Abstract
Background and objectives
Once-daily losartan reduces BP in a dose-dependent manner and is well tolerated in hypertensive children aged 6–16 years. This study assessed the dose-response relationship, safety, and tolerability of losartan in hypertensive children aged 6 months to 6 years.
Design, setting, participants, & measurements
This was a 12-week, randomized, open-label, dose-ranging study, with a 2-year extension. Patients were randomized to losartan at the following dosages: 0.1 mg/kg per day (low), 0.3 mg/kg per day (medium), or 0.7 mg/kg per day (high). Losartan was titrated to the next dose level (to a 1.4 mg/kg per day maximum dosage, not exceeding 100 mg/d, which was not one of the three original doses offered at randomization) at weeks 3, 6, and 9 for patients who did not attain their goal BP and were not taking the highest dose. Dose response was evaluated by analyzing the slope of change in sitting systolic BP (SBP; primary end point) and diastolic BP (DBP; secondary end point) after 3 weeks compared with baseline. Adverse events (AEs) were recorded throughout.
Results
Of the 101 patients randomized, 99 were included in the analysis (low dose, n=32; medium dose, n=34; and high dose, n=33). Mean sitting BP decreased from baseline in the low-, medium-, and high-dose groups by 7.3, 7.6, and 6.7 mmHg, respectively, for SBP and 8.2, 5.1, and 6.7 mmHg, respectively, for DBP after 3 weeks. No dose-response relationship was established by the slope analysis on SBP (P=0.75) or DBP (P=0.64). The BP-lowering effect was observed throughout the 2-year extension. The incidence of AEs was low and comparable between groups.
Conclusions
Hypertensive children aged 6 months to 6 years treated with losartan 0.1–0.7 mg/kg per day had clinically significant decreases from baseline in SBP and DBP, yet no dose-response relationship was evident. Losartan, at a dosage up to 1.4 mg/kg per day, was well tolerated.
Keywords: children, clinical trial, hypertension, pediatrics
Introduction
Hypertension in children is defined as systolic BP (SBP) and/or diastolic BP (DBP)≥95th percentile for sex, age, and height on ≥3 occasions (1). It occurs in 1%–10% of children and adolescents and, at younger ages, frequently has a cardiac or renal cause (2,3). Approximately 50% of children with CKD have hypertension (4), which is a risk factor for cardiovascular morbidity and mortality and contributes to increased proteinuria and resultant progression of renal disease (5). Current guidelines state that the goal of therapy is to reduce both SBP and DBP to <95th percentile, or to <90th percentile in the presence of comorbidities or end organ damage, and treatment should progress to the highest recommended dose until the goal is achieved (6). In the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial, intensified BP control effectively delayed the progression of renal disease among children with CKD (7).
Losartan, an angiotensin II receptor blocker (ARB), is an antihypertensive therapy with demonstrated benefit in children. In children aged 1 month to 16 years, the pharmacokinetic parameters of losartan and its active metabolite E-3174 were similar across all age groups after oral administration, and treatment was well tolerated (8). A randomized, double-blind study showed that once-daily losartan reduced BP in a dose-dependent manner and was well tolerated in hypertensive children aged 6–16 years (9). This randomized clinical study explored the dose-response relationship and the safety and tolerability of losartan in hypertensive children aged 6 months to 6 years.
Materials and Methods
Study Design and Participants
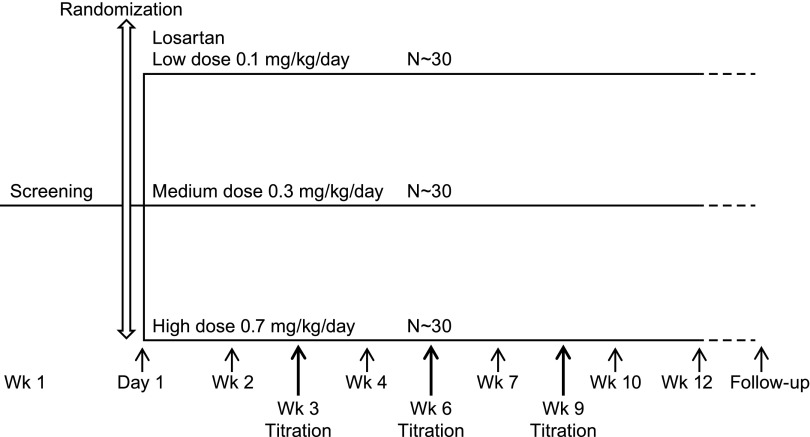
This was a 12-week, randomized, open-label, parallel-group, dose-ranging study of losartan in infants and young children with either newly diagnosed, therapy naïve hypertension or those with inadequate BP control with their current or past antihypertensive regimen (Figure 1). Patients taking other antihypertensive therapies (including angiotensin-converting enzyme inhibitors) before screening did not have a washout period; losartan was added to their existing regimen. Patients who completed the 12-week base study were eligible to continue in the open-label losartan extension, with follow-up visits every 3 months. The duration of follow-up varied and, at the longest, continued until the month 24 visit or until the last patient enrolled completed the 12-week base study, whichever came first.
