Abstract
Background and objectives
Preeclampsia is characterized by hypertension and proteinuria, and increased shedding of podocytes into the urine is a common finding. This finding raises the question of whether preeclamptic nephropathy involves podocyte damage. This study examined podocyte-related changes in a unique sample of renal tissues obtained from women who died of preeclampsia.
Design, setting, participants, & measurements
All patients with preeclampsia who died in The Netherlands since 1990 and had available autopsy tissue were identified using a nationwide database of the Dutch Pathology Registry (PALGA). This resulted in a cohort of 11 women who died from preeclampsia. Three control groups were also identified during the same time period, and consisted of normotensive women who died during pregnancy (n=25), and nonpregnant controls either with (n=14) or without (n=13) chronic hypertension. Glomerular lesions, including podocyte numbers, podocyte proliferation, and parietal cell activation, were measured.
Results
Patients with preeclampsia had prominent characteristic glomerular lesions. The results showed that the number of podocytes per glomerulus did not differ significantly between the patients with preeclampsia and the control groups. However, preeclampsia was associated with a significant increase in intraglomerular cell proliferation (7.3% [SD 9.4] of the glomeruli of patients with preeclampsia had Ki-67–positive cells versus 1.6% [SD 3.3] of the glomeruli of hypertensive controls and 1.1% [SD 1.3] of nonpregnant controls; P=0.004) and activated parietal epithelial cells on a podocyte location (34% [SD 13.1] of the glomeruli of patients with preeclampsia versus 18.0% [SD 15.3] of pregnant controls, 11.9% [SD 13.2] of hypertensive controls, and 10.8% [SD 13.4] of nonpregnant controls; P=0.01).
Conclusions
These findings suggest that the recently described mechanisms of podocyte replacement play a role in preeclampsia. These results provide key new insights into the pathogenesis of preeclamptic nephropathy, and they open new possibilities for developing therapeutic modalities.
Keywords: podocyte, renal epithelial cell, target organ damage, kidney
Introduction
Preeclampsia is a serious pregnancy-related complication that affects up to 8% of all pregnancies, thereby causing significant perinatal and maternal morbidity and mortality worldwide (1). Preeclampsia is believed to arise, at least in part, from an imbalance between the proangiogenic and antiangiogenic factors in the maternal circulation; the maternal kidney is particularly sensitive to this imbalance, as reflected by the occasional finding of severe proteinuria (1,2).
The glomerulus was recognized as the principal site of renal damage in preeclampsia in 1918 (3). The glomerular podocyte later became the focus of attention in relation to proteinuria. Because renal biopsies are rarely performed in pregnant patients with preeclampsia, how the podocyte is affected by preeclampsia has remained largely unknown, although endotheliosis is generally considered a characteristic histopathologic glomerular lesion in preeclampsia. Vikse (4) recently suggested that a previously undetected renal disease might become “overt” in the preeclamptic setting. Preeclampsia is an emerging risk factor for developing CKD—particularly FSGS, which is considered primarily a disease of podocytes—later in life. However, how preeclampsia increases the risk of renal disease is poorly understood.
Our group and others recently reported significantly higher numbers of podocytes in the urine (i.e., podocyturia) of women with preeclampsia compared with pregnant controls (5,6). This podocyturia remained in the patients with preeclampsia up to 1 month after delivery, although their proteinuria resolved (7). Structural changes in the podocyte, including abnormal expression of podocyte-related proteins, were recently reported in a limited number of patients with preeclampsia (8,9). These findings suggest that the podocyte plays a key role in preeclampsia. In particular, the increased shedding of podocytes into the urine of patients with preeclampsia raises the question of whether the origin of preeclamptic renal disease involves podocyte damage. On the basis of the abovementioned studies, we hypothesize that patients with preeclampsia have a higher podocyte turnover. To investigate the association between preeclampsia and glomerular lesions in general, and podocyte-related injury in particular, we collected a unique sample of renal autopsy tissues obtained from patients with preeclampsia and controls.
Materials and Methods
Patient Selection and Nationwide Dutch Pathology Registry Search for Renal Tissue
Autopsy samples were obtained after a nationwide search of the Dutch Pathology Registry (PALGA), a histopathology and cytopathology network and registry that includes all pathology laboratories within The Netherlands (10). We included pregnant patients who died from preeclampsia since 1990 as defined by international guidelines established by the International Society for the Study of Hypertension in Pregnancy (11). Three control groups were also obtained during the same time period. One control group consisted of pregnant women without a hypertensive disorder either before or during the pregnancy. The other two control groups consisted of nonpregnant young women (aged 18–40 years) either with or without a medical history of chronic hypertension. Patients and controls were not matched. We obtained all available paraffin-embedded kidney samples taken from 11 patients with preeclampsia, 25 normotensive pregnant controls, 14 chronic hypertensive nonpregnant controls, and 13 normotensive nonpregnant controls. The patients’ clinical characteristics were obtained from their autopsy reports. The cause of death in each pregnant patient was confirmed by reviewing the records of the National Maternal Mortality Committee of the Dutch Society of Obstetrics and Gynecology (12). A thorough review of the autopsy reports, including kidney weight, confirmed that the controls had no evidence of underlying renal disease. All tissues were coded and handled anonymously in accordance with the Dutch National Ethics Guidelines (Dutch Federation of Medical Scientific Societies Code for Proper Secondary Use of Human Tissue). This study was approved by the Leiden University Medical Center Ethics Committee (P12.107).
