Abstract
Background and objectives
Patients with CKD have increased cardiovascular morbidity and mortality. This study investigated the prognostic value of common clinical echocardiographic parameters.
Design, setting, participants, & measurements
There were 289 unselected consecutive patients who had a transthoracic echocardiogram between January and June 2003. Patients with stage 3 or 4 CKD (n=49) were compared with those with eGFR≥60 ml/min per 1.73 m2, n=240). Left ventricular volume, ejection fraction and mass, left atrial volume, and function parameters were measured. The primary endpoint, determined a priori, was a composite of cardiac death, myocardial infarction, and congestive cardiac failure.
Results
Patients were followed for a median 5.6 years. The incidence of the primary endpoint was higher in patients with CKD (29% versus 12%, P=0.001), who were older and had a higher prevalence of hypertension and ischemic heart disease. Indexed left ventricular mass (LVMI) and left atrial volume (LAVI) were higher in patients with CKD. Furthermore, patients with LAVI>32 ml/m2 had significantly lower event-free survival than patients with normal (<28 ml/m2) or mildly dilated LAVI (28–32 ml/m2) (P<0.001). Multivariate analysis showed that age (odds ratio [OR], 1.19; 95% confidence interval [95% CI], 1.08 to 1.31; P=0.001) and LVMI (OR, 3.66; 95% CI, 2.47 to 5.41; P<0.001) were independently associated with LAVI>32 ml/m2. Multivariate Cox regression analysis demonstrated that CKD (hazard ratio [HR], 1.13; 95% CI, 1.01 to 1.26; P=0.04), hypertension (HR, 2.18; 95% CI, 1.05 to 4.54; P=0.04), and a larger LAVI (HR, 1.35; 95% CI, 1.02 to 1.77; P=0.04) were independent predictors of the primary endpoint.
Conclusions
Patients with CKD were at higher risk for cardiovascular events. LAVI was significantly larger in the CKD group and was a predictor of adverse cardiac events.
Keywords: cardiovascular disease, CKD, congestive heart failure, echocardiography, left ventricular hypertrophy
Introduction
CKD is a global health problem. Based on the clinical practice guidelines established by the National Kidney Foundation, approximately 20 million adults in the United States alone have CKD, with 8 million classified as having moderate or severe kidney disease (1). Australian data demonstrate a similar prevalence of CKD (2), and CKD is shown to be an important risk factor for cardiovascular disease (CVD). Other reports have demonstrated that both the incidence and severity of obstructive coronary artery disease increase as GFR declines (3).
Patients with intermediate stages of CKD (stages 3–4) have higher cardiovascular mortality than individuals with normal renal function (4–6). Cardiorenal damage is thought to be a result of organ “cross-talk” perpetuated by pathophysiologic mechanisms common to both groups (7,8). The interplay of these mechanisms amplifies structural and functional cardiorenal derangement, leading to the progression of both cardiac and renal disease (9).
Previous studies in patients with end stage renal failure on dialysis demonstrated the prognostic value of indexed left atrial volume (LAVI) (10,11). However, the prognostic value of echocardiographic parameters, particularly the LAVI, has not been well characterized in patients with CKD. We therefore examined an unselected patient group that comprised 49 patients with stage 3 or 4 CKD and 240 referent participants without CKD who were referred for a transthoracic echocardiogram (TTE). We sought to examine the prognostic value, if any, of routine echocardiographic parameters, including LAVI, indexed left ventricular mass (LVMI), and left ventricular ejection fraction (LVEF), in addition to clinical risk factors to predict CVD mortality and major adverse cardiac events including nonfatal myocardial infarction (MI) and congestive cardiac failure (CCF).
Materials and Methods
Patient Selection
This is a retrospective, observational study from a single center. The study group comprised consecutive patients who had a TTE at the Liverpool Hospital echocardiography laboratory (Sydney, NSW, Australia) between January and June 2003. There were 289 patients who were aged ≥18 years and met the following inclusion criteria: sinus rhythm with no known history of atrial fibrillation, no previous cardiothoracic bypass or valvular surgery, no greater than mild mitral or aortic valvular stenosis/regurgitation, not pregnant, no congenital cardiac anomalies, and stable eGFR values (<10% change in eGFR) for at least 6 months prior for all patients with CKD, thereby ensuring no acute deterioration as a cause for their reduced renal function before the TTE. This study was approved by the Liverpool Hospital Ethics Committee (2007/070).
