly greater response rates in patients with baseline ISS stage I and female sex, the 95% CIs overlapped as with the other factors, indicating a lack of statistically significant effects. These findings are notable as they suggest that carfilzomib could be used in broad patient populations in the relapsed refractory setting, including patients with renal impairment, unfavorable cytogenetic abnormalities, and advanced age. Other studies likewise support the use of carfilzomib in patients traditionally considered to be at higher risk, including those with substantial renal impairment.34
Hematologic AEs are common in patients with refractory MM,45 and this protocol allowed patients to enter the study with NCI-CTC grade 2 anemia, neutropenia, and thrombocytopenia as phase 1 studies with carfilzomib had shown minimal effects on these parameters. Although these hematologic events were commonly observed with carfilzomib treatment, they were infrequently dose limiting and the severity was low with respect to conventional chemotherapy.37,46 Thrombocytopenia was transient and consistent with proteasome inhibition of platelet budding observed with bortezomib.46 In addition, severe myelosuppression was uncommon and could be managed by dose interruption or reduction and/or growth factor support. Clinically significant grade 3 or 4 cytopenias were likewise infrequent and transient.
Treatment-emergent PN was uncommon, occurring in 12.4% of patients overall, much less than with thalidomide47 and substantially better than the 38% seen in the recent study evaluating subcutaneous bortezomib.48,49 Up to 53% of patients receiving bortezomib48–50 and > 70% of patients receiving thalidomide47 experience treatment-related PN of any grade, which is cumulative and may be dose-limiting. These findings are supported by carfilzomib's lack of neurodegeneration in vitro and less neurotoxicity in animal studies.20,21 The low incidence of new-onset or of worsening PN suggests that carfilzomib may be better tolerated by patients who previously experienced PN with other antimyeloma therapies.
Other nonhematologic AEs, including gastrointestinal events (eg, nausea and vomiting) and constitutional symptoms (eg, fatigue), although frequent, were often mild and non–treatment-limiting. Additional AEs commonly of concern in analogous patient populations, such as renal impairment and acute renal failure, occurred infrequently and were primarily mild to moderate in severity. Of the acute renal failure or renal failure events that qualified as serious AEs, the majority were attributable to disease progression and only 5 were considered to be possibly or probably related to carfilzomib. In a cross-trial analysis of 526 patients from PX-171-003-A0, PX-171-003-A1, PX-171-004, and PX-171-005, treatment-emergent renal events resulting in carfilzomib discontinuation were uncommon.51 Mild to moderate dyspnea was seen in approximately one-third of patients, although it most often occurred early in the course of treatment and either spontaneously resolved without intervention or was readily manageable with 4 mg of dexamethasone as premedication and/or dose reduction when not associated with underlying disease, such as respiratory infections, congestive heart failure, or chronic obstructive pulmonary disease. The precise etiology of the dyspneic events remains to be more fully elucidated. The rate of cardiac AEs observed during this study was not greater than that expected in a population of patients with advanced-stage MM nor was it greater than previously observed in other studies in MM.30,37 This may be a class effect of proteasome inhibitors, as bortezomib has been associated with heart failure events.52 However, this would be more appropriately addressed in the setting of the larger randomized studies of carfilzomib. Interestingly, although carfilzomib and bortezomib displayed similar cardiovascular toxicity profiles in animals at the maximum tolerated doses, cardiotoxicity was not a DLT in humans for either drug (Onyx data on file). No cumulative toxicities, including cardiac events, were observed up to 12 cycles in this study, suggesting that prolonged carfilzomib administration for long-term control of MM may be feasible.
After completing 12 cycles of carfilzomib, 31 patients continued into extension protocol PX-171-010 and 10 remain on study as of July 12, 2012. In addition, of 9 patients who chose not to enroll in PX-171-010, 2 patients continued in response with no new chemotherapy. Both findings are quite notable and further demonstrate that prolonged administration of carfilzomib without cumulative toxicities is possible and highlight that carfilzomib treatment may lead to long-term remission. Further results and implications from the extension study will be presented in a future publication.