Histology, Immunohistochemistry, and Immunofluorescence
Sections of renal tissue samples were stained with hematoxylin and eosin, periodic acid–Schiff, silver, and phosphotungstic acid-hematoxylin using standard methods. Immunohistochemistry was used to identify and count podocytes based on staining for Wilms’ tumor-1 (WT-1), a podocyte-specific transcription factor (13). To confirm the origin of swollen endothelial cells, we performed CD31 staining experiments. Because cell proliferation can affect the number of podocytes, we also performed immunohistochemical staining for Ki-67, a marker of cell proliferation (14). The sections were stained with antibodies against WT-1 (rabbit anti-human polyclonal antibody sc-192 lot number D2104, 1:250; Santa Cruz Biotechnology), or CD31 (1:400; Dako) or Ki-67 (1:200; Thermo Fisher Scientific), and staining was visualized using the appropriate secondary antibodies and diaminobenzidine as the chromagen.
For the first double-staining experiment, we used an antibody against CD44 (1:200; Abcam, Inc.), which is expressed by activated parietal epithelial cells (15) and an antibody against CD45 (leukocyte common antigen, 1:800; Ancell). Staining was visualized using goat anti-mouse Alexa 488 IgG2a and Alexa 546 Ig1. For the second double-staining experiment, we used antibodies against CD44 (1:200; Abcam, Inc.) and Ki-67 (1:200; Thermo Fisher Scientific). Staining was visualized using the goat anti-mouse Alexa 488 IgG2 (1:200) and Alexa 546 Ig1 (1:200) secondary antibodies. We also performed a third double-staining experiment using antibodies against WT-1 (1:250; Santa Cruz Biotechnology) and CD44 (1:200; Abcam, Inc.). WT-1 was visualized using rabbit EnVision horseradish peroxidase (Dako), and CD44 was visualized using the FITC-labeled goat anti-mouse IgG antibody (1:200).
Quantification of Histology
Sections were examined and scored by an experienced renal pathologist who was blinded with respect to the patients’ clinical data. At least 50 glomeruli per section were examined, and the following parameters were scored: presence of endotheliosis, double contours of the glomerular basement membrane (tram tracking), swelling of podocytes (prominent presence of podocytes), mesangial changes, glomerulitis, and FSGS. As previously described by Strevens et al. (16), endotheliosis was scored semiquantitatively as follows: 0 (no endotheliosis), 1 (<20% of the lumen was obliterated), 2 (20%–80% of the lumen was obliterated), or 3 (>80% of the lumen was obliterated). Global sclerosis was recorded as a percentage of the total number of glomeruli scored. Phosphotungstic acid-hematoxylin–confirmed microthrombi, interstitial fibrosis and tubular atrophy, and acute tubular necrosis were scored as either absent or present. To quantify vessel changes, the presence of hyalinosis and intimal fibrosis of arteries was scored. Finally, signs of ischemia, congestion, and edema were also evaluated.
Morphometry and Quantification of Immunohistochemistry and Immunofluorescence
Because differences between the groups with respect to glomerular surface area can affect the absolute number of podocytes, slides stained with periodic acid–Schiff were used to measure the surface area of the glomerular tuft and Bowman’s capsule of 30 randomly selected glomeruli (using ImageJ 1.47d software, downloaded from http://rsb.info.nih.gov/ij; the National Institutes of Health). For the immunohistochemical- and immunofluorescence-stained sections, at least 30 glomeruli per section were analyzed by two observers who were blinded with respect to the patients’ clinical data. The number of WT-1–positive nuclei per glomerular cross-section was counted using ImageJ 1.47d software. Because estimates of podocyte number vary widely between studies (17), we measured podocytes in a control group containing nonpregnant, nonhypertensive women as a measure of the number of podocytes per glomerular cross-section in healthy adult women. Ki-67 staining was quantified by counting the number of Ki-67–positive cells within the glomeruli (both intraglomerular and lining Bowman’s capsule). Finally, the presence of CD44-positive cells in the glomeruli was scored along the inner lining of Bowman’s capsule—in an anatomic parietal epithelial cell location—and covering the glomerular basement membrane on a podocyte location (i.e., not counting endothelial cells) as previously described by Fatima et al. (18). The podocyte location was confirmed by colocalization between CD44 and WT-1. In addition, the number of cellular bridges (i.e., bridges between Bowman’s capsule and the glomerular tuft) was scored in both CD44-stained and silver-stained samples. CD44-positive leukocytes, which were confirmed by costaining with CD45, were excluded from the scoring analysis.
Statistical Analyses
Categorical variables were compared using the chi-squared test. Differences in quantitative parameters between groups were assessed using either one-way ANOVA (for normally distributed data) or the nonparametric Kruskal–Wallis test (for non-normally distributed data). Correlations were calculated using either a Spearman’s coefficient (for ordinal data) or Pearson’s coefficient (for numerical data). All analyses were performed using the SPSS statistical software package (version 20.0; IBM Corp, Armonk, NY). Differences with a P value <0.05 were considered statistically significant.
Results
Clinical Data
Table 1 summarizes clinical characteristics of the four groups. The hypertensive nonpregnant control group was significantly older than the other study groups (median age 41.8 years versus 32.5 years for the women with preeclampsia, 31.0 years for the pregnant controls, and 33.5 years for the nonpregnant controls; P=0.03). No other significant differences were observed with respect to the other clinical characteristics.
