Abstract
Glomerular diseases developing in the kidney allograft are more often recurrences of the original disease affecting the native kidneys. However, in an undefined number of cases de novo, glomerular diseases unrelated to the original disease in the native kidneys can develop in the transplanted kidney. The clinical presentation and histologic features of de novo diseases are often similar to those features observed in patients with primary or secondary GN in the native kidneys. However, in transplanted kidneys, the glomerular, vascular, and tubulointerstitial changes are often intertwined with structural abnormalities already present at the time of transplant or caused by antibody- or cell-mediated allograft rejection, immunosuppressive drugs, or superimposed infection (most often of a viral nature). The pathophysiology of de novo glomerular diseases is quite variable. In rare cases of de novo minimal change disease, circulating factors increasing the glomerular permeability likely participate. Maladaptive hemodynamic changes and tissue fibrosis caused by calcineurin inhibitors or other factors may be involved in the pathogenesis of de novo FSGS. The exposure of cryptic podocyte antigens may favor the development of de novo membranous nephropathy. Many cases of de novo membranoproliferative GN are related to hepatitis C virus infection. Patients with Alport syndrome lacking antigenic epitopes in their glomerular basement membrane may develop antibodies against these glomerular basement membrane antigens expressed in the transplanted kidney. Infection may cause acute GN to have a heterogeneous clinical presentation and outcome. De novo pauci-immune GN in renal transplant is rare. Preexisting or acquired intolerance to glucose may, in the long term, cause diabetic nephropathy. The prognosis of de novo diseases depends on the type of GN, the severity of lesions caused by the alloimmune response, or the efficacy of immunosuppressive therapy. In most cases, the management of de novo glomerular diseases is empirical or elusive.
Keywords: renal transplantation, transplant pathology, GN
Introduction
Post-transplant glomerular diseases represent a frequent complication of kidney transplantation that may worsen the graft function and impair long-term graft survival. In a Canadian review, glomerular diseases were diagnosed in 15.7% of renal transplant patients at 15 years. Post-transplant GN occurred in 24.3% of allografted patients whose original renal disease resulted from biopsy-proven GN compared with 10.5% of those patients with other types of renal or systemic diseases. Patients who developed glomerular diseases had significantly reduced graft survival (1). Post-transplant glomerular disease is generally divided into recurrences of the same original disease that affected the native kidney of the recipient and de novo disease, in which the post-transplant disease is unrelated to the original disease. This differentiation may be difficult, because only 15%–20% of recipients have had biopsies of native kidneys before transplantation. Also, the incidence of de novo GN post-transplant is hard to assess, because immunofluorescence and electron microscopy are not routinely performed in all centers (2). Another important issue is that information on time-zero (preimplantation) biopsy and protocol biopsies was available only in rare cases. Therefore, it is possible that some patients labeled as having de novo disease actually received kidneys already affected by GN. Establishing the role of immunosuppression, hypertension, infection, rejection, genetic differences, or similarity between donor and recipient is also a difficult task.
In this review, we will examine the most frequent types of de novo glomerular disease and the current knowledge about this particular complication of transplanted kidneys.
Minimal Change Disease
De novo minimal change disease (MCD) in the renal allograft is quite rare. Many of the cases reported as de novo MCD disease failed to meet stringent criteria for this diagnosis, and it is possible that some cases represented an early manifestation of FSGS. Markowitz et al. (3) described one case and reported eight other cases that fulfilled the criteria for MCD. Truong and colleagues (4) reported five additional cases (3,5). Other anecdotal reports of de novo MCD have been reported, including cases occurring in ABO-incompatible transplants. Nephrotic-range proteinuria usually develops immediately or shortly after transplantation, but a patient developed de novo MCD 8 years after transplantation (6). AKI occurred in three patients. On biopsy, the glomeruli are typically normal by light microscopy, although some may reveal mild focal segmental mesangial sclerosis, hypercellularity, deposition of IgM/C3, or accumulation of mononuclear inflammatory cells in some glomerular capillaries (3,5).
The pathogenesis of de novo MCD is unknown. It is possible to speculate that the disorder may be triggered by an activation of innate and/or adaptive immunity, with dysfunction of T cells producing cytokines and circulating factors that alter the permeability of glomerular capillary wall, such as cardiotrophin-like cytokine-1 (7) or circulating soluble urokinase-plasminogen receptor (8). The original stimulus for such posited events remains uncertain, but viral-induced activation is a possibility. A role for costimulatory molecule B7–1 (CD80) in podocytes as an inducible modifier of glomerular permselectivity has been suggested. Overexpression of B7–1 (CD80) in podocytes has been found in experimental kidney diseases with nephrotic syndrome (9). Whether B7–1 (CD80) contributes to foot processes fusion and proteinuria also in transplanted kidneys is unknown. It is also possible that, in transplant patients, the disease may be triggered by a reaction to drugs. In one transplant recipient, de novo MCD developed while the patient was treated with sirolimus and reversed after withdrawal of sirolimus, suggesting a direct damage of this drug to podocytes (10).
The prognosis of de novo MCD is usually good. Under various treatments, including increased steroids, sustained remission of nephrotic syndrome was achieved in most cases. After remission, the grafts functioned well without or with minimal proteinuria for several years.
In summary, de novo MCD represents an important but hitherto underemphasized cause of post-transplant nephrotic syndrome that is potentially reversible and does not seem to adversely affect the long-term renal transplant outcome (Table 1).
Table 1.
Main characteristics of the more frequent de novo GN after transplantation
| Disease | Presentation | Time of Onset | Difference with Native GN | Treatment | Prognosis |
|---|---|---|---|---|---|
| MCD | NS | Early after transplant | Mild mesangial sclerosis, hypercellularity | Steroids | Good |
| FSGS | Proteinuria, rarely in nephrotic range | Months or years after transplant | NS is rare; signs of rejection or calcineurin inhibitor toxicity at biopsy | Removal of associated events | Usually poor, particularly in collapsing GN |
| MN | Proteinuria sometimes in nephrotic range | Late after transplant | Associated with transplant complications; IgG1 deposits instead of IgG4 | No specific treatment | Slowly progressive |
| MPGN | Proteinuria, hematuria, NS, nephritic sediment | Months or years after transplant | Often associated with HCV infection, sometimes with other diseases | Steroids+cytotoxic drugs if crescentic GN (?) | Slowly progressive; poor with many crescents |
| IgAN | Hematuria, proteinuria | Macroscopic hematuria is infrequent | Steroids+cytotoxic drugs if crescentic GN (?) | Usually good but poor in patients with crescentic GN |
MCD, minimal change disease; NS, nephrotic syndrome; MN, membranous nephropathy; MPGN, membranoproliferative GN; HCV, hepatitis C virus; IgAN, IgA nephritis.