In conclusion, treatment with carfilzomib produced clinically significant responses with an acceptable safety profile in heavily pretreated patients with relapsed and refractory MM. Given the limited number of therapeutic options available to patients with advanced-stage MM and the diminished prospects for retreatment once an agent has been used, there is significant need in this patient population.6,53,54 The activity of single-agent carfilzomib, even at the lower doses tested in this population, and the apparent lack of cross-resistance in patients with bortezomib- and immunomodulator-refractory disease, underscore the potential for using carfilzomib in treatment of patients with relapsed and refractory MM. The low rates and mild severity of myelosuppression and neuropathy in this study and prior phase 1 experience with carfilzomib are encouraging and strongly suggest its potential for use in conjunction with other effective antimyeloma agents without substantial risk of serious overlapping toxicities.55 The ongoing international, randomized, multicenter phase 3 ASPIRE trial is comparing lenalidomide plus low-dose dexamethasone with or without carfilzomib in patients who have received 1-3 prior regimens for relapsed MM. The results of this and other ongoing studies should provide insight into optimal use of carfilzomib in all patients with MM.
Acknowledgments
The authors thank all of the patients who contributed to this study and their families; the investigators and staff from the additional participating study sites, including Dr Andrew Belch (University of Alberta), Dr David Hurd (Wake Forest University), Dr Nashat Gabrail (Gabrail Cancer Center), Dr Sarit Assouline and Dr Martin Gyger (Jewish General Hospital), Dr James Mason (Scripps Clinic), Dr Maurizio Zangari (University of Utah), Dr Jesus Berdeja and Dr Daniel Couriel (Sarah Cannon Cancer Center), Dr Lowell Hart (Florida Cancer Specialists), Dr Thaddeus Bekker (Southern Cancer Center), and Dr Laurent Gressot (Waldron Medical Research); all of the participating research nurses and data coordinators; the Independent Steering Committee members (Dr Paul Richardson, Dr Brian Durie, and Dr Rueben Niesvizky) for their work on validating the data from the study; Jessica Taylor (Onyx Pharmaceuticals, Inc) for clinical operations lead for the study; Yu-Lin Chang (Onyx Pharmaceuticals, Inc) for statistical support; Dr Thomas Renau (Onyx Pharmaceuticals, Inc) for critical review of the manuscript for scientific accuracy; and Dr Yvonne E. Yarker and Dr Brian E. Szente (Fishawack Communications) for medical writing and editing services.
This work was supported by Onyx Pharmaceuticals, Inc and the Multiple Myeloma Research Consortium.
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.S.S., R.V., A.K.S., S.W., and S.J. designed and performed research, contributed vital new reagents or analytical tools, and analyzed data; T.M., M.W., and F.J.R. performed research and analyzed data; A.J.J. performed research, contributed vital new reagents or analytical tools, and analyzed data; S.L. and R.Z.O. designed and performed research and analyzed data; S.T., V.K., N.B., A.C.-K., F.B., G.S., J.Z., K.S., and E.S. performed research; M.A. designed and performed research; and L.K. and A.F.W. designed research, contributed vital new reagents or analytical tools, and analyzed data.
Conflict-of-interest disclosure: D.S.S. received consultancy and honoraria from Millennium and Celgene and was on the Board of Directors or advisory committee for Millennium and Celgene. T.M. received honoraria from Celgene and was a consultant for Onyx Pharmaceuticals. M.W. received research funding from Onyx Pharmaceuticals. R.V. was a consultant for and received research funding from Onyx Pharmaceuticals. A.J.J. was a consultant for Ortho Biotech, Celgene, Millennium, Onyx Pharmaceuticals, Bristol-Myers Squibb, and Exelixis; received honoraria from Ortho Biotech, Celgene, Millennium, Bristol-Myers Squibb, and Exelixis; was on the Speakers Bureau for Ortho Biotech, Celgene, and Millennium and on the Board of Directors for Millennium, Onyx Pharmaceuticals, and Bristol-Myers Squibb; and was on the advisory committee for Onyx Pharmaceuticals and Bristol-Myers Squibb. S.L. was a consultant for Millennium, Celgene, Novartis, Bristol-Myers Squibb, Onyx Pharmaceuticals, and Merck. V.K. received honoraria from Celgene, Janssen Ortho, and Roche. N.B. received honoraria and was on the Speakers Bureau for Celgene. M.A. was a consultant for Millennium and Novartis, was on the Board of Directors or advisory committee for Millennium and Novartis, and received research funding from Millennium and Allergan. G.S. was on the advisory committee for Onyx Pharmaceuticals. J.Z. was a consultant for Millennium and Amgen, was on the Speakers Bureau for Millennium and Celgene, and received research funding from Millennium and honoraria from Medtronics. A.K.S. was a consultant and received research funding from Celgene, Millennium, Novartis, Bristol-Myers Squibb, and Onyx Pharmaceuticals. E.S. was a consultant and on the Speakers Bureau for Celgene and Millennium. L.K. was a consultant for VLST Biotech, Threshold, and Onyx Pharmaceuticals. A.F.W. was employed by and held Equity Ownership in Onyx Pharmaceuticals. R.Z.O. received honoraria from Onyx Pharmaceuticals and was on the Board of Directors or advisory committee for Onyx Pharmaceuticals. S.J. received honoraria for Millennium, Celgene, Onyx Pharmaceuticals, and Merck, and was on the Board of Directors or advisory committee for Ortho Biotech, Imedex, Medicom World Wide, Optum Health Education, and PER Group. The remaining authors declare no competing financial interests.