Table 1.
Patient characteristics
| Characteristic | PE (n=11) | PC (n=25) | HC (n=14) | NPC (n=13) |
|---|---|---|---|---|
| Age, yr | 32.5 (29.5–35.8) | 31.0 (28.3–36.6) | 41.8 (34.4–43.5)a | 33.5 (24.6–40.0) |
| Gestational age, wk | 35.7 (34.1–39.0) | 33.4 (16.7–40.0) | NA | NA |
| Parity, mean (SD) | 0.6 (1.0) | 0.8 (1.0) | NA | NA |
| Proteinuria, g/24 h | 0.36 (0.3–6.1) | NA | NA | NA |
| BP, mmHg | ||||
| Systolic | 160.0 (141.3–191.3)b | 125.0 (113.5–137.5) | NA | NA |
| Diastolic | 106.0 (87.5–120.0)c | 90.0 (70.0–90.0) | NA | NA |
| Antihypertensive therapy, n (%) | 4 (36) | NA | 4 (100)d | NA |
| Comorbidities, n (%) | ||||
| Sickle cell anemia | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| WPW syndrome | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Asthma | 0 (0) | 2 (8) | 0 (0) | 0 (0) |
| Hyperhomocysteinemia | 0 (0) | 2 (8) | 0 (0) | 0 (0) |
| Hypertension | 1 (9) | 0 (0) | 0 (0) | 0 (0) |
| Epilepsy | 1 (9) | 0 (0) | 0 (0) | 0 (0) |
| Breast cancer | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| Renal insufficiency | 0 (0) | 0 (0) | 2 (14) | 0 (0) |
| Depression | 0 (0) | 0 (0) | 2 (14) | 0 (0) |
| Obesity | 0 (0) | 0 (0) | 3 (21) | 1 (8) |
| Cause of death, n (%) | ||||
| Preeclampsia related | 11 (100) | 0 (0) | 0 (0) | 0 (0) |
| Ectopic pregnancy | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Thromboembolism | 0 (0) | 6 (24) | 2 (14) | 0 (0) |
| Amniotic fluid embolism | 0 (0) | 2 (8) | 0 (0) | 0 (0) |
| Arrhythmias | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Cardiac arrest | 0 (0) | 1 (4) | 2 (14) | 0 (0) |
| Unknown | 0 (0) | 2 (8) | 1 (7) | 0 (0) |
| Malignancy | 0 (0) | 1 (4) | 1 (7) | 0 (0) |
| Aorta dissection | 0 (0) | 3 (12) | 4 (29) | 0 (0) |
| Infection | 0 (0) | 5 (20) | 1 (7) | 0 (0) |
| Cardiomyopathy | 0 (0) | 2 (8) | 0 (0) | 0 (0) |
| Pheochromocytoma | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Cerebral bleeding | 0 (0) | 0 (0) | 3 (21) | 0 (0) |
| Suicide | 0 (0) | 0 (0) | 0 (0) | 5 (38) |
| High energy trauma | 0 (0) | 0 (0) | 0 (0) | 7 (54) |
| Drowning | 0 (0) | 0 (0) | 0 (0) | 1 (8) |
| Death-autopsy interval, h | 18.0 (6.0–32.3) | 24.0 (20.5–24.0) | 24.0 (12.0–48.0) | 24.0 (24.0–60.0) |
Data are given as the median (IQR) unless otherwise specified. PE, preeclampsia; PC, normotensive pregnant control; HC, hypertensive nonpregnant control; NPC, normotensive nonpregnant control; NA, not applicable; WPW, Wolff–Parkinson–White.
P=0.03.
P=0.001.
P=0.01.
Data on hypertensive therapy of the hypertensive controls were available from four patients.
Renal Histology
The majority (81%) of the women with preeclampsia had prominent glomerular lesions. Endotheliosis was present in 55% of the women with preeclampsia. Although endotheliosis is generally considered to be the principal feature of preeclamptic glomerular changes, it was also observed, albeit to a significantly lesser extent (P=0.003), in both the pregnant control group (12%) and the hypertensive nonpregnant control group (15%). Both tram tracking (36% of the patients with preeclampsia; P<0.001) and podocyte swelling (18% of the patients with preeclampsia; P=0.02) were present in the preeclampsia group only. With the exception of one patient with preeclampsia, endotheliosis and tram tracking were not simultaneously present. The presence of podocyte changes significantly correlated with the presence of endotheliosis (correlation coefficient, 0.39; P=0.001). The presence of endotheliosis was not correlated with clinical characteristics. No correlation was found between renal histopathologic lesions and gestational age. The hypertensive nonpregnant controls had significantly more severe ischemic glomerular lesions than the patients with preeclampsia; Table 2 summarizes these and other lesions. Supplemental Figures 1 and 2 show typical examples of renal histology from all four study groups.
Table 2.