FSGS
FSGS was the leading form of de novo glomerular disease in a Canadian review (1). Although recurrent FSGS usually presents early post-transplant as a nephrotic syndrome, de novo FSGS is often detected more than 12 months after transplantation and associated with variable amounts of proteinuria (including nephrotic syndrome), hypertension, and progressive deterioration of renal allograft function.
Compensatory glomerular hyperfiltration in residual nephrons caused by nephron loss or low nephron dose in the transplanted kidney (size discrepancies between the graft and the recipient) has been implicated in the pathogenesis of de novo FSGS. Hypertension, diabetes, rejection, BK polyomavirus (11) or parvovirus B19 (12) infection, and any other condition leading to loss of renal mass can be involved in the pathogenesis of FSGS. Thus, many cases of de novo noncollapsing FSGS that occur in patients with graft dysfunction may actually represent cases of secondary FSGS.
Most patients with de novo FSGS have been receiving calcineurin inhibitors (CNIs) for post-transplant immunosuppression. The form of FSGS associated with CNI nephrotoxcity generally develops some months after transplantation and is characterized by proteinuria, hypertension, and progressive deterioration of renal allograft function. At renal biopsy, the picture is dominated by chronic lesions with arteriolar occlusion, interstitial nephritis, striped interstitial fibrosis, tubular atrophy, and focal or global glomerular sclerosis. The profound vasoconstriction together with the typical microvascular lesions caused by CNI may induce arteriolar ischemia with consequent histologic lesions. A central role is played by TGF-β, a multipotent protein that regulates cellular growth, differentiation, and extracellular matrix formation. CNI can increase the expression of TGF-β (13). An overexpression of TGF-β in podocytes may lead to podocyte apoptosis and detachment from the glomerular basement membrane (GBM), leading to synechia and glomerulosclerosis.
Rare cases of de novo FSGS have also been reported in transplant recipients converted from CNI to high doses of sirolimus in the presence of proteinuria. The lesions resembled those lesions seen in FSGS of classic type. At immunohistochemistry, some podocytes in glomeruli with FSGS lesions had absent or diminished expression of the podocyte-specific epitopes synaptopodin and p57 and had acquired expression of cytokeratin and PAX2, reflecting a immature fetal phenotype (14). Such a pattern of epitope expression provides evidence for podocyte dysregulation. This finding was confirmed by experiments on human podocytes exposed to sirolimus. Vascular endothelial growth factor synthesis and protein kinase B phosphorylation were decreased. No loss of podocyte differentiation markers was observed, with the exception of a dose-dependent decrease of Wilms’ tumor 1, a transcription factor essential for maintaining podocyte integrity (15).
De novo noncollapsing FSGS usually develops months or even years after transplantation. The prognosis is poor, with a renal survival of only 40% at 5 years after diagnosis in cases associated with chronic allograft nephropathy (16,17). Attempts to withdraw CNI by introducing mycophenolate salts or mammalian target of rapamycin (mTOR) inhibitors may obtain improvement or stabilization of renal function if the conversion is performed early, but this maneuver may increase the risk of rejection and result in graft failure if creatinine clearance is lower than 40 ml/min or proteinuria is already present (18). Graft failure caused by de novo FSGS does not represent a contraindication to retransplant. However, efforts should be made to identify the factors responsible for FSGS and take appropriate therapeutic measures to prevent its development after retransplantation, such as minimization of CNI or antiviral prophylaxis (Table 2).
Table 2.
The risk of recurrence of de novo GN after retransplantation is unknown
| Disease | Indications to Retransplant |
|---|---|
| MCD | In view of the favorable prognosis, there is no contraindication to retransplant. |
| FSGS | If FSGS was caused by calcineurin inhibitor or mTOR inhibitor toxicity, there is no contraindication to retransplant, but the dosage of the offending drug should be minimized. If FSGS was associated with antibody-mediated rejection, the risk of recurrence is increased. Circulating antibodies should be removed before retransplant. |
| Collapsing nephropathy | The risk of recurrence is probably high. Antiviral treatment and/or removal of circulating antibodies before retransplant are recommended according to the possible role played by virus infection or antibody-mediated rejection in the first transplant. |
| MN | In view of the slow progression, there is no contraindication to retransplant. |
| MPGN | The risk of recurrence is high in carriers of HCV, active autoimmune disease, or monoclonal gammopathy. These risk factors should be removed or inactivated before retransplant. |
| IgAN | No contraindication to retransplant. |
| Alport syndrome | No contraindication to retransplant. If the first graft was lost because of anti-GBM nephritis, plasmapheresis and rituximab before retransplant are suggested. |
Indications and suggestions are indicated. mTOR, mammalian target of rapamycin; GBM, glomerular basement membrane.
Collapsing Glomerulopathy
Apart from the described cases of FSGS, we will consider collapsing glomerulopathy (CG), which is a pathologically and clinically distinct variant of FSGS. De novo CG has been reported to occur in about 0.6% of renal allografts (19) and develop in a mean of 4–5 years after transplantation. Patients usually present with marked proteinuria and progressive graft dysfunction. The characteristic glomerular lesions are segmental and/or global collapse of the glomerular capillary tufts with prominent podocytes filling Bowman's space, often associated with severe tubulointerstitial injury and obliterative vascular changes (20). These histologic findings are often associated with acute rejection, diabetic nephropathy, and/or immune complex GN.
