Abstract
Background and objectives
Although patients undergoing maintenance hemodialysis have exceptionally high hospitalization rates, the risk factors for hospitalizations are unclear. This study sought to examine hospitalization rates among hemodialysis patients in the United States according to both race/ethnicity and age.
Design, setting, participants, & measurements
US Renal Data System data on 563,281 patients beginning maintenance hemodialysis between 1995 and 2009 were analyzed. Rates of hospital admission and number of hospital days for all-cause and cause-specific hospitalizations during the first year of dialysis were compared among blacks, whites, and Hispanics in the entire cohort and subgroups stratified by age.
Results
After multiple adjustments, compared with whites, Hispanics overall had lower rates of both all-cause hospital days (adjusted rate ratio [aRR], 0.91; 95% confidence interval [95% CI], 0.90 to 0.93; P<0.001) and hospital admissions (aRR, 0.89; 95% CI, 0.88 to 0.90; P<0.001), whereas blacks had a lower rate of all-cause admissions (aRR, 0.95; 95% CI, 0.94 to 0.96; P<0.001). The racial/ethnic differences, however, varied by age. Hispanics exhibited the lowest rates of hospital days and admissions for all age groups≤70 years, but those >80 years had higher rates than their white counterparts. The adjusted black-to-white rate ratios exhibited a U-shaped pattern with age, with higher rates for blacks in the younger and older age groups. Hospitalization rates for dialysis-related infections were markedly higher in blacks and Hispanics than whites, which were consistent in all age groups for blacks (aRRs for hospital days ranged from 1.09 to 1.36) and all ages>60 years for Hispanics (aRRs ranged from 1.20 to 1.38).
Conclusions
There are significant racial/ethnic differences in hospitalization rates within first year of dialysis, which are not consistent across the age groups and also differ by causes of hospitalization. Overall, blacks and Hispanics had lower rates of all-cause hospital admissions than whites. However, younger and older blacks and older Hispanics were at greatest risk.
Keywords: Racial and ethnic differences, hospital days, USRDS, cardiovascular diseases, dialysis-related infection
Introduction
Hospitalization is frequent and costly among patients undergoing maintenance dialysis. Across the United States, approximately 400,000 patients with ESRD receive maintenance dialysis each year and spend an average of approximately 15 days in hospitals (1). In 2010, inpatient services accounted for 38% of the total $32.9 billion Medicare ESRD dollars, in addition to non-Medicare spending (1). Yet, our understanding of dialysis patient subgroups at highest risk remains incomplete.
Racial and ethnic differences in outcomes, such as nutritional and inflammatory measures (2), quality of life (3), and mortality (4,5), among hemodialysis patients are well known. There are also racial and ethnic differences in the processes of care, including dialysis dose (6,7), anemia treatment (8,9), hemodialysis vascular access (10–13), and pre-ESRD clinical care (12,14). Prior studies examining hospitalization in dialysis patients usually used race, among others, as an explanatory factor or control variable (15–21). To our knowledge, no studies have assessed the association of hospitalizations with race/ethnicity and age at a national level.
We recently reported that all-cause mortality in patients undergoing maintenance hemodialysis is lowest in Hispanics, intermediate in non-Hispanic blacks, and highest in non-Hispanic whites, except for those ≤30 years (22). One may postulate a similar pattern for hospitalization because mortality and hospitalization share many risk factors. However, because racial and ethnic minorities have generally received lower quality of care, they may require hospitalizations more frequently. More detailed investigations of the differences in hospitalizations among racial/ethnic groups may help to inform clinical practices and generate new hypotheses to facilitate future intervention studies to reduce hospitalizations for this high-risk subpopulation.
We therefore undertook a national population study to examine all-cause hospitalizations among patients receiving long-term hemodialysis, including hospital days and admission rates, and whether race/ethnicity and age modify these rates. Our second objective was to examine these rates for three common cause-specific hospitalizations: cardiovascular diseases, all-cause infections, and dialysis-related infection.
Materials and Methods
Data Sources and Study Population
The Institutional Review Board at University of Virginia approved this study. Using the US Renal Data System (23), we identified all adult patients (≥18 years) who were beginning maintenance dialysis for the first time in the United States between April 1, 1995, and June 30, 2009, and had no prior kidney transplantation. We included patients who survived the first 90 days since ESRD onset and received in-center hemodialysis at day 91 for ≥60 days. Of 942,184 patients identified, we excluded 17,580 (1.9%) patients who switched to peritoneal dialysis within 1 year after 90 days of ESRD so that we would obtain a more homogeneous study population; we excluded 50,727 patients (5.4%) because of missing covariates. To ensure the completeness of hospitalization data, only patients who had Medicare as the primary payer throughout the 1-year period after the 90 days (65.5%) were included. The final cohort included 563,281 patients (55.4% white, 32.7% black, and 11.9% Hispanic).