Assessment of Renal Function
All study patients had renal function evaluation at the time of TTE. The eGFR was calculated by the simplified Modification of Diet in Renal Disease equation (A. Levey, T. Greene, J. Kusek, G. Beck, MDRD Study Group, unpublished observations). Patients with normal renal function and those with stages 1–2 CKD (eGFR≥60 ml/min per 1.73 m2) comprised the referent group (n=240). Patients with CKD (n=49) had an eGFR<60 ml/min per 1.73 m2 (i.e., stage 3 or 4 CKD). Patients on RRT were excluded (n=19).
Echocardiography
A comprehensive, two-dimensional, spectral and color flow Doppler echocardiogram was performed in all patients at baseline using commercially available equipment (Phillips Sonos 7500 and GE Vivid 7 System). Images were digitally archived on a departmental server. The TTE was performed with the subject in the left lateral decubitus position, according to the recommendations of the European Association of Echocardiography and the American Society of Echocardiography (12,13). Standard views were obtained from parasternal, apical, and subcostal windows. Biplane left ventricular (LV) end diastolic and end systolic volumes were obtained from the apical four- and two-chamber views; LVEF was measured using the modified biplane Simpson's method (14). LV diastolic function was assessed using pulsed wave transmitral Doppler signals obtained with the sample-volume placed at the mitral leaflets tips to measure peak early (E) and late (A) velocities, as well as the E/A ratio. The deceleration time was measured as the slope of the E wave. On the basis of published criteria, LV diastolic dysfunction was classified into grades as normal, impaired relaxation, pseudo-normal, and restrictive filling (13). LV mass was calculated using the area-length method (15). Biplane maximum left atrial (LA) volume was measured at end systole using the area-length method from the apical four- and two-chamber views (16). LA function was assessed by measurement of the A-wave velocity time integral (VTI) and its fraction of the total mitral inflow VTI (the atrial fraction). LA ejection force was calculated as previously reported (17). The LA volume and LV mass were indexed to the body surface area (BSA). All echocardiographic measurements were repeated from digitized loops by investigators who were blinded to the patients’ clinical details as well as group allocation. An average of three measurements was used for final analyses. Age was expressed as 1 SD of the interrater SD (1 SD=5 years of age), eGFR was expressed as 1 SD of the interrater SD (1 SD=10 eGFR), LAVI was expressed as 1 SD of the interrater SD (1 SD=10 LAVI), and LVMI was expressed as 1 SD of the interrater SD (1 SD=34 LVMI). Interobserver and intraobserver variability was evaluated in 10 randomly selected participants. A single observer measured the same parameters 4 weeks apart, blinded to the initial measurements. A second observer measured the same participants, once again blinded to the initial measurements and a coefficient of variation was calculated.
Baseline Data and Patient Follow-Up
Demographic and clinical data at baseline, including age, sex, and traditional cardiovascular risk factors, were recorded for all patients included in the study. Medications at baseline were collected for each patient. Long-term clinical outcomes were followed up at a median of 5.6 years (range, 5.5–5.7) after index TTE, from hospital records, and/or by telephone follow-up from patients or their primary care physicians. Information regarding death was obtained from the New South Wales death registry, and corroborated with medical records. CKD is associated with the higher incidence of LV diastolic dysfunction and coronary artery disease (18). The primary clinical outcome, determined a priori, was a composite of cardiac death, acute MI (AMI), and CCF. If a patient reported occurrence of AMI or heart failure, this was corroborated from medical records using prespecified criteria. The diagnosis of AMI was based on the presence of new Q waves in at least two contiguous leads and an elevated creatine kinase myocardial band isoform fraction. In the absence of pathologic Q waves, the diagnosis of AMI was based on an increase in the creatine kinase level to more than twice the upper limit of the normal range with an elevated level of creatine kinase myocardial band isoform or troponin I. CCF was defined as a clinical syndrome with New York Heart Association class III or IV symptoms with signs of heart failure, with heart failure documented as the primary diagnosis for the hospitalization.
