Abstract
Background and objectives
Kidney injury molecule-1 (KIM-1) has been suggested as a clinically relevant highly specific biomarker of acute kidney tubular damage. However, community-based data on the association between urinary levels of KIM-1 and the risk for cardiovascular mortality are lacking. This study aimed to investigate the association between urinary KIM-1 and cardiovascular mortality.
Design, setting, participants, & measurements
This was a prospective study, using the community-based Uppsala Longitudinal Study of Adult Men (N=590; mean age 77 years; baseline period, 1997–2001; median follow-up 8.1 years; end of follow-up, 2008).
Results
During follow-up, 89 participants died of cardiovascular causes (incidence rate, 2.07 per 100 person-years at risk). Models were adjusted for cardiovascular risk factors (age, systolic BP, diabetes, smoking, body mass index, total cholesterol, HDL cholesterol, antihypertensive treatment, lipid-lowering treatment, aspirin treatment, and history of cardiovascular disease) and for markers of kidney dysfunction and damage (cystatin C–based eGFR and urinary albumin/creatinine ratio). Higher urinary KIM-1/creatinine (from 24-hour urine collections) was associated with a higher risk for cardiovascular mortality (hazard ratio per SD increase, 1.27; 95% confidence interval [95% CI], 1.05 to 1.54; P=0.01). Participants with a combination of high KIM-1/creatinine (upper quintile, ≥175 ng/mmol), low eGFR (≤60 ml/min per 1.73 m2), and microalbuminuria/macroalbuminuria (albumin/creatinine ratio≥3 g/mol) had a >8-fold increased risk compared with participants with low KIM-1/creatinine (<175 ng/mmol), normal eGFR (>60 ml/min per 1.73 m2), and normoalbuminuria (albumin/creatinine ratio<3 g/mol) (hazard ratio, 8.56; 95% CI, 4.17 to 17.56; P<0.001).
Conclusions
These findings suggest that higher urinary KIM-1 may predispose to a higher risk of cardiovascular mortality independently of established cardiovascular risk factors, eGFR, and albuminuria. Additional studies are needed to further assess the utility of measuring KIM-1 in the clinical setting.
Keywords: albuminuria, tubule cells, epidemiology, outcomes
Introduction
There is growing recognition of the clinical importance of the interplay between renal damage and the development of cardiovascular disease (CVD) (1). Two different aspects of kidney pathology, low eGFR and albuminuria, were recently shown to provide additive and independent predictive information for the development of CVD beyond the established CVD risk factors (2,3). However, the role of kidney tubular damage in the development of CVD is less studied.
Kidney injury molecule-1 (KIM-1) is a transmembrane protein that is primarily expressed in epithelial cells in damaged regions of the proximal tubuli (4,5). KIM-1 is suggested to be involved in the modulation of tubular damage and repair in response to AKI (6,7), but KIM-1 may also play a role in CKD progression (8–12).
Urinary levels of KIM-1 are suggested as a clinically relevant biomarker of acute tubular injury (5) because they rise more rapidly and are more specific to tubular damage than the urinary albumin/creatinine ratio (ACR) or low eGFR. Urinary levels of KIM-1 are also elevated in chronic renal diseases (13).
Previous studies showed that higher urinary KIM-1 levels are associated with higher mortality risk in patients with overt kidney disease (14) or heart failure (15), but there is limited evidence of an association between KIM-1 and cardiovascular mortality in the community.
In this study, we hypothesized that individuals with kidney tubular damage are at higher risk for cardiovascular mortality. Accordingly, this study aimed to investigate the association between a specific marker of kidney tubular damage (urinary KIM-1/creatinine) and the risk of cardiovascular and total mortality in a community-based sample of elderly men and whether this association was independent of established cardiovascular risk factors, eGFR, and ACR.
Materials and Methods
Study Sample
The Uppsala Longitudinal Study of Adult Men (ULSAM) began in 1970. The ULSAM study design and selection criteria were previously described (3,16,17). Further details are available on the ULSAM website (http://www.pubcare.uu.se/ULSAM) and in recent publications on KIM-1 in the ULSAM cohort (18,19). Our analyses are based on the fourth examination cycle (1997–2001), when 838 men (mean age 77.5 years) were investigated. Of these, urine samples and KIM-1 measurements were available in 627 individuals. Thirty-seven participants had missing data on covariates, leaving 590 individuals with data on all covariates in this study sample. All participants gave written informed consent, and the ethics committee of Uppsala University approved the study protocol.
