Abstract
Background and objectives
Patients with CKD receiving maintenance dialysis are at risk for upper gastrointestinal bleeding. However, the risk of upper gastrointestinal bleeding in patients with early CKD who are not receiving dialysis is unknown. The hypothesis was that their risk of upper gastrointestinal bleeding is negatively linked to renal function. To test this hypothesis, the association between eGFR and risk of upper gastrointestinal bleeding in patients with stages 3–5 CKD who were not receiving dialysis was analyzed.
Design, setting, participants, & measurements
Patients with stages 3–5 CKD in the CKD program from 2003 to 2009 were enrolled and prospectively followed until December of 2012 to monitor the development of upper gastrointestinal bleeding. The risk of upper gastrointestinal bleeding was analyzed using competing-risks regression with time-varying covariates.
Results
In total, 2968 patients with stages 3–5 CKD who were not receiving dialysis were followed for a median of 1.9 years. The incidence of upper gastrointestinal bleeding per 100 patient-years was 3.7 (95% confidence interval, 3.5 to 3.9) in patients with stage 3 CKD, 5.0 (95% confidence interval, 4.8 to 5.3) in patients with stage 4 CKD, and 13.9 (95% confidence interval, 13.1 to 14.8) in patients with stage 5 CKD. Higher eGFR was associated with a lower risk of upper gastrointestinal bleeding (P=0.03), with a subdistribution hazard ratio of 0.93 (95% confidence interval, 0.87 to 0.99) for every 5 ml/min per 1.73 m2 higher eGFR. A history of upper gastrointestinal bleeding (P<0.001) and lower serum albumin (P=0.004) were independently associated with higher upper gastrointestinal bleeding risk.
Conclusions
In patients with CKD who are not receiving dialysis, lower renal function is associated with higher risk for upper gastrointestinal bleeding. The risk is higher in patients with previous upper gastrointestinal bleeding history and low serum albumin.
Keywords: CKD, gastrointestinal complications, GFR
Introduction
Upper gastrointestinal bleeding (UGIB) is a common disorder in patients with CKD (1). In patients with CKD, UGIB-related hospitalization is associated with a 3-fold increase in in-hospital mortality (2). Risk factors of UGIB include diabetes mellitus, coronary artery disease (CAD) (3), cirrhosis (4,5), use of nonsteroidal anti-inflammatory drugs (NSAIDs) (5), and history of UGIB in patients with CKD (6). Most of the available studies on UGIB in CKD are in stage 5 patients receiving maintenance dialysis (1,3,5). The risk of UGIB in patients with early CKD not receiving dialysis is unknown, but in a recent report, the relative risk of UGIB in this population was 1.95 for patients with stages 4 and 5 CKD (5). Therefore, we hypothesize that the risk of UGIB is negatively linked to renal function, which is indicated by eGFR in patients with CKD not receiving dialysis. To test this hypothesis, we analyzed the association between eGFR and risk of UGIB in patients with stages 3–5 CKD not receiving dialysis.
Materials and Methods
All patients with stages 3–5 CKD in the outpatient-based CKD program of the China Medical University Hospital from June of 2003 to December of 2009 were analyzed. All patients were followed to the date of the first instance of UGIB; the initiation of RRT, including hemodialysis, peritoneal dialysis, or kidney transplant; loss to follow-up; death; or September of 2012. Internal review board approval (DMR 99-IRB-301) was obtained for the review of medical records, and the need for informed consent was waived.
UGIB was defined as melena, hematemesis, rectorrhagia, or the presence of red blood in gastric lavage fluid together with observation of a bleeding lesion or a lesion likely to have bled on endoscopy of the upper digestive tract (4). A past history of UGIB was recorded on the basis of patients’ medical records at enrollment. CAD was defined as a positive exercise test, angiographic findings of at least one stenosis of >50%, or positive findings on scintigraphy (7). Diabetes mellitus was defined as use of insulin, use of a hypoglycemic agent, or a fasting plasma glucose level of 126 mg/dl or more (8). Hypertension was defined as taking antihypertensives without regard to the actual measurement of BP or having a systolic BP reading >140 mmHg or a diastolic BP reading >90 mmHg (9). Helicobacter pylori infection was defined as the presence of the organism on endoscopic antral biopsies or a positive Campylobacter-like organism test (10).