Outcomes, Follow-Up, and Covariates
The primary outcomes were total hospital days and admissions per year for all-cause and cause-specific hospitalizations, including cardiovascular diseases, all-cause infection, and dialysis-related infection. First all-cause hospitalization and the composite outcome of first all-cause hospitalization or death were examined in sensitivity analyses.
The follow-up period was 1 year from day 91 of ESRD, 3 days before kidney transplant (to avoid counting a kidney transplantation hospitalization [23]), loss to follow-up, or death or December 31, 2009, whichever came first. Overlapping or consecutive hospitalizations that occurred within 1 day were combined into a single hospitalization. A hospitalization that occurred before day 91 of ESRD was not counted as an admission in the present analysis, but any hospitalization day within the study period was counted in the total hospital days for that patient. The time-at-risk for total hospital days included all days within the study period, while the time-at-risk for hospital admissions excluded hospital days. If a hospitalization began before day 91 of ESRD and the discharge occurred within the study period, the time-at-risk for the first event began on the day of discharge.
The classification of cause-specific hospitalizations was based on the principal diagnostic codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (see Supplemental Material) (1).
Race and ethnicity were reported in the Centers for Medicare & Medicaid Services Medical Evidence form (CMS-2728). The adjusted analyses included the following covariates at the ESRD onset: age, sex, health insurance (private, Medicare, or Medicaid/none), body mass index, cause of ESRD, eGFR, lifestyle behaviors (smoking, alcohol, drug dependence), immobility, and presence/absence of each comorbid condition (hypertension, diabetes, all cardiac diseases, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer), as well as pre-ESRD use of erythropoietin-stimulating agents and employment status at 6 months before ESRD. Health insurance status at the ESRD onset may be an indicator of the patient’s socioeconomic status and/or access to care before ESRD. Additionally, we included two pre-ESRD care indicators, nephrologist care and vascular access type used for the first outpatient dialysis session, in sensitivity analysis for the subset of patients who have these data available since 2005 (n=184,732).
Statistical Analyses
We calculated unadjusted rates by dividing the total number of events (hospital days or admissions) by total patient-years at risk for each racial/ethnic subgroup. We examined the unadjusted and adjusted differences among racial/ethnic subgroups for the entire cohort and then for each of seven age groups at ESRD onset (18–30, 31–40, 41–50, 51–60, 61–70, 71–80, >80 years). For primary outcomes (hospital days and admission), overdispersed Poisson regression was used to account for multiple hospitalizations per patient. We report rate ratios (RRs) for blacks or Hispanics versus whites. The adjusted analyses included the covariates described above. Statistical interactions between racial/ethnic groups and age groups were tested for all primary outcomes.
We performed several sensitivity analyses, including additional adjustment for the two pre-ESRD care indicators, and used Cox regression to examine the composite outcome of the first all-cause hospitalization or death and the first all-cause hospitalization. We also examined the results additionally adjusted for census regions (Northeast, Midwest, South, and West). We performed additional analyses using the study sample that included patients with Medicare as their primary payer at day 91 of ESRD (i.e., using intent-to-treat principle) and patients who subsequently switched to peritoneal dialysis with time censored at the switch. All analyses were performed using SAS statistical package (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
Table 1 presents the patient characteristics of these three racial/ethnic groups by three broad age groups at ESRD onset (18–40, 41–70, and >70 years). Some important differences within and across the age groups are noted. For example, blacks and Hispanics in the age group of 18–40 years had a lower prevalence of diabetes, cardiovascular diseases, and immobility than their white counterparts. In the group aged>70 years, conversely, they had a higher prevalence of diabetes and immobility than whites. In all age groups, blacks and Hispanics were less likely than whites to have received erythropoietin-stimulating agents and pre-ESRD nephrologist care and to have used arteriovenous fistula (AVF) for their first dialysis.
Table 1.
