Abstract
Background and objectives
Novel markers may help to improve risk prediction in CKD. One potential candidate is tissue advanced glycation end product accumulation, a marker of cumulative metabolic stress, which can be assessed by a simple noninvasive measurement of skin autofluorescence. Skin autofluorescence correlates with higher risk of cardiovascular events and mortality in people with diabetes or people requiring RRT, but its role in earlier CKD has not been studied.
Design, setting, participants, & measurements
A prospective cohort of 1741 people with CKD stage 3 was recruited from primary care between August 2008 and March 2010. Participants underwent medical history, clinical assessment, blood and urine sampling for biochemistry, and measurement of skin autofluorescence. Kaplan–Meier plots and multivariate Cox proportional hazards models were used to investigate associations between skin autofluorescence (categorical in quartiles) and all-cause mortality.
Results
In total, 1707 participants had skin autofluorescence measured; 170 (10%) participants died after a median of 3.6 years of follow-up. The most common cause of death was cardiovascular disease (41%). Higher skin autofluorescence was associated significantly with poorer survival (all-cause mortality, P<0.001) on Kaplan–Meier analysis. Univariate and age/sex-adjusted Cox proportional hazards models showed that the highest quartile of skin autofluorescence was associated with all-cause mortality (hazard ratio, 2.64; 95% confidence interval, 1.71 to 4.08; P<0.001 and hazard ratio, 1.84; 95% confidence interval, 1.18 to 2.86; P=0.003, respectively, compared with the lowest quartile). This association was not maintained after additional adjustment to include cardiovascular disease, diabetes, smoking, body mass index, eGFR, albuminuria, and hemoglobin.
Conclusions
Skin autofluorescence was not independently associated with all-cause mortality in this study. Additional research is needed to clarify whether it has a role in risk prediction in CKD.
Keywords: CKD, glycation, mortality
Introduction
In the context of CKD, accumulation of advanced glycation end products (AGEs) has been identified as a novel risk factor for cardiovascular disease (CVD) (1). AGEs are a heterogeneous group of compounds formed by the reaction of free amino groups on proteins, lipids, and nucleic acids with reactive carbonyl groups on reducing sugars. They accumulate by endogenous formation through nonenzymatic reaction over time (the Maillard reaction) or from reactive carbonyl products generated by oxidative stress (dicarbonyl stress). Accumulation also occurs from exogenous sources, principally food cooked at high temperature, and AGEs formed by smoking. In addition, AGEs are normally excreted by the kidneys and therefore, accumulate with decreased renal function (2–6). In CKD, AGE accumulation may be exacerbated by increased formation, because dicarbonyl and oxidative stress are increased with reduced renal function, and there is increased availability of precursor compounds (such as oxidized ascorbic acid arising in people on hemodialysis) (7). AGE formation is a marker of cumulative metabolic stress, adversely influencing the ageing process and development and progression of chronic disease across the life course (8–11).
Serum AGEs are subject to fluctuation and have been shown to be a poor indicator of AGE accumulation in tissue compared with skin biopsy (12). Assessment of AGE accumulation in practice has been simplified by devices that measure skin autofluorescence (AF). AF allows for noninvasive assessment of tissue AGE deposition by exploiting the close correlation between collagen-linked fluorescence and AGE content observed in skin biopsies (13). Skin AF measurement has been validated against levels of specific AGE molecules in patients with diabetes and CKD and healthy controls (14–18). Increased skin AF is associated with AGE accumulation and development of a range of vascular complications as well as all-cause and cardiovascular mortality in people with diabetes (14–18). In people with end-stage kidney disease on dialysis, skin AF is associated with arterial stiffness (19). Skin AF is also independently associated with cardiovascular and all-cause mortality in hemodialysis patients (20,21).
Potential for reversibility of AGE accumulation has been observed in patients moving from dialysis to renal transplant (22). In earlier CKD, increased skin AF has been shown to be associated with a wide range of poor prognostic factors, including anemia, proteinuria, diabetes, age, and eGFR, in cross-sectional analysis (2). Dietary modification to reduce exogenous AGEs may be important (4). However, the relationship between elevated skin AF and subsequent adverse outcomes in earlier stages of CKD is not yet known. In this cohort study of people with CKD stage 3 in primary care, we aimed to evaluate the association between skin AF and all-cause mortality.