Clinical and Biochemical Evaluation at Baseline
Cystatin C was measured by latex enhanced reagent (NLatexCystatin C;Siemens, Deerfield, IL). 24-Hour urine were collected, aliquoted and stored at −70° until analysis (mean freezer time 10.5 years [range 9.3-12.7 years]). Urinary KIM-1 levels (the concentration of the cleaved extracellular KIM-1 domain) were analyzed with the commercial sandwich ELISA kit, (DY1750 R&D Systems, Minneapolis, MN, USA). The total coefficient of variation for the assay was approximately 7%. Urine albumin was measured by nephelometry (Urine albumin, Dade Behring, Deerfield IL, USA) using a Behring BN ProSpec® analyzer (Dade Behring). Urine creatinine was analyzed with a modified kinetic Jaffe reaction on an Architect Ci8200® analyzer (Abbott, Abbot Park, IL, USA) and creatinine related urine albumin (ACR) was calculated. ACR was also dichotomized at 3 g/mol (the threshold for micro-albuminuria used in clinical practice in Sweden). KIM-1/creatinine was also calculated. eGFR in ml/min per 1.73 m2 was calculated from serum cystatin C results in mg/L by the formula y = 77.24x-1.2623 (20). Serum and urine neutrophil gelatinase-associated lipocalin (NGAL) were analyzed with a commercial sandwich ELISA kit, (DY1757, R&D Systems, Minneapolis, MN).
Outcome
The Swedish Cause-of-Death register was used to obtain the outcomes total and cardiovascular mortality. Cardiovascular mortality was defined as International Classification of Diseases (10th Revision) codes I00–I99 (Supplemental Table 1).
Statistical Analyses
The associations of urinary KIM-1/creatinine with cardiovascular and total mortality were investigated utilizing Cox proportional hazard regression using the following multivariable models. Model A was adjusted for age (age was adjusted for in all models by using age as the timeline). Model B was adjusted for age and established cardiovascular risk factors, including known CVD at baseline, antihypertensive treatment (yes/no), lipid-lowering treatment (yes/no), low-dose aspirin treatment (yes/no), current smoking, diabetes, systolic BP, body mass index, total cholesterol, and HDL cholesterol. Finally, model C was adjusted for age, established cardiovascular risk factors (as in model B), and markers of kidney damage and dysfunction (eGFR and ACR). We also performed analyses adjusting for urinary neutrophil gelatinase–associated lipocalin (NGAL)/creatinine and serum NGAL.
In our primary analyses, we modeled KIM-1/creatinine as a continuous variable (expressed as a 1-SD increase). We also performed secondary threshold models (upper quintile versus below the upper quintile of KIM-1/creatinine). Proportional hazards assumptions were confirmed by Schoenfeld tests, and linearity assumptions were confirmed by inspecting Martingale residuals. To gain additional insight into potential nonlinearity of the associations, we examined the Cox regression models using cubic splines with four degrees of freedom.
To evaluate the interplay between KIM-1/creatinine and other aspects of kidney pathology in the risk of cardiovascular mortality, we divided the participants into the following five groups. Group 1 comprised participants with normal eGFR (>60 ml/min per 1.73 m2), normal ACR (<3 g/mol), and normal KIM-1/creatinine (quintile 1–4, <175 ng/mmol [referent]). Group 2 included participants with normal eGFR (>60 ml/min per 1.73 m2) and normal ACR (<3 g/mol) but with high KIM-1/creatinine (quintile 5, ≥175 ng/mmol). Group 3 comprised participants with either low eGFR (≤60 ml/min per 1.73 m2), high ACR (≥3 g/mol), or both, but with normal KIM-1/creatinine (quintile 1–4, <175 ng/mmol). Group 4 included participants with either low eGFR (≤60 ml/min per 1.73 m2) or high ACR (≥3 g/mol), but with high KIM-1/creatinine (quintile 5, ≥175 ng/mmol). Finally, group 5 included participants with low eGFR (≤60 ml/min per 1.73 m2), high ACR (≥3 g/mol), and high KIM-1/creatinine (quintile 5, ≥175 ng/mmol)
We also performed tests for effect modification by hypertension, diabetes, prevalent CVD, microalbuminuria, and eGFR by including multiplicative interaction terms of these variables and urinary KIM-1/creatinine. We also performed analyses after exclusion of participants with a previous diagnosis of heart failure at baseline or during follow-up (n=100) to limit the possibility of reverse causation owing to heart failure as an explanation of our findings. In secondary analyses, multiple imputation methods were used to account for the potential influence of missing data. Cumulative incidence curves were compared with Kaplan–Meier failure curves, and Fine and Gray analyses were performed to address the issue of competing risk from non-CVD mortality (21,22). In secondary analyses, covariate factors were replaced by a propensity score to ensure that our adjusted models were not influenced by overfitting (23).
Laplace regression was used to calculate the difference in years until 10% of the participants died of cardiovascular mortality in individuals with low eGFR (≤60 ml/min per 1.73 m2), microalbuminuria/macroalbuminuria (≥3 g/mol), and high KIM-1/creatinine (≥175 ng/mmol), respectively, versus those without these traits (24).
We also investigated the association between KIM-1 excretion per 24 hours (KIM-1/24 hours) and the risk for cardiovascular mortality.
A two-sided P value <0.05 was regarded as significant in all analyses. The STATA 11.2 statistical software package (Stata Corp, College Station, TX) was used for all analyses.
Results
Table 1 shows the baseline characteristics of included participants (participants excluded because of missing data on KIM-1/creatinine had similar values as those included; P>0.14 for all; data not shown). The Spearman correlation coefficients were −0.14 between KIM-1/creatinine (P<0.001) and eGFR and 0.41 between KIM-1/creatinine and albumin/creatinine (P<0.001).
