Abstract
Background and objectives
Levels of asymmetric dimethylarginine, an inhibitor of nitric oxide synthase, are elevated in kidney disease and associated with mortality in white European hemodialysis populations. Nitric oxide production and degradation are partially genetically determined and differ by racial background. No studies have measured asymmetric dimethylarginine in African Americans on dialysis and assessed whether differences exist in its association with mortality by race.
Design, setting, participants, & measurements
Asymmetric dimethylarginine was measured in 259 patients on maintenance hemodialysis assembled from 2004 to 2012 in Boston area outpatient centers. Cox proportional hazards models were used to determine the association between asymmetric dimethylarginine and all-cause mortality, and an interaction with race was tested.
Results
Mean (SD) age was 63 (17) years, 46% were women, and 22% were African American. Mean asymmetric dimethylarginine in non–African Americans was 0.79 µmol/L (0.16) versus 0.70 µmol/L (0.11) in African Americans (P<0.001); 130 patients died over a median follow-up of 2.3 years. African Americans had lower mortality risk than non–African Americans (hazard ratio, 0.27; 95% confidence interval, 0.15 to 0.50) that was robust to adjustment for age, comorbidity, and asymmetric dimethylarginine (hazard ratio, 0.35; 95% confidence interval, 0.17 to 0.69). An interaction was noted between race and asymmetric dimethylarginine (P=0.03), such that asymmetric dimethylarginine was associated with higher mortality in non–African Americans (adjusted hazard ratio, 1.29; 95% confidence interval, 1.06 to 1.57 per 1 SD higher asymmetric dimethylarginine) but not in African Americans (adjusted hazard ratio, 0.57; 95% confidence interval, 0.28 to 1.18). Additional adjustment for fibroblast growth factor 23 partially attenuated the association for non–African Americans (adjusted hazard ratio, 1.22; 95% confidence interval, 0.98 to 1.50).
Conclusions
African Americans have lower asymmetric dimethylarginine levels and lower hazard for mortality compared with non–African Americans. Levels of asymmetric dimethylarginine did not explain lower hazard for mortality in non–African American patients. High asymmetric dimethylarginine was a risk factor for mortality exclusively in non–African Americans. Mechanisms explaining these relationships need to be evaluated.
Keywords: mortality risk, hemodialysis, nitric oxide
Introduction
Levels of asymmetric dimethylarginine (ADMA), an inhibitor of nitric oxide synthase, are known to be elevated in patients with kidney disease (1,2). ADMA decreases blood vessel compliance and promotes atherogenesis (3). High ADMA levels have previously been shown to be an independent risk factor for mortality in both patients with CKD and patients on hemodialysis (4–6). However, these previous studies have predominantly been conducted in Europe, resulting in findings that are only generalizable to Caucasian patients.
Multiple reports have shown that African American patients on hemodialysis have better survival than non–African American patients on hemodialysis (7–10). Although it has been suggested that younger age, faster progression toward ESRD, and a lower prevalence of comorbidity may result in overall healthier African American patients on dialysis, these factors do not fully explain the magnitude of this phenomenon. Nitric oxide generation and degradation are strongly influenced by gene polymorphisms, which may be conserved by racial background (11–13). African Americans are more likely to benefit from treatments for heart failure that target nitric oxide pathways, with genetic differences perhaps explaining this treatment effect (14,15). Despite these findings, levels of ADMA in patients on hemodialysis have not been previously evaluated as a potential mechanism for different mortality rates by race. We, therefore, measured baseline ADMA and l-arginine, a precursor of nitric oxide, in a cohort of patients on prevalent hemodialysis. We then evaluated the baseline factors associated with ADMA levels, evaluated whether ADMA explained any of the difference in mortality rates between African American and non–African American patients, and explored its association with mortality in African Americans and non–African Americans.
Materials and Methods
Study Population
This study is a secondary analysis of data from the Cognition and Dialysis study cohort, the details of which have been described in greater detail elsewhere (16). Briefly, patients receiving chronic in-center hemodialysis at five Dialysis Clinic Inc. (DCI) units or one hospital-based unit (St. Elizabeth’s Medical Center) in the greater Boston area were evaluated for study enrollment from January 28, 2004, to May 31, 2012. Reflecting the primary study goal of assessing cognitive performance, eligibility criteria included English fluency as well as sufficient visual and hearing acuity to complete cognitive testing. Individuals with advanced dementia on the basis of medical record review were excluded. Nonvascular access–related hospitalization within 1 month, delirium, receipt of hemodialysis for <1 month, and single-pool Kt/V<1.0 were temporary exclusion criteria. The Tufts Medical Center/Tufts University Institutional Review Board approved the study, and all participants who underwent cognitive testing signed informed consent forms. The clinical and research activities being reported are consistent with the Declaration of Helsinki.