Materials and Methods
Participants
Participants were recruited from 32 general practitioner surgeries for the Renal Risk in Derby (RRID) study, a prospective cohort study of CKD stage 3 in a primary care setting. Detailed methods for the RRID study have been published elsewhere (2). Eligible participants were 18 years or over, met the Kidney Disease Outcomes Quality Initiative criteria for CKD stage 3 (eGFR=30–59 ml/min per 1.73 m2 on two occasions at least 3 months apart, including the most recent value before the baseline visit), were able to attend their general practitioner surgery, and gave informed consent. People with previous transplant or terminal illness were excluded. Screening and baseline visits were combined because of the large proportion of elderly participants and the logistical challenges of conducting study visits in multiple primary care centers. First study visits occurred from August of 2008 to March of 2010. The questionnaire information was checked, anthropomorphic measurements were taken, urinalysis was performed, and blood specimens were taken. All participants provided written informed consent. The study was approved by Nottingham Research Ethics Committee 1 and included on the National Institute for Health Research Clinical Research Portfolio (Study ID 6632).
Definitions
Previous cardiovascular event was defined as participant-reported myocardial infarction, stroke, transient ischemic attack, revascularization, or amputation caused by peripheral vascular disease or aortic aneurysm. Smoking status was categorized as never smoked, ex-smoker, and current smoker. Socioeconomic status (SES) was defined by two methods. The first method was the Indices of Multiple Deprivation score, a social deprivation score comprising a composite measure of seven domains that show a strong relationship to health in all geographical locations (23). The second method was self-reported education status, an important indicator of SES in elderly populations (24). Education status was categorized into three groups (1, no formal qualifications; 2, school or equivalent qualifications; 3, degree or equivalent). Self-reported ethnicity information was collected. Because of the small number of nonwhite participants in this study, it was categorized into white and other for analysis. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (25). Albuminuria was defined as the albumin-to-creatinine ratio (uACR)≥2.5 mg/mmol in men and uACR≥3.5 mg/mmol in women in at least two of three urine specimens. uACR was fitted as a continuous variable in regression analyses as log of the mean of three uACR values. Body mass index (BMI) was calculated from weight in kilograms divided by height squared in meters. Central obesity was defined as a waist-to-hip ratio of ≥0.9 for men or ≥0.8 for women (26). Diabetes was defined by self-report of having a previous clinical diagnosis in line with World Health Organization criteria (27).
Skin AF
Skin AF was assessed on the left forearm using an AGE Reader device (DiagnOptics, Groningen, The Netherlands). Three readings were taken, and the average was calculated. Care was taken to avoid areas of skin that were tattooed or colored with cosmetics, were heavily freckled, or had vessels near to the surface of the skin. It was not possible to conduct skin AF readings on very dark or black skin. According to the manufacturer, the AGE Reader and its software have been validated in patients with skin reflection>6% (Fitzpatrick classes 1–4). In patients with darker skin color (Fitzpatrick classes 5 and 6 [dark brown or black]), a correction is made to the skin AF value if the ultraviolet reflectance is between 6% and 8%. If ultraviolet reflectance is below 6%, the AGE Reader gives a warning that the signal is too low for valid results. Skin AF measurement is not operator-dependent. Values are expressed in arbitrary units (AU). Coefficient of variation for 10 skin AF readings obtained on a single patient by single operator was 7%. Ten readings performed by 10 different operators yielded a coefficient of variation of 8% (2). A single device was used for all of the skin AF measurements in the study. This device was maintained and calibrated by the manufacturer according to their recommendations.
Outcomes
Participants were registered with the Health and Social Care Information Centre to obtain date and cause of death. The observation period was from the date of recruitment to February 24, 2013. Cause of death was as recorded on the death certificate. Causes of death were independently reviewed by three investigators and classified as cardiovascular, cancer, infection, or other. Classification differences were resolved by discussion.
