Abstract
The introduction of trastuzumab into clinical practice changed the natural course of HER2-positive breast cancer. Currently, treatment with trastuzumab represents the standard of care for HER2-positive breast cancer and this treatment has been approved in the adjuvant, neoadjuvant, and metastatic settings. Besides trastuzumab, two other anti-HER2 agents—lapatinib and pertuzumab—have been approved for the treatment of HER2-positive advanced breast cancer. Strong biologic data support the concept of dual HER2 blockade, with different anti-HER2 agents demonstrating complementary mechanisms of action. Several neoadjuvant and metastatic studies performed in HER2-positive breast cancer using dual HER2 blockade have been proven to outperform anti-HER2 monotherapies. These dual combinations of agents represent a promising therapeutic strategy that is now reaching clinical practice. In this review we describe the results of studies utilizing dual blockade in patients with HER2-positive breast cancer.
Keywords: Dual targeted therapy for breast cancer, Trastuzumab, Pertuzumab, Lapatinib, Everolimus, Metastatic breast cancer
Function of human epidermal growth factor receptors and their inhibitors
In the past four decades the development of strategies for the treatment of breast cancer has focused on understanding the expression, regulation, and function of critical signaling pathways involved in cancer initiation and progression. This process allowed the identification of different subgroup of patients with distinct biology and clinical outcome. Examples of these include the successful use of hormonal therapy for women with hormone-sensitive tumors [1] and the use of anti-human epidermal growth factor receptor 2 (HER2) therapies for women with HER2-overexpressing tumors [2].
Human epidermal growth factor receptor 2 is a 185-kDa transmembrane oncoprotein (p185) encoded by the HER2/neu gene and occurs in 20–25 % of breast cancers [3]. HER2 overexpression or amplification was initially reported to be associated with decreased disease-free survival (DFS) and overall survival (OS) [4]. HER2, also known as ErbB2, is a tyrosine kinase receptor. It is a member of the HER growth factor receptor family. This family of receptors comprise four distinct receptors, the epidermal growth factor receptor (EGFR) or ErbB1, HER2 (or ErbB2), HER3 (or ErbB3), and HER4 (or ErbB4) [5]. Homo- or heterodimerization of these receptors results in phosphorylation of residues from the intracellular domain of the receptor. This results in the recruitment of signaling molecules from the cytoplasm and activation of several signaling pathways. With the exception of HER2, which has no ligand, the HER proteins exist at the plasma membrane in an inactivated state that activates on ligand binding. HER2 is constitutively active and can undergo ligand-independent dimerization [6]. The two most studied HER2 downstream signaling pathways are the RAS/Raf/mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K)/Akt cascades [7]. One of the most successful strategies in the development of targeted therapy in oncology has involved the production of inhibitors of cell membrane growth factor receptors. Monoclonal antibodies directed against extracellular epitopes expressed in tumor cells and small tyrosine kinase inhibitors (TKIs) directed to a number of targets including extracellular receptor binding and inhibitor of intracellular signaling pathways represent two complementary approaches to growth factor receptor inhibition. Some of these agents have shown remarkable activity and have already become part of the standard of care in patients with HER2-positive breast cancer.
Current therapeutic options for patients whose tumors are HER2-positive include trastuzumab (Herceptin®) (a humanized monoclonal antibody that binds to HER2), lapatinib (Tykerb®) (a small-molecule inhibitor of HER2 tyrosine kinase activity), and pertuzumab (Perjeta®) (a recombinant humanized monoclonal antibody that disrupts HER2 dimerization).
The US Food and Drug Administration (FDA) approved trastuzumab for use in metastatic HER2-positive breast cancer [2] in September 1998, and subsequently in early-stage HER2-positive breast cancer [8, 9] in January 2008. Most recently, trastuzumab acquired another indication in the metastatic, unresectable HER2-positive gastric and gastroesophageal junction cancer [10].
Lapatinib was FDA-approved for use in metastatic HER2-positive breast cancer in March 2007 in combination with capecitabine for the treatment of patients with advanced metastatic breast cancer whose tumors overexpressed HER2 and who had received prior therapy including an anthracycline, a taxane, and trastuzumab [11]. Subsequently, in January 2010, the FDA granted approval to lapatinib plus letrozole (Femara®) for the treatment of postmenopausal women with hormone receptor-positive, HER2-positive tumors for whom hormonal therapy is indicated.
Pertuzumab was approved by FDA in June 2012 for use in combination with trastuzumab and docetaxel for the treatment of patients with HER-2-positive metastatic breast cancer who had not received prior anti-HER2 therapy or chemotherapy for metastatic disease [12].
Many other agents, including trastuzumab emtansine (T-DM1), and neratinib, have shown significant activity in clinical trials but are not yet approved for clinical practice. These agents are not discussed in this review.