Baseline Demographics and Clinical Characteristics
Demographic, clinical, and laboratory characteristics were ascertained at the time of study enrollment. Demographic data (age and sex) were obtained through participant report, review of medical charts, and the DCI and St. Elizabeth’s Medical Center databases. Education (<12th grade, high school graduate, or ≥2 years of college) and smoking history (never, current, or past smoker) were obtained through a standardized patient questionnaire. Race assignment was determined by patient self-classification. Medical history, including history of cardiovascular disease (a composite of a history of either coronary artery disease or peripheral vascular disease), stroke, heart failure (HF), and presence of diabetes, was defined by patient history or documentation in the patient’s electronic or paper charts. The cause of ESRD, time since start of hemodialysis (dialysis vintage), and any activated vitamin D use within 2 weeks of cognitive testing were obtained from the DCI or St. Elizabeth electronic record along with the mean monthly predialysis systolic and diastolic BPs. Predialysis blood tests, including serum phosphorus, serum albumin, and single-pool Kt/V, were obtained on the day of study enrollment. High-sensitivity C-reactive protein (CRP) and fibroblast growth factor 23 (FGF-23) levels (C-terminal ELISA assay; Immutopics, Inc.) were measured at a later date from stored frozen samples taken at study enrollment. All DCI laboratory tests were measured in a central laboratory in Nashville, Tennessee.
Measurement of ADMA
Samples were available for testing in 259 patients. Each sample was collected predialysis, centrifuged, and frozen at −80°C, and it had not been previously thawed before testing. ADMA and l-arginine were measured by HPLC in batched assays at the University of Padua (17). Control samples were run with each batch to assess the reproducibility of test results. The coefficient of variation for the control samples was 5.5% for ADMA and 7.7% for l-arginine, similar to previously reported results using this method (18).
Outcomes
We obtained survival status on all patients enrolled in the study by review of the electronic medical record and/or contact with each individual dialysis unit. Survival time was defined as the period of time elapsed from initial study enrollment and blood sample measurement to death, receipt of kidney transplantation (censoring event), or end of study follow-up (March 31, 2013).
Statistical Analyses
Descriptive characteristics of the study population were reported as proportions for categorical and binary variables, means with SDs for continuous normally distributed variables, and medians with interquartile ranges for skewed variables. To better assess differences across ADMA level, the study population was divided into quartiles. Linear trends across quartiles were assessed using linear regression for continuous variables and the Cochrane–Armitage test for binary variables.
Baseline Correlates of ADMA Level
The association of baseline characteristics with ADMA level was assessed using univariate and multivariable linear regression. Continuous variables were expressed per SD to better allow for comparison across variables. A history of cardiovascular disease was forced into the multivariable models given its known association with ADMA levels, whereas selection of the remaining terms was on the basis of a P value of <0.10 for linear trend across quartiles of ADMA.
Mortality Analyses
Race and Mortality.
The association between race and all-cause mortality was assessed using Cox proportional hazards models; a univariate model explored the association between race and time to all-cause mortality, whereas a parsimonious model adjusted for age and sex. An expanded model also adjusted for dialysis vintage, vascular access type, history of cardiovascular disease, diabetes, and HF, and a final model added adjustment for ADMA.
ADMA and Mortality.
A second set of models explored the univariate association between ADMA level and all-cause mortality, whereas sequentially adjusted multivariable models first evaluated the addition of age and sex, and an expanded model included additional adjustment for dialysis-specific factors potentially associated with mortality, including diabetes, dialysis vintage, vascular access type, history of cardiovascular disease, and HF. Hazard ratios (HRs) are presented per 1 SD-higher ADMA. To assess for differences by race, we included an interaction term for race and ADMA level in the final multivariable model. Using this model, an interaction plot was created to show the predicted HR for mortality by level of continuous ADMA compared with the reference value of mean ADMA level and non–African American stratified by African American and non–African American.
We performed several sensitivity analyses. First, to assess for any overt deviation from linearity, the association of quartiles of ADMA with mortality was assessed. Second, to explore whether any additional potential confounding variables may affect the relationship between ADMA and mortality in both the overall cohort and the ADMA–race interaction model, we additionally adjusted for variables that showed a trend to being different across quartiles of ADMA (P<0.10): FGF-23, phosphorus, diastolic BP, and body mass index. Three additional factors that may be associated with mortality, albumin, high-sensitivity CRP, and activated vitamin D use were also included in these models. Finally, because some have argued that the l-arginine-to-ADMA ratio is better correlated with nitric oxide production capacity (19), we assessed the relationship between the l-arginine-to-ADMA ratio (higher ratio means more nitric oxide production capacity) and all-cause mortality in similar models. We also tested for an interaction between l-arginine-to-ADMA ratio, race, and mortality.