Of 497 (16.8%) patients who underwent endoscopy, 126 (25.4%) patients had positive Campylobacter-like organism tests. The primary care physician who enrolled the patient diagnosed CKD. A study nurse reviewed all the medical records and consulted a physician over uncertainties. The study nurse and physician were blinded to the aim of the study, and other physicians performed the analysis. Body mass index, systolic BP, diastolic BP, hemoglobin, platelet count, BUN, and creatinine were measured at enrollment. Serum uric acid, calcium, phosphorus, albumin, cholesterol, triglyceride, and fasting blood glucose were measured within 3 months. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (11). The equation for white or other race was used, because the studied patients were all Chinese. The time-varying analysis used an average of three eGFR measurements. Medications that may be associated with a higher risk of UGIB, including NSAIDs, aspirin, and warfarin, were also reviewed. Patient-reported use of NSAIDs before 3 months of enrollment was recorded in the CKD program database and considered in the analysis.
Data are reported as mean±SD, median (interquartile range), or frequency (percentage). All continuous variables were tested using skewedness and kurtosis test for their normality. Data were analyzed using the t test for parametric variables, the Kolmogorov–Smirnov test for nonparametric variables, and one-way ANOVA with a Holm–Sidak post hoc analysis or chi-squared test for categorical variables. Risk factors of UGIB were analyzed using univariate competing-risks regression followed by multivariate competing-risks regression with time-varying covariates. The competing-risks regression was used, because the Cox regression may overestimate the risk of UGIB when the competing events were not analyzed (12).
Subdistribution hazard ratio (SHR) and 95% confidence interval (95% CI) were calculated. All patients were followed from the date of enrollment to the date of the first instance of UGIB, competing events, or September of 2012. The primary outcome was defined as UGIB; the competing events included death, initiation of dialysis, kidney transplant, or loss to follow-up. All analyses were performed using Stata, version 12 SE (StataCorp.); P<0.05 was considered statistically significant.
Results
Patient Characteristics
The patients’ clinical characteristics are presented in Table 1. In total, 2968 patients with stages 3–5 CKD (1354 women and 1614 men; average age, 65.5±13.3 years) not receiving dialysis were analyzed. In a median of 1.9 (95% CI, 0.8 to 3.3) years of follow-up, 386 (13%) patients developed UGIB, 762 (25.7%) patients were lost to follow-up, and 754 (25.4%) patients started RRT. Of 2968 patients, 806 patients had stage 3 CKD, 877 patients had stage 4 CKD, and 1285 patients had stage 5 CKD without RRT. The incidence of UGIB was 3.7 (95% CI, 3.5 to 3.9) per 100 patient-years in stage 3 CKD, 5.0 (95% CI, 4.8 to 5.3) per 100 patient-years in stage 4 CKD, and 13.9 (95% CI, 13.1 to 14.8) per 100 patient-years in stage 5 CKD. The incidence of overall mortality was 1.7 (95% CI, 1.6 to 1.8) per 100 patient-years in stage 3 CKD, 2.8 (95% CI, 2.6 to 2.9) per 100 patient-years in stage 4 CKD, and 4.1 (95% CI, 3.9 to 4.4) per 100 patient-years in stage 5 CKD. Among patients with stage 3 CKD, 76 (9.4%) patients progressed to stage 5 CKD (44 of 76 patients started RRT), and 86 (10.7%) patients progressed to stage 4 CKD. Among 877 patients with stage 4 CKD, 283 (32.3%) patients progressed to stage 5 CKD; 157 of these patients started RRT during follow-up. Patients with stage 4 CKD were older than patients with stages 3 and 5 CKD. The body mass indices of patients with stages 4 and 5 CKD were significantly lower than the indices of patients with stage 3 CKD. Systolic BP, BUN, creatinine, and serum phosphorus were higher with higher stage of CKD. Hemoglobin, serum calcium, and serum albumin values were lower with the progression of CKD. The percentage of patients taking NSAIDs, aspirin, and warfarin and the prevalence of H. pylori infection were not different among patients with the various stages of CKD.
Table 1.