Statistical Analyses
Variables are reported as the mean and SD if normally distributed or the median and interquartile range if not normally distributed. Chi-squared tests for trend (for categorical variables) and one-way ANOVAs (for continuous variables) were used to compare variables across quartiles of skin AF. A Kaplan–Meier plot was used to compare all-cause mortality by quartile of skin AF. Cox regression models were developed with skin AF fitted as a categorical variable (quartiles) with subsequent addition of sociodemographic (age and sex) and then clinical variables (CVD, diabetes, hypertension, smoking, BMI, central obesity, total-to-HDL cholesterol ratio, eGFR, uACR, and hemoglobin). Skin AF was treated as a categorical variable because of a nonlinear relationship being identified between skin AF and all-cause mortality. The final model included variables with a P value <0.10 on univariate analysis. The primary outcome was all-cause mortality. Interactions between skin AF and diabetes were assessed because of the potential for differential variation of skin AF by diabetes status (18). Despite meeting the inclusion criteria (and therefore, having a clinical diagnosis of CKD stage 3), some participants were found to have baseline eGFR≥60 ml/min per 1.73 m2 (possibly because of strict meat-fasted status being observed before the baseline measurement). Sensitivity analyses were, therefore, conducted in only those participants whose baseline eGFR was <60 ml/min per 1.73 m2. Sensitivity analyses were also conducted with cardiovascular mortality as the outcome of interest. SPSS version 19.0 was used for analysis, and P<0.05 was considered statistically significant.
Results
Of 1741 people recruited to the RRID study, 1707 (98%) people had valid measures of skin AF; 34 participants were excluded, because skin AF readings could not be obtained because of dark skin color (n=17) or technical failure (n=17). Despite meeting the inclusion criteria, 410 (24%) participants had eGFR≥60 ml/min per 1.73 m2 at baseline (mean eGFR at baseline in this group=68.4; SD=6.1). The study population was predominantly white (>98%) and elderly (67% over 70 years old), and a high proportion met the study criteria for hypertension (88%). Table 1 shows the baseline characteristics. Mean skin AF was 2.73 AU (SD=0.61), and skin AF was normally distributed.
Table 1.
Baseline characteristics
| Characteristic | Overall | Lowest Quartile of Skin AF | Second Quartile of Skin AF | Third Quartile of Skin AF | Highest Quartile of Skin AF | P Value |
|---|---|---|---|---|---|---|
| Sexa | ||||||
| Men | 671 (39.3) | 156 (36.4) | 161 (35.9) | 164 (38.5) | 190 (47.1) | 0.001 |
| Women | 1036 (60.7) | 273 (63.6) | 288 (64.1) | 262 (61.5) | 213 (52.9) | |
| Age, yra | ||||||
| <60 | 125 (7.3) | 55 (12.8) | 39 (8.7) | 19 (4.5) | 12 (3.0) | <0.001 |
| 60–69 | 439 (25.7) | 137 (31.9) | 123 (27.4) | 98 (23.1) | 81 (20.1) | |
| 70–79 | 738 (43.2) | 164 (38.2) | 196 (43.7) | 190 (44.6) | 122 (46.7) | |
| 80+ | 405 (23.7) | 73 (17.0) | 91 (20.3) | 119 (27.9) | 188 (30.3) | |
| Age, yr | 73±9 | 70±10 | 72±9 | 74±8 | 75±8 | |
| Ethnicity | ||||||
| White | 1681 (98.5) | 416 (97.0) | 448 (99.8) | 419 (98.4) | 398 (98.8) | 0.11 |
| Other | 26 (1.5) | 13 (3.0) | 1 (0.2) | 7 (1.6) | 5 (1.2) | |