Results
Baseline Characteristics
The mean (SD) age of the study population was 63 (17) years, 118 (46%) patients were women, and 56 (22%) patients were African Americans. Most patients had arteriovenous fistulas as a form of vascular access (66%), and the median (25th–75th) dialysis vintage was 14 (6.4–33.5) months. The mean (SD) ADMA level was 0.77 (0.15) µmol/L, and the mean l-arginine level was 139.0 (44.6) µmol/L, yielding a mean l-arginine-to-ADMA ratio of 189.5 (69.5). ADMA levels were 0.79 (0.16) µmol/L in non–African American patients versus 0.70 (0.11) µmol/L in African American patients (P<0.001), whereas l-arginine and the l-arginine-to-ADMA ratio did not differ by race (Supplemental Table 1).
Correlates of ADMA
Patients in the higher quartiles of ADMA were more likely to be non–African American, have a history of HF and a history of diabetes, and have higher levels of phosphorus and FGF-23 levels (Table 1). In a multivariable model adjusting for age, sex, race, history of HF, history of cardiovascular disease, serum phosphorus, and FGF-23, African American race was associated with ADMA; African Americans had a −0.10 µmol/L (95% confidence interval [95% CI], −0.05 to −0.14) lower ADMA level compared with non–African Americans (Table 2). Women had higher ADMA (0.06 µmol/L; 95% CI, 0.02 to 0.09), whereas patients with diabetes, history of HF, and higher FGF-23 level also had higher ADMA levels. Age and history of cardiovascular disease were not associated with ADMA in multivariable analysis.
Table 1.
Demographics and clinical characteristics by quartile of asymmetric dimethylarginine
| Qs of ADMA (n=259) | ADMA Q1 (n=66) | ADMA Q2 (n=62) | ADMA Q3 (n=67) | ADMA Q4 (n=64) | Trend P Value |
|---|---|---|---|---|---|
| ADMA (µmol/L) | 0.58±0.06 | 0.71±0.03 | 0.80±0.03 | 0.98±0.08 | — |
| l-Arginine (µmol/L) | 127.1±36.3 | 133.7±41.9 | 145.7±44.1 | 149.6±52.0 | 0.004 |
| l-Arginine-to-ADMA ratio | 219.6±60.9 | 188.8±60.5 | 181.6±56.3 | 152.7±49.3 | <0.001 |
| Age (yr) | 61.7±16.8 | 60.8±18.5 | 63.4±16.5 | 66.6±15.0 | 0.08 |
| Women | 36 | 47 | 48 | 52 | 0.09 |
| African American | 32 | 26 | 24 | 5 | <0.001 |
| HF | 26 | 29 | 37 | 47 | <0.01 |
| Stroke | 9 | 18 | 25 | 14 | 0.26 |
| CVD | 41 | 37 | 42 | 47 | 0.42 |
| Diabetes | 39 | 39 | 54 | 55 | 0.03 |
| Active vitamin D use (%) | 58 | 53 | 67 | 60 | 0.41 |
| Primary cause of ESRD | |||||
| Diabetes | 35 | 26 | 33 | 39 | 0.84 |
| Hypertension | 15 | 19 | 22 | 17 | |
| Other | 15 | 16 | 19 | 9 | |
| Unknown | 14 | 23 | 15 | 11 | |
| GN | 21 | 16 | 10 | 23 | |
| Smoking history | |||||
| Never | 32 | 40 | 43 | 41 | 0.50 |
| Past | 61 | 45 | 46 | 52 | |
| Current | 8 | 15 | 10 | 6 | |
| Dialysis access | |||||
| Fistula | 71 | 55 | 69 | 70 | 0.81 |
| Graft | 2 | 10 | 9 | 3 | |
| Catheter | 27 | 35 | 22 | 27 | |
| Vintage (mo) | 15.0 (5.0–37.5) | 13.1 (6.5–35.4) | 15.0 (7.8–29.1) | 14.0 (4.9–30.9) | 0.88 |
| Systolic BP (mmHg) | 140±18 | 143±22 | 141±20 | 140±24 | 0.95 |
| Diastolic BP (mmHg) | 74±10.6 | 75±15 | 73±11 | 70±12 | 0.09 |
| Kt/V | 1.54±0.20 | 1.50±0.25 | 1.52±0.27 | 1.49±0.25 | 0.23 |
| Albumin (g/dl) | 3.8±0.3 | 3.8±0.3 | 3.8±0.4 | 3.8±0.3 | 0.80 |
| Phosphorus (mg/dl) | 5.2±1.4 | 5.7±1.7 | 5.9±1.4 | 5.6±1.5 | 0.03 |
| CRP (mg/L) | 4.5 (1.6–16.4) | 4.7 (1.8–9.6) | 5.0 (2.3–11.9) | 7.1 (2.6–16.6) | 0.51 |
| FGF-23 (RU) | 1750 (931–5660) | 1806 (936–7739) | 3655 (1333–8682) | 4151 (1784–9038) | 0.004 |
Presented as mean±SD, median (interquartile range), or percentage. Q, quartile; ADMA, asymmetric dimethylarginine; HF, heart failure; CVD, cardiovascular disease (composite of history of peripheral vascular disease or coronary artery disease); CRP, high-sensitivity C-reactive protein; FGF-23, fibroblast growth factor 23; RU, relative units.