Table 1.
Baseline characteristics of all men and stratified by quintiles of KIM-1/creatinine
| Variable | All | Q1–Q4 | Q5 | P Value (Q1–Q4 versus Q5) |
|---|---|---|---|---|
| Participants (n) | 590 | 472 | 118 | |
| Age (yr) | 77.5±0.8 | 77.5±0.8 | 77.5±0.7 | 0.72 |
| BMI (kg/m2) | 26.3±4.5 | 26.2±3.4 | 26.7±3.8 | 0.15 |
| Systolic BP (mmHg) | 150±21 | 150±20 | 153±24 | 0.20 |
| Cholesterol (mmol/L) | 97.3±18 | 97.3±18 | 95.5±20 | 0.17 |
| HDL cholesterol (mmol/L) | 23.4±5.4 | 23.4±5.4 | 23.4±5.4 | 0.10 |
| eGFR (ml/min per 1.73 m2) | 74±17 | 75±16 | 69±20 | |
| Albumin/creatinine ratio (g/mol) | 0.7 (0.3–2.0) | 0.6 (0.3–1.6) | 1.6 (0.7–1.5) | <0.001 |
| KIM-1/creatinine ratio (ng/mmol) | 98 (55–152) | 78 (49–115) | 228 (199–301) | <0.001 |
| eGFR (<60 ml/min per 1.73 m2) | 124 (21) | 82 (17) | 42 (36) | <0.001 |
| Microalbuminuria/macroalbuminuria (>3 g/mol) | 110 (19) | 67 (14) | 43 (36) | <0.001 |
| Current smoking | 42 (7) | 28 (6) | 14 (12) | 0.03 |
| Diabetes | 78 (13) | 54 (11) | 24 (20) | 0.01 |
| Antihypertensive medication | 281 (48) | 215 (46) | 66 (66) | 0.04 |
| Lipid-lowering medication | 110 (19) | 80 (17) | 30 (25) | 0.03 |
| NSAID | 32 (5) | 22 (5) | 10 (9) | 0.10 |
| Low-dose aspirin | 166 (28) | 122 (26) | 44 (37) | 0.01 |
| History of cardiovascular disease | 153 (26) | 116 (25) | 37 (31) | 0.13 |
| History of ischemic heart disease | 109 (18) | 79 (17) | 30 (25) | 0.03 |
| History of stroke | 54 (9) | 43 (9) | 11 (9) | 0.94 |
| History of heart failure | 25 (4) | 18 (4) | 7 (6) | 0.31 |
Data are presented as the mean±SD for continuous variables, median (25th to 75th percentiles), and n (%) for categorical variables. History of cardiovascular disease was defined as a history of hospitalization for ischemic heart disease, cerebrovascular disease, or heart failure before baseline. BMI, body mass index; KIM-1, kidney injury molecule-1; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
During follow-up (median 8.1 years; range, 0.3–10.8 years), 198 individuals died; of which, 89 deaths were the result of cardiovascular causes (Table 2).
Table 2.
The association between KIM-1/creatinine and cardiovascular and total mortality: incidence rates and multivariable cox regression
| Mortality | Events/ At Risk (n) | Incidence Rate per 100 Person-Years at Risk (95% CI) | Hazard Ratio (95% CI) | ||
|---|---|---|---|---|---|
| Model A | Model B | Model C | |||
| Cardiovascular mortality | |||||
| Continuous models | |||||
| 1-SD increase (91 ng/mmol) | 1.50 (1.29 to 1.75)a | 1.45 (1.22 to 1.71)a | 1.27 (1.05 to 1.54)b | ||
| Threshold models | |||||
| Q1–Q4 (<175 ng/mmol) | 59/472 | 16.6 (12.9 to 21.4) | Referent | Referent | Referent |
| Q5 (≥175 ng/mmol) | 30/118 | 38.1 (26.7 to 54.5) | 2.34 (1.51 to 3.64)a | 2.08 (1.33 to 3.27)b | 1.72 (1.07 to 2.76)c |
| Total mortality | |||||
| Continuous models | |||||
| 1-SD increase | 1.30 (1.16 to 1.47)a | 1.24 (1.10 to 1.41)a | 1.12 (0.98 to 1.29) | ||
| Threshold models | |||||
| Q1–Q4 (<175 ng/mmol) | 148/472 | 41.6 (35.4 to 48.9) | Referent | Referent | Referent |
| Q5 (≥175 ng/mmol) | 50/118 | 63.6 (48.2 to 83.9) | 1.56 (1.13 to 2.14)b | 1.42 (1.02 to 1.97)c | 1.20 (0.85 to 1.69) |
Data are presented as the incident rate (95% CI) or hazard ratio (95% CI) unless otherwise specified. Model A is adjusted for age. Model B is adjusted for age and established cardiovascular risk factors such as known cardiovascular disease at baseline, antihypertensive treatment, lipid-lowering treatment, low-dose aspirin treatment, current smoking, diabetes, systolic BP, body mass index, total cholesterol, and HDL cholesterol. Model C is adjusted for age, established cardiovascular risk factors, eGFR, and albumin/creatinine ratio. 95% CI, 95% confidence interval.