The pathogenesis is unknown. Among other factors, hemodynamic disturbances may play a role in the development of the pattern of CG in renal allografts. Cases of de novo CG have been observed in transplant patients with cytomegalovirus or parvovirus B19 infection (21,22) and most recently, associated with post-transplant antibodies to angiotensin II type 1 receptor occurring as a part of antibody-mediated rejection (23).
The prognosis of CG is quite poor. Graft loss occurred within 3 years in all 10 patients reported in the work by Swaminathan et al. (24). There is no specific treatment for the disorder at present. Antiviral treatment may be suggested in patients with evidence of viral infection. If B7–1 (CD80) is expressed in podocyte, perhaps a trial of abatacept might be justified (25), but the effects of this agent on de novo FSGS are unknown. Little information is available about the results of retransplantation in patients who lost a previous graft because of CG. A pretransplant screening for antibodies to angiotensin II type 1 receptor and an aggressive antiviral treatment may be recommended in case of retransplantation.
Membranous Nephropathy
De novo membranous nephropathy (MN) develops in around 2% of adults receiving renal allografts (26). In a number of cases, de novo MN is associated with new-onset hepatitis C virus (HCV) infection, Alport syndrome, ureteral obstruction, or even recurrent IgA nephritis (IgAN) (27). De novo MN usually occurs months or years after transplantation, but rarely, it can develop soon after transplantation. The clinical presentation is variable from asymptomatic to nephrotic-range proteinuria. The light microscopic lesions are similar to those lesions observed in idiopathic MN, although some mesangial proliferation, numerous foam cells in the intima of hyperplastic arteries, and/or signs of antibody-mediated rejection may be seen (28). By immunofluorescence microscopy, there are diffuse granular deposits of IgG along the subepithelial aspect of the GBM, which can be segmental in a few cases. In a small series, the subclasses of deposited IgG were studied. IgG4 was found in recurrent MN, whereas IgG1 staining was dominant in de novo MN (29).
It has been hypothesized that de novo MN might represent a peculiar form of immune response triggered by exposure of hidden antigens, probably different from those antigens observed in idiopathic MN. A recent study has shown that, although recurrent MN is strongly correlated with positive glomerular staining for phospholipase A2 receptor, in post-transplant de novo MN, phospholipase A2 receptor staining was almost always negative (30). Theoretically, a viral, mechanical, or alloimmune injury causing damage of podocytes might cause a release of cytoplasmic- or membrane-associated podocyte proteins, which can be recognized by the immune system as foreign antigens with production of antibodies and formation of subepithelial immune complexes (27). Because MN may also be associated with malignancy or infection, the workup for post-transplant MN should include a careful search for underlying cancer or viral infection.
Whether de novo MN has a deleterious impact on the outcome of transplant is still controversial. In adult series, some investigators found a high rate of allograft failure, with signs of chronic allograft rejection in graft biopsies (31), and others reported that the allograft survival was not affected by the development of de novo MN (32). There is no formal contraindication to retransplantation.
Membranoproliferative GN
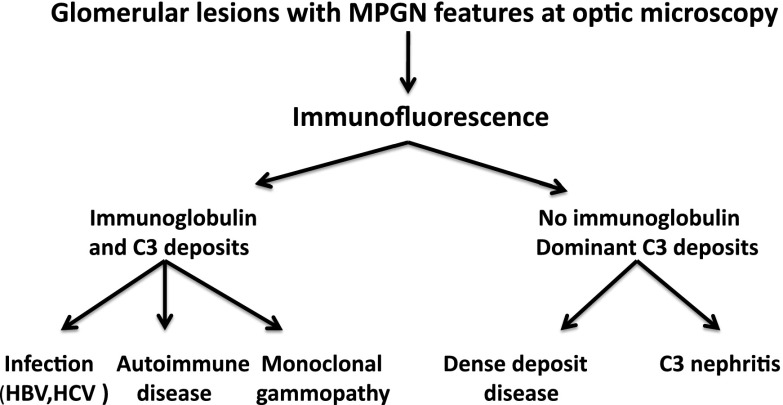
The currently recommended classification of membranoproliferative GN (MPGN) is mainly based on immunofluorescence findings (Figure 1). Cases with mesangial and capillary complement deposits and lack of Ig deposition on immunofluorescence are classified as C3 glomerulopathy (either dense deposit disease or C3 GN). Cases with capillary and mesangial deposits of Igs are classified as Ig-mediated MPGN (and can be of the monoclonal, oligoclonal, or polyclonal varieties depending on the nature of the Ig deposits). Secondary forms of MPGN can be associated with thrombotic microangiopathy, viral hepatitis, autoimmune diseases, or monoclonal gammopathy (33).
Figure 1.
Glomerular lesions with membranoproliferative GN (MPGN) features at optical microscopy are today classified according to the results of immunofluorescence studies. Cases with glomerular deposition of Igs (IgG or IgM) are caused by circulating Igs or immune complexes and associated with other pathologic diseases, such as hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, autoimmune diseases (systemic lupus erythematosus), or monoclonal gammopathy, including myeloma. In these cases, complement is frequently activated by the classic pathway. Cases without or with faint deposits of Igs are characterized by dominant deposits of C3. These cases are usually caused by congenital/hereditary mutations or acquired inhibitors (e.g., autoantibodies or monoclonal proteins) of factors regulating the alternative pathway of complement cascade. These latter disorders are called C3 glomerulopathies and can be subdivided into C3 GN and dense deposit disease according to electron microscopy findings.
In patients with C3 glomerulopathy, the disease can be related to mutations of factors regulating the alternative pathway of complement cascade. After transplantation, recurrence is frequent, but new-onset (de novo) cases of C3 glomerulopathy have not been reported. De novo Ig-mediated MPGN develops uncommonly after transplantation and is associated with HCV in about one half of cases (34). In a French series, only 13 of 399 renal transplant recipients developed a de novo MPGN (35). Renal biopsy showed the typical MPGN pattern of mesangial hypercellularity accompanied by broadening of the peripheral capillary loops caused by reduplication of the GBM. At immunofluorescence, there was subendothelial and mesangial deposition of Igs and complement. Glomerular subendothelial electron-dense deposits with fibrillar appearance may be seen in the case of cryoglobulinemia, most likely a consequence of HCV (36).