Table 2.
Association between baseline characteristics and asymmetric dimethylarginine
| Baseline Characteristics | Univariate | Multivariablea | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Age (per 1 SD increase) | 0.02 (<−0.01 to 0.03) | 0.14 | 0.01 (−0.01 to 0.03) | 0.45 |
| Men versus women | −0.05 (−0.08 to −0.01) | 0.02 | −0.06 (−0.09 to −0.02) | 0.002 |
| African American versus non–African American | −0.09 (−0.04 to −0.13) | <0.001 | −0.1 (−0.14 to −0.05) | <0.001 |
| Diabetes | 0.05 (0.02 to 0.09) | <0.01 | 0.06 (0.02 to 0.1) | <0.01 |
| HF | 0.06 (0.02 to 0.09) | <0.01 | 0.04 (0.01 to 0.08) | 0.03 |
| History of CVD | 0.02 (−0.02 to 0.06) | 0.36 | −0.01 (−0.06 to 0.03) | 0.50 |
| Phosphorus (per 1 SD increase) | 0.02 (<−0.01 to 0.03) | 0.06 | 0.01 (−0.01 to 0.03) | 0.55 |
| Log FGF-23 (per doubling) | 0.01 (<0.01 to 0.02) | 0.01 | 0.02 (0.01 to 0.03) | 0.004 |
CVD, cardiovascular disease (composite of history of peripheral vascular disease or coronary artery disease); 95% CI, 95% confidence interval.
Multivariable model is adjusted for all listed covariates.
All-Cause Mortality
There were 130 deaths (118 deaths in non–African Americans and 12 deaths in African Americans) over a median follow-up time of 2.3 (interquartile range=1.2–3.8) years, with death rates of 23.2 and 7.1 per 100 person-years among non–African Americans and African Americans, respectively; 21 (10%) non–African American patients underwent kidney transplantation and were censored, in contrast to 11 (20%) of African American patients. Compared with non–African American patients, African American patients had a significantly lower hazard for mortality (HR, 0.27; 95% CI, 0.15 to 0.50) in unadjusted models (Supplemental Table 2). A model with adjustment for age and sex (HR, 0.42; 95% CI, 0.23 to 0.79) and an expanded model with additional adjustment for cause of ESRD, dialysis vintage, history of cardiovascular disease, and vascular access type (HR, 0.32; 95% CI, 0.16 to 0.62) showed slight attenuation of the relationship between African American race and mortality. Similar results were seen when adjusting for ADMA in the final multivariable model (HR, 0.35; 95% CI, 0.17 to 0.69).
Association of ADMA with Mortality
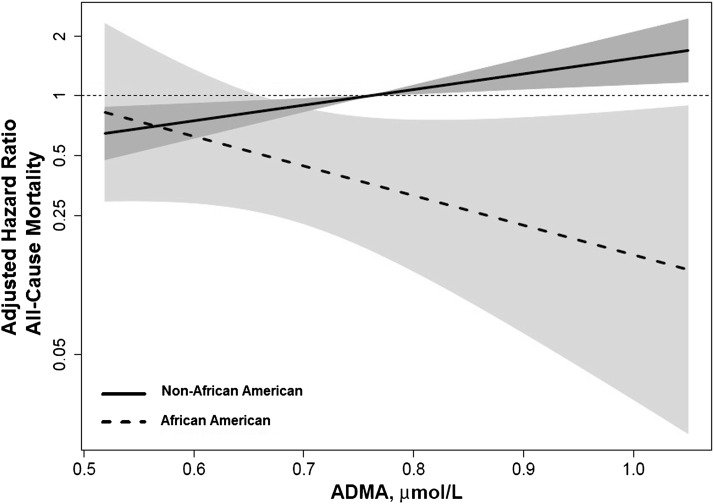
In univariate analysis, each 1 SD (0.15 µmol/L)-higher ADMA was associated with a 22% higher risk for all-cause mortality for the total cohort. The risk remained relatively unchanged in a parsimonious model adjusting for age and sex as well as in an expanded model adjusting for age, sex, dialysis vintage, vascular access type, history of cardiovascular disease, history of congestive HF, and diabetes (Table 3). There was a significant interaction between race and ADMA level (P=0.03) (Figure 1), such that ADMA was associated with mortality in non–African Americans (adjusted HR, 1.29; 95% CI, 1.0 to 1.57 per 1 SD-higher ADMA) but not in African Americans (adjusted HR, 0.57; 95% CI, 0.28 to 1.18 per 1 SD-higher ADMA) (Table 3).