Figure 1.
Study design. A 12-week, randomized, open-label, parallel-group, dose-ranging study of losartan
Boys and girls aged 6 months to <7 years were enrolled. For children aged≥1 year, hypertension was defined as SBP and/or DBP ≥95th percentile by National High Blood Pressure Education Program standards (1) for the patient’s sex, age, and height (or local standards, if required by ethical review committee and/or local regulations). For children aged<1 year, hypertension was defined as SBP≥95th percentile according to charts based on sex and age from the Report of the Second Task Force on Blood Pressure Control in Children. Patients with SBP and/or DBP≥90th percentile and evidence of end organ damage (left ventricular hypertrophy, retinal vascular changes, etc.) or comorbidities (CKD, overweight [≥95th percentile for age], hyperlipidemia, or diabetes mellitus) may have been enrolled at the investigator’s discretion. Patients were required to have an eGFR≥30 ml/min per 1.73 m2 calculated by the Schwartz formula based on the baseline plasma creatinine value (10).
The first 50 patients enrolled were aged≥1 year to obtain safety data in older patients; the decision to proceed with enrollment of patients aged<1 year was made by the independent Data Safety Monitoring Board after review of these data. This study was designed to include at least 25 children aged 6–23 months (young cohort).
All patients were randomly allocated to open-label losartan, starting at the following dosages: 0.1 mg/kg per day (low), 0.3 mg/kg per day (medium), or 0.7 mg/kg per day (high) (Figure 1) via an interactive voice response system. All doses were supplied as losartan dry powder in a sachet formulation for in situ reconstitution as a suspension. The study did not include a placebo or comparator treatment group. Patients were stratified by the presence of comorbidities or evidence of end organ damage that warranted a lower BP goal. The stratification was accomplished by the interactive voice response system. Losartan was titrated to the next dose level (up to a maximum dosage of 1.4 mg/kg per day, not to exceed 100 mg/d, which was not one of the three original doses offered at randomization) at weeks 3, 6, and 9 for patients who did not attain their goal BP and were not taking the highest dose. With the exception of another ARB, investigators were permitted to add and titrate other open-label antihypertensive medications (including angiotensin-converting enzyme inhibitors) for children who reached 1.4 mg/kg per day of losartan and did not attain goal BP. Sitting BP (or supine BP if the child could not sit) was monitored at each visit using a standardized oscillometric BP device (Datascope Duo; Datascope Corp., Mahwah, NJ).
This study, registered on ClinicalTrials.gov (NCT00756938), was conducted as Merck Losartan Protocol 337 and was part of Merck’s commitment toward fulfilling the Pediatric Investigation Plan agreed with the European Medicines Agency (EMA). The three-arm design (without placebo) was accepted by the Pediatric Committee of the EMA. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. Informed consent was obtained from parents or guardians.
End Points
The primary efficacy end point was the slope of change in sitting SBP after the first 3 weeks of treatment compared with baseline as a function of dose, and the principal secondary efficacy end point was the slope of change in sitting DBP using the same parameters. Exploratory end points included the change from baseline in SBP and DBP at 3-week intervals (base phase) and at 3-month intervals (extension phase), as well as the percentages of patients who attained goal SBP or DBP by week 3, who were uptitrated to the next dose at week 3, and who required additional antihypertensive medication. Changes from baseline in SBP by week 3 were evaluated for prespecified subgroups defined by sex, age (<1 year or ≥1 year), race, prior use of antihypertensive medication (yes or no), presence of comorbidities/end organ damage (yes or no), position during BP measurement (sitting or supine), and age cohort (6 to ≤23 months or >23 months).