The occurrence of a de novo MPGN pattern of injury in a transplant renal biopsy is often related to the development of a systemic disease. The pathogenesis is unknown, but it is possible that, in HCV-positive patients, the histologic changes are caused by glomerular deposits of HCV and anti-HCV Igs (34). Some of these patients also have cryoglobulinemia, but in a number of patients, the search for circulating cryoglobulins is persistently negative (37). In other instances, new-onset MPGN may be associated with a histologic and clinical pattern of de novo thrombotic microangiopathy, a condition that may be triggered by rejection, CNI toxicity, or viral infection (see below).
At presentation, most patients show proteinuria>1 g/d, and about one half of them have a nephrotic syndrome. Patients with signs of thrombotic microangiopathy at biopsy may show the clinical and laboratory features of hemolytic–uremic syndrome.
Patients with non-nephrotic proteinuria and normal graft function may have a slow and largely silent course, but in other cases, the development of MPGN accelerates loss of the graft (37). Steroids and reinforcement of immunosuppression have been used with inconsistent and usually poor results. In the case of graft failure, retransplant is not contraindicated. However, risk factors should be searched for and eradicated whenever possible. In this setting, a trial with IFN, ribaviran, new protease inhibitors, and/or RNA polymerase inhibitors should be attempted in carriers of HCV before considering retransplantation.
Fibrillary GN/Immunotactoid Nephritis
De novo fibrillary GN after transplantation is very rare (38). The disease may develop after transplantation and lead to progressive graft dysfunction. A case of de novo immunotactoid nephritis occurred in a transplant recipient affected by cytomegalovirus infection. The morphologic lesions and clinical features reversed after recovery from the viral infection (39).
IgAN
A number of cases of de novo IgAN have been described (40–43). The allograft biopsy usually discloses intracapillary proliferation, with crescentic GN in many cases. Immunofluorescence shows granular deposits of IgA and C3 along glomerular capillary walls and mesangial areas. In view of the high frequency of asymptomatic (lanthanic) IgAN, particularly in Asian population, it is possible to speculate that some patients with de novo post-transplant IgAN received a kidney that already had latent IgA deposition before transplantation. If so, many cases of de novo IgAN would be better classified as inadvertently transmitted disease rather than de novo disease. Actually, in a Japanese study in which 0-hour allograft biopsies were performed, mesangial IgA deposition was present in 82 (16.1%) of 510 biopsied allografts (41).
The course of the de novo IgAN may be relatively favorable in cases with mild mesangial cell proliferation. However, the prognosis of patients with crescents at biopsy is poor (43). No specific therapy is recommended for patients with mild to moderate de novo IgAN. We feel that, in patients with rapidly progressive course (often accompanied by crescents), an aggressive treatment with methylprednisolone pulses and cyclophosphamide and plasma exchange in some cases may be tried, although with little chances of success.
Infectious Proliferative GN
A score of de novo bacterial or fungal infection-related GNs in renal transplant recipients has been reported (44,45). The only exception was the infection caused by a streptococcal organism, but in most cases, the etiology of infections was variable with different characteristics of presentation. The histologic picture at biopsy was also heterogeneous. Some cases showed proliferative and exudative endocapillary GN with granular deposits of C3 at immunofluorescence and typical subendothelial electron-dense deposits. However, many other cases showed necrotizing GN associated with frequent cellular crescents and tubulointerstitial changes. AKI was the clinical presentation in the majority of cases. The prognosis and treatment depend on the clinical–pathologic characteristics of the disease. Some but not all patients recovered renal function with appropriate antimicrobial therapy. Most patients have also been treated with steroids, mainly methylprednisolone pulses, that seemed to be effective in few cases.
Anti-GBM Nephritis in Alport Syndrome
In normal subjects, α3(IV) collagen produced by bone marrow-derived podocytes integrates into the GBM and associates with other α-chains to form type IV collagen triple helical networks. In Alport syndrome, mutations in GBM-associated type IV collagen genes (COL; COL4A3, COL4A4, or COL4A5) lead to absence of all three GBM collagen triple helical chains with consequent basement membrane structural defects, proteinuria, and progressive renal failure (46). The Alport patients who have undergone kidney transplantation may develop transient IgG linear deposition along GBM without circulating anti-GBM antibodies, but 3%–12% of them, mainly those patients with a juvenile type of X-linked Alport syndrome and truncation mutations of the COLIVA5 gene, may develop anti-GBM disease (47,48). This complication may manifest immediately after transplantation or later but usually within the first 1 year. The immunization of Alport patients is caused by the presence in the transplanted kidney of antigenic epitopes that are lacking in the native kidneys. The epitopes recognized by the anti-GBM antibodies in post-transplant de novo anti-GBM disease in X-linked Alport syndrome are noncryptic, unlike those epitopes of the classic Goodpasture disease in native kidneys (49,50).
Clinically, de novo anti-GBM disease in a recipient with X-linked Alport syndrome is characterized by the onset of a rapidly progressive GN. The diagnosis is based on the detection of circulating anti-GBM antibodies and renal biopsy, which shows a diffuse crescentic nephritis by optical microscopy with strongly positive linear IgG staining of the GBM on immunofluorescence. Anti-GBM antibodies in post-transplant Alport syndrome recognize epitopes on COLIVA3, COLIVA4, and COLIVA5 that are different from those epitopes associated with classic Goodpasture disease and may be difficult to detect in routine assays for anti-GBM antibody (49,50).
The prognosis of Alport patients with de novo anti-GBM disease is poor. Intensive plasma-exchange therapy with steroids and cytotoxic agents may be attempted to remove the anti-GBM antibodies, but only a few patients respond to treatment. With the exception of the few patients who develop anti-GBM nephritis, the outcome of renal transplant in patients with Alport syndrome is good. An analysis of 14 registries in Europe showed that patients with Alport syndrome had better renal graft and patient survival than matched controls (51). In those few patients who lost the allograft because of anti-GBM disease, plasmapheresis and rituximab might be tried immediately before retransplant and in the perioperative period.