P<0.001.
P<0.01.
P<0.05.
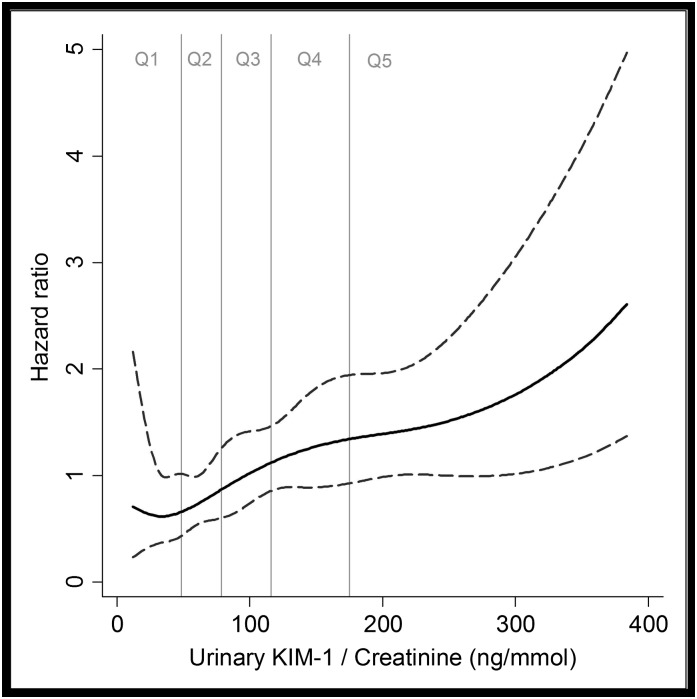
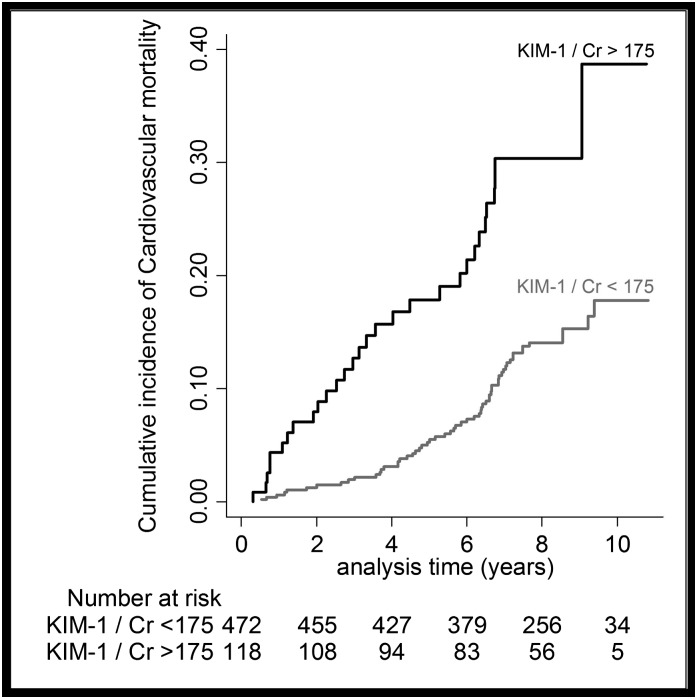
In Cox proportional hazard models, higher urinary levels of KIM-1/creatinine were significantly associated with higher risk for CVD mortality. The associations were only mildly effected by adjustments for age, cardiovascular risk factors, eGFR, and ACR (models A–C; Table 2). Examination of regression splines suggests a linear increase in hazard for CVD mortality with increasing KIM-1/creatinine (Figure 1). In secondary analysis, participants with KIM-1/creatinine levels in the highest quintile (≥175 ng/mmol) were at almost a doubled risk for CVD mortality (Figure 2, Table 2). The association between urinary KIM-1/creatinine and total mortality was generally weaker compared with the association between KIM-1/creatinine and CVD mortality (Table 2).
Figure 1.
Cubic regression splines of the unadjusted association between urinary KIM-1/creatinine and cardiovascular mortality. Black line indicates estimated hazard ratios (with 95% confidence intervals). KIM-1, kidney injury molecule-1; Q, quintile.
Figure 2.
The cumulative incidence of cardiovascular mortality. Analyzed by above versus below urinary KIM-1/creatinine of 175 ng/mmol (Q5 versus Q1–Q4). Cr, creatinine.
All aspects of kidney pathology (KIM-1/creatinine, eGFR, and ACR) predicted cardiovascular mortality independently of each other when included in the same model, even after further addition of cardiovascular risk factors as well as serum and urinary NGAL (Table 3).
Table 3.