Patient characteristics by age and race/ethnicity
| Characteristics | Age 18–40 yr (n=40,670) | Age 41–70 yr (n=284,748) | Age >70 yr (n=237,863) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| White | Black | Hispanic | White | Black | Hispanic | White | Black | Hispanic | |
| Patients (n) | 13,138 | 21,399 | 6133 | 131,126 | 111,278 | 42,344 | 168,051 | 51,346 | 18,466 |
| Mean age (yr) | 32.6±6.0 | 32.7±5.7 | 32.0±6.3 | 60.5±7.8 | 57.5±8.4 | 58.0±8.0 | 78.5±5.2 | 77.6±5.2 | 77.3±5.0 |
| Men (%) | 63.6 | 57.8 | 59.9 | 55.9 | 50.8 | 56.2 | 55.7 | 38.4 | 47.1 |
| Mean BMI (kg/m2) | 26.7±7.8 | 28.9±8.7 | 27.4±7.5 | 29.2±8.0 | 28.8±8.0 | 28.0±7.0 | 26.0±6.0 | 26.2±6.6 | 25.9±6.0 |
| Mean eGFR (ml/min per 1.73 m2) | 8.4±4.3 | 7.9±4.2 | 7.5±4.1 | 9.7±4.5 | 9.2±4.4 | 9.0±4.3 | 10.5±4.7 | 10.3±4.8 | 10.2±4.7 |
| Current smoker (%) | 14.3 | 7.7 | 3.8 | 9.6 | 8.1 | 2.9 | 3.1 | 2.8 | 1.5 |
| No insurance at ESRD onset (%) | 24.1 | 34.9 | 34.4 | 8.9 | 16.1 | 20.2 | 1.0 | 1.7 | 3.9 |
| Cause of ESRD (%) | |||||||||
| Diabetes | 42.0 | 23.8 | 35.0 | 54.8 | 50.9 | 73.7 | 34.2 | 44.8 | 59.4 |
| Hypertension | 11.3 | 36.7 | 19.1 | 19.1 | 33.5 | 13.9 | 40.0 | 42.9 | 27.7 |
| GN | 18.3 | 15.3 | 21.0 | 7.4 | 5.2 | 4.1 | 6.7 | 3.1 | 3.4 |
| Others | 24.1 | 20.8 | 18.4 | 15.5 | 8.1 | 6.1 | 13.9 | 6.4 | 6.4 |
| Unknown | 4.3 | 3.4 | 6.5 | 3.3 | 2.3 | 2.2 | 5.2 | 2.9 | 3.1 |
| Comorbidity | |||||||||
| Hypertension (%) | 73.7 | 81.0 | 75.8 | 78.9 | 86.2 | 82.8 | 79.5 | 86.0 | 81.8 |
| Diabetes (%) | 42.3 | 27.2 | 36.1 | 59.5 | 57.3 | 74.0 | 42.0 | 53.0 | 61.8 |
| Cardiac failure (%) | 12.9 | 14.3 | 10.9 | 36.6 | 30.9 | 30.7 | 44.6 | 38.2 | 39.8 |
| Atherosclerotic heart disease (%) | 6.3 | 2.9 | 3.0 | 31.4 | 16.9 | 20.0 | 40.5 | 24.3 | 30.2 |
| Other cardiac diseases (%) | 3.4 | 3.4 | 2.3 | 11.2 | 6.7 | 5.8 | 16.7 | 9.9 | 10.2 |
| Cerebrovascular disease (%) | 3.1 | 2.6 | 2.1 | 10.9 | 10.5 | 7.3 | 12.1 | 13.3 | 10.1 |
| Peripheral vascular disease (%) | 6.2 | 2.7 | 3.9 | 19.4 | 10.7 | 13.6 | 20.3 | 14.0 | 16.4 |
| Chronic obstructive pulmonary disease (%) | 1.9 | 0.9 | 0.7 | 12.5 | 5.3 | 3.3 | 13.5 | 7.0 | 6.4 |
| Cancer (%) | 1.5 | 0.7 | 0.7 | 6.4 | 3.6 | 2.2 | 11.0 | 7.8 | 4.8 |
| Immobility (%) | 2.9 | 1.4 | 1.5 | 5.6 | 4.2 | 3.9 | 5.7 | 8.0 | 7.3 |
| Pre-ESRD care | |||||||||
| ESA use before ESRD (%) | 25.0 | 17.4 | 19.3 | 30.4 | 24.5 | 24.8 | 33.3 | 28.8 | 28.9 |
| Nephrologist care before ESRD (%)a | 55.3 | 43.9 | 42.4 | 61.1 | 53.6 | 49.1 | 64.4 | 57.6 | 54.3 |
| Vascular access for first hemodialysis session (%)a | |||||||||
| AVF | 13.9 | 8.5 | 9.5 | 17.0 | 13.4 | 11.9 | 16.7 | 12.4 | 11.7 |
| Graft | 2.8 | 3.1 | 2.0 | 3.7 | 5.7 | 3.1 | 4.1 | 7.4 | 4.5 |
| Catheter | 81.2 | 86.8 | 87.1 | 77.2 | 79.4 | 83.6 | 77.3 | 78.5 | 82.2 |
| Others | 2.0 | 1.6 | 1.5 | 2.1 | 1.5 | 1.3 | 1.9 | 1.7 | 1.6 |
Means are expressed with SDs. BMI, body mass index; ESA, erythropoietin-stimulating agents; AVF, arteriovenous fistula.
For patients who completed the new Centers for Medicare & Medicaid Services Medical Evidence form since 2005.