Pauci-Immune GN
De novo pauci-immune (ANCA-positive) GN in renal transplant is rare. In three ANCA-positive cases, the disease developed more than 10 years after renal transplantation (52,53). Patients presented with hematuria, proteinuria, and a rapidly progressive deterioration of renal function. Despite treatment with methylprednisolone pulses and cyclophosphamide, renal function did not recover. Another case of de novo ANCA-negative extracapillary necrotizing pauci-immune GN has been reported. GN developed 5 months after transplantation after a prolonged bowel paralysis and sepsis from a Staphylococcus epidermidis infection. The patient was treated with methylprednisolone pulses and cyclophosphamide and eventually recovered a good function of the allograft (54). There is no information about the results of retransplant in patients who lost their allografts because of de novo pauci-immune GN.
Diabetic Nephropathy
De novo nephropathy caused by new-onset diabetes mellitus after transplantation seems to occur as frequently as recurrent diabetic nephropathy. Histologic diabetic nephropathy develops in a mean of 5.9 years after new-onset diabetes (55). However, the longer the duration of post-transplant diabetes, the higher the risk of developing diabetic nephropathy. The histologic abnormalities resemble those abnormalities observed in the classic diabetic nephropathy in native kidneys. Initially, there is thickening of GBM and the tubular basement membrane. An increase in mesangial matrix develops later. Over time, the extracellular matrix accumulates with formation of a nodular mesangial expansion surrounded by large round zones around the periphery of the nodule and compression of the associated glomerular capillaries that eventually lead to glomerular sclerosis and obliteration of capillary lumina. Afferent and efferent arteriolar hyalinosis and tubulointerstitial lesions also occur in parallel with glomerular lesions (56). In renal transplant, these lesions are often associated with vascular and tubulointerstitial changes caused by allograft rejection, viral infection, or CNI nephrotoxicity.
The risk factors identified for type 2 diabetes may also be involved in the development of new-onset diabetes after transplantation. However, the type and dosage of immunosuppressive drugs also play a major pathogenetic role. The diabetogenic effect of glucocorticoids is well established and related to both the dose used and the duration of steroid treatment (57). Cyclosporine (58) and tacrolimus (59) are both diabetogenic, but tacrolimus-treated transplant recipients more frequently develop insulin-dependent diabetes (60). The mTOR inhibitors can also favor the development of post-transplant diabetes (61), particularly when associated with tacrolimus (62).
New-onset post-transplant diabetes may worsen both the patient and graft prognosis. In transplant patients with impaired glucose tolerance or diabetes mellitus, these glucose dysmetabolism abnormalities are associated with the progression of histopathological changes, especially in patients with already compromised kidneys (63). Plasma creatinine levels and 12-year graft survival were significantly worse in transplant patients who developed diabetes than in controls (64).
Efforts should be made to prevent the possible development of post-transplant diabetes. Diet, physical activity, weight loss in the obese, and reduction of the doses of steroids and CNI whenever possible are effective measures to minimize glucose intolerance in transplant recipients. Treatment interventions in diabetic nephropathy include glycemic control, treatment of hypertension and hyperlipidemia, cessation of smoking, and protein restriction. In patients with diabetes, improvement in glucose control is the most important therapeutic approach in primary prevention (65). It is reasonable to extrapolate this recommendation to renal transplant recipients. A target of glycated hemoglobin levels around 7% is recommended in most patients with diabetes, but such targets do need to be individualized to avoid the deleterious effects of hypoglycemia (66). A more aggressive treatment aimed to reduce glycated hemoglobin levels to 6.5% may obtain a 21% relative reduction in the risk of nephropathy (67), but the prospects of a long-term benefit should be balanced against the risk of this aggressive approach. A systematic review of intensive glycemic control versus conventional glycemic control based on 28 randomized clinical trials did not show significant differences for all-cause mortality and cardiovascular mortality between the two treatments. In some trials, targeting intensive glycemic control seemed to reduce the risks of nonfatal myocardial infarction, amputation of a lower extremity, and development of a composite outcome of microvascular disease, nephropathy, retinopathy, and risk of retinal photocoagulation. However, intensive glycemic control significantly increased the risk of hypoglycemia and serious adverse events. Health-related quality of life did not show any statistically significant differences. Thus, targeting intensive glycemic control seemed to reduce the risk of microvascular complications but increased the risk of hypoglycemia and serious adverse events (68). Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers might be useful to retard the onset of microalbuminuria and slow the progression of renal disease in transplant patients with diabetic nephropathy, but this approach has never been tested in a randomized clinical trial. Combined use of an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker is presently discouraged because of an increased risk of adverse events (69).
Thrombotic Microangiopathy
Although atypical hemolytic uremic syndrome (HUS) frequently recurs after transplantation, a de novo thrombotic microangiopathy (TMA) is relatively rare. According to the US Renal Data System, after transplantation, only 0.8% of patients develop a de novo TMA (70). However, single center studies reported a higher incidence ranging between 4% and 14% (71,72). TMA usually develops within the first 1 year after transplantation (73).
Most patients with de novo TMA were treated with CNI (74), but cases have also been reported with the use of mTOR inhibitors (75,76). Antibody-mediated rejection is a frequent cause of TMA. In a retrospective review of renal allograft biopsies, Satoskar et al. (77) observed de novo TMA in 59 of 715 biopsies (6.1%), most of them (55%) with C4d-positive biopsy. Among patients with C4d-positive biopsies, 13.6% had TMA compared with only 3.6% patients with C4d-negative biopsies. A number of other factors may be associated with an increased risk of post-transplant de novo TMA, including viral infection, malignancy, drugs such as valacyclovir or clopridogrel, and positivity for antiphospholipid antibodies (78). Progressive TMA may also occur after ABO-incompatible renal transplantation (79). It is likely that the endothelial lesions caused by these factors may be amplified by the use of CNI and mTOR inhibitors or vice versa. CNI increases the expression of vasoconstrictor agents, such as angiotensin, endothelin, and thromboxane A2, while decreasing the production of nitric oxide and prostacyclin (80). Moreover, endothelial cells exposed to cyclosporine in vitro and in vivo release microparticles that activate the alternative pathway of complement. In renal transplant patients, the number of endothelial microparticles in plasma increases 2 weeks after starting tacrolimus, leading to increased C3 deposition on endothelial microparticles in the plasma of some patients. These data show that release of endothelial microparticles caused by vascular injury can trigger intravascular complement activation and favor the development of TMA and HUS (81). However, the repair of endothelial lesions may be hampered by the decreased endothelial cell proliferation and the antiangiogenic activity of mTOR inhibitors (82).