Statistical Analyses
Analyses were performed using SPSS 21 software for Windows (SPSS Inc., Chicago, IL). The Kolmogorov–Smirnov test was used to assess whether continuous variables were normally distributed. Continuous data are presented as the mean±SD. Differences between groups were examined by the t test or Mann–Whitney nonparametric tests. Categorical variables were reported as numbers and percentages, and the chi-squared test or the Fisher’s exact test was used when appropriate. With the exception of the Kolmogorov–Smirnov test, all statistical tests were two-tailed and a P value <0.05 was deemed significant. The correlation between variables was examined by the Spearman rank test. The cumulative survival curves were constructed with the use of the Kaplan–Meier method and groups were compared with the log-rank test. Cox regression analysis was used to construct the final model using a forced-entry approach with application of a stringent α cut-off values (P=0.01) of univariate predictors. We tested the incremental value of LAVI by comparing the global chi-squared values between the models with significant clinical parameters, with and without LAVI. Penalized spline regression analysis was performed to examine the relationship between LAVI and LVMI. The penalized spline regression was performed in R software (version 3.0.0; R Foundation for Statistical Computing, Vienna, Austria). Interobserver and intraobserver variability was analyzed by calculation of the coefficient of variation.
Results
The study cohort comprised 289 consecutive patients referred for TTE. The subgroup of patients with CKD with an eGFR<60 ml/min per 1.73 m2 (stage 3 or 4 CKD; n=49) was compared with a referent group with an eGFR≥60 ml/min per 1.73 m2 (normal or stage 1 or 2 CKD; n=240) (Table 1). The mean eGFR values were 93±22 and 42±16 ml/min per 1.73 m2, respectively. Sex was similarly distributed between the two groups, whereas patients with CKD were older (71±10 versus 55±16 years; P<0.001) and had a higher BSA (1.9±0.2 m2 versus 1.8±0.3 m2; P=0.03). In addition, more patients with CKD had a history of hypertension (78% versus 49%; P<0.001) and ischemic heart disease (39% versus 20%; P=0.01), although there was no difference with respect to other cardiac risk factors (e.g., diabetes mellitus, prior stroke, and hyperlipidemia). A similar proportion of current smokers was observed in both groups. Patients with CKD were treated more often with aspirin (51% versus 37%; P=0.08), β-blockers (39% versus 23%; P=0.03), and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (63% versus 46%; P=0.03). Treatment with clopidogrel and statins were similar in the two groups.
Table 1.
Baseline characteristics of the study cohort (N=289)
| Characteristic | eGFR (ml/min per 1.73 m2) | P Value | |
|---|---|---|---|
| ≥60 (n=240) | <60 (n=49) | ||
| Risk factor | |||
| Age (yr) | 54.5±16.2 | 70.8±9.5 | <0.001 |
| BSA (m2) | 1.86±0.22 | 1.80±0.27 | 0.03 |
| Men | 142 (59) | 26 (53) | 0.43 |
| eGFR (ml/min per 1.73 m2) | 92.8±22.1 | 41.6±15.9 | <0.001 |
| Hypertensiona | 118 (49) | 38 (78) | <0.001 |
| Diabetes mellitus | 66 (28) | 16 (33) | 0.49 |
| Ischemic heart disease | 48 (20) | 19 (39) | 0.01 |
| Prior stroke | 39 (16) | 12 (25) | 0.22 |
| Hyperlipidemia | 89 (37) | 20 (41) | 0.63 |
| Current smoker | 61 (25) | 10 (20) | 0.59 |
| Malignancy | 43 (18) | 3 (6) | 0.05 |
| Medication at discharge | |||
| Aspirin | 88 (37) | 25 (51) | 0.08 |
| Clopidogrel | 31 (13) | 8 (16) | 0.50 |
| β-blockers | 55 (23) | 19 (39) | 0.03 |
| Statins | 96 (40) | 21 (43) | 0.75 |
| ACEI/ARB | 110 (46) | 31 (63) | 0.03 |
| Oral hypoglycemic treatment | 51 (21) | 11 (22) | 0.85 |
| LV parameters | |||
| Diastolic function | |||
| Peak E-wave velocity (cm/s) | 70.0±19.3 | 67.3±22.2 | 0.33 |
| Peak A-wave velocity (cm/s) | 72.1±19.3 | 84.0±22.2 | <0.001 |
| E/A ratio | 1.04±0.42 | 0.88±0.54 | 0.02 |
| E-deceleration time (s) | 0.20±0.06 | 0.21±0.07 | 0.16 |
| Altered diastolic function (IR, PN, or restrictive) | 192 (80) | 48 (98) | <0.01 |
| Systolic function | |||
| LVEF (%) | 64.4±7.4 | 57.7±8.5 | <0.001 |
| LVMI (g/m2) | 114.2±31.4 | 131.6±41.7 | 0.001 |
| LA size and function | |||
| LAVI (ml/m2) | 26.7±9.7 | 32.6±12.3 | 0.002 |
| A-wave VTI (cm) | 7.02±2.53 | 8.28±2.59 | 0.002 |
| Atrial fraction (%) | 37.6±12.5 | 44.0±11.3 | 0.001 |
| LA ejection force (%) | 20.4±13.6 | 21.1±13.4 | 0.78 |
Data are presented as the mean±SD or n (%). BSA, body surface area; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; LV, left ventricular; E, early; A, late; IR, impaired relaxation; PN, pseudo-normal; LVEF, left ventricular ejection fraction; LVMI, indexed left ventricular mass; LA, left atrial; LAVI, indexed left atrial volume; VTI, velocity time integral.