| Qualificationsa | ||||||
| No formal qualifications | 935 (54.8) | 188 (43.9) | 234 (52.1) | 258 (60.6) | 255 (63.4) | <0.001 |
| School or equivalent qualifications | 459 (26.9) | 132 (30.8) | 129 (28.7) | 110 (25.8) | 88 (21.9) | |
| Degree or equivalent qualifications | 311 (18.2) | 108 (25.2) | 86 (19.2) | 58 (13.6) | 59 (14.7) | |
| IMD quintilesa | ||||||
| 1 (most deprived) | 139 (8.1) | 35 (8.2) | 34 (7.6) | 31 (7.3) | 39 (9.7) | 0.01 |
| 2 | 426 (25.0) | 93 (21.8) | 109 (24.3) | 117 (27.5) | 107 (26.6) | |
| 3 | 320 (18.7) | 71 (16.6) | 83 (18.5) | 95 (22.4) | 71 (17.6) | |
| 4 | 441 (25.8) | 123 (28.8) | 117 (26.1) | 92 (21.6) | 109 (27.0) | |
| 5 (least deprived) | 378 (22.1) | 105 (24.6) | 106 (23.6) | 90 (21.2) | 77 (19.1) | |
| History of cardiovascular diseasea | 580 (34.0) | 113 (26.3) | 140 (31.2) | 151 (35.4) | 176 (43.7) | <0.001 |
| Diabetesa | 284 (16.6) | 37 (8.6) | 49 (10.9) | 76 (17.8) | 122 (30.3) | <0.001 |
| Hypertension | 1495 (87.6) | 369 (86.0) | 387 (86.2) | 380 (89.2) | 359 (89.1) | 0.09 |
| Average systolic BP, mmHgb | 134±18 | 133±17 | 133±18 | 134±17 | 136±20 | 0.04 |
| Average diastolic BP, mmHgb | 72±11 | 74±11 | 74±11 | 72±10 | 71±11 | <0.001 |
| Smokinga | ||||||
| Current | 79 (4.6) | 16 (3.7) | 13 (2.9) | 17 (4.0) | 33 (8.2) | <0.001 |
| Ex-smoker | 849 (49.7) | 183 (42.7) | 214 (47.7) | 215 (50.5) | 237 (58.8) | |
| Never | 779 (45.6) | 230 (53.6) | 222 (49.4) | 194 (45.5) | 133 (33.0) | |
| Body mass index, kg/m2 | 29.0±5.1 | 28.6±4.5 | 29.4±5.5 | 29.1±5.0 | 29.1±5.4 | 0.19 |
| Waist-to-hip ratiob | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | <0.001 |
| Total-to-HDL cholesterol ratio | 3.5±1.2 | 3.6±1.2 | 3.6±1.3 | 3.5±1.1 | 3.4±1.2 | 0.20 |
| uACR, mg/gc | 3.0 (0.0–13.3) | 1.5 (0.0–7.7) | 2.4 (0.0–9.4) | 3.8 (0.0–13.9) | 6.2 (0.0–28.9) | <0.001 |
| eGFR groups, ml/min per 1.73 m2a | ||||||
| ≥60 | 410 (24.0) | 130 (30.3) | 127 (28.3) | 87 (20.4) | 66 (16.4) | <0.001 |
| 45–59 | 892 (52.3) | 242 (56.4) | 242 (53.9) | 218 (51.2) | 190 (47.1) | |
| 30–44 | 379 (22.2) | 54 (12.6) | 78 (17.4) | 112 (26.3) | 135 (33.5) | |
| <30 | 26 (1.5) | 3 (0.7) | 2 (0.4) | 9 (2.1) | 12 (3.0) | |
| eGFR, ml/min per 1.73 m2b | 52.5±10.4 | 57.0±10.9 | 55.6±11.2 | 52.0±11.9 | 49.1±11.9 | <0.001 |
| Hemoglobin, g/dlb | 13.2±1.4 | 13.6±1.3 | 13.4±1.3 | 13.0±1.4 | 12.8±1.5 | <0.001 |
| Skin autofluorescence, AUb | 2.7±0.6 | 2.0±0.2 | 2.5±0.1 | 2.9±0.1 | 3.57±0.4 | <0.001 |
Data are number (%), mean±SD, or median (interquartile range). AF, autofluorescence; IMD, Index of Multiple Deprivation; uACR, albumin-to-creatinine ratio; AU, arbitrary unit.
Test for trend across quartiles (P<0.05).
One-way ANOVA (P<0.05).
Log uACR used as uACR not normally distributed. One-way ANOVA (P<0.05).
Overall mean follow-up time was 3.6±0.8 years (1317±287 days); 170 (10%) participants died in the follow-up period, and 1537 (90%) participants remained alive. Those participants who died tended to be older, be men, have fewer educational qualifications, have a history of smoking, and have CVD and/or diabetes. The skin AF of people who died tended to be higher than the skin AF of people who did not die (mean=3.0±0.8 versus 2.7±0.6 AU, respectively). The most common cause of death was CVD (41%) followed by cancer (29%).