The clinical presentation of de novo TMA may be insidious. Some patients may show the typical features of HUS. However, anemia, increased lactate dehydrogenase, decreased haptoglobin, and schistocytosis may be absent. Often, TMA is discovered by renal biopsy performed either for protocol or because of a silent deterioration of graft function. The prognosis depends on the severity of histologic lesions and clinical features. Graft loss is frequent, particularly in patients with systemic signs and symptoms of HUS. Instead, the prognosis is less severe in patients with isolated glomerular TMA (83). Specific antiviral treatment is recommended in case of viral infection. Withdrawal or reduction of CNI or mTOR inhibitors may be followed by remission in milder cases. Plasma exchange in addition to CNI withdrawal can contribute to graft salvage (71,73,84). Recently, cases of remission with eculizumab in post-transplant de novo TMA in transplant have been reported (85–87).
A number of different de novo glomerular diseases may develop in the transplanted kidney, but their recognition and classification are difficult, because our understanding of many diseases has changed; also, the lack of native kidney diagnosis is not determined in many transplant recipients. In many cases, the clinical presentation and histologic features resemble those features of primary or secondary GN that occur in native kidneys. In some cases, such as CNI-related FSGS, infection-related nephritis, anti-GBM nephritis in X-linked Alport syndrome patients, and diabetic nephropathy, the etiology is clear, but in many other instances, the pathophysiology of de novo disease is still far from being elucidated. Thus, it is important to document both the nature of the primary renal disease in the recipient and the possible presence of glomerular lesions in the donated kidney by a zero-time biopsy of the allografted kidney. Electron microscopy may also be helpful in distinguishing a de novo glomerular disease from a recurrence of a primary GN. Moreover, it is of critical importance to identify possible factors responsible for the glomerular lesions, such as cellular- or antibody-mediated rejection, viral infection, malignancy, etc. The prognosis of de novo glomerular diseases may be variable depending on the type of lesions and the concomitant injuries caused by ischemia–reperfusion injury, allograft rejection, drug toxicity, and/or viral or microbial infection. In most cases, the specific treatment of de novo glomerular diseases is empirical and often elusive.
Disclosures
C.P. was a consultant of Novartis Italy until December 31, 2011, and received honoraria for lectures from Novartis. G.M. has no conflict of interest. R.J.G. was a consultant for Novartis, Genentech, Questcor, Eli Lilly, Sanofi-Genzyme, Bristol Myers-Squibb, Astellas, Mitsubishi9-Tanabe, UpToDate, Karger (American Journal of Nephrology and Karger Blogs), Chemicentryx, and BioMarin.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chailimpamontree W, Dmitrienko S, Li G, Balshaw R, Magil A, Shapiro RJ, Landsberg D, Gill J, Keown PA, Genome Canada Biomarkers in Transplantation Group : Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol 20: 843–851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golgert WA, Appel GB, Hariharan S: Recurrent glomerulonephritis after renal transplantation: An unsolved problem. Clin J Am Soc Nephrol 3: 800–807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markowitz GS, Stemmer CL, Croker BP, D’Agati VD: De novo minimal change disease. Am J Kidney Dis 32: 508–513, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Zafarmand AA, Baranowska-Daca E, Ly PD, Tsao CC, Choi YJ, Suki WN, Truong LD: De novo minimal change disease associated with reversible post-transplant nephrotic syndrome. A report of five cases and review of literature. Clin Transplant 16: 350–361, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki Y, Iwata T, Nishikido M, Uramatsu T, Sakai H, Taguchi T: De novo minimal change disease after ABO-incompatible kidney transplantation. Clin Transplant 26[Suppl 24]: 81–85, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Madhan KK, Temple-Camp CR: Late de novo minimal change disease in a renal allograft. Saudi J Kidney Dis Transpl 20: 266–269, 2009 [PubMed] [Google Scholar]
- 7.McCarthy ET, Sharma M, Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainra R, Mulay A, Bell R, Karpinski J, Hoar S, Knoll G, Robertson S, Wang D: Sirolimus use and de novo minimal change nephropathy following renal transplantation. Transplantation 80: 1816, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Li RM, Branton MH, Tanawattanacharoen S, Falk RA, Jennette JC, Kopp JB: Molecular identification of SV40 infection in human subjects and possible association with kidney disease. J Am Soc Nephrol 13: 2320–2330, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Waldman M, Kopp JB: Parvovirus B19 and the kidney. Clin J Am Soc Nephrol 2[Suppl 1]: S47–S56, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Olyaei AJ, de Mattos AM, Bennett WM: Nephrotoxicity of immunosuppressive drugs: New insight and preventive strategies. Curr Opin Crit Care 7: 384–389, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi MN, Helal I, Noël LH, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Letavernier E, Bruneval P, Vandermeersch S, Perez J, Mandet C, Belair MF, Haymann JP, Legendre C, Baud L: Sirolimus interacts with pathways essential for podocyte integrity. Nephrol Dial Transplant 24: 630–638, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Cosio FG, Frankel WL, Pelletier RP, Pesavento TE, Henry ML, Ferguson RM: Focal segmental glomerulosclerosis in renal allografts with chronic nephropathy: Implications for graft survival. Am J Kidney Dis 34: 731–738, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Gupta R, Sharma A, Mahanta PJ, Agarwal SK, Dinda AK: Focal and segmental glomerulosclerosis in renal allograft recipients: A clinico-pathologic study of 37 cases. Saudi J Kidney Dis Transpl 24: 8–14, 2013 [PubMed] [Google Scholar]