All-Cause Hospitalization
A total of 7,026,229 hospital days resulting from 912,723 hospital admissions occurred over a median of 1-year follow-up after 90 days of ESRD (25th–75th percentiles, 9.3–12.0 months of follow-up for hospital days and 8.5–12.0 months for admissions), with average rates of 14.95 hospital days and 2.03 admissions per patient-year. Factors associated with higher risk of hospitalization included female sex, unemployment, Medicare/Medicaid/no insurance (versus employer group insurance), lower body mass index, higher eGFR, and presence of comorbid conditions (e.g., diabetes, cardiovascular diseases, cancer) (see Supplemental Tables 1 and 2). Table 2 shows the unadjusted rates of all-cause hospital days and admissions together with RRs for blacks or Hispanics versus whites. For both hospital days and admissions, unadjusted rates were lowest in Hispanics, intermediate in blacks, and highest in whites in the entire cohort (13.91, 14.94, and 15.20 hospital days per patient-year, respectively, and 1.89, 2.01, and 2.07 admissions per patient-year, respectively). After adjustment for the covariates, Hispanics had 9% fewer hospital days than whites (adjusted RR [aRR], 0.91; 95% confidence interval [95% CI], 0.90 to 0.93; P<0.001), while blacks had similar numbers of hospital days as whites. The adjusted admission rates were 11% lower in Hispanics (aRR, 0.89; 95% CI, 0.88 to 0.90; P<0.001) and 5% lower in blacks (aRR, 0.95; 95% CI, 0.94 to 0.96; P<0.001) than in whites.
Table 2.
Rate ratios for all-cause hospital days and admissions for blacks and Hispanics compared with whites in the entire cohort and seven age groups
| Race/Ethnicity | Overall Patients (Patients with ≥1 Hospitalization), n (%) | All-Cause Hospital Days | All-Cause Admissionsa | ||||
|---|---|---|---|---|---|---|---|
| Rate per Patient-Year | Unadjusted RR (95% CI) | Adjusted RR (95% CI)b | Rate per Patient-Year | Unadjusted RR (95% CI) | Adjusted RR (95% CI)b | ||
| Overall | |||||||
| White | 312,315 (65.0) | 15.20 | 1 (Reference) | 1 (Reference) | 2.07 | 1 (Reference) | 1 (Reference) |
| Black | 184,023 (62.4) | 14.94 | 0.98 (0.97 to 0.99) | 1.01 (0.99 to 1.02)c | 2.01 | 0.97 (0.96 to 0.98) | 0.95 (0.94 to 0.96) |
| Hispanic | 66,943 (60.3) | 13.91 | 0.91 (0.90 to 0.93) | 0.91 (0.90 to 0.93) | 1.89 | 0.91 (0.90 to 0.93) | 0.89 (0.88 to 0.90) |
| Age 18–30 yr | |||||||
| White | 4376 (51.1) | 11.20 | 1 (Reference) | 1 (Reference) | 2.01 | 1 (Reference) | 1 (Reference) |
| Black | 6954 (58.2) | 13.45 | 1.20 (1.10 to 1.31) | 1.29 (1.19 to 1.39) | 2.37 | 1.18 (1.10 to 1.26) | 1.27 (1.19 to 1.35) |
| Hispanic | 2300 (47.6) | 9.16 | 0.82 (0.72 to 0.93) | 0.94 (0.84 to 1.05)c | 1.66 | 0.82 (0.75 to 0.91) | 0.93 (0.85 to 1.01)c |
| Age 31–40 yr | |||||||
| White | 8762 (58.5) | 13.94 | 1 (Reference) | 1 (Reference) | 2.28 | 1 (Reference) | 1 (Reference) |
| Black | 14,445 (58.9) | 13.23 | 0.95 (0.90 to 1.00)c | 1.20 (1.14 to 1.27) | 2.23 | 0.98 (0.94 to 1.02)c | 1.16 (1.11 to 1.22) |
| Hispanic | 3,833 (53.3) | 10.29 | 0.74 (0.68 to 0.80) | 0.87 (0.80 to 0.94) | 1.77 | 0.78 (0.73 to 0.83) | 0.88 (0.83 to 0.94) |
| Age 41–50 yr | |||||||
| White | 18,375 (59.9) | 14.30 | 1 (Reference) | 1 (Reference) | 2.13 | 1 (Reference) | 1 (Reference) |
| Black | 27,344 (59.9) | 13.16 | 0.92 (0.89 to 0.96) | 1.02 (0.98 to 1.06)c | 2.03 | 0.95 (0.92 to 0.98) | 1.01 (0.98 to 1.04)c |
| Hispanic | 8824 (57.0) | 11.71 | 0.82 (0.78 to 0.86) | 0.86 (0.81 to 0.90) | 1.78 | 0.83 (0.80 to 0.87) | 0.86 (0.82 to 0.90) |
| Age 51–60 yr | |||||||