Arterial pressure>130/85 mmHg or normotensive patient with a history of hypertension on antihypertensive treatment.
Echocardiographic parameters at baseline are listed in Table 1. Patients with CKD had a lower LVEF (57.7%±8.5% versus 64.4%±7.4%; P<0.001) albeit within the normal clinical range, and a higher LVMI (132±42 g/m2 versus 114±31 g/m2; P=0.001) than the referent group. Diastolic function evaluation demonstrated that deceleration time and E/A ratio were similar between the two groups. However, a significantly higher proportion of patients with CKD had diastolic dysfunction (impaired relaxation, pseudo-normal, and restrictive filling pattern) than the referent group (98% versus 80%; P<0.01). Assessment of LA parameters demonstrated that LAVI was significantly larger (33±12 ml/m2 versus 27±10 ml/m2; P=0.001) in the CKD group. The A-wave VTI (8.3±2.6 cm versus 7.0±2.5 cm; P=0.002) and the atrial fraction (44.0%±11.3% versus 37.6%±12.5%; P=0.001) were higher in the CKD group. A modest but significant inverse correlation was observed between eGFR and LAVI (ρ=−0.21; P<0.001) by the Spearman rank correlation. The coefficients of variation were 9.2% for LAVI interobserver variability and 4% for intraobserver variability.
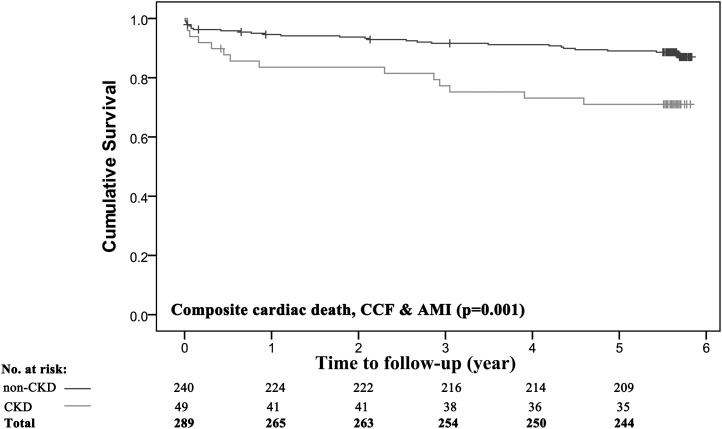
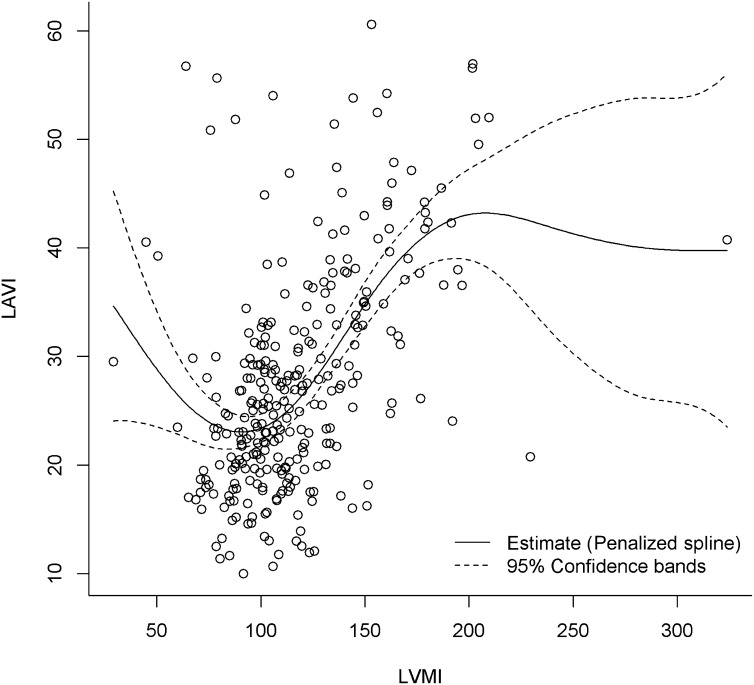
All of the study patients were followed for a median duration of 5.6 years (range, 5.5–5.7), and details of adverse events were obtained for all patients. Kaplan–Meier analysis was performed to evaluate outcomes for the composite endpoint of CCF, nonfatal MI, and cardiac death (Figure 1). The incidence of the composite endpoint was higher in patients with CKD (29% versus 12%; P=0.001). We performed multiple regression analysis using a stringent α cut-off P value of 0.01 for univariate predictors, to avoid overfitting the model. The variables selected in the model were eGFR, hypertension, diabetes mellitus (clinical), and LAVI (echocardiographic). Age was excluded in the model due to concerns of collinearity (e.g., with eGFR and LAVI). LVMI was also excluded from the model to minimize the confounding effect because penalized spline regression demonstrated a linear relationship between LAVI and LVMI (Figure 2). Moreover, it is well known that LVMI is an independent predictor of LAVI.