Renal histologic parameters in patients and control groups
| Histologic Parameter | PE (n=11) | PC (n=25) | HC (n=14) | NPC (n=13) | P Value |
|---|---|---|---|---|---|
| Acute tubular necrosis | 0 (0) | 4 (16) | 3 (21) | 3 (23) | 0.41 |
| Congestion | 0 (0) | 0 (0) | 0 (0) | 3 (23) | 0.01 |
| Endotheliosis | 6 (55) | 3 (12) | 2 (14) | 0 (0) | 0.003 |
| <20% of the lumen | 1 (17) | 3 (100) | 1 (50) | NA | |
| 20%–80% of the lumen | 3 (50) | 0 | 1 (50) | NA | |
| >80% of the lumen | 2 (33) | 0 | 0 | NA | |
| FSGS | 1 (9) | 2 (8) | 5 (36) | 0 (0) | 0.03 |
| Global sclerosis>1% | 1 (9) | 0 (0) | 5 (36) | 1 (8) | 0.04 |
| Glomerulitis | 0 (0) | 6 (24) | 1 (7) | 2 (15) | 0.23 |
| Hyalinosis | 1 (9) | 4 (16) | 7 (50) | 10 (77) | <0.001 |
| Interstitial fibrosis tubular atrophy | 0 (0) | 0 (0) | 2 (14) | 0 (0) | 0.06 |
| Intima fibrosis | 2 (18) | 7 (28) | 11 (79) | 5 (39) | 0.01 |
| Ischemia | 0 (0) | 1 (4) | 3 (21) | 1 (8) | 0.17 |
| Mesangium changes | 1 (9) | 0 (0) | 4 (29) | 1 (8) | 0.04 |
| Microthrombi | 1 (9) | 0 (0) | 1 (7) | 0 (0) | 0.20 |
| Edema | 1 (9) | 0 (0) | 0 (0) | 1 (8) | 0.34 |
| Podocyte changes | 2 (18) | 0 (0) | 0 (0) | 0 (0) | 0.02 |
| Tram tracking | 4 (36) | 0 (0) | 0 (0) | 0 (0) | <0.001 |
Values are expressed as n (%). All patients with FSGS were classified as the “not otherwise specified” variant.
Morphometric Analyses
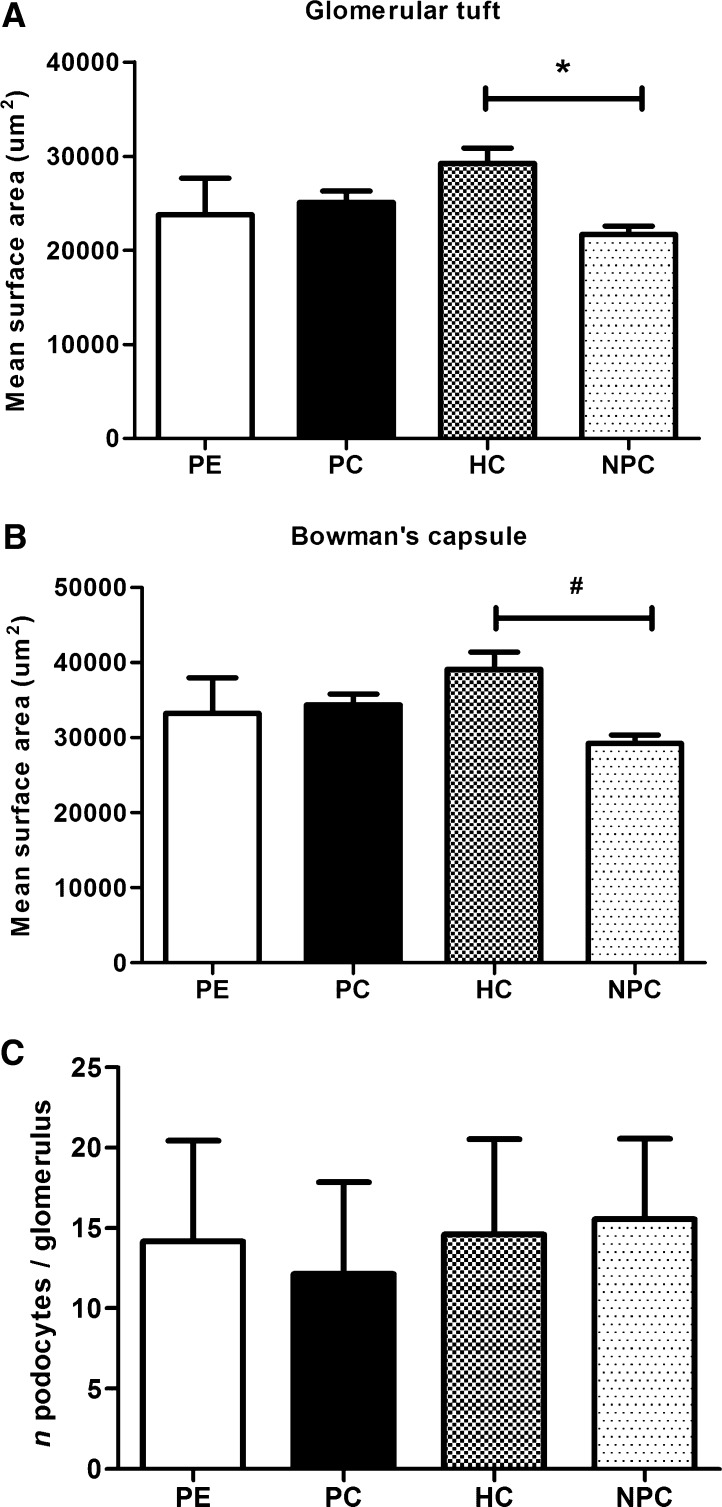
The glomerular surface area did not differ significantly between the preeclampsia and control groups. However, the chronic hypertensive nonpregnant controls had significantly larger surface areas in the glomerular tuft and Bowman’s capsule than the normotensive nonpregnant controls (Figure 1, A and B). Similar results were obtained when we calculated glomerular volume using the Weibel–Gomez method (19).