Safety was assessed by physical examinations, vital signs measurements, laboratory evaluations (serum chemistry, hematology), and adverse event (AE) monitoring at every study visit. The investigator evaluated all AEs to ascertain the duration, maximum intensity, seriousness, action taken, and relationship to study medication. AEs of special interest (AESIs) included investigator-reported renal dysfunction, angioedema, hyperkalemia, hypotension, and abnormal liver function tests (aspartate aminotransferase or alanine transaminase≥3 times the upper limit of normal [ULN] and total bilirubin≥2 times the ULN and, at the same time, alkaline phosphatase<2 times the ULN).
The following predefined limits of change were used for the assessment of laboratory parameters: hemoglobin, ≥12%; serum sodium, >5 mEq/L; serum creatinine, >30%; serum potassium, >10%; aspartate aminotransferase, >20 U/L; and alanine transaminase, >20 U/L. All changes in predefined test limits were recorded and evaluated, but may not have met the criteria for an AE.
Statistical Analyses
The primary analysis population for efficacy was the full analysis set, including all patients who received at least one dose of study medication and had a baseline and a post-treatment observation. The primary end point was assessed using an analysis of covariance (ANCOVA) model for the change from baseline with terms for dose (as a continuous covariate: 0.1, 0.3, or 0.7 mg/kg per day), weight, and presence of comorbidities/end organ damage (yes or no). The primary hypothesis was assessed by testing whether the slope for dose in the above regression model was zero. To estimate the change from baseline in SBP for each dose regimen by week 3, a similar ANCOVA model with dose included as a factor was used. Similar analyses were performed for the change from baseline in DBP. A longitudinal data analysis model was used to provide supportive analyses for the change from baseline in SBP and DBP after 3 weeks.
With 30 analyzable patients in each dose regimen, the study had approximately 80% power to detect a positive linear dose-response relationship at the 0.05 level (2-sided) if the difference in change from baseline in SBP between the most extreme doses was 8 mmHg (assuming a SD for the change from baseline in SBP equal to 11 mmHg).
Safety analyses were based on the all-patients-as-treated population. Counts and percentages of AEs were tabulated by actual dose received at the time of the event over 12 weeks. Overall counts and percentages of AEs that occurred during the extension were provided.
Results
Patient Population
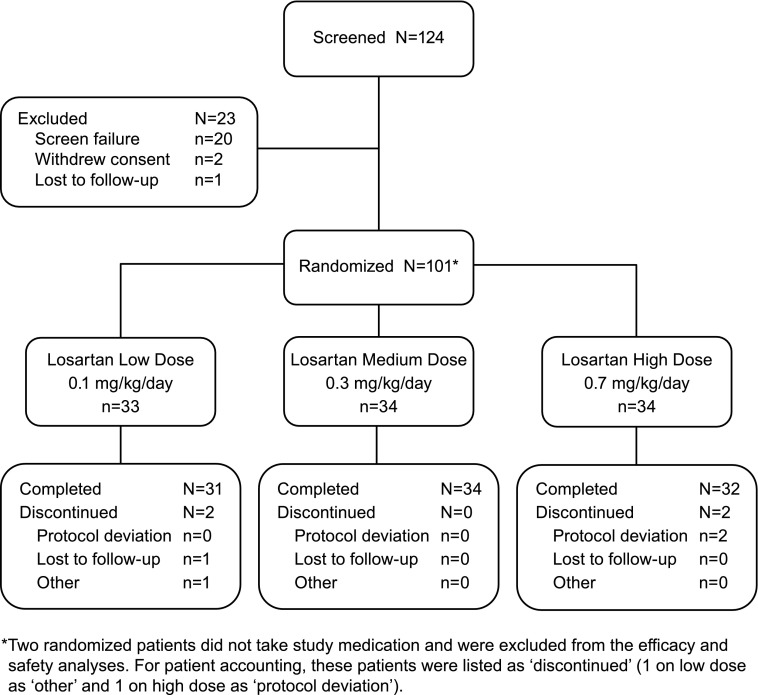
We screened 124 patients, and 101 patients were randomized in 28 centers worldwide (Figure 2). Ninety-nine patients received at least one dose of randomized study medication and were included in the efficacy and safety analyses. Ninety patients (89.1%) entered into and 53 (58.9%) completed the extension phase. Across both phases, the mean duration of study medication taken was 263 days (range, 7–828).
Figure 2.
Patient disposition.
Baseline characteristics were generally similar between the treatment groups (Table 1). Most patients (66.3%) had a renal cause for their hypertension. Of the 99 treated patients, 36 had no evidence of CKD and 63 patients met the criteria for CKD as follows: stage 1 CKD, n=45; stage 2 CKD, n=12; stage 3 CKD, n=5; and stage 4 CKD, n=1 (randomized in error and subsequently discontinued at week 2 of the study). Other causes included metabolism and nutrition disorders, specifically obesity, and other or unknown reasons. The young cohort comprised 26.7% of the study population.