The association between urinary KIM-1/creatinine and cardiovascular mortality, adjusted for established cardiovascular risk factors, eGFR, urinary albumin/creatinine ratio, and serum and urinary NGAL
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| KIM-1/creatinine (ng/mmol) | 1.27 (1.05 to 1.54) | 0.01 |
| BMI (kg/m2) | 0.88 (0.70 to 1.11) | 0.28 |
| Systolic BP (mmHg) | 0.96 (0.77 to 1.19) | 0.71 |
| Cholesterol (mmol/L) | 0.90 (0.72 to 1.13) | 0.37 |
| HDL cholesterol (mmol/L) | 1.20 (0.93 to 1.54) | 0.16 |
| eGFR (ml/min per 1.73 m2) | 0.98 (0.97 to 0.99) | 0.01 |
| Albumin/creatinine ratio (g/mol) | 1.16 (1.01 to 1.32) | 0.03 |
| Current smoking | 0.75 (0.35 to 1.63) | 0.46 |
| Diabetes | 2.15 (1.24 to 3.73) | 0.01 |
| Antihypertensive medication | 1.47 (0.88 to 2.46) | 0.14 |
| Lipid-lowering medication | 0.80 (0.45 to 1.41) | 0.43 |
| Low-dose aspirin | 1.06 (0.64 to 1.75) | 0.82 |
| History of cardiovascular disease | 2.06 (1.23 to 3.46) | 0.01 |
| Serum NGAL (µg/L) | 1.02 (0.83 to 1.25) | 0.88 |
| Urinary NGAL/creatinine (µg/mmol) | 0.97 (0.79 to 1.18) | 0.74 |
Data are multivariable hazard ratios when all variables were simultaneously included in the model. Hazard ratios for continuous variables are expressed per SD increase. BMI, body mass index; NGAL, neutrophil gelatinase–associated lipocalin.
The association between urinary KIM-1/creatinine and cardiovascular mortality was essentially unaltered when missing data on covariates were imputed, and based on all 627 individuals with KIM-1 measurements (data not shown).
Among participants with KIM-1/creatinine levels in the upper quintile (≥175 ng/mmol), the first 10% died of cardiovascular mortality 4.3 years earlier (95% confidence interval [95% CI], 6.2 to 2.4; P<0.001) compared with those with lower KIM-1/creatinine levels (<175 ng/mmol). The corresponding numbers were 3.2 years earlier (95% CI, 4.9 to 1.5; P<0.001) for eGFR≤60 ml/min per 1.73 m2 versus eGFR>60 ml/min per 1.73 m2 and 3.5 years earlier (95% CI, 4.7 to 2.3; P<0.001) for microalbuminuria/macroalbuminuria (ACR≥3 g/mol) versus no microalbuminuria/macroalbuminuria.
As seen in Table 4, participants with low eGFR (≤60 ml/min per 1.73 m2), microalbuminuria/macroalbuminuria (ACR>3 g/mol), and high KIM-1/creatinine (≥175 ng/mmol) had a >8-fold increase in risk for cardiovascular mortality, and participants with either low eGFR or microalbuminuria/macroalbuminuria and high KIM-1/creatinine had a 3-fold increase in risk compared with participants with normal eGFR, normoalbuminuria, and low KIM-1/creatinine. A similar risk (approximately 3-fold increase) was seen in participants with low eGFR and/or microalbuminuria/macroalbuminuria but with low KIM-1/creatinine. Participants with normal eGFR and normoalbuminuria but with high KIM-1/creatinine had a >2-fold increased risk. All of these findings remained significant and were mildly attenuated after adjustments for established cardiovascular risk factors. The associations with total mortality were not as strong.
Table 4.
Data portraying the interplay between KIM-1/creatinine, eGFR, and albuminuria and the risk for total and cardiovascular mortality: Cox regression
| Groups According to eGFR, Albuminuria, and KIM-1/Creatinine Statusa | Total Mortality | Cardiovascular Mortality | ||||
|---|---|---|---|---|---|---|
| Events/ At Risk (n) | Hazard Ratio (95% CI) | Events/ At Risk (n) | Hazard Ratio (95% CI) | |||
| Model A | Model B | Model A | Model B | |||
| Normal eGFR, normal ACR and normal KIM-1/creatinine | 82/342 | Referent | Referent | 30/342 | Referent | Referent |
| Normal eGFR, normal ACR, and high KIM-1/creatinine | 19/52 | 1.70 (1.03 to 3.80)b | 1.60 (0.97 to 2.65) | 10/52 | 2.45 (1.20 to 5.01)b | 2.29 (1.11 to 4.70)b |
| Low eGFR, high ACR or both, and normal KIM-1/creatinine | 66/131 | 2.42 (1.75 to 3.25)c | 2.18 (1.55 to 3.06)c | 29/131 | 2.93 (1.76 to 4.90)c | 2.35 (1.38 to 4.01)d |
| Low eGFR or high ACR, and high KIM-1/creatinine | 19/45 | 2.14 (1.30 to 3.52)d | 1.84 (1.09 to 3.12)b | 10/45 | 3.09 (1.51 to 6.33)d | 2.44 (1.14 to 5.19)b |
| Low eGFR, high ACR and high KIM-1/creatinine | 12/20 | 3.69 (2.01 to 6.77)c | 3.31 (1.76 to 6.23)c | 10/20 | 8.56 (4.18 to 17.56)c | 6.87 (3.21 to 14.73)c |
Model A is adjusted for age. Model B is adjusted for age and established cardiovascular risk factors such as known cardiovascular disease at baseline, antihypertensive treatment, lipid-lowering treatment, low-dose aspirin treatment, current smoking, diabetes, systolic BP, body mass index, total cholesterol, and HDL cholesterol. ACR, albumin/creatinine ratio.