Table 3.
Association of asymmetric dimethylarginine level and mortality in overall cohort and with interaction with race
| Asymmetric Dimethylarginine | Unadjusted HR (95% CI) | Model 1a HR (95% CI) | Model 2b HR (95% CI) |
|---|---|---|---|
| Total cohort | |||
| Continuous | 1.22 (1.03 to 1.44) | 1.25 (1.04 to 1.49) | 1.22 (1.01 to 1.47) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.23 (0.73 to 2.08) | 1.24 (0.73 to 2.10) | 1.12 (0.66 to 1.93) |
| Quartile 3 | 1.22 (0.73 to 2.04) | 1.19 (0.71 to 2.00) | 1.12 (0.67 to 1.90) |
| Quartile 4 | 1.91 (1.15 to 3.17) | 1.97 (1.17 to 3.34) | 1.84 (1.08 to 3.12) |
| Interaction with race | |||
| African Americanc | 0.65 (0.30 to 1.44) | 0.66 (0.31 to 1.40) | 0.57 (0.28 to 1.18) |
| Non–African Americanc | 1.16 (0.98 to 1.38) | 1.30 (1.08 to 1.56) | 1.29 (1.06 to 1.57) |
HR per 1 SD change in asymmetric dimethylarginine (0.15 µmol/L), except for quartile analysis. HR, hazard ratio.
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, dialysis vintage, vascular access type, history of cardiovascular disease, history of congestive heart failure, and diabetes.
Models include interaction term between African American race and asymmetric dimethylarginine.
Figure 1.
Interaction plot between ADMA, all-cause mortality, and race. Final multivariable model was adjusted for age, sex, dialysis vintage, vascular access type, history of cardiovascular disease, history of heart failure, and diabetes. Hazard ratios are compared with a reference mean ADMA level in non–African Americans (0.79 µmol/L). Shading represents the 95% confidence interval for each race. AMDA, asymmetric dimethylarginine.
Sensitivity Analyses
The fourth quartile of ADMA was associated with significantly higher risk than the first quartile, with the second and third quartiles also showing higher hazards but not being statistically different from the first quartile (Table 3). A model with additional adjustment for serum phosphorus, albumin, high-sensitivity CRP, diastolic BP, body mass index, and activated vitamin D use revealed similar HRs to the main model. Additional adjustment for FGF-23 partially attenuated the HR for mortality in non–African Americans (adjusted HR per 1 SD-higher ADMA, 1.22; 95% CI, 0.98 to 1.50) but did not change the HR for African Americans (adjusted HR, 0.55; 95% CI, 0.26 to 1.15 per 1 SD-higher ADMA). Notably, the interaction with race remained significant (P=0.04).
The l-arginine-to-ADMA ratio was inversely associated with all-cause mortality in univariate analysis (HR, 0.78; 95% CI, 0.66 to 0.93 per 1 SD higher) and a parsimonious model adjusting for age and sex (HR, 0.78; 95% CI, 0.65 to 0.93). The relationship was attenuated and nonsignificant in an expanded model adjusting for age, sex, cause of ESRD, dialysis vintage, history of cardiovascular disease, and vascular access type (HR, 0.85; 95% CI, 0.70 to 1.03). The P value for the interaction of l-arginine-to-ADMA and race was 0.16.
Discussion
In a cohort of patients on maintenance hemodialysis, we noted that ADMA levels were lower in African American than non–African American patients. African American race was strongly associated with a lower hazard for mortality. This finding was only slightly attenuated in multivariable analyses, including adjustment for ADMA. We also noted an interaction between African American race and ADMA, such that high ADMA was associated with mortality only in non–African American participants, although this finding was partially attenuated after additional adjustment for FGF-23. In contrast to ADMA alone, the l-arginine-to-ADMA ratio was inversely associated with mortality in univariate and demographic-adjusted analyses but not the expanded multivariable analyses.