Table 1.
Baseline patient characteristics by treatment group
| Characteristic | Dosage per Day | Totala | ||
|---|---|---|---|---|
| Low (0.1 mg/kg) | Medium (0.3 mg/kg) | High (0.7 mg/kg) | ||
| Participants (n) | 33 | 34 | 34 | 101 |
| Sex | ||||
| Boys | 20 (60.6) | 18 (52.9) | 20 (58.8) | 58 (57.4) |
| Girls | 13 (39.4) | 16 (47.1) | 14 (41.2) | 43 (42.6) |
| Race | ||||
| White | 21 (63.6) | 20 (58.8) | 26 (76.5) | 67 (66.3) |
| Asian | 8 (24.2) | 8 (23.5) | 3 (8.8) | 19 (18.8) |
| African American | 0 (0.0) | 1 (2.9) | 4 (8.8) | 4 (4.0) |
| Otherb | 4 (12.1) | 5 (14.7) | 2 (5.9) | 11 (10.9) |
| Age (yr) | ||||
| <1 | 2 (6.1) | 4 (11.8) | 4 (11.8) | 10 (9.9) |
| 1–6 | 31 (93.9) | 30 (88.2) | 30 (88.2) | 91 (90.1) |
| Age cohorts (mo) | ||||
| 6–23 | 13 (39.4) | 6 (17.6) | 8 (23.5) | 27 (26.7) |
| >23 | 20 (60.6) | 28 (82.4) | 26 (76.5) | 74 (73.3) |
| Median (mo) | 39.0 | 45.0 | 41.5 | 41.0 |
| 25th and 75th percentile | 19–63 | 27–63 | 24–60 | 22–63 |
| Duration of hypertension (mo) | ||||
| Median | 4.0 | 2.0 | 4.0 | 3.0 |
| 25th and 75th percentile | 1–9 | 1–8 | 1–9 | 1–9 |
| Antihypertensive medicationc | 11 (33.3) | 11 (32.4) | 12 (35.3) | 34 (33.7) |
| Agents acting on the RAS | 6 (18.2) | 6 (17.6) | 10 (29.4) | 22 (21.8) |
| Calcium channel blockers | 5 (15.2) | 6 (17.6) | 2 (5.9) | 13 (12.9) |
| β-Blocking agents | 2 (6.1) | 4 (11.8) | 4 (11.8) | 10 (9.9) |
| Diuretics | 2 (6.1) | 3 (8.8) | 1 (2.9) | 6 (5.9) |
| Other antihypertensive agents | 1 (3.0) | 2 (5.9) | 1 (2.9) | 4 (4.0) |
| DBP (mmHg) | ||||
| Mean (SD) | 68.8 (6.1) | 69.4 (8.6) | 68.6 (7.4) | 68.9 (7.4) |
| Range | 55–81 | 55–90 | 51–86 | 51–90 |
| SBP (mmHg) | ||||
| Mean (SD) | 111.3 (8.8) | 113.7 (8.2) | 111.2 (7.2) | 112.1 (8.1) |
| Range | 89–130 | 102–129 | 98–129 | 89–130 |
Data are expressed as n (%), unless otherwise noted. Every patient is counted once for each applicable medication. Patients who received multiple medications with a category are counted once for that category. DBP, diastolic BP; RAS, renin-angiotensin system; SBP, systolic BP.
Two randomized patients did not take study medication, and were excluded from the efficacy and safety analyses.
Includes American Indian or Alaska Native and multiracial.
Includes patients who received prior medication, irrespective of whether the treatment continued during the trial.
BP
Base Phase.