Figure 1.
Morphometric analysis and podocyte number. (A and B) Glomerular surface areas were calculated for the glomerular tuft (A; *P=0.003) and Bowman’s capsule (B; #P=0.002) in patients with preeclampsia and the control groups. (C) Number of WT-1–positive podocytes per glomerulus in the women with preeclampsia and the control groups. The error bars represent SDs. HC, hypertensive nonpregnant control; NPC, normotensive nonpregnant control; PC, normotensive pregnant control; PE, preeclampsia; WT-1, Wilms’ tumor-1.
Podocyte Numbers
No significant difference in the number of podocytes was found between the patients with preeclampsia and the control groups (Figure 1C). Supplemental Figure 3A shows a typical example of WT-1 staining. WT-1 staining was not correlated with either the renal histopathologic lesions or patient characteristics.
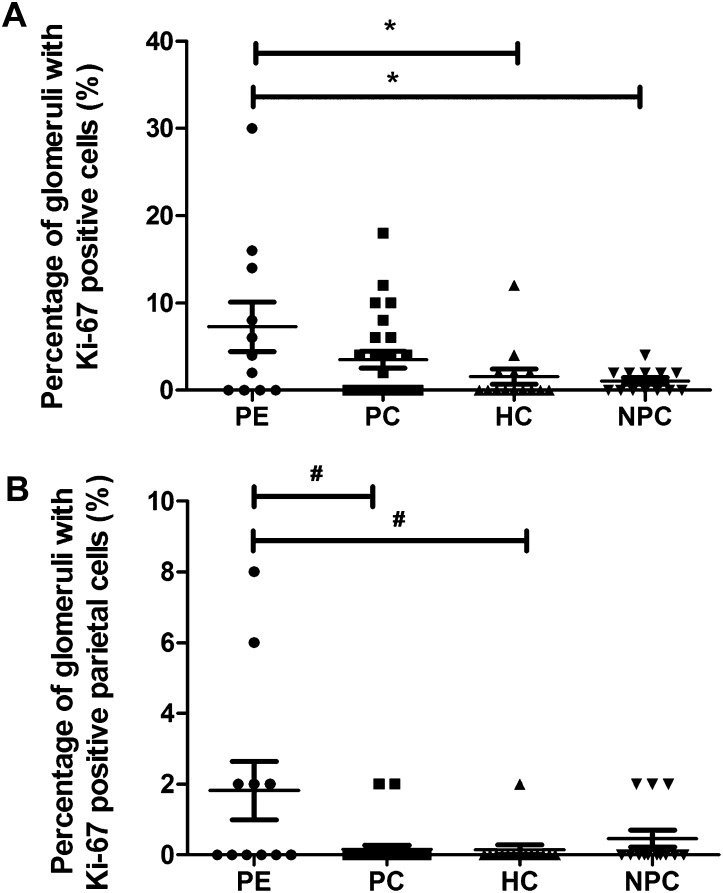
Cellular Proliferation
The women with preeclampsia had significantly more intraglomerular Ki-67–positive cells (mean 7.3% [SD 9.4]) compared with the hypertensive (1.6% [SD 3.3]) and normotensive nonpregnant control groups (1.1% [SD 1.3]; P=0.004; Figure 2A). Furthermore, the women with preeclampsia had significantly more Ki-67–positive parietal epithelial cells (1.8% [SD 2.8]) than the pregnant controls (0.2% [SD 0.6]) and the hypertensive nonpregnant controls (0.1% [SD 0.5]; P=0.02; Figure 2B). Supplemental Figure 3B shows a typical example of Ki-67 staining. Ki-67 and CD44 staining was colocalized (Supplemental Figure 4).
Figure 2.
Ki-67 analysis. (A) The percentage of glomeruli with Ki-67–positive cells (*P=0.004). (B) Percentage of glomeruli with Ki-67–positive parietal epithelial cells (#P=0.02). In A and B, each symbol represents an individual patient or control. The lines represent the mean value, and the error bars represent the SD.
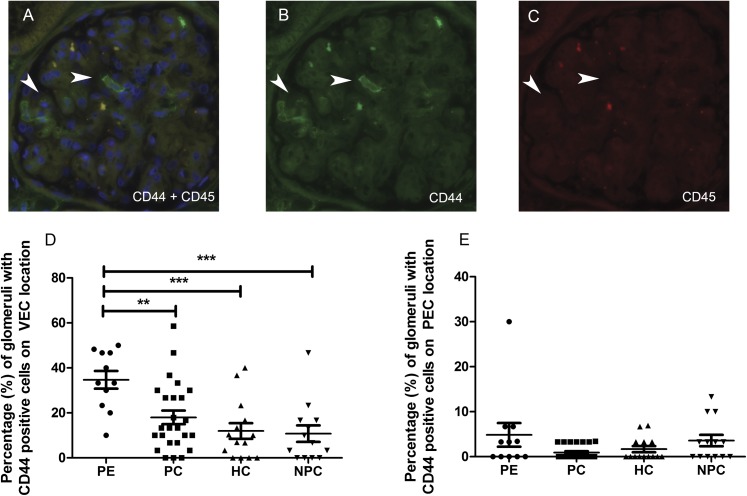
CD44-Positive Cells
We scored the presence and location of CD44-positive/CD45-negative cells within the glomeruli for the four study groups (Figure 3, A–C). The podocyte location was confirmed by colocalization of CD44 and WT-1 staining (Supplemental Figure 5). The number of CD44-positive cells on a podocyte location was significantly higher in the women with preeclampsia than in all three control groups (Figure 3D). Moreover, the presence of CD44-positive cells was significantly associated with the presence of Ki-67–positive intraglomerular cells (correlation coefficient, 0.271; P=0.03). Although a trend was observed between preeclampsia and CD44-positive cells on a parietal epithelial cell location, this association did not reach statistical significance (4.8% [SD 8.9] of the glomeruli of patients with preeclampsia showed positivity versus 0.84% [SD 1.4] of the pregnant controls, 1.6% [SD 2.6] of the hypertensive controls, and 3.5% [SD 4.6] of the nonpregnant controls; P=0.07; Figure 3E). The presence of cellular bridges was significantly associated with the presence of FSGS (correlation coefficient, 0.30; P=0.02). Supplemental Figure 6 shows an example of a cellular bridge.