Clinical characteristics of patients with stages 3–5 CKD
| Patients' Characteristics | CKD Stage | ||
|---|---|---|---|
| 3 (n=806) | 4 (n=877) | 5 (n=1285) | |
| Age, yr | 66±19a | 70±18a,b | 66±18b |
| Men, n (%) | 581 (72.1)b | 469 (53.5) | 564 (43.9)b |
| Follow-up, yr | 3.6 (2.6–4.9)a,c | 2.9 (1.6–4.3)a,b | 1.8 (0.8–3.3)b,c |
| Lost to follow-up | 201 (24.9) | 257 (29.3) | 304 (23.7) |
| Underlying disease | |||
| Diabetes mellitus | 182 (31.7)a,c | 297 (39.5)a | 425±40.6c |
| Chronic GN | 195 (33.9)a | 229 (30.5)a,b | 341±32.6b |
| Hypertension | 98 (17.0)c | 116 (15.4) | 125±11.9c |
| History of upper gastrointestinal bleeding | 41 (5.1)a | 89 (10.2)a | 96±7.5 |
| H. pylori infection | 34 (4.2) | 34 (3.9) | 58±4.5 |
| Coronary artery disease | 97 (12.0) | 122 (13.9) | 156±12.2 |
| Nonsteroidal anti-inflammatory drugs | 40 (5.0) | 62 (7.1) | 55±4.3 |
| Aspirin | 113 (14.0) | 103 (11.7) | 127±9.9 |
| Warfarin | 17 (2.1) | 25 (2.9) | 22±1.7 |
| eGFR, ml/min per 1.73 m2 | 40±12a,c | 22±7a,b | 8±5b,c |
| Body mass index, kg/m2 | 24.6±4.8a,c | 23.8±4.8a | 23.4±4.9c |
| Systolic BP, mmHg | 131±22a,c | 135±25a,b | 140±25b,c |
| Diastolic BP, mmHg | 80±15 | 80±13 | 80±15 |
| Hemoglobin, g/dl | 12.7±3.2a,c | 10.7±2.4a,b | 9.2±2.2b,c |
| Platelet count, 1000/μl | 212 (200–225) | 210 (186–223) | 209 (174–233) |
| BUN, mg/dl | 24±10a,c | 37±19a,b | 66±36b,c |
| Creatinine, mg/dl | 1.6±0.4a,c | 2.6±0.9a,b | 5.7±3.3b,c |
| Uric acid, mg/dl | 8.2±2.6 | 8.3±2.5 | 8.0±2.6 |
| Calcium, mg/dl | 9.2±0.7c | 9.1±0.6b | 8.8±0.9b,c |
| Phosphorus, mg/dl | 4.1±1.2a,c | 4.4±1.3a,b | 5.5±1.6b,c |
| Albumin, g/dl | 3.8±0.6a,c | 3.6±0.8a,b | 3.3±0.8b,c |
| Cholesterol, mg/dl | 190 (165–220)a,c | 180 (155–214)a | 179 (153–212)c |
| Triglyceride, mg/dl | 133 (89–198) | 127 (91–184) | 125 (88–174) |
| Fasting blood glucose, mg/dl | 111 (98–143)c | 116 (100–147) | 113 (97–146)c |
Data are reported as mean±SD, median (interquartile range), or frequency (percentage).
P<0.05 in CKD 4 versus CKD 3.
P<0.05 in CKD 5 versus CKD 4.
P<0.05 in CKD 5 versus CKD 3.
Characteristics of Patients with UGIB
The characteristics of patients with and without UGIB are summarized in Table 2. Patients who developed UGIB were older than patients who did not develop UGIB, and previous history of UGIB was found in 17.2% of patients who developed UGIB and 6.2% of patients without UGIB (P<0.001). Diabetes mellitus was the underlying cause of kidney disease in 45.7% of patients with UGIB and 36.9% of patients without UGIB (P=0.002). The prevalence of hypertension as an underlying cause of kidney disease was lower in patients with UGIB than patients without UGIB. The prevalence of H. pylori infection was higher in UGIB patients. The prevalence of CAD was not different between patients with and without UGIB. The eGFR, hemoglobin, serum calcium, and serum albumin values were lower in patients with UGIB, whereas BUN, serum phosphorus, fasting blood glucose, and serum creatinine values were higher in patients with UGIB. The percentages of patients who took NSAIDs, aspirin, and warfarin were not different between patients with and without UGIB.
Table 2.