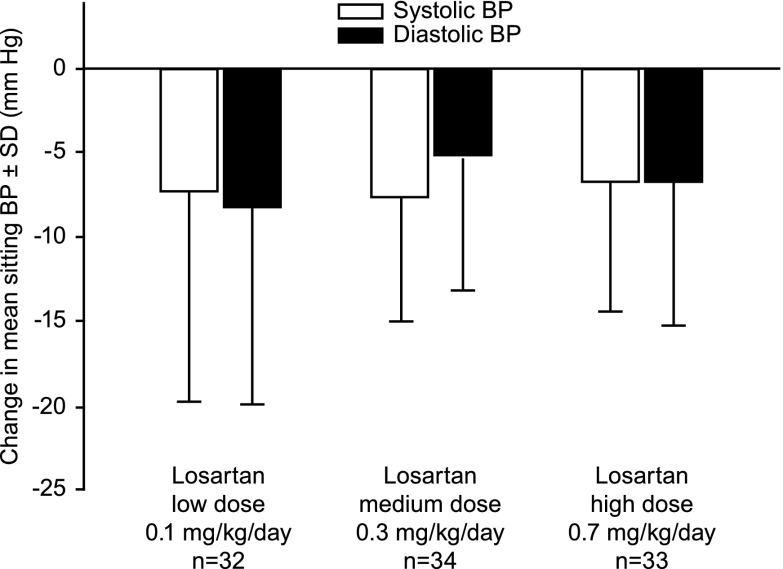
Reductions from baseline to week 3 in mean sitting SBP and DBP were observed in all dose groups (Figure 3, Table 2). No dose-response relationships were established by the slope analysis on SBP or DBP. The estimated slopes of dose for change from baseline were 1.2 mmHg/mg per kg per day (95% confidence interval, −6.4 to 8.9; P=0.75) for SBP and 1.8 mmHg/mg per kg per day (95% confidence interval, −5.9 to 9.5; P=0.64) for DBP. The supportive longitudinal data analysis showed that the least-squares mean changes from baseline in SBP and DBP were similar across the treatment groups and were comparable with those from the ANCOVA models (data not shown).
Figure 3.
Change from baseline to week 3±SD in mean systolic BP and diastolic BP in the base study.
Table 2.
Summary of change from baseline in SBP and DBP as a function of dose
| BP (mg/kg per d) | n | Baseline (mmHg) | Week 3 (mmHg) | Change from Baseline | Least-Squares Change from Baseline (mmHg)a |
|---|---|---|---|---|---|
| SBP | |||||
| Low (0.1) | 32 | 111.2 (8.8) | 103.9 (12.8) | −7.3 (12.5) | −7.41 (−10.8 to −4.1) |
| Medium (0.3) | 34 | 113.7 (8.2) | 106.1 (9.4) | −7.7 (7.5) | −7.6 (−10.9 to −4.3) |
| High (0.7) | 33 | 111.2 (7.2) | 104.5 (9.8) | −6.7 (7.9) | −6.8 (−10.1 to −3.4) |
| DBP | |||||
| Low (0.1) | 32 | 68.8 (6.1) | 60.6 (10.4) | −8.3 (11.8) | −8.3 (−11.7 to −5.0) |
| Medium (0.3) | 34 | 69.3 (8.6) | 64.2 (9.2) | −5.2 (8.1) | −5.3 (−8.6 to −2.0) |
| High (0.7) | 33 | 68.6 (7.4) | 61.9 (6.4) | −6.7 (8.6) | −6.7 (−10.0 to −3.4) |
Data are presented as the mean (SD) or mean change (95% confidence interval).
From analysis of covariance model with terms for dose as a factor, weight as a continuous covariate, and presence of comorbidities/end organ damage.
Consistent changes from baseline in SBP during the 12-week base phase were observed in the medium-dose group as follows: −7.1, −7.9, −9.4, and −9.6 mmHg at weeks 3, 6, 9, and 12, respectively. Consistent changes were also observed in the low- and high-dose groups through week 9, but a lesser antihypertensive effect was observed at week 12. Consistent changes from baseline in DBP during the 12-week base phase were observed overall, but no consistent changes were observed at the 3-week intervals within each dose group. During the base phase, 27 patients (26.7%) had continued their prior concomitant antihypertensive medications, and 10 patients (9.9%) had initiated therapy with a new antihypertensive medication (Supplemental Table 1).
By week 3, 51 of 99 patients (51.5%) reached their goal BP (56.3%, 44.1%, and 54.5% of patients in the low-, medium-, and high-dose groups, respectively). The percentages of patients who required uptitration in the low-, medium-, and high-dose groups, respectively, were as follows: 46.9%, 41.2%, and 33.3% by week 3; 34.4%, 29.4%, and 12.1% by week 6; and 3.1%, 5.9%, and 3.0% by week 12. Eight patients (8.1%) required additional antihypertensive medication during the 12-week base phase (0 in the low-, 4 in the medium-, and 4 in the high-dose groups). The changes from baseline in BP were similar across all patient subgroups examined, and suggested no trend toward a dose response (data not shown).
Extension Phase.