HER2-inhibitors and tumor resistance
Trastuzumab consists of two antigen-specific sites that bind to the juxtamembrane portion of the extracellular domain of the HER2 receptor and that prevent the activation of its intracellular tyrosine kinase [13]. Although the exact mechanism by which trastuzumab exerts its antitumor activity is unknown, several possibilities have been proposed, including activation of antibody-dependent cellular cytotoxicity, blockage of proteolytic cleavage of the HER2 extracellular domain, inhibition of intracellular signal transduction, inhibition of tumor-induced angiogenesis, and inhibition of repair of cancer treatment-induced DNA damage [14]. The success of trastuzumab is based on the dependence of HER2-positive breast cancer cells on HER2, a feature exemplifying oncogene addiction.
Pertuzumab binds to subdomain II of the HER2 extracellular domain [15] and prevents HER2 from dimerizing with other ligand-activated HER2 receptors, most notably HER3 [16, 17]. Like trastuzumab, pertuzumab stimulates antibody-dependent, cell-mediated cytotoxicity [18]. Whereas trastuzumab prevents ligand-independent HER2 signaling, pertuzumab interferes with ligand-dependent HER3-mediated signaling. Preclinical studies suggested that the combination of pertuzumab and trastuzumab in cancer cells expressing high levels of HER2 protein is associated with significant antitumor activity [18, 19].
In a phase III trial of first-line trastuzumab plus various chemotherapy regimens compared with chemotherapy alone, there was significant improvement in OS (25.1 vs. 20.3 months) and overall response rate (ORR) (50 vs. 32 %) [2]. This study resulted in approval of trastuzumab with paclitaxel for the first-line treatment of HER2-overexpressing metastatic breast cancer on the basis of a 62 % reduction in the risk of disease progression [(hazard ratio) HR 0.38; 95 % CI 0.27–0.53], and significantly longer median progression-free survival (PFS) (6.9 vs. 3.0 months; P < 0.001). However, approximately one-half of patients have primary resistance to trastuzumab, and among those who respond, the majority of patients eventually develop progressive disease while receiving trastuzumab-based therapy [14].
In the adjuvant setting, several independent randomized studies have shown that the addition of trastuzumab to chemotherapy reduced the rate of recurrence by over 50 % among women with HER2-positive early breast cancer [20]. On the basis of these results, trastuzumab in combination with standard chemotherapy was approved by the FDA in 2006 for use in the adjuvant setting.
Dual anti-HER2 targeted therapy for metastatic breast cancer
Preclinical models demonstrated the interaction of lapatinib with trastuzumab as synergistic and resulting in enhanced apoptosis in ErbB-positive advanced breast cancer [21] including cancers progressing on prior trastuzumab-based therapy [22]. In a group of patients heavily pretreated with chemotherapy and trastuzumab, the combination of trastuzumab plus lapatinib was associated with significantly longer median PFS than lapatinib alone (HR 0.73; 95 % CI 0.57–0.93; P = 0.008) [23].
In a small study of 29 patients with HER2-positive advanced breast cancer that had progressed during trastuzumab-based therapy, pertuzumab as a single agent resulted in an objective response rate of 3.4 % and a clinical benefit rate of 10.3 % [24]. When trastuzumab was added to pertuzumab in patients with disease progression during this single-agent pertuzumab therapy, the ORR and clinical benefit rate (CBR) were 17.6 and 41.2 %, respectively. This increased clinical activity motivated further study of the combination of trastuzumab and pertuzumab.
Another phase II study in patients with HER2-positive advanced breast cancer who experienced progression during trastuzumab therapy demonstrated that the addition of pertuzumab to trastuzumab resulted in an ORR of 24.2 % and a CBR of 50 % [25].
The Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) trial was a phase III study in which 808 patients with HER2-positive metastatic breast cancer were randomized in a 1:1 ratio to receive as first-line therapy for metastatic disease either placebo plus trastuzumab plus docetaxel (control group) or pertuzumab plus trastuzumab plus docetaxel (pertuzumab group) [12]. The primary endpoint was PFS assessed by the investigator; secondary endpoints were ORR and OS. The median PFS was 12.4 months in the control group and 18.5 months in the pertuzumab group (HR 0.62; 95 % CI 0.51–0.75; P < 0.001). The ORR was 69.3 and 80.2 %, in the control group and pertuzumab group, respectively. The absolute difference in objective response rate was 10.8 % (95 % CI 4.2–17 %; P = 0.001). More deaths occurred in the control group than in the pertuzumab group [96 (23.6 %) vs. 69 (17.2 %); HR 0.64; 95 %, CI 0.47–0.88; P < 0.005].
These clinical results suggest that targeting HER2-positive breast cancer with two different monoclonal antibodies (pertuzumab plus trastuzumab) that have a complementary mechanism of action results in a more comprehensive blockade than treatment with either antibody alone and highlights the importance of preventing the ligand-dependent formation of HER2 dimers in order to silence HER2 signaling to the greatest extent possible. A trial of pertuzumab plus trastuzumab in patients with newly diagnosed HER2-positive breast cancer, the Adjuvant Pertuzumab and Herceptin in Initial Therapy of Breast Cancer (APHINITY) trial (NCT01358877), is currently under way.