Clinical characteristics of patients with and without upper gastrointestinal bleeding
| Patients' Characteristics | Upper Gastrointestinal Bleeding (−; n=2584) | Upper Gastrointestinal Bleeding (+; n=384) | P Value |
|---|---|---|---|
| Age, yr | 67±19 | 68±17 | 0.02 |
| Men, n (%) | 1399 (54.1) | 215 (56.0) | 0.46 |
| Follow-up, yr | 1.9 (2.5) | 1.8 (2.4) | 0.99 |
| Underlying disease | |||
| Diabetes mellitus | 761 (36.9) | 143 (45.7) | 0.002 |
| Chronic GN | 671 (32.6) | 94 (30.0) | 0.53 |
| Hypertension | 314 (15.2) | 25 (8.0) | 0.001 |
| History of upper gastrointestinal bleeding | 160 (6.2) | 66 (17.2) | <0.001 |
| H. pylori infection | 54 (2.1) | 72 (18.8) | <0.001 |
| Coronary artery disease | 318 (12.3) | 57 (14.8) | 0.16 |
| Nonsteroidal anti-inflammatory drugs | 132 (5.2) | 23 (6.0) | 0.47 |
| Aspirin | 300 (11.5) | 45 (11.7) | 0.95 |
| Warfarin | 52 (2.0) | 12 (3.1) | 0.16 |
| eGFR, ml/min per 1.73 m2 | 18.4±23.0 | 13.9±16.8 | <0.001 |
| Body mass index, kg/m2 | 23.9±4.9 | 23.9±5.1 | 0.40 |
| Systolic BP, mmHg | 136±25 | 140±26 | 0.08 |
| Diastolic BP, mmHg | 80±15 | 80±14 | 0.23 |
| Hemoglobin, g/dl | 10.3±3.2 | 9.9±2.8 | <0.001 |
| Platelet count, 1000/μl | 203 (86–221) | 205 (170–241) | 0.92 |
| BUN, mg/dl | 42±38 | 49±38 | <0.001 |
| Creatinine, mg/dl | 2.9±3.3 | 3.6±3.6 | <0.001 |
| Uric acid, mg/dl | 8.1±2.6 | 8.3±2.8 | 0.08 |
| Calcium, mg/dl | 9.1±0.7 | 8.9±0.8 | 0.04 |
| Phosphorus, mg/dl | 4.7±1.7 | 4.9±1.6 | 0.04 |
| Albumin, g/dl | 3.5±0.8 | 3.3±0.8 | <0.001 |
| Cholesterol, mg/dl | 183 (157–216) | 179 (149–210) | 0.34 |
| Triglyceride, mg/dl | 126 (89–183) | 128 (90–185) | 0.50 |
| Fasting blood glucose, mg/dl | 112 (97–143) | 123 (102–160) | 0.03 |
Data are reported as mean±SD, median (interquartile range), or frequency (percentage).
Confounders of UGIB
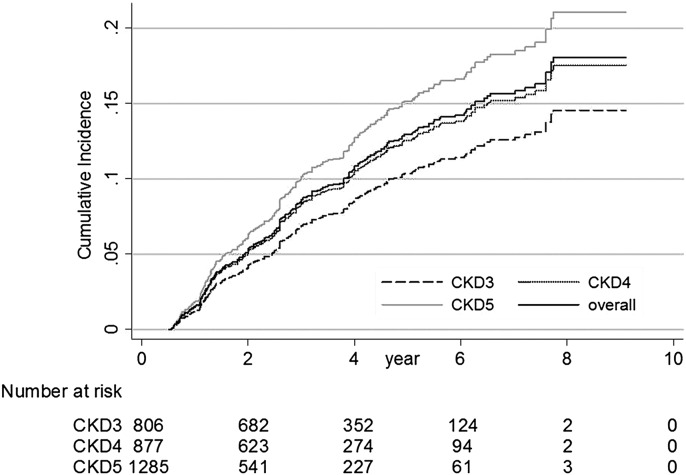
The SHRs of risk factors for UGIB are presented in Table 3. In univariate analysis, a higher eGFR was associated with a lower risk of UGIB (P<0.001), with an SHR of 0.89 (95% CI, 0.86 to 0.93) for every 5 ml/min per 1.73 m2 higher eGFR. The adjusted SHR was 0.90 (95% CI, 0.84 to 0.96; P<0.001) in multivariate competing-risks regression. Age, history of UGIB, diabetes, and serum albumin were associated with UGIB in both univariate and multivariate analyses. The adjusted SHR was 1.09 (95% CI, 1.00 to 1.19; P=0.01) for every 10 additional years, 1.94 (95% CI, 1.36 to 2.83) for history of UGIB, 1.30 (95% CI, 1.03 to 1.63) for diabetes, and 0.80 (95% CI, 0.67 to 0.96) for every 1 g/dl higher serum albumin (SHR, 0.66; 95% CI, 0.57 to 0.76 in univariate analysis). H. pylori infection, hemoglobin, and BUN were significantly associated with UGIB in univariate analysis but not multivariate analysis. The use of NSAIDs, aspirin, or warfarin was not associated with higher UGIB risk. The UGIB risk of all patients, according to CKD stage, was analyzed using competing-risks regression with adjustments for age, sex, diabetes, history of UGIB, history of H. pylori infection, hemoglobin, BUN, and serum albumin (Figure 1). The cumulative incidence of UGIB was higher in patients with advanced CKD stages; it was lowest in patients with stage 3 CKD and highest in patients with stage 5 CKD.