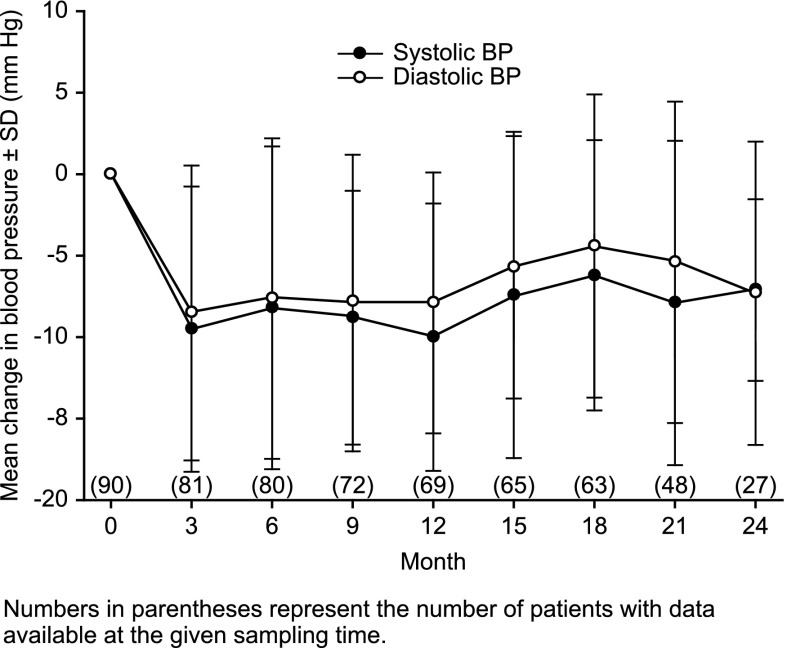
A consistent BP-lowering effect was observed for the overall population throughout the 2-year extension (Figure 4). Overall, 34 patients (37.8%) were receiving concomitant antihypertensive medications (either initiated before enrollment or newly initiated during the study). A post hoc analysis demonstrated a consistent BP-lowering response in the subgroup of patients who did not receive concomitant antihypertensive medications with that of the overall population (data not shown).
Figure 4.
Change from baseline in mean systolic BP and diastolic BP.
Safety
Base Phase.
Table 3 provides a summary of AEs during the first 12 weeks of the study by dose received (low, medium, high, and highest [1.4 mg/kg per day]) at the time of the event.
Table 3.
AE summary by dose received at time of event over 12 weeks in the base study
| AE Type | Dosage per Day | Total | |||
|---|---|---|---|---|---|
| Low (0.1 mg/kg) | Medium (0.3 mg/kg) | High (0.7 mg/kg) | Highest (1.4 mg/kg) | ||
| Participants (n) | 34 | 54 | 63 | 33 | 99 |
| Clinical | |||||
| Any clinical AE | 20 (57.1) | 30 (55.6) | 38 (59.4) | 21 (65.6) | 79 (79.8) |
| Any drug-related clinical AEa | 0 (0.0) | 2 (3.7) | 2 (3.2) | 1 (3.0) | 3 (3.0) |
| Any serious clinical AE | 3 (8.8) | 2 (3.7) | 2 (3.2) | 0 (0.0) | 7 (7.1) |
| Any serious drug-related clinical AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Died | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cause of discontinuation of study medicationb | |||||
| Clinical AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Clinical drug-related AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serious clinical AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serious drug-related clinical AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Laboratory | |||||
| Any laboratory AE | 1 (2.9) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 2 (2.0) |
| Any drug-related laboratory AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any serious laboratory AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any serious drug-related laboratory AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cause of discontinuation of study medicationb | |||||
| Laboratory AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Drug-related laboratory AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serious laboratory AE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Serious drug-related laboratory AEa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AEs of clinical interest | 1 (2.9) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 2 (2.0) |
| GFR decreased | 1 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Hypotension | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (1.0) |
Data are shown as the number of participants in the AE category and percentages are calculated based on the number of participants who received at least one dose of the specified study treatment dose and with follow-up during the base study. Patients could have been counted in more than one dose group according to each dose to which they were exposed during the 12-week randomized phase of the trial; each participant was counted only once in the total column. AE, adverse event.
Determined by the investigator to be possibly, probably, or definitely related to the drug. If unknown, the AE was considered to be drug related.
Study medication withdrawn.