Figure 1.
Cumulative survival in the entire cohort among patients with and without CKD. The study population comprised 240 individuals without CKD (≥60 ml/min per 1.73 m2) and 49 patients with CKD (eGFR<60 ml/min per 1.73 m2). Kaplan–Meier survival curves for the composite endpoint of cardiac death, CCF, or nonfatal AMI are shown. P values were calculated using the log-rank test. AMI, acute myocardial infarction; CCF, congestive cardiac failure.
Figure 2.
Penalized spline regression between LVMI and LAVI. The degree of freedom was 4.25. LAVI, indexed left atrial volume; LVMI, indexed left ventricular mass.
The final model demonstrated that reduced eGFR (hazard ratio [HR], 1.13; 95% confidence interval [95% CI], 1.01 to 1.26; P=0.04), hypertension (HR, 2.18; 95% CI, 1.05 to 4.54; P=0.04), and LAVI (HR, 1.35; 95% CI, 1.02 to 1.77; P=0.04) were the independent predictors of the composite outcome for the entire study group (Table 2). The global chi-squared value for the model with hypertension and eGFR for determining the composite endpoint was 18.22, and this increased significantly (P=0.01) to 24.43 when LAVI was forced into the model (Supplemental Figure 1).
Table 2.
Cox regression analyses for the composite endpoint of cardiac death, CCF, and AMI
| Predictor | Univariate Analyses | Multivariate Analyses | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| eGFR (ml/min per 1.73 m2) | 1.20 (1.08 to 1.33) | 0.001 | 1.13 (1.01 to 1.26) | 0.04 |
| Hypertensiona | 3.04 (1.50 to 6.16) | 0.002 | 2.18 (1.05 to 4.54) | 0.04 |
| LAVI (ml/m2) | 1.48 (1.15 to 1.90) | 0.003 | 1.35 (1.02 to 1.77) | 0.04 |
| Diabetes mellitus | 2.21 (1.21 to 4.03) | 0.01 | 1.83 (0.10 to 3.38) | 0.05 |
| LVEF (%) | 0.96 (0.93 to 0.99) | 0.01 | ||
| LVMI (g/m2) | 1.47 (1.20 to 1.79) | <0.001 | ||
| Age (yr) | 1.04 (1.01 to 1.06) | 0.001 | ||
| β-Blocker | 1.91 (1.03 to 3.55) | 0.04 | ||
| ACEI/ARB | 1.87 (1.01 to 3.47) | <0.05 | ||
| Aspirin | 1.79 (0.99 to 3.26) | 0.06 | ||
| Men | 1.23 (0.67 to 2.29) | 0.51 | ||
| BSA (m2) | 0.83 (0.22 to 3.13) | 0.78 | ||
| Atrial fraction (%) | 1.00 (0.98 to 1.02) | 0.99 | ||
CCF, congestive cardiac failure; AMI, acute myocardial infarction; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HR, hazard ratio; 95% CI, 95% confidence interval.