- 18.Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R, Li H, Scarola J, Neylan JF, Sirolimus CONVERT Trial Study Group : Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 87: 233–242, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Meehan SM, Pascual M, Williams WW, Tolkoff-Rubin N, Delmonico FL, Cosimi AB, Colvin RB: De novo collapsing glomerulopathy in renal allografts. Transplantation 65: 1192–1197, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Sharma A, Agarwal SK, Dinda AK: Collapsing glomerulopathy in renal allograft biopsies: A study of nine cases. Indian J Nephrol 21: 10–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson L, Boriskin Y, McPhee I, Holwill S, Rice P: Acute cytomegalovirus infection complicated by collapsing glomerulopathy. Nephrol Dial Transplant 18: 187–189, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Moudgil A, Shidban H, Nast CC, Bagga A, Aswad S, Graham SL, Mendez R, Jordan SC: Parvovirus B19 infection-related complications in renal transplant recipients: Treatment with intravenous immunoglobulin. Transplantation 64: 1847–1850, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Alachkar N, Gupta G, Montgomery RA: Angiotensin antibodies and focal segmental glomerulosclerosis. N Engl J Med 368: 971–973, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Swaminathan S, Lager DJ, Qian X, Stegall MD, Larson TS, Griffin MD: Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant 21: 2607–2614, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW, 3rd, Mundel P: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369: 2416–2423, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aline-Fardin A, Rifle G, Martin L, Justrabo E, Bour JB, D’Athis P, Tanter Y, Mousson C: Recurent and de novo membranous glomerulopathy after kidney transplantation. Transplant Proc 41: 669–671, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Ponticelli C, Glassock RJ: De novo membranous nephropathy (MN) in kidney allografts. A peculiar form of alloimmune disease? Transpl Int 25: 1205–1210, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Monga G, Mazzucco G, Basolo B, Quaranta S, Motta M, Segoloni G, Amoroso A: Membranous glomerulonephritis (MGN) in transplanted kidneys: Morphologic investigation on 256 renal allografts. Mod Pathol 6: 249–258, 1993 [PubMed] [Google Scholar]
- 29.Kearney N, Podolak J, Matsumura L, Houghton D, Troxell M: Patterns of IgG subclass deposits in membranous glomerulonephritis in renal allografts. Transplant Proc 43: 3743–3746, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Larsen CP, Walker PD: Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation 95: 1259–1262, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Truong L, Gelfand J, D’Agati V, Tomaszewski J, Appel G, Hardy M, Pirani CL: De novo membranous glomerulonephropathy in renal allografts: A report of ten cases and review of the literature. Am J Kidney Dis 14: 131–144, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Schwarz A, Krause PH, Offermann G, Keller F: Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine A. Am J Kidney Dis 17: 524–531, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, Carreno M, Esquenazi V, Miller J: De novo membranoproliferative glomerulonephritis in hepatitis C virus-infected renal allograft recipients. Transplantation 59: 1676–1682, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Hammoud H, Haem J, Laurent B, Alamartine E, Diab N, Defilippis JP, Berthoux P, Berthoux F: Glomerular disease during HCV infection in renal transplantation. Nephrol Dial Transplant 11[Suppl 4]: 54–55, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Faguer S, Kamar N, Boulestin A, Esposito L, Durand D, Blancher A, Rostaing L: Prevalence of cryoglobulinemia and autoimmunity markers in renal-transplant patients. Clin Nephrol 69: 239–243, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Cruzado JM, Carrera M, Torras J, Grinyó JM: Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant 1: 171–178, 2001 [PubMed] [Google Scholar]
- 38.Isaac J, Herrara GA, Shihab FS: De novo fibrillary glomerulopathy in the renal allograft of a patient with systemic lupus erythematosus. Nephron 87: 365–368, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Rao KV, Hafner GP, Crary GS, Anderson WR, Crosson JT: De novo immunotactoid glomerulopathy of the renal allograft: Possible association with cytomegalovirus infection. Am J Kidney Dis 24: 97–103, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Moriyama T, Nitta K, Suzuki K, Honda K, Horita S, Uchida K, Yumura W, Tanabe K, Toma H, Nihei H, Yamaguchi Y: Latent IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transplant 19[Suppl 14]: 41–48, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y: Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Ji S, Liu M, Chen J, Yin L, Sha G, Chen H, Liu Z, Li L: The fate of glomerular mesangial IgA deposition in the donated kidney after allograft transplantation. Clin Transplant 18: 536–540, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Kowalewska J, Yuan S, Sustento-Reodica N, Nicosia RF, Smith KD, Davis CL, Alpers CE: IgA nephropathy with crescents in kidney transplant recipients. Am J Kidney Dis 45: 167–175, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Moroni G, Papaccioli D, Banfi G, Tarantino A, Ponticelli C: Acute post-bacterial glomerulonephritis in renal transplant patients: Description of three cases and review of the literature. Am J Transplant 4: 132–136, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Plumb TJ, Greenberg A, Smith SR, Butterly DW, Pham TT, Fields TA, Howell DN: Postinfectious glomerulonephritis in renal allograft recipients. Transplantation 82: 1224–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrne MC, Budisavljevic MN, Fan Z, Self SE, Ploth DW: Renal transplant in patients with Alport’s syndrome. Am J Kidney Dis 39: 769–775, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Rutgers A, Meyers KE, Canziani G, Kalluri R, Lin J, Madaio MP: High affinity of anti-GBM antibodies from Goodpasture and transplanted Alport patients to alpha3(IV)NC1 collagen. Kidney Int 58: 115–122, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG: Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 363: 343–354, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Temme J, Kramer A, Jager KJ, Lange K, Peters F, Müller GA, Kramar R, Heaf JG, Finne P, Palsson R, Reisæter AV, Hoitsma AJ, Metcalfe W, Postorino M, Zurriaga O, Santos JP, Ravani P, Jarraya F, Verrina E, Dekker FW, Gross O: Outcomes of male patients with Alport syndrome undergoing renal replacement therapy. Clin J Am Soc Nephrol 7: 1969–1976, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asif A, Toral C, Diego J, Miller J, Roth D: De novo ANCA-associated vasculitis occurring 14 years after kidney transplantation. Am J Kidney Dis 35: E10, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Schultz DR, Diego JM: Antineutrophil cytoplasmic antibodies (ANCA) and systemic vasculitis: Update of assays, immunopathogenesis, controversies, and report of a novel de novo ANCA-associated vasculitis after kidney transplantation. Semin Arthritis Rheum 29: 267–285, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Tabata H, Honda K, Moriyama T, Itabashi M, Taneda S, Takei T, Tanabe K, Teraoka S, Yamaguchi Y, Oda H, Nitta K: Two cases of ANCA-associated vasculitis in post-transplant kidney: Relapse and de novo. Clin Transplant 23[Suppl 20]: 49–53, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Bhalla V, Nast CC, Stollenwerk N, Tran S, Barba L, Kamil ES, Danovitch G, Adler SG: Recurrent and de novo diabetic nephropathy in renal allografts. Transplantation 75: 66–71, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Fioretto P, Mauer M: Histopathology of diabetic nephropathy. Semin Nephrol 27: 195–207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrøm J, Leivestad T, Egeland T, Fauchald P: Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation 64: 979–983, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Sumrani NB, Delaney V, Ding ZK, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH: Diabetes mellitus after renal transplantation in the cyclosporine era—an analysis of risk factors. Transplantation 51: 343–347, 1991 [DOI] [PubMed] [Google Scholar]