Table 3.
Subdistribution hazard ratio of risk factors for upper gastrointestinal bleeding in univariate and multivariate competing-risks regression with time-varying covariates
| Risk Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| SHR | 95% Confidence Interval | Adjusted SHR | 95% Confidence Interval | |
| eGFR (every 5-ml/min per 1.73 m2 incremental increase) | 0.89 | 0.86 to 0.93 | 0.90 | 0.84 to 0.96 |
| Age (every 10-yr incremental increase) | 1.12 | 1.03 to 1.21 | 1.09 | 1.00 to 1.19 |
| Men | 1.04 | 0.85 to 1.29 | 1.29 | 0.85 to 1.63 |
| History of upper gastrointestinal bleeding | 2.67 | 2.00 to 3.56 | 1.94 | 1.36 to 2.83 |
| H. pylori | 2.56 | 1.65 to 3.97 | 1.63 | 0.93 to 2.90 |
| Diabetes mellitus | 1.41 | 1.14 to 1.73 | 1.30 | 1.03 to 1.63 |
| Nonsteroidal anti-inflammatory drugs | 1.03 | 0.65 to 1.65 | 1.52 | 0.65 to 3.61 |
| Aspirin | 1.03 | 0.75 to 1.42 | 1.08 | 0.77 to 1.54 |
| Warfarin | 1.66 | 0.91 to 3.03 | 1.59 | 0.79 to 3.19 |
| Hemoglobin (every 1-g/dl incremental increase) | 0.90 | 0.86 to 0.95 | 0.95 | 0.88 to 1.02 |
| BUN (every 10-mg/dl incremental increase) | 1.05 | 1.03 to 1.08 | 1.01 | 0.97 to 1.06 |
| Albumin (every 1-g/dl incremental increase) | 0.66 | 0.57 to 0.76 | 0.80 | 0.67 to 0.96 |
SHR, subdistribution hazard ratio.
Figure 1.
Cumulative incidence of upper gastrointestinal bleeding (UGIB) in patients with stages 3–5 CKD using competing-risks regression with adjustments for age, sex, diabetes, history of UGIB, H. pylori infection, hemoglobin, BUN, and albumin (UGIB was the primary outcome; competing events included death, initiation of dialysis, kidney transplant, or loss to follow-up).
Confounders of UGIB in Patients without a History of UGIB
Confounders of UGIB were further analyzed in patients without a history of UGIB (Table 4). eGFR (P<0.01), age (P=0.03), H. pylori infection (P=0.04), and serum albumin (P=0.01) were independently associated with UGIB risk. The adjusted SHR was 0.91 (95% CI, 0.85 to 0.98) for every 5 ml/min per 1.73 m2 higher eGFR, 1.12 (95% CI, 1.02 to 1.23) for every 10 additional years, 2.26 (95% CI, 1.02 to 5.00) for H. pylori infection, and 0.78 (95% CI, 0.63 to 0.95) for every 1 mg/dl higher serum albumin.
Table 4.