Normal eGFR, ≥60 ml/min per 1.73 m2; low eGFR, ≤60 ml/min per 1.73 m2; normal ACR, <3 g/mol; microalbuminuria/macroalbuminuria, ACR≥3 g/mol; normal KIM-1/creatinine, <175 ng/mmol; and high KIM-1/creatinine, ≥175 ng/mmol.
P<0.05.
P<0.001.
P<0.01.
The results did not substantially change after excluding participants with heart failure at baseline or during follow-up (model B: hazard ratio for 1-SD increase in KIM-1, 1.36; 95% CI, 1.08 to 1.72; P=0.01). The multiplicative interaction terms for prevalent CVD, hypertension, diabetes, low eGFR (≤60 ml/min per 1.73 m2), or microalbuminuria were nonsignificant (P>0.27 for all).
A comparison between cumulative incidence curves and Kaplan–Meier failure curves, as well as Fine and Gray analyses, indicated that our results would not be compromised by competing risk from non-CVD mortality (data not shown), nor were they likely to be a result of overfitting because the findings remained unaltered in models in which the covariate factors were replaced by a propensity score (data not shown).
In the 24-hour urine collections, increments of KIM-1 concentration without creatinine standardization were also significantly associated with cardiovascular mortality in multivariable models A–C (Supplemental Table 2).
The risk estimates for cardiovascular mortality for the total amount of excreted KIM-1/24 hours varied substantially in the whole cohort compared with participants with >1000 ml urine/24 hours or participants with >1500 ml urine/24 hours (hazard ratios ranged from 1.08 per SD increase of KIM-1/24 hours [whole sample] to 2.97 [participants with >1.500 ml urine]). Conversely, the KIM-1/creatinine estimate was similar in all different urine volume subgroups (Supplemental Table 3).
Discussion
In a cohort of free-living elderly men, higher urinary KIM-1/creatinine was associated with a higher risk for cardiovascular mortality. These associations remained robust after adjustments for established cardiovascular risk factors and established markers for kidney damage and dysfunction (ACR and eGFR). Participants who had a combination of high KIM-1, low eGFR, and microalbuminuria/macroalbuminuria had the highest cardiovascular risk. Interestingly, our data indicate that urinary KIM-1 also identifies a subgroup of individuals with higher cardiovascular risk in those with no other signs of kidney damage or dysfunction. Our data confirm and extend previous studies suggesting a link between kidney tubular damage and the development of CVD (25).
Comparison with the Literature
Higher urinary levels of KIM-1 are suggested to provide prognostic information regarding mortality risk in patients with renal disease (13,14) or heart failure (15). For instance, in a study of hospitalized patients with ARF, higher urinary KIM-1 was associated with a higher risk of dialysis requirement or death (14). In kidney transplant patients, higher urinary KIM-1 was associated with an increased risk of graft loss (13,26). In patients with overt heart failure, higher urinary KIM-1 was associated with a higher risk for mortality and heart failure morbidity (15). Moreover, we recently reported that higher urinary KIM-1 is associated with an increased risk for incident heart failure hospitalizations in the community-based ULSAM cohort (19). Our findings of an association between urinary KIM-1 and total mortality is in accordance with two recent community-based studies (27,28); however, in one of these, no association between KIM-1 and cardiovascular mortality was found (28). Perhaps differences in the protocol for urine collection (spot sample versus 24-hour collection), or differences in age and gender distribution of the study samples may explain the discrepancy between the previous study and the present study.
Both circulating and urinary levels of NGAL, another tubular damage biomarker, were also shown to be an independent predictor of cardiovascular mortality (29). Yet, in the present study, the independent association between KIM-1/creatinine and cardiovascular mortality was not influenced after adding both urinary and circulating NGAL to the multivariate model. Interestingly, in this model, neither urinary nor circulating NGAL was a significant predictor of cardiovascular mortality (Table 3), perhaps indicating that KIM-1/creatinine is superior to NGAL as a prognostic marker.
Potential Mechanisms
Previous experimental studies illustrated different functions of KIM-1 in various renal diseases, including protective functions in AKI and damaging functions in CKD (5–7). Although it is not possible to establish causality in our observational study, there are some potential pathways that could explain these associations.
First, higher urinary levels of KIM-1 have been reported in patients with impaired eGFR and microalbuminuria or macroalbuminuria (26,30), two aspects of kidney disease that are shown to be closely associated with an increased cardiovascular risk (2,3). The fact that KIM-1 predicted cardiovascular death beyond these established markers of kidney damage and dysfunction indicates that KIM-1 portrays an aspect of kidney pathology that is not fully reflected by levels of eGFR or albuminuria. It is also possible that KIM-1 predicts the deterioration of kidney function, which in turn leads to an increased cardiovascular risk (8,31).