ADMA, a potent inhibitor of nitric oxide synthase, has been previously shown to adversely affect blood vessel compliance as well as promote atherosclerosis (3,20). ADMA levels are known to increase as kidney function declines because of both decreased renal clearance and impairment of enzymatic breakdown (1). We found several factors associated with high ADMA, including non–African American race, women, diabetes, history of HF, and FGF-23 level. Two prior studies have examined factors associated with ADMA. A study of 820 patients with stages 3 and 4 CKD showed that higher ADMA levels were associated with prevalent cardiovascular disease and reduced GFR (6). A second study of 90 patients on maintenance hemodialysis noted an association between CRP and ADMA (21). In comparison, our study showed no relationship between baseline cardiovascular disease or CRP and ADMA, although the related conditions, diabetes and HF, did show associations, perhaps indicating a shared pathophysiology. The differences in the findings may be because of the differences in the study populations, including different stages of CKD (6), different prevalence of cardiovascular disease and diabetes (4), and different demographics of the study population (4). The association between a history of HF and ADMA may be consistent with a recent study showing that ADMA may be useful in diagnosing acute HF (22). Finally, we noted an association between higher FGF-23 level and higher ADMA, a finding seen in a previous study of patients with stages 3 and 4 CKD (23) but not previously reported in patients on hemodialysis.
We noted lower levels of ADMA in African Americans versus non–African Americans in univariate and multivariable analyses. Among patients with normal kidney function, there are conflicting reports about racial differences in ADMA level. A study of 30 healthy black Africans and 28 age-matched white Europeans showed higher ADMA in black versus white patients (0.34 versus 0.25 µmol/L) (24), whereas a significantly larger study of over 900 older United States residents showed that black patients had modestly but statistically significant lower ADMA levels compared with white patients (0.60 versus 0.63 µmol/L) (25). We are not aware of prior studies of ADMA that have formally reported racial differences in patients with kidney disease, because most prior studies were conducted in Europe or Japan and did not include participants of African ancestry (4,26,27).
We confirmed that African American race was associated with a lower risk for mortality compared with non–African American race. Although the African American group was younger and had a lower prevalence of cardiovascular disease, adjustment for these differences did not change the risk for mortality. Furthermore, adjustment for ADMA level only slightly attenuated this association, suggesting that the lower ADMA levels in African Americans do not significantly explain this mortality difference. African American race has been associated with longer survival on dialysis in multiple previous studies (7–10). Several possible mechanisms have been proposed for this phenomenon, including a younger average age at start of dialysis, fewer comorbid conditions, and, perhaps, a more rapid decline in kidney function and, therefore, survivor bias in those patients who reach ESRD. However, even when these factors are considered, there still seems to be a survival benefit of being African American, indicating that additional research is needed. In support of this finding, a recent study of patients with CKD also showed improved survival in African Americans, even after adjusting for case-mix differences, suggesting that a survival advantage may exist even before the onset of ESRD (28).
In this study, high ADMA was associated with a higher risk of mortality in non–African American patients. ADMA levels have been previously associated with a higher risk for mortality in both patients with CKD and patients on hemodialysis. Two previous studies in cohorts of patients with CKD showed that high ADMA was associated with a modestly higher risk for mortality (5,6). Notably, this association remained unchanged in each study after adjustment for a history of cardiovascular disease and vascular disease risk factors. Extending this finding to hemodialysis, a study of 225 patients also showed that high ADMA was an independent risk factor for mortality (4). Finally, ADMA has been shown to be independently associated with a higher risk for mortality in a cohort of community-dwelling participants without evident kidney disease (29). In our cohort, we also found that the association between ADMA and mortality in non–African American patients persisted even after adjustment for multiple comorbid conditions and laboratory values. Additional adjustment for FGF-23, however, partially attenuated the relationship. One prior study found an association between ADMA and FGF-23 and reported that ADMA attenuated the relationship between FGF-23 and flow-mediated dilation of the forearm (23). It was suggested by Yilmaz et al. (23) that ADMA and FGF-23 may share a common pathway for inducing vascular dysfunction. Therefore, it remains unclear whether the attenuation seen in our findings is caused by either a shared pathway (where one would not want to adjust for the FGF-23) or true confounding (where adjustment is appropriate).