| White | 36,311 (60.8) | 15.29 | 1 (Reference) | 1 (Reference) | 2.04 | 1 (Reference) | 1 (Reference) |
| Black | 37,226 (59.3) | 13.51 | 0.88 (0.86 to 0.91) | 0.91 (0.89 to 0.94) | 1.82 | 0.89 (0.87 to 0.91) | 0.90 (0.88 to 0.92) |
| Hispanic | 15,005 (58.8) | 12.48 | 0.82 (0.78 to 0.85) | 0.84 (0.81 to 0.87) | 1.74 | 0.86 (0.83 to 0.88) | 0.87 (0.84 to 0.89) |
| Age 61–70 yr | |||||||
| White | 76,440 (64.3) | 15.44 | 1 (Reference) | 1 (Reference) | 2.02 | 1 (Reference) | 1 (Reference) |
| Black | 46,708 (62.1) | 14.95 | 0.97 (0.95 to 0.99) | 0.97 (0.95 to 0.99) | 1.87 | 0.92 (0.91 to 0.94) | 0.92 (0.90 to 0.94) |
| Hispanic | 18,515 (61.7) | 14.65 | 0.95 (0.92 to 0.98) | 0.94 (0.91 to 0.97) | 1.89 | 0.93 (0.91 to 0.96) | 0.93 (0.91 to 0.96) |
| Age 71–80 yr | |||||||
| White | 110,970 (67.0) | 15.46 | 1 (Reference) | 1 (Reference) | 2.06 | 1 (Reference) | 1 (Reference) |
| Black | 37,778 (66.6) | 17.33 | 1.12 (1.10 to 1.14) | 1.08 (1.06 to 1.11) | 2.08 | 1.01 (0.99 to 1.03)c | 0.99 (0.97 to 1.01)c |
| Hispanic | 13,896 (63.9) | 16.53 | 1.07 (1.04 to 1.10) | 1.02 (0.99 to 1.06)c | 2.08 | 1.01 (0.98 to 1.04)c | 0.99 (0.96 to 1.02)c |
| Age >80 yr | |||||||
| White | 57,081 (68.4) | 15.16 | 1 (Reference) | 1 (Reference) | 2.13 | 1 (Reference) | 1 (Reference) |
| Black | 13,568 (71.2) | 19.53 | 1.29 (1.25 to 1.33) | 1.22 (1.18 to 1.26) | 2.39 | 1.12 (1.09 to 1.15) | 1.08 (1.05 to 1.11) |
| Hispanic | 4570 (67.2) | 18.84 | 1.24 (1.18 to 1.31) | 1.16 (1.10 to 1.23) | 2.38 | 1.12 (1.07 to 1.17) | 1.08 (1.03 to 1.13) |
All P values <0.001 unless otherwise specified. RR, rate ratio; 95% CI, 95% confidence interval.
2914 patients who stayed in hospitals throughout the study period were excluded.
Adjusted for age, sex, body mass index, eGFR, employment, health insurance, cause of ESRD (diabetes, hypertension, glomerulonephritis, other, unknown), the presence of comorbid conditions (hypertension, diabetes, cardiac failure, atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease, other cardiac diseases, chronic obstructive pulmonary disease, cancer), lifestyle behaviors (smoking, alcohol use, drug dependence), immobility, and pre-ESRD use of erythropoiesis-stimulating agents.
Nonsignificant at the two-sided significance level of 0.05
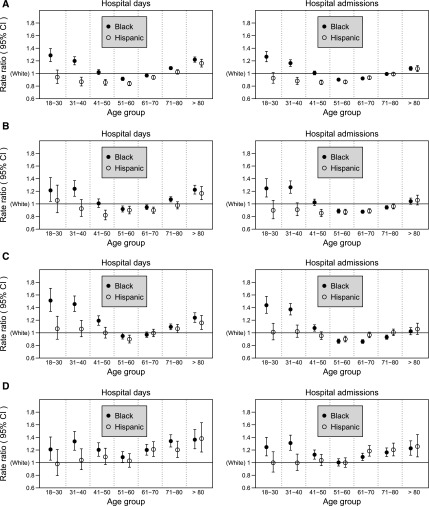
The differences in rates of hospital days and admissions among the races/ethnicities, however, varied depending on age (both P for interaction<0.001). The pattern of lowest rates in Hispanics, intermediate in blacks, and highest in whites was evident only in the middle two age groups (51–60 and 61–70 years), whereas among younger (≤40 years) or older (>70–80 years) patients, blacks had higher rates than whites (Figure 1A, Table 2). Thus, the adjusted black-to-white RRs for the hospital days exhibited a U-shaped pattern with age, and their RRs were 1.29, 1.20, 1.02, 0.91, 0.97, 1.08, and 1.22 from younger to older groups. This U-shaped pattern was less prominent in hospital admissions (Figure 1A, Table 2). Hispanics exhibited the lowest rates of both hospital days and admissions for all age groups≤70 years (aRRs ranging from 0.84 to 0.94 versus whites), whereas in the oldest age group (>80 years), their rates exceeded those in whites (aRRs ranging from 1.08 to 1.16). These patterns by race/ethnicity and age were consistent over the study time periods, with the exception that in the 18- to 30-year age group the higher black-to-white RR for hospital days during 1995–1998 (1.61) was smaller in recent years (1.27 during 2007–2009).