Second, CVD and CKD share several common risk factors, including hypertension, hypercholesterolemia, diabetes, inflammation, oxidative stress, and renin-angiotensin-aldosterone system activation (32,33). However, the multivariable models in this study indicate that these are not major pathways that explain our findings.
Finally, it is possible that the results of this study are the result of reverse causation (i.e., that higher urinary KIM-1 levels are a consequence of prevalent CVD, perhaps because of atherosclerosis in the small vessels of the kidney or by prevalent heart failure). For instance, patients with heart failure have higher urinary KIM-1 compared with healthy controls (34), and higher KIM-1 predicts mortality and hospitalizations in this patient group (15,35). However, in this study, the association between urinary KIM-1 and cardiovascular mortality was similar in participants free from CVD at baseline, after further exclusion of participants that developed heart failure during follow-up, which would argue against reverse causation because of prevalent cardiovascular disease or heart failure as an explanation of our findings.
Clinical Implications
Because KIM-1 expression is virtually absent in normal kidneys (36) but rises rapidly in response to tubular damage (37), KIM-1 is proposed as a clinically useful marker for acute tubular kidney damage (14). In fact, recent studies suggest that the assessment of biomarkers reflecting tubular damage should be included in the diagnostic criteria for AKI (38). However, in this study, urinary KIM-1 predicted cardiovascular mortality in a community-based sample in which the prevalence of individuals with AKI likely was very low. Thus, our data indicate that urinary KIM-1 levels should not merely be considered as a marker for acute tubular damage but perhaps also a marker for chronic tubular damage that predisposes to an increased risk for cardiovascular mortality. Whether tubular damage should be considered in the diagnostic criteria for chronic kidney damage remains to be established. The clinical utility of measuring urinary KIM-1 or other markers of kidney tubular damage, such as urinary NGAL, cystatin, IL-18, fatty-acid binding proteins, microglobulins, or N-acetyl-β-d-glucosaminidase (39), for risk prediction purposes in patients and in the community needs to be evaluated in further large-scale studies. Previous studies suggested that treatment with diuretics or pharmacologic inhibition of the renin-angiotensin-aldosterone system reduces urinary levels of KIM-1 (40,41). However, it is not known whether this reduction of KIM-1 levels corresponds to a reduction of cardiovascular risk.
KIM-1 without creatinine standardization and the total excreted amount of KIM-1/24 hours also predicted cardiovascular mortality, but the strength of the association with KIM-1/24 hours varied substantially in the whole cohort compared with participants with >1000 ml urine/24 hours or participants with >1500 ml urine/24 hours. The highest risk estimate for KIM-1/24 hours was seen in those who most likely had the highest validity of the 24-hour collection of urine. By contrast, the KIM-1/creatinine risk estimate was similar in all subgroups. This finding raises the question of how best to measure urinary KIM-1 in clinical practice. Of course, this is also an issue of feasibility because it is difficult to obtain reliable 24-hour urine collections in clinical practice. We emphasize that given the limited size of the different strata, no firm conclusions should be drawn on the basis of this exploratory subgroup analysis.
Strengths and Limitations
Strengths of the study include the community-based sample, the longitudinal study design with long follow-up time, and the detailed characterization of the study participants. Other strengths include the high quality and completeness of ascertainment of population registers in Sweden (42,43). The main limitation of this study is that we only had access to data in elderly men; thus, extrapolations of these findings to women, other ethnicities, and other age groups must be done with caution. Further studies are needed to evaluate our secondary aim, as well as age- and sex-specific thresholds for what should be considered a normal KIM-1/creatinine, and to evaluate the clinical relevance of the suggested threshold for urinary KIM-1/creatinine in this study (175 ng/mmol). We had access to only single eGFR, ACR, and KIM-1/creatinine measurements. In addition, biomarker levels measured in urine may be biased by the large variation in urine volume or concentration; however, we minimized the risk of this by standardizing the KIM-1 levels for urinary creatinine. We did not have access to serum creatinine levels and thus could not calculate eGFR based on the new combined equation (44). Another limitation is the fact that some of our multivariable models were adjusted for a large number of confounders, which could potentially lead to bias and uncertain risk estimates because of overfitting. Some of the groups that we studied were also fairly small and the point estimates should thus be interpreted with caution. However, the consistency of the results across all multivariable models and in models in which we replaced the covariate factors with a propensity score would argue against overfitting as an explanation of our findings (23).
Our results show that kidney tubular damage may predispose to a higher risk for cardiovascular mortality in the community-based setting. Further studies are needed to firmly establish a causal pathophysiologic role for KIM-1 in the development of CVD, and to evaluate the utility of measuring urinary KIM-1 in the clinical setting.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by the Swedish Research Council (grants 2006-6555, 2012-1727, and 2012-2215), Swedish Heart-Lung Foundation, Thuréus Foundation, Marianne and Marcus Wallenberg Foundation, Dalarna University, and Uppsala University.
The funding sources did not play any role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; and in preparation, review, or approval of the manuscript. J.Ä. is the guarantor of this work, had full access to all of the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11901113/-/DCSupplemental.