We also found an inverse relationship between the l-arginine-to-ADMA ratio and all-cause mortality in our univariate and parsimonious models, although the association was attenuated when adjusting for comorbid conditions. Although no studies have previously assessed this relationship in patients with kidney disease, a previous study of patients with HF noted that the l-arginine-to-ADMA ratio was independently associated with mortality (30). l-Arginine acts as a substrate for nitric oxide synthase in the production of nitric oxide; thus, it has been argued that the ratio of l-arginine to ADMA may be reflective of nitric oxide production capacity (19).
We noted a significant interaction of ADMA with race, such that ADMA was only associated with mortality within non–African American patients. High ADMA within African American patients was associated with lower risk for mortality, although this result was not statistically significant. Potential mechanisms for these differences by race could reflect the mechanism of the action of ADMA as an inhibitor of nitric oxide. Racial differences in nitric oxide production have been described in patients without kidney disease that have been attributed to gene polymorphisms that alter the function nitric oxide synthase (11,31,32). Additionally, ADMA level is strongly influenced by dimethylarginine dimethylaminhydrolase (DDAH), an enzyme responsible for ≤80% of the metabolism of ADMA (33). Genetic polymorphisms in DDAH genes have been associated with variation in ADMA levels in humans (12), and animal studies involving knockout/gain of function of DDAH have raised/lowered ADMA levels with a subsequent effect on vascular health. There is additional evidence that, in addition to influencing ADMA levels, DDAH may also directly influence vascular health, independent of ADMA (34). We hypothesize that DDAH polymorphisms may be responsible for both the lower ADMA levels that we observed in African American patients and, possibly, the lack of association between ADMA and mortality. Perhaps reflecting these findings, African Americans also seem to have a different response than other races to nitric oxide–related treatments (14).
There are several limitations to our study. First, the patient population in our cohort was selected as part of a study evaluating cognition, which may have resulted in a population that is not fully generalizable. Our cohort, by design, excluded patients acutely ill as well as patients with severe cognitive impairment, which may bias to a healthier population. However, we note that our cohort is comparable in terms of demographics (age, sex, and race) and vascular disease prevalence with the overall hemodialysis population in the United States (35). Second, the statistical power to detect an association between ADMA and mortality within African Americans is limited by a smaller number of outcomes in this subgroup. As a result, we are limited to stating that an association with ADMA was detected only within non–African American patients. In addition, we do not have a direct measure of residual renal function, which may partially affect ADMA levels. To partially address this limitation, we included dialysis vintage in the adjusted analyses, because those patients with longer vintage are likely have less residual kidney function. Third, we acknowledge that, given the observational nature of the study, both residual and unmeasured confounding are possible. This study also has several strengths, including a detailed ascertainment of medical history and comprehensive laboratory testing on all cohort patients. Given the design of the study, we also have detailed follow-up of participants, allowing for accurate ascertainment of the time of death and censoring events.
In conclusion, we showed lower levels of ADMA in African American versus non–African American patients on hemodialysis. ADMA level was associated with all-cause mortality, a finding that was exclusive to non–African American patients on hemodialysis. We also confirmed a lower hazard for mortality in African American patients versus non–African American patients, and this difference was not explained by ADMA levels. Given the modest sample size, these exploratory findings should be replicated in larger cohorts of patients on hemodialysis with diverse racial/ethnic backgrounds. The mechanism underlying our results should also be further evaluated.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Dialysis Clinic, Inc. (DCI) and, in particular, the staff and patients at the five DCI units in the Boston area and St. Elizabeth’s Dialysis Unit, whose generous cooperation made this study possible. This work was prepared as part of the master’s thesis of D.A.D at the Tufts Clinical and Translational Science Institute (CTSI), and therefore, we thank the Tufts CTSI for guidance in completing this work.
The study was funded by an American Society of Nephrology Research Fellowship Grant (to D.A.D.) and National Institutes of Health Grants K23-DK71636 (to D.E.W.), R21-DK068310 (to M.J.S.), and R01-DK078204 (to M.J.S.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00770114/-/DCSupplemental.