Arterial pressure>130/85 mmHg or normotensive patient with a history of hypertension on antihypertensive treatment.
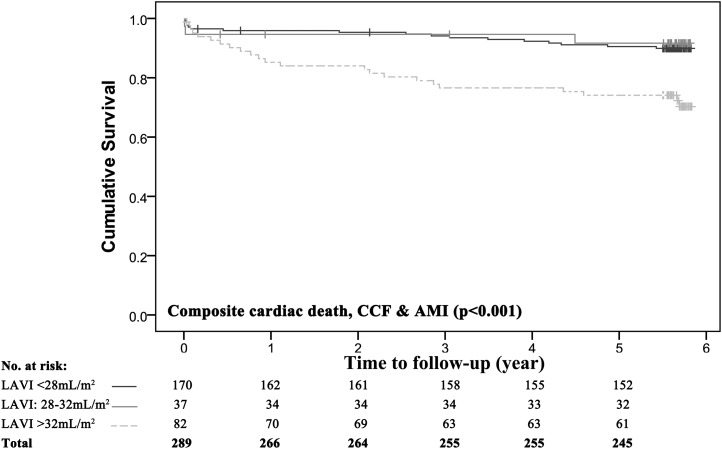
We further divided the study group based on American Society of Echocardiography guidelines for normal and mildly enlarged LAVI (19,20) into patients with LAVI values <28 ml/m2, 28–32 ml/m2, and >32 ml/m2. Multivariate logistic regression analysis was performed to identify the independent determinants of LAVI>32 ml/m2 (Table 3). The univariate predictors entered into the model were age, sex, BSA, eGFR, hypertension, CCF, ischemic heart disease, atrial fraction, LVEF, and LVMI. Age (odds ratio, 1.19; 95% CI, 1.08 to 1.31; P=0.001) and LVMI (odds ratio, 3.66; 95% CI, 2.47 to 5.41; P<0.001) were the only independent determinants of LAVI>32 ml/m2. Kaplan–Meier analyses were performed to evaluated the effect of LAVI on survival in the entire study group and demonstrated that patients with LAVI>32 ml/m2 had significantly lower event-free survival than patients with normal (<28 ml/m2) and mildly dilated LAVI (28–32 ml/m2; log-rank P<0.001) (Figure 3).
Table 3.
Multivariate logistic regression analyses for predictors of LAVI>32 ml/m2
| Predictor | Univariate Analyses | Multivariate Analyses | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (yr) | 1.27 (1.15 to 1.39) | <0.001 | 1.19 (1.08 to 1.31) | 0.001 |
| LVMI (ml/m2) | 4.04 (2.76 to 5.90) | <0.001 | 3.66 (2.47 to 5.41) | <0.001 |
| CCF | 0.25 (0.09 to 0.69) | <0.01 | ||
| Hypertensiona | 1.85 (1.09 to 3.13) | 0.02 | ||
| Ischemic heart disease | 2.05 (1.15 to 3.64) | 0.02 | ||
| eGFR (ml/min per 1.73 m2) | 1.16 (1.06 to 1.28) | 0.002 | ||
| LVEF (%) | 0.93 (0.90 to 0.96) | <0.001 | ||
| Men | 1.18 (0.70 to 1.99) | 0.54 | ||
| BSA (m2) | 0.51 (0.16 to 1.61) | 0.25 | ||
| Atrial fraction (%) | 1.01 (0.99 to 1.03) | 0.53 | ||
OR, odds ratio.
Arterial pressure>130/85 mmHg or normotensive patient with a history of hypertension on antihypertensive treatment.
Figure 3.
Cumulative survival in the entire cohort stratified by LAVI. There were 170 individuals with an LAVI<28 ml/m2, 37 with an LAVI of 28–32 ml/m2, and 82 with a LAVI>32 ml/m2. Kaplan–Meier survival curves are shown for the composite endpoint of cardiac death, CCF, or nonfatal AMI. P values were calculated using the log-rank test.