Figure 1.
Adjusted rate ratios of hospital days and admissions for blacks and Hispanics compared with whites by age group. (A) All causes. (B) Cardiovascular diseases. (C) All-cause infection. (D) Dialysis-related infection. The error bars represent 95% confidence intervals (95% CIs). All P values for the interaction between race/ethnicity and age were <0.001, except for P=0.05 for the hospital days due to dialysis-related infection.
Cause-Specific Hospitalizations
Cardiovascular diseases and all-cause infections combined accounted for the majority of all hospital days (26.7% and 30.1%, respectively) and hospital admissions (29.4% and 23.7%). Approximately one third of all-cause infectious hospitalizations, in turn, were attributable to dialysis-related infection. While rates of hospitalization due to cardiovascular diseases increased with older age, rates due to the dialysis-related infection somewhat decreased with age (Supplemental Figure 1).
The pattern of racial/ethnic differences in cardiovascular hospitalization across age groups was similar to that of all-cause hospitalizations (Figure 1, A and B). Blacks≤40 years of age had markedly higher rates (40%–50% greater) (Figure 1C, Supplemental Table 3) of all-cause infectious hospitalization than their white counterparts. Blacks and Hispanics had higher rates of hospitalization due to dialysis-related infection than whites, which was consistent in all age groups for blacks (aRRs for hospital days ranged from 1.09 to 1.36) and all age groups>60 years for Hispanics (aRRs ranged from 1.20 to 1.38), compared with whites (Figure 1D, Supplemental Table 3).
Sensitivity Analyses
Our sensitivity analyses for the first all-cause hospitalization and the composite of the first all-cause hospitalization or death show that the patterns across age groups were mostly consistent with those of all-cause admissions (Figure 1A), except for the oldest age group (>80 years), in which the rates in Hispanics were slightly lower (hazard ratio, 0.97), rather than higher, than in whites. Additional adjustment for pre-ESRD nephrologist care and vascular access type for the subset of patients since 2005 did not alter the main findings. However, the RRs in the younger and older age groups attenuated after this additional adjustment (e.g., black-to-white RR for infectious hospital days in ages 18–30 years, 1.35 [P=0.001], with the additional adjustment; 1.43 [P=0.001] without additional adjustment). The results of other sensitivity analyses were similar to the main results.
Discussion
Mortality and hospitalization are unacceptably high among patients with ESRD undergoing long-term dialysis (1). We recently reported that dialysis mortality is lowest in Hispanics, intermediate in blacks, and highest in whites across all age groups except for the youngest age group (18–30 years), in which mortality rates in blacks exceed those in whites (22). However, little is known about possible differences of hospitalization among racial/ethnic groups and by age group. In this national study of 563,281 hemodialysis patients, we found that overall, racial/ethnic minorities had lower rates of all-cause hospitalization than whites, which was consistent with their overall lower mortality rates reported in prior studies (22,24). However, we unexpectedly found that the relative risks for hospitalization in the racial minorities compared with whites were much smaller than their respective mortality differences. Specifically, the relative risks for all-cause hospital admissions for blacks and Hispanics, compared with whites, were 0.95 and 0.89, respectively, whereas their relative risks of mortality were 0.77 and 0.70 in the similar population in our prior study (22). This might have been due to the effect of more equity in insurance coverage by day 91 for patients receiving dialysis, thus reducing disparities in access to care. In addition, in contrast to the mortality difference that was consistent across age groups except for the youngest group, racial and ethnic differences in hospitalization rates in the present study varied substantially by age group. The overall pattern of the lowest all-cause hospitalization rate in Hispanics, intermediate in blacks, and highest in whites was evident only in the middle age groups (51–60 and 61–70 years) and did not apply to either extreme of age. The black-to-white RRs exhibited a U-shaped relationship with age, with greater, rather than lower, rates for blacks than whites in the younger and older ends of age (Figure 1A). Rates of cause-specific hospitalization also varied across age groups. For example, blacks≤40 years of age had 40%–50% higher hospitalization rates due to infections compared with their white counterparts (Figure 1C).