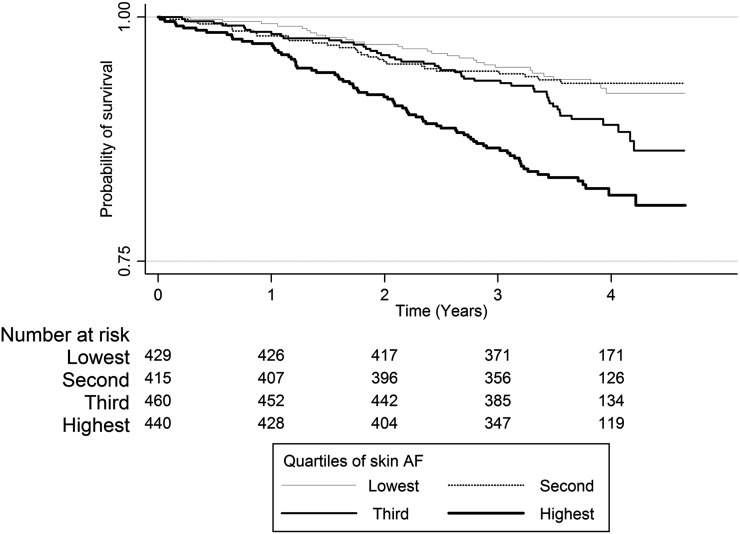
The prevalence of several risk factors for death increased across quartiles of skin AF (Table 1), and a Kaplan–Meier plot showed significantly poorer survival in people in the highest quartile of skin AF (log rank=32.3, P<0.001) (Figure 1). On univariate analyses, skin AF, men, age, history of CVD, diabetes, smoking (current or previous), decreasing eGFR, albuminuria, and lower hemoglobin were associated with increased risk of all-cause mortality. After age/sex adjustment, the relationship between skin AF and all-cause mortality was attenuated (from 2.64 [95% confidence interval (95% CI), 1.71 to 4.08] to 1.84 [95% CI, 1.18 to 2.86]; P=0.003) for the highest compared with the lowest quartile of skin AF.
Figure 1.
The Kaplan–Meier plot shows cumulative survival (all-cause mortality) by quartile of skin autofluorescence. Log rank=32.3; P<0.001. Note that the x axis does not intersect the y axis at zero. Skin autofluorescence (AF) is measured in arbitrary units.
Age, history of CVD, decreasing eGFR, and albuminuria remained significantly associated with increased risk of all-cause mortality in the final model, but skin AF did not (Table 2). No associations were identified between SES and either all-cause or cardiovascular mortality. No interactions were identified.
Table 2.
Hazard ratios for all-cause mortality in the study population
| Variable | Univariate | Final Modela | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Skin autofluorescence (versus lowest quartile) | ||||
| Quartile 2 | 0.98 (0.58 to 1.63) | <0.001b | 0.82 (0.49 to 1.38) | 0.39b |
| Quartile 3 | 1.61 (1.01 to 2.57) | 0.95 (0.59 to 1.53) | ||
| Highest quartile | 2.64 (1.71 to 4.08) | 1.21 (0.76 to 1.93) | ||
| Sex (men versus women) | 1.95 (1.45 to 2.63) | <0.001 | 1.27 (0.91 to 1.78) | 0.16 |
| Age, yr | 1.09 (1.07 to 1.12) | <0.001 | 1.06 (1.04 to 1.09) | <0.001 |
| Cardiovascular disease (versus no cardiovascular disease) | 3.15 (2.33 to 4.26) | <0.001 | 2.17 (1.58 to 2.98) | <0.001 |
| Diabetes (versus no diabetes) | 1.54 (1.09 to 2.19) | 0.02 | 1.10 (0.75 to 1.62) | 0.62 |
| Hypertension (versus no hypertension) | 1.24 (0.76 to 2.03) | 0.38 | ||
| Smoking (versus never smokers) | ||||
| Current smoker | 1.50 (0.74 to 3.02) | 0.01b | 2.06 (0.99 to 4.31) | 0.15 |
| Ex-smoker | 1.67 (1.22 to 2.29) | 1.17 (0.83 to 1.65) | ||
| Body mass index, kg/m2 | 0.97 (0.94 to 1.00) | 0.05 | 0.99 (0.96 to 1.03) | 0.77 |
| Central obesity (versus not centrally obese) | 0.89(0.60 to 1.32) | 0.56 | ||
| Total-to-HDL cholesterol ratio | 0.99 (0.87 to 1.12) | 0.87 | ||
| eGFR, ml/min per 1.73 m2 (continuous) | 0.94 (0.93 to 0.95) | <0.001 | 0.97 (0.95 to 0.99) | <0.001 |
| Log average uACR, mg/mmol | 1.35 (1.22 to 1.51) | <0.001 | 1.15 (1.03 to 1.28) | 0.02 |
| Hemoglobin, g/dl | 0.80 (0.72 to 0.89) | <0.001 | 0.94 (0.84 to 1.05) | 0.30 |
| Qualifications (versus none) | ||||
| School level | 1.56 (1.00 to 2.46) | 0.12b | ||
| Degree or equivalent | 1.27 (0.76 to 2.11) | |||
| IMD quintiles (versus least deprived) | ||||
| 1 (most deprived) | 1.06 (0.55 to 2.05) | 0.75b | ||
| 2 | 1.26 (0.80 to 1.98) | |||
| 3 | 1.22 (0.75 to 1.99) | |||
| 4 | 1.33 (0.86 to 2.07) | |||
All variables are continuous except sex, cardiovascular disease, diabetes, hypertension, smoking status, and central obesity. HR, hazard ratio; 95% CI, 95% confidence interval.