In the young cohort, 24 of 27 patients (88.9%) had clinical AEs, with 8 of 14 (57.1%), 9 of 15 (60.0%), 10 of 14 (71.4%), and 4 of 5 (80.0%) in the low, medium, high, and highest dose groups, respectively. Two of 27 patients (7.4%) had serious AEs (one patient had two separate hospitalizations for a thermal burn injury and pyelonephritis and the other patient was hospitalized with a diagnosis of asthenia), both in the low-dose group, and 1 of 27 patients (3.7%) had a drug-related AE (an event of hypotension). Two patients, both in the young cohort, had AESIs, one with hypotension (noted above) and the other with decreased GFR.
The percentages of patients with changes exceeding predefined laboratory test limits were 7 of 98 (7.1%) for serum sodium, 23 of 96 (24.0%) for serum potassium, 14 of 98 (14.3%) for serum creatinine, and 8 of 98 (8.2%) for hemoglobin, with no clinically significant differences between the treatment groups. No patients had evidence of abnormal liver function tests.
Extension Phase.
Overall, 75 of 90 patients (83.3%) had clinical AEs, and 5 of 90 patients (5.6%) had drug-related AEs. One patient discontinued therapy because of an AE of allergic dermatitis, which was determined to be “not drug related” by the investigator.
Four AESIs occurred, all in the young cohort (one hypotension, two renal dysfunction [one ARF], and one hyperkalemia). The ARF developed several days after the patient completed the extension and was attributed to severe sepsis. This patient was not receiving any other antihypertensive medication at the time of the AE. The hyperkalemia (serum potassium 5.5 mmol/L) was associated with a difficult and prolonged blood draw; a repeat level 1 month later was 4.9 mmol/L, with no adjustment of the losartan dose.
Discussion
This study is one of the largest antihypertensive medication trials in children aged 6 months to 6 years, a cohort whose BP may be difficult to control and who would benefit from having access to an ARB. Treatment with losartan produced clinically meaningful decreases from baseline to week 3 in SBP and DBP across all dose groups, an effect that was consistently observed at week 12 and month 21, at which time more than half of the originally randomized cohort remained in the trial. These findings are similar to results from a previous trial in older children, in which average dosages of approximately ≥0.75 mg/kg per day provided a consistent antihypertensive effect (9).
No linear dose response for BP was observed at 3 weeks between the low-dose versus the medium-dose or high-dose regimens. This finding is discordant with data from a study of losartan in older children (aged 6–16 years), showing a dose response in the medium-dose (0.75 mg/kg) and high-dose (1.44 mg/kg) regimens compared with the low-dose regimen (0.07 mg/kg) after 3 weeks (9). The absence of a dose response could not be explained by the addition of antihypertensive therapies to control BP, because no meaningful differences were observed across dose groups. One explanation may be that very young children, particularly those with renal causes of their hypertension, may be more sensitive to the effects of ARBs, and the dosages used here represent the upper end of the dose-response curve. Similar results were reported in a study of the ARB valsartan in 90 children aged 1–6 years (4). The authors of this study suggested that young children may be uniquely sensitive to treatment, and a lower starting dose might have facilitated demonstration of a dose response (4). Renin levels are higher in younger children, possibly contributing to greater sensitivity to renin-angiotensin-aldosterone system blockade (11); however, this association could not be tested because renin was not measured in the present study.
One interpretation is that all losartan doses were equally effective in lowering BP; however, the absence of a placebo arm is an important limitation that must be considered. Nonetheless, it is unlikely that placebo would produce a persistent antihypertensive effect throughout the 12-week base phase in these children, who predominantly had a primary renal cause of their hypertension. Considering the statistically significant antihypertensive efficacy of losartan in older children (9), coupled with the BP-lowering effects observed in children aged 1–6 years with valsartan (4), it is reasonable to conclude that despite the lack of a dose response, losartan lowers BP in children aged<6 years.
To permit a relevant risk assessment by drug exposure, AEs were summarized for the actual losartan dose received at the time of the event, not for the originally randomized dose. All losartan doses were generally well tolerated. The majority of clinical AEs were reflective of the routine illnesses experienced in young children or related to the underlying cause of their hypertension, especially recurrent urinary tract infections. The safety profile is consistent with studies of losartan in older children with hypertension (9) and CKD (12). The safety profile was similar in the young cohort to that of the overall population, except that the six AESIs observed over the entire treatment period occurred in this subgroup. Although the results from this small group (n=27) should be interpreted with caution, physicians should closely monitor very young children during treatment with losartan.