The higher hospitalization risk for younger blacks was consistent with prior findings of higher mortality risk among younger blacks (22,24,25). This consistency would not be unexpected because mortality and hospitalization generally share common risk factors, including their general lower socioeconomic status, lower likelihood of being insured and employed, limited social support networks, and lack of trust in medical systems that may influence health behaviors and reduce effective access to medical care, despite receiving Medicare coverage at day 91 of dialysis (26,27). On a positive note, the black-to-white RR for hospital days among younger patients during 2007–2009 (1.27) was much lower than during 1995–1998 (1.61), and might reflect improvements in utilization or quality of care over time, possibly due to the ESRD continuous performance metrics (28).
However, the greater risk of hospitalization for blacks in the older age groups in the present study was in contrast to our prior study showing the lower mortality risk in older blacks than their white counterparts (22). It is possible that the higher rates of hospitalization accompanied by lower mortality in this older age could be related to the competing risk of death, as lower mortality for blacks increases the likelihood of observing hospitalization before death in the older black population. We also noticed that black patients>80 years had 22% longer hospital days than whites but had only 8% greater admission rates (Table 2). So, another possibility was that older blacks stayed in hospitals longer and received more care than whites, which could explain their lower risk of death. Further research is needed to elucidate the biologic and system-level factors in diverse elderly populations that may influence hospitalizations, mortality, and other clinical outcomes.
Previously, we found that Hispanics have the lowest mortality among these three racial/ethnic groups in all age groups (22). In the present study, Hispanics≤70 years of age had the lowest rate of all-cause and cardiovascular hospitalizations. However, those >80 years had higher risk of all-cause and cardiovascular hospitalization than their white counterparts (Figure 1, A and B). In addition, their rate of hospitalization due to dialysis-related infection was higher than that in whites for all ages>60 years, possibly because of a lower likelihood of AVF placement (Figure 1D) (29).
Similar to previous studies (30), this study has confirmed the higher risk of hospitalization due to dialysis-related infection among racial and ethnic minorities. Additionally, the risk of hospitalization for dialysis-related infection appeared to be independent of age (Supplemental Figure 1), indicating that the contributing factors might be less related to age but more related to processes of care. We also found a consistent attenuation of the RRs in younger and older ages after additional adjustments for pre-ESRD nephrologist care and AVF access. This result reinforces the need to increase pre-ESRD care for racial minorities, which had lower proportions of receiving such care than whites in the present patient population (Table 1); this may lead to reduced rates of hospitalization due to dialysis-related infection, as suggested in the literature (31,32).
There are limitations to our current findings. First, we did not include non-Medicare patients or patients with Medicare as secondary payer because their hospitalizations might not be complete in the US Renal Data System Medicare claims database. The characteristics of patients included with those excluded were mostly similar, but had relatively higher prevalence of comorbid conditions, which might be due to larger numbers of older patients in the current Medicare cohort. Second, our models did not adjust for detailed clinical assessment and laboratory data, which is common in observational studies relying on administrative datasets. However, the national data with more than a half-million patients provides confidence in the reported differences in hospitalization by race/ethnicity and age. Third, the classification of cause-specific hospitalizations was based on the principal diagnosis of the International Classification of Diseases, Ninth Revision, Clinical Modification code only, which might not have captured all hospitalizations of that type. However, the inconsistency across racial/ethnic groups and ages would be highly unlikely. Finally, both admission rates and hospital days may change over time; thus, our results for the first year of dialysis may not be representative of the entire course of ESRD.
In summary, the present study appears to be the first to document the important differences in hospitalization rates among racial and ethnic groups, which vary across age groups in a national cohort. While overall rate of all-cause hospitalization was greatest among whites, age-specific subgroups at greater risk of hospitalization, irrespective of the cause, were noted, including black patients in their younger and older ages, and Hispanic patients in their older age. Furthermore, both blacks and Hispanics are at greater risk of hospitalization for dialysis-related infection than whites. Further studies are needed to explore in more detail issues such as health beliefs and behaviors, social networks and other subtleties that may add critical insights to these observations, as well as the assessment of biomarkers associated with resiliency such as telomeres/telomerase activity and those of inflammation and oxidative stress. This could lead to novel interventions to reduce hospitalizations and costs in the dialysis population.
Disclosures
None.
Supplementary Material
Acknowledgments
This work is funded by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Disease grant 5R01-DK084200-04. In addition, K.N. is supported in part by NIH grants P20-MD000182, 3P30-AG021684, and UL1-TR000124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Part of this work was presented at the Annual Meeting of the American Society of Nephrology, November 5–10, 2013, Atlanta, GA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12621213/-/DCSupplemental.