Adjusted for age, sex, cardiovascular disease, diabetes, smoking, body mass index, eGFR, albuminuria, and hemoglobin.
P value for trend.
Sensitivity analyses, including only people with baseline eGFR<60 ml/min per 1.73 m2 (n=1297), showed no significant differences from the associations identified in the whole study population. Examining the relationship between skin AF and cardiovascular mortality (n=69) in the whole study population showed a univariate association with skin AF (hazard ratio, 2.09; 95% CI, 1.08 to 4.05; P=0.04) for the highest compared with the lowest quartile of skin AF. After adjustment for age, sex, CVD, diabetes, smoking, BMI, eGFR, albuminuria, and hemoglobin, there was a similar loss of association between skin AF and cardiovascular mortality as for all-cause mortality (skin AF: hazard ratio, 2.11; 95% CI, 0.95 to 4.71; P=0.09) for the highest compared with the lowest quartile of skin AF.
Discussion
This cohort study of people with CKD has shown that higher levels of skin AF were associated with all-cause mortality but that this association was lost after adjusting for established risk factors, including eGFR and uACR. A similar relationship was seen for cardiovascular mortality (although the smaller numbers of events limited power). To our knowledge, this study is the first investigation of the association of skin AF with mortality in early to moderate CKD.
It has been suggested that AGEs may have an important role in the pathogenesis of heart failure and other cardiovascular disorders (28). In support of this suggestion, an association has been shown between AGE accumulation measured as skin AF and peripheral arterial disease independent of diabetes status, and increasing AGEs (measured as serum pentosidine) have been associated with poor prognosis in people with heart failure (29,30). In the context of CKD, Hartog et al. (16) showed correlation between skin AF and poorer diastolic function in a cross-sectional study of peritoneal dialysis and hemodialysis patients, and Meerwaldt et al. (20) identified independent associations between skin AF and cardiovascular as well as all-cause mortality in a prospective cohort of 109 patients on hemodialysis. Our findings in a larger prospective cohort of people with earlier CKD do not support the finding of an independent association between skin AF and mortality. In the baseline analysis of this cohort, independent associations were shown between skin AF and several cardiovascular and renal progression risk factors, including age, eGFR, and uACR (2). In these follow-up analyses, we have shown an association between all-cause mortality and skin AF, but it was not maintained independently from these known risk factors. Additional research is required to evaluate whether AGE accumulation represents a mechanism whereby CKD contributes to the pathogenesis of CVD. Risk factors for CVD and CKD progression are more prevalent in lower SES groups (31). Although we showed associations between SES and increased cardiovascular risk at baseline (31), we did not detect associations between mortality and measures of SES in these follow-up analyses.
There are several proposed mechanisms by which AGE accumulation may influence mortality (28).