- 59.van Hooff JP, Christiaans MH, van Duijnhoven EM: Tacrolimus and posttransplant diabetes mellitus in renal transplantation. Transplantation 79: 1465–1469, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC: Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. BMJ 331: 810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston O, Rose CL, Webster AC, Gill JS: Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol 19: 1411–1418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peddi VR, Wiseman A, Chavin K, Slakey D: Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando) 27: 97–107, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Helanterä I, Ortiz F, Räisänen-Sokolowski A, Koskinen P: Impact of glucose metabolism abnormalities on histopathological changes in kidney transplant protocol biopsies. Transpl Int 23: 374–381, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 29: 1963–1972, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA: Diabetes mellitus after renal transplantation: As deleterious as non-transplant-associated diabetes? Transplantation 65: 380–384, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Executive Summary : Executive summary: Standards of medical care in diabetes—2009. Diabetes Care 32[Suppl 1]: S6–S12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F, ADVANCE Collaborative Group : Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, Wetterslev J: Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 11: CD008143, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Reynolds JC, Agodoa LY, Yuan CM, Abbott KC: Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis 42: 1058–1068, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Karthikeyan V, Parasuraman R, Shah V, Vera E, Venkat KK: Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant 3: 1289–1294, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Zarifian A, Meleg-Smith S, O’donovan R, Tesi RJ, Batuman V: Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int 55: 2457–2466, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Caires RA, Marques ID, Repizo LP, Sato VA, Carmo LP, Machado DJ, de Paula FJ, Nahas WC, David-Neto E: De novo thrombotic microangiopathy after kidney transplantation: Clinical features, treatment, and long-term patient and graft survival. Transplant Proc 44: 2388–2390, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Ponticelli C: De novo thrombotic microangiopathy. An underrated complication of renal transplantation. Clin Nephrol 67: 335–340, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Pratap B, Abraham G, Srinivas CN, Bhaskar S: Post-renal transplant hemolytic uremic syndrome following combination therapy with tacrolimus and everolimus. Saudi J Kidney Dis Transpl 18: 609–612, 2007 [PubMed] [Google Scholar]
- 76.Sartelet H, Toupance O, Lorenzato M, Fadel F, Noel LH, Lagonotte E, Birembaut P, Chanard J, Rieu P: Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant 5: 2441–2447, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Satoskar AA, Pelletier R, Adams P, Nadasdy GM, Brodsky S, Pesavento T, Henry M, Nadasdy T: De novo thrombotic microangiopathy in renal allograft biopsies-role of antibody-mediated rejection. Am J Transplant 10: 1804–1811, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Ponticelli C, Banfi G: Thrombotic microangiopathy after kidney transplantation. Transpl Int 19: 789–794, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Miura M, Fujita H, Suzuki A, Kubota KC, Fukasawa Y, Shimoda N, Tsuchihashi S, Tamaki T: A case of progressive thrombotic microangiopathy after ABO-incompatible renal transplantation. Clin Transplant 25[Suppl 23]: 19–22, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Burdmann EA, Andoh TF, Yu L, Bennett WM: Cyclosporine nephrotoxicity. Semin Nephrol 23: 465–476, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Renner B, Klawitter J, Goldberg R, McCullough JW, Ferreira VP, Cooper JE, Christians U, Thurman JM: Cyclosporine induces endothelial cell release of complement-activating microparticles. J Am Soc Nephrol 24: 1849–1862, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keller K, Daniel C, Schöcklmann H, Endlich KH, Kerjaschki D, Johnson RJ, Hugo C: Everolimus inhibits glomerular endothelial cell proliferation and VEGF, but not long-term recovery in experimental thrombotic microangiopathy. Nephrol Dial Transplant 21: 2724–2735, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Zand MS: De novo thrombotic microangiopathy in renal transplant recipients: A comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis 41: 471–479, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Mor E, Lustig S, Tovar A, Bar-Nathan N, Shharabani E, Shapira Z, Yusim A: Thrombotic microangiopathy early after kidney transplantation: Hemolytic uremic syndrome or vascular rejection? Transplant Proc 32: 686–687, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Wilson CH, Brown AL, White SA, Goodship TH, Sheerin NS, Manas DM: Successful treatment of de novo posttransplant thrombotic microangiopathy with eculizumab. Transplantation 92: e42–e43, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Chandran S, Baxter-Lowe L, Olson JL, Tomlanovich SJ, Webber A: Eculizumab for the treatment of de novo thrombotic microangiopathy post simultaneous pancreas-kidney transplantation—a case report. Transplant Proc 43: 2097–2101, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Stewart ZA, Collins TE, Schlueter AJ, Raife TI, Holanda DG, Nair R, Reed AI, Thomas CP: Case report: Eculizumab rescue of severe accelerated antibody-mediated rejection after ABO-incompatible kidney transplant. Transplant Proc 44: 3033–3036, 2012 [DOI] [PubMed] [Google Scholar]