In conclusion, losartan produced a consistent BP-lowering effect across all doses examined throughout the 12-week base and 2-year extension phases of this study in hypertensive children aged 6 months to 6 years. No dose-response effect was observed during the first 3 weeks of losartan therapy. Losartan was generally well tolerated at doses up to 1.4 mg/kg, extending its previously established safety profile to young children.
Disclosures
R.M., C.L., E.P.S., C.M.S., and R.O.B. are current or former employees of Merck & Co., Inc. and may own stock or stock options in the company. N.J.A.W. reports participation on advisory boards for Merck, Abbvie, Astellas, and Takeda. T.G.W. reports receiving consulting fees and/or honoraria from Merck and Takeda. S.S. reports participation on advisory boards for Merck. W.M.D. is an employee of Parexel International, the contract research organization that provided clinical trial services, as contracted by Merck, in the conduct of this study.
Supplementary Material
Acknowledgments
The authors thank Kathleen Newcomb and Jennifer Rotonda (both from Merck & Co., Inc., Whitehouse Station, NJ) for administrative and editorial support of the manuscript.
Merck & Co., Inc. provided all funding and study drugs for this study.
Selected data from this article were presented at the 16th Congress of the International Pediatric Nephrology Association, Shanghai, China, August 30–September 3, 2013.
The study investigators are as follows: S. Miceli (Argentina); R.D.P. Meneses, E.D.A. Burdmann, V. Koch, J.P.M. Ribeiro Neto, and N.L. Bresolin (Brazil); L.F. Gonzalez (Chile); J.J. Vanegas (Colombia); R.L. Meda and C.M. Patal (Guatemala); G. Reusz (Hungary); A. Dubey (India); A. Jankauskiene and N. Roznova (Lithuania); A. Hirth (Norway); F. Valdes, B. Sablan, and A. Gutierrez-Marbella (Philippines); M. Craiu and A.V. Craciun (Romania); I. Zamora (Spain); N. Webb and S. Marks (United Kingdom); and C. Cottrill, J Flynn, L. Greenbaum, R. Haws, and J. Sherbotie (United States).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Present address: Dr. Lam, New York-Presbyterian Hospital/Columbia University College of Physicians and Surgeons, New York, New York.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11111113/-/DCSupplemental.
References
- 1.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents : Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program. Pediatrics 98: 649–658, 1996 [PubMed] [Google Scholar]
- 2.Prineas RJ, Elkwiry EM: Epidemiology and measurement of high blood pressure in children and adolescents. In: Pediatric and Adolescent Hypertension, edited by Loggi JMH, Oxford, Blackwell Scientific Publications, 1992, pp 91–103 [Google Scholar]
- 3.Sinaiko AR: Hypertension in children. N Engl J Med 335: 1968–1973, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Flynn JT, Meyers KE, Neto JP, de Paula Meneses R, Zurowska A, Bagga A, Mattheyse L, Shi V, Gupte J, Solar-Yohay S, Han G, Pediatric Valsartan Study Group : Efficacy and safety of the angiotensin receptor blocker valsartan in children with hypertension aged 1 to 5 years. Hypertension 52: 222–228, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Mitsnefes M, Ho PL, McEnery PT: Hypertension and progression of chronic renal insufficiency in children: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol 14: 2618–2622, 2003 [DOI] [PubMed] [Google Scholar]
- 6.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[2 Suppl]: 555–576, 2004 [PubMed] [Google Scholar]
- 7.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F, ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Shaw W, Hogg R, Koch V, Wells T, Frishberg Y, Jones D, Delucchi A, Hernandez O, Tenney F, Romero B, Murphy G, Lo M, Hand E, Panebianco D, Shahinfar S: Losartan (Los) and E-3174 (E) pharmacokinetics (PK) in hypertensive children and infants [Abstract]. J Am Soc Nephrol 13: 149A, 2002. 11752032 [Google Scholar]
- 9.Shahinfar S, Cano F, Soffer BA, Ahmed T, Santoro EP, Zhang Z, Gleim G, Miller K, Vogt B, Blumer J, Briazgounov I: A double-blind, dose-response study of losartan in hypertensive children. Am J Hypertens 18: 183–190, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 11.Broughton Pipkin F, Smales OR, O’Callaghan M: Renin and angiotensin levels in children. Arch Dis Child 56: 298–302, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb NJ, Shahinfar S, Wells TG, Massaad R, Gleim GW, Santoro EP, Sisk CM, Lam C: Losartan and enalapril are comparable in reducing proteinuria in children. Kidney Int 82: 819–826, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.