Figure 3.
Double staining of CD44 and CD 45. Sections were costained for CD44 (green) and CD45 (red), and the number of CD44-positive/CD45-negative cells was scored within the glomeruli. (A) Double staining of CD44-positive/CD45-negative cells on a podocyte location (arrowheads). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). (B and C) Note that the CD44-positive cells (B) are CD45-negative (C). (D) The number of CD44-positive cells on a podocyte (visceral epithelial cell) location (**P=0.01; ***P=0.001). (E) The number of CD44-positive cells on a parietal epithelial cell location (P=0.07). In D and E, each symbol represents an individual patient or control. The lines represent the mean value, and the error bars represent the SD. PEC, parietal epithelial cell; VEC, visceral epithelial cell.
Discussion
In our cohort of women with preeclampsia, podocyte changes and tram tracking of the glomerular basement membrane were the most typical preeclampsia-associated lesions, occurring in 18% and 36% of patients, respectively. By contrast, none of the patients in our control groups had either of these lesions. Endotheliosis, a lesion that was previously described as a characteristic of preeclampsia, was present in 55% of the patients with preeclampsia; however, it was also present, albeit at much lower percentages, in the pregnant controls and the hypertensive nonpregnant controls. We also provide the first report that although the number of glomerular podocytes is unaffected in preeclampsia (as determined by histologic evaluation), preeclampsia is characterized by a higher number of activated parietal epithelial cells. A mechanism to explain our findings might come from elegant experiments by Appel et al., who used a rat model for lineage tracing and gene tagging and found that parietal epithelial cells migrate into the glomerular tuft (20). Moreover, our findings strongly suggest that lost podocytes are replaced by progenitor cells of the parietal epithelium in the context of preeclampsia. Importantly, none of the patients had any indication of underlying renal disease, suggesting that these findings are likely attributable to preeclampsia.
Preeclampsia is characterized by an increase in the shedding of podocytes into the urine (5–7), and this can occur before the onset of clinical manifestations (21). Furthermore, the expression of podocyte-specific proteins such as nephrin and glomerular epithelial protein-1 is significantly lower in kidney biopsies from patients with preeclampsia than from controls (8,9). Given the increased shedding of podocytes, our current findings that the number of glomerular podocytes remains stable suggest an increased turnover of podocytes in preeclampsia. Podocytes have traditionally been regarded as highly differentiated, nondividing cells, which would imply that these cells cannot regenerate after podocyte injury and/or loss. However, a recent study reported that the parietal epithelial cells that line Bowman’s capsule can replace injured and lost podocytes (20). On the other hand, a recent mouse study reported that proteinuria inhibits the differentiation of parietal epithelial cells into podocytes by sequestering retinoic acid (22).
It was recently shown that parietal epithelial cells, but not podocytes, upregulate their de novo expression of CD44 (marker of cell migration) after podocyte injury and/or loss (15,23). CD44 can also be expressed by endothelial cells (24). However, in our study, CD44 colocalized with WT-1, and CD44 positivity on a podocyte location was significantly higher in the patients with preeclampsia than in the three control groups. On the basis of the aforementioned study describing parietal epithelial cell migration in a rat model (20), our findings suggest that during preeclampsia, activated parietal epithelial cells can migrate and replace lost podocytes. Additional data to support this mechanism included the increased cell proliferation and the colocalization of Ki-67 and CD44 staining that were observed in the glomeruli of the women with preeclampsia. Together with the observed colocalization between WT-1 and CD44, these findings confirm that the Ki-67–positive cells present on a podocyte location are indeed podocytes.