First, AGEs cross-link extracellular matrix proteins, a mechanism that may be implicated in the development of arterial stiffness associated with old age and diabetes (32,33).Second, AGEs cross-link intracellular proteins, altering their physiologic function. For example, AGEs affects cardiomyocyte function by altering intracellular protein function in animal models (34,35). Third, AGEs bind to cell membrane receptors (particularly the cell receptor for AGEs) and may induce several intracellular cascades, resulting in the release of cytokines (9), inflammation (36), tumor growth (37), neurodegenerative processes (38), and amyloidosis (39). The cell receptor for AGEs has also been implicated in CKD and CVD pathogenesis, and it is associated with arterial stiffness (10–12). Although the details of many such pathophysiological mechanisms have not yet been fully elucidated, these examples suggest that AGEs may influence both cardiovascular and non-CVD processes and mortality.
People with CKD stage 3 represent the majority of the CKD population but are a heterogeneous group with respect to the associated risks of GFR decline, CVD, and death. Current risk stratification models, such as the model proposed by Tangri et al. (40), are potentially useful for prediction of CKD progression but less useful for cardiovascular risk and all-cause mortality predictions. When considering cardiovascular risk, for United Kingdom populations, the QRisk2 score seems to achieve better predictive accuracy for CVD than the Framingham score by incorporating other comorbidities, including CKD (although CKD is treated as a simple dichotomous variable in QRisk2) (41,42). Use of skin AF has been proposed as a tool to aid the identification of people at risk of complications in diabetes (17,43). Although improved cardiovascular risk prediction in people with CKD would help target interventions, such as statins, our study does not provide evidence to support such use of skin AF in CKD stage 3.
Our study has several limitations. Skin AF cannot currently be assessed in people with dark skin, which represents a significant number of patients with CKD, particularly in the United States. A method has been proposed for calculating skin AF independent of skin color, and it may be an important area of investigation for future research (44). This study was an observational study, and we are cautious in ascribing any causal relationship to these results. In addition, our primary outcome was all-cause mortality, and our findings in relation to cardiovascular mortality should be interpreted with caution. Finally, we have not examined the relationship between skin AF and nonfatal CVD events at this stage of follow-up.
In this prospective cohort of people with CKD stage 3, skin AF was associated with all-cause mortality, but this association was lost after adjustment for other known risk factors. Additional research is needed to clarify whether skin AF has a role in improving mortality and cardiovascular risk prediction in CKD.
Disclosures
None.
Acknowledgments
The authors thank the collaborating general practitioner practices and their staff and Professor Alan Kimber for his advice on survival analysis.
This study was supported by a fellowship grant from Kidney Research United Kingdom and the British Renal Society as well as an unrestricted educational grant from Roche Products.
This study was presented in abstract form at the American Society of Nephrology Kidney Week in Atlanta, GA, on November 7–10, 2013 (“Skin Autofluorescence: A Non-Invasive Test to Improve Mortality Risk Prediction in Chronic Kidney Disease Stage 3”).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Noordzij MJ, Lefrandt JD, Smit AJ: Advanced glycation end products in renal failure: An overview. J Ren Care 34: 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 2.McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW: Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin J Am Soc Nephrol 6: 2356–2363, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baynes JW: Role of oxidative stress in development of complications in diabetes. Diabetes 40: 405–412, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H: Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 1043: 461–466, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Treweek JB, Dickerson TJ, Janda KD: Drugs of abuse that mediate advanced glycation end product formation: A chemical link to disease pathology. Acc Chem Res 42: 659–669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H: Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A 94: 6474–6479, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyata T, Wada Y, Cai Z, Iida Y, Horie K, Yasuda Y, Maeda K, Kurokawa K, van Ypersele de Strihou C: Implication of an increased oxidative stress in the formation of advanced glycation end products in patients with end-stage renal failure. Kidney Int 51: 1170–1181, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Baynes JW: From life to death—the struggle between chemistry and biology during aging: The Maillard reaction as an amplifier of genomic damage. Biogerontology 1: 235–246, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Linden E, Cai W, He JC, Xue C, Li Z, Winston J, Vlassara H, Uribarri J: Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol 3: 691–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Agati V, Schmidt AM: RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol 6: 352–360, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Lindsey JB, Cipollone F, Abdullah SM, McGuire DK: Receptor for advanced glycation end-products (RAGE) and soluble RAGE (sRAGE): Cardiovascular implications. Diab Vasc Dis Res 6: 7–14, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Busch M, Franke S, Rüster C, Wolf G: Advanced glycation end-products and