References
- 1.Arnlöv J: Diminished renal function and the incidence of heart failure. Curr Cardiol Rev 5: 223–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nerpin E, Ingelsson E, Risérus U, Sundström J, Larsson A, Jobs E, Jobs M, Hallan S, Zethelius B, Berglund L, Basu S, Arnlöv J: The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant 26: 2820–2827, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV: Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123: 4023–4035, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huo W, Zhang K, Nie Z, Li Q, Jin F: Kidney injury molecule-1 (KIM-1): A novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant Rev (Orlando) 24: 143–146, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H: Kidney injury molecule-1 in renal disease. J Pathol 220: 7–16, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, Racusen L, Rabb H: Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol 298: F1472–F1483, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees AJ, Kain R: Kim-1/Tim-1: From biomarker to therapeutic target? Nephrol Dial Transplant 23: 3394–3396, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Vaidya VS, Ferguson MA, Bonventre JV: Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48: 463–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 13.van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, van Goor H, Stegeman CA, Bonventre JV, Bakker SJ: High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 84: 1625–1630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL: Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 18: 904–912, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Damman K, Masson S, Hillege HL, Maggioni AP, Voors AA, Opasich C, van Veldhuisen DJ, Montagna L, Cosmi F, Tognoni G, Tavazzi L, Latini R: Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J 32: 2705–2712, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Byberg L, Zethelius B, McKeigue PM, Lithell HO: Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia 44: 2134–2139, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J: Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119: 2765–2771, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Carlsson AC, Calamia M, Risérus U, Larsson A, Helmersson-Karlqvist J, Lind L, Arnlöv J: Kidney injury molecule (KIM)-1 is associated with insulin resistance: Results from two community-based studies of elderly individuals. Diabetes Res Clin Pract 103: 516–521, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, Sundström J, Arnlöv J: Urinary kidney injury molecule 1 and incidence of heart failure in elderly men. Eur J Heart Fail 15: 441–446, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Larsson A, Malm J, Grubb A, Hansson LO: Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest 64: 25–30, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 22.Lin DY: Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med 16: 901–910, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Cepeda MS, Boston R, Farrar JT, Strom BL: Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol 158: 280–287, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Wändell P, Carlsson AC, Sundquist J, Johansson SE, Bottai M, Sundquist K: Effects of prescribed antithrombotics and other cardiovascular pharmacotherapies on all-cause mortality in patients with diabetes and atrial fibrillation - a cohort study from Sweden using propensity score analyses. Diabetol Metab Syndr 6: 2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels LB, Barrett-Connor E, Clopton P, Laughlin GA, Ix JH, Maisel AS: Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J Am Coll Cardiol 59: 1101–1109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauta FL, Bakker SJ, van Oeveren W, Navis G, van der Heide JJ, van Goor H, de Jong PE, Gansevoort RT: Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis 57: 733–743, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Sarnak MJ, Katz R, Newman A, Harris T, Peralta CA, Devarajan P, Bennett MR, Fried L, Ix JH, Satterfield S, Simonsick EM, Parikh CR, Shlipak MG, for the Health ABC Study : Association of urinary injury biomarkers with mortality and cardiovascular events [published online ahead of print February 7, 2014]. J Am Soc Nephrol 10.1681/ASN.2013070713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Seaghdha CM, Hwang SJ, Larson MG, Meigs JB, Vasan RS, Fox CS: Analysis of a urinary biomarker panel for incident kidney disease and clinical outcomes. J Am Soc Nephrol 24: 1880–1888, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helmersson-Karlqvist J, Larsson A, Carlsson AC, Venge P, Sundström J, Ingelsson E, Lind L, Arnlöv J: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with mortality in a community-based cohort of older Swedish men. Atherosclerosis 227: 408–413, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV: Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int 79: 464–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurman JM: Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 123: 7–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schupp N, Kolkhof P, Queisser N, Gärtner S, Schmid U, Kretschmer A, Hartmann E, Oli RG, Schäfer S, Stopper H: Mineralocorticoid receptor-mediated DNA damage in kidneys of DOCA-salt hypertensive rats. FASEB J 25: 968–978, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, Wilson PW: Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 290: 891–897, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL: Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 96: 1297–1302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, von Bary C, Endemann D, Banas B, Mack M, Böger CA, Riegger G, Luchner A: Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 13: 1104–1110, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M: Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Bonventre JV: Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol Dial Transplant 24: 3265–3268, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57: 1752–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F: Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28: 436–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damman K, Ng Kam Chuen MJ, MacFadyen RJ, Lip GY, Gaze D, Collinson PO, Hillege HL, van Oeveren W, Voors AA, van Veldhuisen DJ: Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol 57: 2233–2241, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Waanders F, Vaidya VS, van Goor H, Leuvenink H, Damman K, Hamming I, Bonventre JV, Vogt L, Navis G: Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: A post hoc analysis of a randomized controlled trial. Am J Kidney Dis 53: 16–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almgren T, Wilhelmsen L, Samuelsson O, Himmelmann A, Rosengren A, Andersson OK: Diabetes in treated hypertension is common and carries a high cardiovascular risk: Results from a 28-year follow-up. J Hypertens 25: 1311–1317, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO: External review and validation of the Swedish national inpatient register. BMC Public Health 11: 450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.