Correspondence: David S. Siegel, John Theurer Cancer Center, Hackensack University Medical Center, 92 Second St, 3rd Floor, Hackensack, NJ 07601; e-mail: dsiegel@hackensackumc.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26(1):73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Offidani M, Leoni P, Corvatta L, et al. Outcome and toxicity in the modern era of new drugs for multiple myeloma: a reappraisal for comparison with future investigational trials. Clin Lymphoma Myeloma Leuk. 2010;10(5):353–360. doi: 10.3816/CLML.2010.n.068. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.van de Donk NW, Lokhorst HM, Dimopoulos M, et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37(4):266–283. doi: 10.1016/j.ctrv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Richardson PG, Laubach J, Mitsiades C, et al. Tailoring treatment for multiple myeloma patients with relapsed and refractory disease. Oncology (Williston Park) 2010;24(3 Suppl 2):22–29. [PubMed] [Google Scholar]
- 8.Mitsiades CS, Davies FE, Laubach JP, et al. Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. J Clin Oncol. 2011;29(14):1916–1923. doi: 10.1200/JCO.2010.34.0760. [DOI] [PubMed] [Google Scholar]
- 9.Jung T, Catalgol B, Grune T. The proteasomal system. Mol Aspects Med. 2009;30(4):191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Rolfe M, Chiu MI, Pagano M. The ubiquitin-mediated proteolytic pathway as a therapeutic area. J Mol Med. 1997;75(1):5–17. doi: 10.1007/s001090050081. [DOI] [PubMed] [Google Scholar]
- 11.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 12.Wan F, Lenardo MJ. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20(1):24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HG, Wang J, Yang X, Hsu HC, Mountz JD. Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene. 2004;23(11):2009–2015. doi: 10.1038/sj.onc.1207373. [DOI] [PubMed] [Google Scholar]
- 14.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8(6):508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 15.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12(10):2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 16.Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13(18):5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 17.Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67(13):6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110(9):3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parlati F, Lee S, Aujay M, et al. Carfilzomib: a selective inhibitor of the chymotrypsin-like activity of the constitutive proteasome and immunoproteasome has anti-tumor activity on multiple myeloma, lymphoma, and leukemia cells with minimal effects on normal cells. Haematologica. 2009;94(Suppl 2) 0373. [Google Scholar]
- 20.Arastu-Kapur S, Anderl JL, Kraus M, et al. Non-proteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17(9):2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- 21.Kirk CJ, Jiang J, Muchamuel T, et al. The selective proteasome inhibitor carfilzomib is well tolerated in experimental animals with dose intensive administration [abstract]. Blood (ASH Annual Meeting Abstracts) 2008;112(11) Abstract 2765. [Google Scholar]
- 22.Bruna J, Udina E, Ale A, et al. Neurophysiological, histological and immunohistochemical characterization of bortezomib-induced neuropathy in mice. Exp Neurol. 2010;223(2):599–608. doi: 10.1016/j.expneurol.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Poruchynsky MS, Sackett DL, Robey RW, Ward Y, Annunziata C, Fojo T. Proteasome inhibitors increase tubulin polymerization and stabilization in tissue culture cells: a possible mechanism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibition. Cell Cycle. 2008;7(7):940–949. doi: 10.4161/cc.7.7.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor OA, Stewart AK, Vallone M, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15(22):7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsina M, Trudel S, Furman RR, et al. A phase 1 single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18(17):4830–4840. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- 26.Jagannath S, Vij R, Stewart K, et al. Final results of PX-171-003-A0, part 1 of an open label, single-arm, phase II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM) [abstract]. J Clin Oncol. 2009;27(15 Suppl) Abstract 8504. [Google Scholar]