The penalized spline regression was performed between LVMI and LAVI (Figure 2), and the estimated degree of freedom for the penalized spline was 4.25. For LAVI values between 20 and 40 ml/m2, its relationship with LVMI is linear (corresponds to LVMI values of 80–160 g/m2). This range for LAVI (20–40 ml/m2) would reflect a significant proportion of the population that is evaluated in clinical practice (i.e., normal, mild, or moderately dilated LAVI). Importantly, in patients with CKD as opposed to end stage renal failure, we would likely only see this degree of LA enlargement and, therefore, the relationship remains clinically relevant. Between LVMI values from 80 to 160 g/m2, where the majority of the data lie, the relationship between LVMI and LAVI appeared linear and most stable.
Discussion
This study evaluated clinical risk and the additional value of echocardiographic parameters measured in routine clinical practice in predicting adverse CVD events in 289 unselected patients referred for a TTE. Forty-nine patients had CKD and these individuals were older and had a higher incidence of hypertension and ischemic heart disease, a higher indexed LVMI, more diastolic dysfunction, and a larger LAVI. Patients with CKD had a higher incidence of adverse outcomes at a mean follow-up of 5 years. Stratification by LAVI demonstrated that patients with a LAVI>32 ml/m2 were at the highest risk of adverse events.
The risk of CVD is increased in CKD (21,22) and a significantly higher incidence of the composite outcome (29% versus 12%; P=0.01) was observed in the CKD subgroup. Traditional risk factors such as hypertension, smoking, diabetes, and dyslipidemia are common in both groups (23). Although the Framingham risk score is a powerful tool to predict cardiovascular events (24), two studies (25,26) demonstrated that this clinical risk score underestimates adverse events and provides relatively poor predictive accuracy, especially in the CKD population. Thus, it is important to consider other markers of adverse cardiovascular outcomes in this high-risk group. Echocardiography provides quantitative information on LV structure, function, and hypertrophy. LV hypertrophy is common in patients with renal disease and correlates with the degree of renal functional impairment (27). Although two-dimensional echocardiography is widely utilized for LV mass assessment, it has limited reproducibility (28,29).
Hypertension and consequent LV hypertrophy predispose to the development of diastolic dysfunction (30) with consequent LA enlargement. Thus, LVMI and LAVI would have a significant relationship, and LVMI was shown to be an independent predictor of LAVI. The relationship between LAVI and LVMI was additionally explored using penalized spline regression, demonstrating a linear relationship between LVMI values of 80 and 160 g/m2 corresponding to a LAVI range from 25 to 40 ml/m2 (Figure 2). LAVI was previously demonstrated to be a marker of severity and chronicity of diastolic dysfunction (31). Thus, quantification of LAVI could be especially relevant in patients with CKD. In fact, LAVI was reported to be an independent predictor of death in patients with ESRD (10) and was considered to be a reproducible echocardiographic parameter (17). We examined predictors of adverse cardiovascular events in the entire study group and demonstrated that eGFR, hypertension, and increased LAVI were independent predictors. Although these findings cannot be extrapolated to the CKD subgroup, we note that LAVI was larger and hypertension was more prevalent in the CKD subgroup.
We observed a modest yet significant correlation between eGFR and LAVI. Other studies have reported that a LAVI>32 ml/m2 is an important marker for risk stratification and this has been used as a guide for surveillance and therapy in high-risk patient groups including after AMI and heart failure (20,32,33). Similarly, when patients were stratified by LAVI in this study, those with LAVI>32 ml/m2 had significantly lower event-free survival than patients with LAVI≤32 ml/m2 (log-rank P<0.001; Figure 3). Interestingly, the mean LAVI of the CKD group was 32.6 ml/m2 and lower survival was observed in the CKD versus referent group.