Subdistribution hazard ratio of risk factors for upper gastrointestinal bleeding in patients without a history of upper gastrointestinal bleeding using multivariate competing-risks regression with time-varying covariates
| Risk Factors | Adjusted SHR | 95% Confidence Interval | P Value |
|---|---|---|---|
| eGFR (every 5-ml/min per 1.73 m2 incremental increase) | 0.91 | 0.85 to 0.98 | <0.01 |
| Age (every 10-yr increase) | 1.12 | 1.01 to 1.23 | 0.03 |
| H. pylori infection | 2.26 | 1.02 to 5.00 | 0.04 |
| Diabetes mellitus | 1.23 | 0.95 to 1.58 | 0.11 |
| Hemoglobin (every 1-g/dl incremental increase) | 0.97 | 0.91 to 1.05 | 0.47 |
| BUN (every 10-mg/dl incremental increase) | 0.97 | 0.92 to 1.03 | 0.30 |
| Albumin (every 1-g/dl incremental increase) | 0.78 | 0.63 to 0.95 | 0.01 |
Discussion
Competing-risks regression analysis of this observational cohort study of patients with stages 3–5 CKD who were not receiving dialysis showed that every 5 ml/min per 1.73 m2 increase in eGFR was associated with a 10% lower UGIB risk. The risk of UGIB was higher when the risk of UGIB was estimated using Cox regression, because patients who died, started dialysis, or were lost to follow-up were not taken into consideration. Therefore, a competing-risks regression analysis may be a better method for investigating the risk of UGIB in this study. Our findings were consistent with the findings of a previous study (5) showing a higher incidence of UGIB in patients with stages 4 and 5 CKD not receiving dialysis. This study further showed a negative association between renal function and UGIB. This association may be explained by the presence of elevated serum gastrin levels (13,14), which are higher in patients with CKD (15), and/or a higher frequency vascular ectasia (16).
Antithrombotic (17) or antiplatelet medications (18) that are commonly used in patients with CAD also may contribute to the higher risk of UGIB in patients with CKD, given the strong association between CKD and CAD. We reviewed the use of aspirin and warfarin in our patients, but these medications were not linked to a higher risk of UGIB; this finding is consistent with the other studies that were unable to show an association between UGIB and antithrombotic medications (3). BUN and hemoglobin were significantly associated with UGIB in univariate competing-risks regression but not multivariate competing-risks regression that includes renal function in the model. eGFR was positively correlated with hemoglobin and negatively correlated with BUN. The absence of statistical significance for BUN and hemoglobin in multivariate analysis may be explained by their association with eGFR. The SHR of eGFR was similar in univariate (0.89) and multivariate (0.90) competing-risks regressions, suggesting that eGFR is the main effect variable. The association between lower serum albumin values and higher UGIB risk is also supported by a previous study in patients with CKD receiving dialysis (19).
A history of UGIB is an important risk factor but has not been taken into consideration in previous studies (1–3,6,20–22). Our study showed that patients with CKD and a history of UGIB had a 2-fold higher risk of UGIB. In addition, H. pylori infection has been associated with a higher risk of UGIB (23,24), and H. pylori eradication therapy decreases the risk of recurrent UGIB (25). Forty-two (1.4%) patients were receiving H. pylori eradication therapy; this therapy did not significantly affect UGIB risk. This lack of effect may be because the number of patients receiving H. pylori eradication therapy was not sufficient to generate statistical significance.
It is unclear if the risk of UGIB in patients with stage 3 CKD in our study was higher than that in a control Chinese population. However, the reported incidence (3.7 per 100 patient-years) of UGIB in patients with stage 3 CKD was higher than the incidence reported in the general population (5). Patients with stages 1 and 2 CKD were not analyzed in this study, because there were insufficient enrollees in these stages. In addition, patients who were lost to follow-up were older and predominately in stages 5 (39.9%), 4 (33.7%), and 3 (26.4%) CKD.
The limitations of this study were its observational design and inclusion of data from only one Chinese hospital; studies of various ethnic groups and multiple institutions are required to determine if our results can be generalized to other populations. Despite its limitations, this study clearly showed a strong association between renal function and UGIB independent of confounding factors that were considered. Additional studies are needed to determine if treatment of CKD with agents, such as angiotensin II blockers, statins, phosphate binders, or charcoal absorbent, will decrease the UGIB risk in patients with CKD.