References
- 1.Vallance P, Leone A, Calver A, Collier J, Moncada S: Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Anderstam B, Katzarski K, Bergström J: Serum levels of NG, NG-dimethyl-L-arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol 8: 1437–1442, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Cooke JP, Ghebremariam YT: Dietary nitrate, nitric oxide, and restenosis. J Clin Invest 121: 1258–1260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoccali C, Bode-Böger S, Mallamaci F, Benedetto F, Tripepi G, Malatino L, Cataliotti A, Bellanuova I, Fermo I, Frölich J, Böger R: Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 358: 2113–2117, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C: Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Young JM, Terrin N, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Sarnak MJ, Menon V: Asymmetric dimethylarginine and mortality in stages 3 to 4 chronic kidney disease. Clin J Am Soc Nephrol 4: 1115–1120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI: Revisiting survival differences by race and ethnicity among hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910–2918, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol 5: 1231–1242, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Yan G, Norris KC, Yu AJ, Ma JZ, Greene T, Yu W, Cheung AK: The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol 8: 953–961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNamara DM, Tam SW, Sabolinski ML, Tobelmann P, Janosko K, Venkitachalam L, Ofili E, Yancy C, Feldman AM, Ghali JK, Taylor AL, Cohn JN, Worcel M: Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: Results from the A-HeFT trial. J Card Fail 15: 191–198, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Fogarty RD, Abhary S, Javadiyan S, Kasmeridis N, Petrovsky N, Whiting MJ, Craig JE, Burdon KP: Relationship between DDAH gene variants and serum ADMA level in individuals with type 1 diabetes. J Diabetes Complications 26: 195–198, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Pope AJ, Karuppiah K, Cardounel AJ: Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol Res 60: 461–465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Jr., Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN, African American Heart Failure Trial Investigators : Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 351: 2049–2057, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Taylor AL, Ziesche S, Yancy CW, Carson P, Ferdinand K, Taylor M, Adams K, Olukotun AY, Ofili E, Tam SW, Sabolinski ML, Worcel M, Cohn JN, African American Heart Failure Trial Investigators : Early and sustained benefit on event-free survival and heart failure hospitalization from fixed-dose combination of isosorbide dinitrate/hydralazine: Consistency across subgroups in the African American Heart Failure Trial. Circulation 115: 1747–1753, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Sarnak MJ, Tighiouart H, Scott TM, Lou KV, Sorensen EP, Giang LM, Drew DA, Shaffi K, Strom JA, Singh AK, Weiner DE: Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 80: 471–480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanova M, Artusi C, Boffa GM, Zaninotto M, Plebani M: HPLC determination of plasma dimethylarginines: Method validation and preliminary clinical application. Clin Chim Acta 411: 1632–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Böger R, CREED Investigators : Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62: 339–345, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Bode-Böger SM, Scalera F, Ignarro LJ: The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther 114: 295–306, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Böger SM: ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 37: 2024–2029, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Malatino LS, Böger R, CREED Investigators : Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol 13: 490–496, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Tutarel O, Denecke A, Bode-Böger SM, Martens-Lobenhoffer J, Lovric S, Bauersachs J, Schieffer B, Westhoff-Bleck M, Kielstein JT: Asymmetrical dimethylarginine—more sensitive than NT-proBNP to diagnose heart failure in adults with congenital heart disease. PLoS ONE 7: e33795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 78: 679–685, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Melikian N, Wheatcroft SB, Ogah OS, Murphy C, Chowienczyk PJ, Wierzbicki AS, Sanders TA, Jiang B, Duncan ER, Shah AM, Kearney MT: Asymmetric dimethylarginine and reduced nitric oxide bioavailability in young Black African men. Hypertension 49: 873–877, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sydow K, Fortmann SP, Fair JM, Varady A, Hlatky MA, Go AS, Iribarren C, Tsao PS, ADVANCE Investigators : Distribution of asymmetric dimethylarginine among 980 healthy, older adults of different ethnicities. Clin Chem 56: 111–120, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Böger RH, Zoccali C: ADMA: A novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease. Atheroscler Suppl 4: 23–28, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kumagai H, Sakurai M, Takita T, Maruyama Y, Uno S, Ikegaya N, Kato A, Hishida A: Association of homocysteine and asymmetric dimethylarginine with atherosclerosis and cardiovascular events in maintenance hemodialysis patients. Am J Kidney Dis 48: 797–805, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Quarles LD, Lott EH, Lu JL, Ma JZ, Molnar MZ, Kalantar-Zadeh K: Survival advantage in black versus white men with CKD: Effect of estimated GFR and case mix. Am J Kidney Dis 62: 228–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS: Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 119: 1592–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderssohn M, Rosenberg M, Schwedhelm E, Zugck C, Lutz M, Lüneburg N, Frey N, Böger RH: The L-Arginine-asymmetric dimethylarginine ratio is an independent predictor of mortality in dilated cardiomyopathy. J Card Fail 18: 904–911, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Li R, Lyn D, Lapu-Bula R, Oduwole A, Igho-Pemu P, Lankford B, Morgan J, Nkemdechi S, Liu G, Pack C, Silvestrov N, von Deutsch DA, Song Q, Abukhalaf IK, Ofili E: Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 17: 560–567, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Tanus-Santos JE, Desai M, Flockhart DA: Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics 11: 719–725, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P: Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23: 1455–1459, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P: Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med 13: 198–203, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen S-C, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 63[Suppl]: A7, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.