References
- 1.U.S. Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Noori N, Kovesdy CP, Dukkipati R, Feroze U, Molnar MZ, Bross R, Nissenson AR, Kopple JD, Norris KC, Kalantar-Zadeh K: Racial and ethnic differences in mortality of hemodialysis patients: Role of dietary and nutritional status and inflammation. Am J Nephrol 33: 157–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unruh M, Miskulin D, Yan G, Hays RD, Benz R, Kusek JW, Meyer KB, HEMO Study Group : Racial differences in health-related quality of life among hemodialysis patients. Kidney Int 65: 1482–1491, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Held PJ, Pauly MV, Diamond L: Survival analysis of patients undergoing dialysis. JAMA 257: 645–650, 1987 [PubMed] [Google Scholar]
- 5.Agodoa L, Eggers P: Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial 20: 577–585, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Frankenfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF, Jr: Racial/ethnic analysis of selected intermediate outcomes for hemodialysis patients: Results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis 34: 721–730, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Owen WF, Jr, Chertow GM, Lazarus JM, Lowrie EG: Dose of hemodialysis and survival: Differences by race and sex. JAMA 280: 1764–1768, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Weisbord SD, Fried LF, Mor MK, Resnick AL, Kimmel PL, Palevsky PM, Fine MJ: Associations of race and ethnicity with anemia management among patients initiating renal replacement therapy. J Natl Med Assoc 99: 1218–1226, 2007 [PMC free article] [PubMed] [Google Scholar]
- 9.Lacson E, Jr, Rogus J, Teng M, Lazarus JM, Hakim RM: The association of race with erythropoietin dose in patients on long-term hemodialysis. Am J Kidney Dis 52: 1104–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Wasse H, Hopson SD, McClellan W: Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol 32: 234–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sehgal AR: Outcomes of renal replacement therapy among blacks and women. Am J Kidney Dis 35[Suppl 1]: S148–S152, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Yan G, Cheung AK, Ma JZ, Yu AJ, Greene T, Oliver MN, Yu W, Norris KC: The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin J Am Soc Nephrol 8: 610–618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arce CM, Mitani AA, Goldstein BA, Winkelmayer WC: Hispanic ethnicity and vascular access use in patients initiating hemodialysis in the United States. Clin J Am Soc Nephrol 7: 289–296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ifudu O, Dawood M, Iofel Y, Valcourt JS, Friedman EA: Delayed referral of black, Hispanic, and older patients with chronic renal failure. Am J Kidney Dis 33: 728–733, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group : Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J Am Soc Nephrol 14: 1863–1870, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS, HEMO Study Group : Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Kassam H, Adeniyi M, Martinez M, Agaba EI, Onime A, Servilla KS, Raj DS, Murata GH, Tzamaloukas AH: Hospital admissions in elderly patients on chronic hemodialysis. Int Urol Nephrol 43: 1229–1236, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Abbott KC, Agodoa LY: Hospitalizations for valvular heart disease in chronic dialysis patients in the United States. Nephron 92: 43–50, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Rocco MV, Soucie JM, Reboussin DM, McClellan WM: Risk factors for hospital utilization in chronic dialysis patients. Southeastern Kidney Council (Network 6). J Am Soc Nephrol 7: 889–896, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Hedayati SS, Grambow SC, Szczech LA, Stechuchak KM, Allen AS, Bosworth HB: Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis 46: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Yan G, Norris KC, Yu AJ, Ma JZ, Greene T, Yu W, Cheung AK: The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol 8: 953–961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 24.Rhee CM, Lertdumrongluk P, Streja E, Park J, Moradi H, Lau WL, Norris KC, Nissenson AR, Amin AN, Kovesdy CP, Kalantar-Zadeh K: Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol 39: 183–194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, Montgomery RA, Segev DL: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norris K, Nissenson AR: Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 19: 1261–1270, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Kovesdy CP, Norris KC: Racial survival paradox of dialysis patients: Robust and resilient. Am J Kidney Dis 60: 182–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eknoyan G, Levin NW, Steinberg EP: The dialysis outcomes quality initiative: History, impact, and prospects. Am J Kidney Dis 35[Suppl 1]: S69–S75, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Lilly MP, Lynch JR, Wish JB, Huff ED, Chen SC, Armistead NC, McClellan WM: Prevalence of arteriovenous fistulas in incident hemodialysis patients: Correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis 59: 541–549, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Xue H, Ix JH, Wang W, Brunelli SM, Lazarus M, Hakim R, Lacson E, Jr: Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am J Kidney Dis 61: 123–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astor BC, Eustace JA, Powe NR, Klag MJ, Sadler JH, Fink NE, Coresh J: Timing of nephrologist referral and arteriovenous access use: the CHOICE Study. Am J Kidney Dis 38: 494–501, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, Owen W, Jr: Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 55: 711–716, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.