In conclusion, in patients with CKD, a higher renal function was associated with a lower risk of UGIB. The association between renal function and risk of UGIB was independent of a history of UGIB, H. pylori infection, age, and serum albumin levels.
Disclosures
None.
Acknowledgments
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004) and China Medical University Hospital (DMR-101-019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Yang JY, Lee TC, Montez-Rath ME, Paik J, Chertow GM, Desai M, Winkelmayer WC: Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol 23: 495–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sood P, Kumar G, Nanchal R, Sakhuja A, Ahmad S, Ali M, Kumar N, Ross EA: Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am J Nephrol 35: 216–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasse H, Gillen DL, Ball AM, Kestenbaum BR, Seliger SL, Sherrard D, Stehman-Breen CO: Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int 64: 1455–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bernard B, Cadranel JF, Valla D, Escolano S, Jarlier V, Opolon P: Prognostic significance of bacterial infection in bleeding cirrhotic patients: A prospective study. Gastroenterology 108: 1828–1834, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Luo JC, Leu HB, Huang KW, Huang CC, Hou MC, Lin HC, Lee FY, Lee SD: Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. CMAJ 183: E1345–E1351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN: Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc 71: 44–49, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J, PROactive investigators : Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 366: 1279–1289, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ: Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 165: 863–867, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Lavie P, Herer P, Hoffstein V: Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ 320: 479–482, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slomianski A, Schubert T, Cutler AF: [13C]urea breath test to confirm eradication of Helicobacter pylori. Am J Gastroenterol 90: 224–226, 1995 [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 13.Lindström E, Chen D, Norlén P, Andersson K, Håkanson R: Control of gastric acid secretion:the gastrin-ECL cell-parietal cell axis. Comp Biochem Physiol A Mol Integr Physiol 128: 505–514, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Konturek JW, Konturek SJ, Domschke W: Cholecystokinin in the control of gastric acid secretion and gastrin release in response to a meal at low and high pH in healthy subjects and duodenal ulcer patients. Scand J Gastroenterol 30: 738–744, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Korman MG, Laver MC, Hansky J: Hypergastrinaemia in chronic renal failure. BMJ 1: 209–210, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalasani N, Cotsonis G, Wilcox CM: Upper gastrointestinal bleeding in patients with chronic renal failure: Role of vascular ectasia. Am J Gastroenterol 91: 2329–2332, 1996 [PubMed] [Google Scholar]
- 17.Elliott MJ, Zimmerman D, Holden RM: Warfarin anticoagulation in hemodialysis patients: A systematic review of bleeding rates. Am J Kidney Dis 50: 433–440, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, Califf RM, Topol EJ, CREDO Investigators : The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: Results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J 155: 687–693, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Chen YT, Yang WC, Lin CC, Ng YY, Chen JY, Li SY: Comparison of peptic ulcer disease risk between peritoneal and hemodialysis patients. Am J Nephrol 32: 212–218, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Müller T, Barkun AN, Martel M: Non-variceal upper GI bleeding in patients already hospitalized for another condition. Am J Gastroenterol 104: 330–339, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Åhsberg K, Höglund P, Staël von Holstein C: Mortality from peptic ulcer bleeding: The impact of comorbidity and the use of drugs that promote bleeding. Aliment Pharmacol Ther 32: 801–810, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Lin SC, Wu KL, Chiu KW, Lee CT, Chiu YC, Chou YP, Hu ML, Tai WC, Chiou SS, Hu TH, Changchien CS, Chuah SK: Risk factors influencing the outcome of peptic ulcer bleeding in end stage renal diseases after initial endoscopic haemostasis. Int J Clin Pract 66: 774–781, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Kang JY, Ho KY, Yeoh KG, Guan R, Wee A, Lee E, Lye WC, Leong SO, Tan CC: Peptic ulcer and gastritis in uraemia, with particular reference to the effect of Helicobacter pylori infection. J Gastroenterol Hepatol 14: 771–778, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y: Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int 75: 96–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng GY, Lin HJ, Fang CT, Yang HB, Tseng GC, Wang PC, Hung TL, Deng YC, Cheng YT, Huang CH: Recurrence of peptic ulcer in uraemic and non-uraemic patients after Helicobacter pylori eradication: A 2-year study. Aliment Pharmacol Ther 26: 925–933, 2007 [DOI] [PubMed] [Google Scholar]