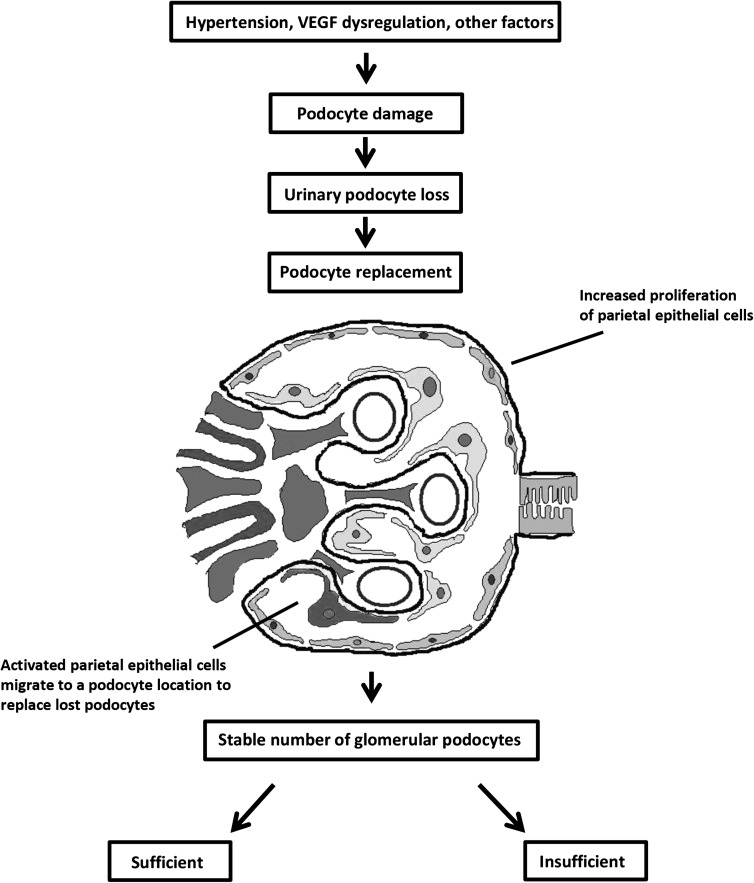
The replacement of lost podocytes by activated parietal epithelial cells is a compensatory mechanism that, if successful, is accompanied by remodeling the glomerular architecture (25). However, under certain conditions, this replacement mechanism cannot compensate fully, thereby leading to renal damage that is histologically characterized by FSGS. For example, in a mouse model of FSGS (23), an excessive proliferative response of parietal epithelial cells was involved in the progression of sclerotic lesions (15,23,26). There is also evidence that in renal transplants, increased CD44 staining, an indicator of activated parietal epithelial cells, distinguishes early recurrent FSGS (which manifests with podocyte foot process effacement only) from minimal change disease (18). These authors speculated that CD44 expression in nephrotic patients without sclerosis has positive value in predicting progressive podocyte damage, including FSGS (18). In our study, the patients with preeclampsia did not have significantly more FSGS in their kidneys; nevertheless, the significant correlation between FSGS and CD44-positive cellular bridges (connecting Bowman’s capsule and the glomerular tuft) supports the previous notion that CD44-positive cells are involved in the formation of sclerotic lesions (18,23). The results reported by Fatima et al. (18) provide insight into the implications of our current findings, because women with preeclampsia have a higher risk of developing FSGS later in life (27). Moreover, we found that the women with preeclampsia in our study had higher CD44 positivity on a podocyte location compared with the control groups. We therefore speculate that this increased CD44 positivity in preeclamptic kidneys is a sign of progressive podocytopathy and may have predictive value for FSGS in the long term. Our findings within the preeclamptic kidney during pregnancy might also explain the reported increased risk for women with preeclampsia to develop ESRD later in life (4,28). However, it bears mentioning that because we investigated autopsy material, we were unable to analyze any possible correlation between CD44 positivity during pregnancy and the development of sclerotic lesions later in life. Figure 4 illustrates the sequence of events and the aforementioned putative mechanisms that underlie podocyte replacement and subsequent podocytopathy during preeclampsia.
Figure 4.
Schematic overview of the putative mechanism underlying podocyte replacement in preeclampsia. Hypertension, dysregulation of VEGF, and other factors cause damage and loss of podocytes during preeclampsia, resulting in increased shedding of podocytes into the urine. In preeclampsia, an increase in turnover of podocytes results from increased proliferation of parietal epithelial cells and higher numbers of activated parietal epithelial cells on a podocyte location. As a result of this increased turnover, the absolute number of glomerular podocytes is stable during preeclampsia. The replacement of lost podocytes by activated parietal epithelial cells is a compensatory mechanism that, if sufficient, leads to the resolution of clinical symptoms, including proteinuria. However, if compensation is not sufficient, this mechanism can trigger persistent podocytopathy, with progressive proteinuria and renal function loss later in life, histologically characterized by FSGS. VEGF, vascular endothelial growth factor.
Our study has some limitations. Because we investigated autopsy material of patients after the clinical development of preeclampsia, it remains uncertain whether the proliferative podocyte changes are a cause or consequence of the syndrome. Furthermore, because autopsy material includes post mortem changes, the observations in this study might differ from the living state of patients with preeclampsia and controls.
An important lingering question is what early mechanism causes podocyte injury and loss during preeclampsia. In our study, preeclampsia was characterized by damage to all three layers of the glomerular filtration barrier. Although endotheliosis was observed, albeit relatively rarely, in the pregnant controls and hypertensive nonpregnant controls, endotheliosis was most prevalent among the women with preeclampsia, which is consistent with a previous report (16). A dysregulation of, and the resulting imbalance between, proangiogenic and antiangiogenic factors is believed to cause endotheliosis. In particular, increased levels of the antiangiogenic factor soluble Fms-like tyrosine kinase-1 can prevent vascular endothelial growth factor (VEGF) from maintaining the renal endothelium (29). Because VEGF is essential for the interaction between endothelial cells and podocytes (30), dysregulation of VEGF also affects the podocyte. The notion that dysregulation of these factors plays a role in the renal manifestations of preeclampsia is supported by studies showing that endotheliosis is a key feature of glomerular injury in a podocyte-specific VEGF-knockout mouse (29). The same study also reported that similar lesions were observed in patients who were treated with the VEGF inhibitor bevacizumab (29). On the basis of these observations, it is highly likely that angiogenic imbalance plays a causative role in the renal manifestations of preeclampsia. However, preeclampsia may arise from a variety of causative factors other than angiogenic imbalance alone (31).