the kidney. Eur J Clin Invest 40: 742–755, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ: Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 47: 1324–1330, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR: Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med 314: 403–408, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, Smit AJ: Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 29: 2654–2659, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hartog JW, Hummel YM, Voors AA, Schalkwijk CG, Miyata T, Huisman RM, Smit AJ, Van Veldhuisen DJ: Skin-autofluorescence, a measure of tissue advanced glycation end-products (AGEs), is related to diastolic function in dialysis patients. J Card Fail 14: 596–602, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ: Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 30: 107–112, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lutgers HL, Gerrits EG, Graaff R, Links TP, Sluiter WJ, Gans RO, Bilo HJ, Smit AJ: Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia 52: 789–797, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, Emoto M, Tahara H, Kakiya R, Tabata T, Miyata T, Nishizawa Y: Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism 57: 1452–1457, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Meerwaldt R, Hartog JWL, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans ROB, Smit AJ: Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 16: 3687–3693, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Arsov S, Trajceska L, van Oeveren W, Smit AJ, Dzekova P, Stegmayr B, Sikole A, Rakhorst G, Graaff R: Increase in skin autofluorescence and release of heart-type fatty acid binding protein in plasma predicts mortality of hemodialysis patients. Artif Organs 37: E114–E122, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Crowley LE, Johnson CP, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW, Leung JC: Tissue advanced glycation end product deposition after kidney transplantation. Nephron Clin Pract 124: 54–59, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Jordan H, Roderick P, Martin D: The Index of Multiple Deprivation 2000 and accessibility effects on health. J Epidemiol Community Health 58: 250–257, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy E, Holt G: The socioeconomic status of older adults: How should we measure it in studies of health inequalities? J Epidemiol Community Health 55: 895–904, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto-Sietsma SJ, Navis G, Janssen WMT, de Zeeuw D, Gans ROB, de Jong PE, PREVEND Study Group : A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41: 733–741, 2003 [DOI] [PubMed] [Google Scholar]
- 27.The World Health Organization and International Diabetes Federation : The Definition and Diagnosis of Diabetes Mellitus and Intermediate Glycaemia, Geneva, Switzerland, WHO Press, 2006 [Google Scholar]
- 28.Hegab Z, Gibbons S, Neyses L, Mamas MA: Role of advanced glycation end products in cardiovascular disease. World J Cardiol 4: 90–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP, Kamphuisen PW, Zeebregts CJ, Lefrandt JD: Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol 33: 131–138, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Koyama Y, Takeishi Y, Arimoto T, Niizeki T, Shishido T, Takahashi H, Nozaki N, Hirono O, Tsunoda Y, Nitobe J, Watanabe T, Kubota I: High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail 13: 199–206, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, Taal MW: Socio-economic disparities in the distribution of cardiovascular risk in chronic kidney disease stage 3. Nephron Clin Pract 122: 58–65, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Brownlee M: Advanced protein glycosylation in diabetes and aging. Annu Rev Med 46: 223–234, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Zieman SJ, Kass DA: Advanced glycation endproduct crosslinking in the cardiovascular system: Potential therapeutic target for cardiovascular disease. Drugs 64: 459–470, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Bidasee KR, Nallani K, Henry B, Dincer UD, Besch HR, Jr.: Chronic diabetes alters function and expression of ryanodine receptor calcium-release channels in rat hearts. Mol Cell Biochem 249: 113–123, 2003 [PubMed] [Google Scholar]
- 35.Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D: Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol 270: H1529–H1537, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM: RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 97: 889–901, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM: Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405: 354–360, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM: RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382: 685–691, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Yan SD, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, Soto C, Schmidt AM, Stern D, Kindy M: Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med 6: 643–651, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Collins GS, Altman DG: An independent and external validation of QRISK2 cardiovascular disease risk score: A prospective open cohort study. BMJ 340: c2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, Brindle P: Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ 336: 1475–1482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerrits EG, Lutgers HL, Kleefstra N, Graaff R, Groenier KH, Smit AJ, Gans RO, Bilo HJ: Skin autofluorescence: A tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 31: 517–521, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Koetsier M, Nur E, Chunmao H, Lutgers HL, Links TP, Smit AJ, Rakhorst G, Graaff R: Skin color independent assessment of aging using skin autofluorescence. Opt Express 18: 14416–14429, 2010 [DOI] [PubMed] [Google Scholar]