Our study has several limitations. This is a single-center retrospective study with a relatively small sample size and small number of events. A larger prospectively recruited cohort is required to validate our findings and is currently underway. Consecutive patients who underwent a TTE in our institution between January and June 2003 were screened using the exclusion criteria mentioned, but only 289 patients qualified. Thus, a relatively small proportion (approximately 17%) of patients with CKD (n=49) were identified. Although there was no intentional selection bias during the screening process for eligible patients, patients with CKD in this study were selected from among those referred for a TTE. Proteinuria is likely a risk factor for the composite outcome and predictor of adverse outcomes; therefore, the inability to adjust for this confounder because of the retrospective nature of data collection is a limitation. We could not perform risk stratification specifically in the CKD subgroup because of the limited number of patients with CKD to provide adequate statistical power.
The results of our study suggest that LAVI may provide additional prognostic value to clinical risk factors in unselected patients referred for TTE including a subgroup with CKD. Patients with CKD were at higher risk for adverse cardiovascular events and had a larger LAVI compared with the referent group. These preliminary results warrant confirmation in prospective longitudinal studies specifically involving patients with CKD.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06700613/-/DCSupplemental.
References
- 1.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, Atkins RC: Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol 14[Suppl 2]: S131–S138, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR: Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol 28: 354–360, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A: Renal function and intensive lowering of blood pressure in hypertensive participants of the Hypertension Optimal Treatment (HOT) study. J Am Soc Nephrol 12: 218–225, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Mann JFE, Gerstein HC, Dulau-Florea I, Lonn E: Cardiovascular risk in patients with mild renal insufficiency. Kidney Int Suppl 63: S192–S196, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Ruilope LM, van Veldhuisen DJ, Ritz E, Luscher TF: Renal function: The Cinderella of cardiovascular risk profile. J Am Coll Cardiol 38: 1782–1787, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 1527–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B: The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J 26: 11–17, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Patel RK, Jardine AG, Mark PB, Cunningham AF, Steedman T, Powell JR, McQuarrie EP, Stevens KK, Dargie HJ, Jardine AG: Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis 55: 1088–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C: Left atrial volume in end-stage renal disease: A prospective cohort study. J Hypertens 24: 1173–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS: Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10: 165–193, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, American College of Cardiology Foundation. American Heart Association : 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. J Am Coll Cardiol 53: e1–e90, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W, American Society of Echocardiography’s Nomenclature and Standards Committee. Task Force on Chamber Quantification. American College of Cardiology Echocardiography Committee. American Heart Association. European Association of Echocardiography. European Society of Cardiology : Recommendations for chamber quantification. Eur J Echocardiogr 7: 79–108, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Khoo CW, Krishnamoorthy S, Lim HS, Lip GYH: Assessment of left atrial volume: A focus on echocardiographic methods and clinical implications. Clin Res Cardiol 100: 97–105, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Manning WJ, Silverman DI, Katz SE, Douglas PS: Atrial ejection force: A noninvasive assessment of atrial systolic function. J Am Coll Cardiol 22: 221–225, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Cerasola G, Nardi E, Palermo A, Mulè G, Cottone S: Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: A review. J Nephrol 24: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB: Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J Am Coll Cardiol 47: 1018–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS: Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol 96: 832–836, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - A position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Burbury K, Wheeler D: Premature cardiovascular disease in chronic renal failure. Lancet 356: 147–152, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ: The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 50: 217–224, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ducloux D, Kazory A, Chalopin J-M: Predicting coronary heart disease in renal transplant recipients: A prospective study. Kidney Int 66: 441–447, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kasiske BL, Chakkera HA, Roel J: Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol 11: 1735–1743, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Schiffrin EL, Lipman ML, Mann JF: Chronic kidney disease: Effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Mondelli JA, Di Luzio S, Nagaraj A, Kane BJ, Smulevitz B, Nagaraj AV, Greene R, McPherson DD, Rigolin VH: The validation of volumetric real-time 3-dimensional echocardiography for the determination of left ventricular function. J Am Soc Echocardiogr 14: 994–1000, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Gopal AS, Shen Z, Sapin PM, Keller AM, Schnellbaecher MJ, Leibowitz DW, Akinboboye OO, Rodney RA, Blood DK, King DL: Assessment of cardiac function by three-dimensional echocardiography compared with conventional noninvasive methods. Circulation 92: 842–853, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Döring A, Broeckel U, Riegger G, Schunkert H: Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J 24: 320–328, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB: Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90: 1284–1289, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA: Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study). Am J Cardiol 97: 83–89, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA: Left atrial volume: A powerful predictor of survival after acute myocardial infarction. Circulation 107: 2207–2212, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.