- 27.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT: European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 28.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 29.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-responder bias. Stat Med. 1984;3(1):35–44. doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 30.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 31.Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13(6):741–748. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 33.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 34.Singhal S, Siegel DS, Martin T, et al. Integrated safety from phase 2 studies of monotherapy carfilzomib in patients with relapsed and refractory multiple myeloma (MM): an updated analysis [abstract]. Blood (ASH Annual Meeting Abstracts) 2011;118(21) Abstract 1876. [Google Scholar]
- 35.Siegel D, Kaufman J, Wang M, et al. A summary of safety and efficacy data achieved with long-term carfilzomib (CFZ) treatment in patients with relapsed and/or refractory multiple myeloma (R/R MM) [abstract]. Haematologica. 2011;96(Suppl 2):126. Abstract 0302. [Google Scholar]
- 36.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 38.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22(2):231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 39.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikhael JR. A phase I/II trial of cyclophosphamide, carfilzomib, thalidomide, and dexamethasone (CYCLONE) in patients with newly diagnosed multiple myeloma [abstract]. J Clin Oncol. 2012;30(15) Abstract 8010. [Google Scholar]
- 41.Sonneveld P, Hacker E, Zweegman S, et al. Carfilzomib combined with thalidomide and dexamethasone (CARTHADEX) as induction treatment prior to high-dose melphalan (HDM) in newly diagnosed patients with multiple myeloma (MM): a trial of the European Myeloma Network EMN [abstract]. Blood (ASH Annual Meeting Abstracts) 2011;118(21) Abstract 633. [Google Scholar]
- 42.Squifflet P, Michiels S, Siegel DS, Vij R, Ro SK, Buyse M. Multivariate modelling reveals evidence of a dose-response relationship in phase 2 studies of single-agent carfilzomib [abstract]. Blood (ASH Annual Meeting Abstracts) 2011;118(21) Abstract 1877. [Google Scholar]
- 43.Papadopoulos KP, Lee P, Singhal S, et al. A phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56 mg/m2 expansion cohort of PX-171-007 [abstract]. Blood (ASH Annual Meeting Abstracts. 2011;118(21) Abstract 2930. [Google Scholar]
- 44.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gay F, Palumbo A. Management of disease- and treatment-related complications in patients with multiple myeloma. Med Oncol. 2010;27(Suppl 1):S43–S52. doi: 10.1007/s12032-010-9542-z. [DOI] [PubMed] [Google Scholar]
- 46.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohty B, El-Cheikh J, Yakoub-Agha I, Moreau P, Harousseau JL, Mohty M. Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica. 2010;95(2):311–319. doi: 10.3324/haematol.2009.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 49.Arnulf B, Pylypenko H, Grosicki S, et al. Updated survival analysis of a randomized, phase 3 study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma [published online ahead of print June 11, 2012]. Haematologica. doi: 10.3324/haematol.2012.067793. doi: 10.3324/haematol.2012.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 51.Harvey D, Lonial S, Patel P, McCulloch L, Niesvizky R, Kaufman J. Carfilzomib dose and schedule need not be adjusted for baseline renal dysfunction, including patients on hemodialysis [abstract]. Haematologica. 2012;97(Suppl 2) Abstract 0844. [Google Scholar]
- 52.Millennium Pharmaceuticals. Velcade Prescribing Information. Cambridge, MA: Millennium Pharmaceuticals; 2012. [Google Scholar]
- 53.Richards T, Weber D. Advances in treatment for relapses and refractory multiple myeloma. Med Oncol. 2010;27(Suppl 1):S25–S42. doi: 10.1007/s12032-009-9407-5. [DOI] [PubMed] [Google Scholar]
- 54.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Multiple Myeloma. Version 1.2012. Fort Washington, PA: National Comprehensive Cancer Network; 2012. [Google Scholar]
- 55.Niesvizky R, Bensinger W, Martin T, et al. An update on the phase 1b/2 dose-escalation study of carfilzomib with lenalidomide and low-dose dexamethasone (CRd) in patients with relapsed or refractory multiple myeloma [abstract 0295]. Haematologica. 2011;96(Suppl 2):122. [Google Scholar]