In conclusion, building on our previous report that preeclampsia is characterized by increased shedding of podocytes into the urine (6), this is the first report that the absolute number of glomerular podocytes is actually unchanged during preeclampsia. Our results indicate that podocytopathy plays a central role in preeclamptic nephropathy, thereby contributing, at least in part, to the increased risk of developing FSGS later in life. This notion is consistent with previous reports that podocytopathy plays a role in other forms of FSGS, and it lends credence to speculation that targeting the podocyte may have therapeutic value (32,33). Whether the activation of parietal epithelial cells is a mechanism to compensate for ongoing podocyte injury and loss, or whether these cells contribute to glomerular injury, or both, remains to be investigated. Nevertheless, unraveling the mechanisms of podocyte damage and parietal epithelial cell recruitment in the setting of preeclampsia may lead to novel approaches for treating renal injury.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Floor Luken for her excellent technical support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12811213/-/DCSupplemental.
See related editorial, “The Role of the Podocyte in Preeclampsia,” on pages 1337–1340.
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R: Pre-eclampsia. Lancet 376: 631–644, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP: Preeclampsia: A renal perspective. Kidney Int 67: 2101–2113, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Lohlein M: Zur Pathogenese der Nierenkrankheiten; Nephritis und Nephrose mit besonderes Berücksichtigung der Nephropathia gravidarum. Dtsch Med Wochenschr 44: 1187–1189, 1918 [Google Scholar]
- 4.Vikse BE: Pre-eclampsia and the risk of kidney disease. Lancet 382: 104–106, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC, Rose CH, Gavrilova L, Craigo P, Bailey KR, Achenbach J, Schiffer M, Grande JP: Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol 196: e1–e7, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kelder TP, Penning ME, Uh HW, Cohen D, Bloemenkamp KW, Bruijn JA, Scherjon SA, Baelde HJ: Quantitative polymerase chain reaction-based analysis of podocyturia is a feasible diagnostic tool in preeclampsia. Hypertension 60: 1538–1544, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Aita K, Etoh M, Hamada H, Yokoyama C, Takahashi A, Suzuki T, Hara M, Nagata M: Acute and transient podocyte loss and proteinuria in preeclampsia. Nephron Clin Pract 112: c65–c70, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Garovic VD, Wagner SJ, Petrovic LM, Gray CE, Hall P, Sugimoto H, Kalluri R, Grande JP: Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant 22: 1136–1143, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Gu X, Groome LJ, Wang Y: Decreased nephrin and GLEPP-1, but increased VEGF, Flt-1, and nitrotyrosine, expressions in kidney tissue sections from women with preeclampsia. Reprod Sci 16: 970–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA: Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 29: 19–24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM: The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 20: IX–XIV, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, de Boer K, Pel M, Vermeulen G, Visser W, van Roosmalen J, Netherlands Maternal Mortality Committee : Rise in maternal mortality in the Netherlands. BJOG 117: 399–406, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B: Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development 119: 1329–1341, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Scholzen T, Gerdes J: The Ki-67 protein: From the known and the unknown. J Cell Physiol 182: 311–322, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, Willner J, Olsen S: Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG 110: 831–836, 2003 [PubMed] [Google Scholar]
- 17.Lemley KV, Bertram JF, Nicholas SB, White K: Estimation of glomerular podocyte number: A selection of valid methods. J Am Soc Nephrol 24: 1193–1202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatima H, Moeller MJ, Smeets B, Yang HC, D’Agati VD, Alpers CE, Fogo AB: Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 7: 1852–1858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weibel ER, Gomez DM: A principle for counting tissue structures on random sections. J Appl Physiol 17: 343–348, 1962 [DOI] [PubMed] [Google Scholar]
- 20.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craici IM, Wagner SJ, Bailey KR, Fitz-Gibbon PD, Wood-Wentz CM, Turner ST, Hayman SR, White WM, Brost BC, Rose CH, Grande JP, Garovic VD: Podocyturia predates proteinuria and clinical features of preeclampsia: Longitudinal prospective study. Hypertension 61: 1289–1296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, Lombardi D, Giocaliere E, Della Bona M, Villanelli F, Parente E, Ballerini L, Sagrinati C, Wanner N, Huber TB, Liapis H, Lazzeri E, Lasagni L, Romagnani P: Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol 24: 1756–1768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chipps TJ, Appukuttan B, Pan Y, Smith JR: CD44 isoforms in human retinal and choroidal endothelial cells. Graefes Arch Clin Exp Ophthalmol 251: 1245–1246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vikse BE, Hallan S, Bostad L, Leivestad T, Iversen BM: Previous preeclampsia and risk for progression of biopsy-verified kidney disease to end-stage renal disease. Nephrol Dial Transplant 25: 3289–3296, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM: Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sison K, Eremina V, Baelde H, Min W, Hirashima M, Fantus IG, Quaggin SE: Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol 21: 1691–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rana S, Karumanchi SA, Lindheimer MD: Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension 63: 198–202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barisoni L, Schnaper HW, Kopp JB: Advances in the biology and genetics of the podocytopathies: Implications for diagnosis and therapy. Arch Pathol Lab Med 133: 201–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.