Dual anti-HER2 targeted therapy for the neoadjuvant setting
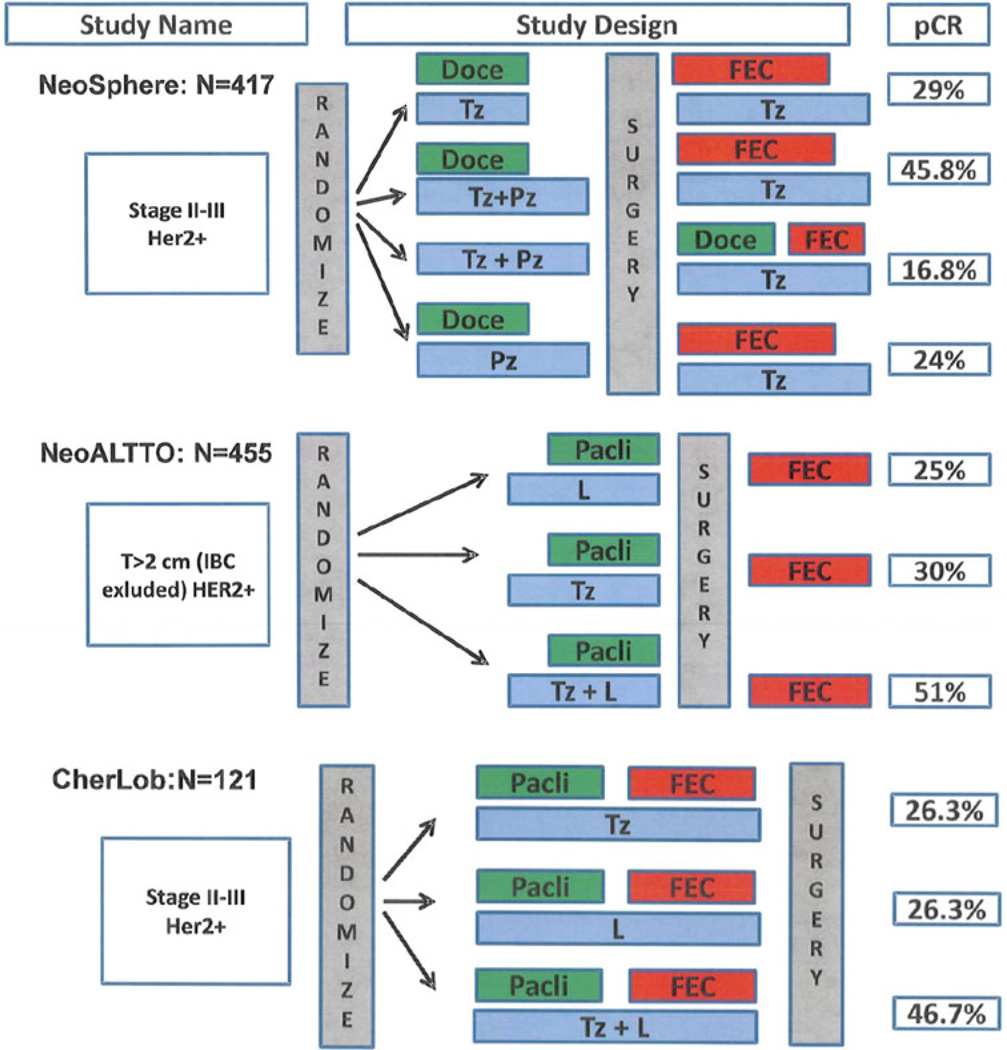
Outcomes for women with early-stage HER2-positive breast cancer have improved markedly since the introduction of trastuzumab. The 5-year survival rate for women with node-positive disease treated with multiagent chemotherapy and trastuzumab exceeds 80 % [9, 26]. Recently, three published clinical trials have tested the hypothesis that in HER2-positive breast cancer, neoadjuvant therapy with two HER2-directed agents with non-overlapping mechanisms of action has greater efficacy than therapy with a single HER2-directed agent (Table 1; Fig. 1).
Table 1.
Pathologic complete response (pCR) rates with anti-HER2 therapy in the neoadjuvant setting
| Trial | Taxane + trastuzumab (%) |
Taxane + pertuzumab (%) |
Taxane + lapatinib (%) |
Taxane + trastuzumab + pertuzumab (%) |
Taxane + trastuzumab + lapatinib (%) |
Trastuzumab + pertuzumab (%) |
|---|---|---|---|---|---|---|
| NeoSphere [27] | ||||||
| Overall | 29 | 24 | – | 46 | – | 17 |
| ER+ | 20 | 17 | – | 26 | – | 6 |
| ER− | 37 | 30 | – | 63 | – | 27 |
| NeoALTTO [28] | ||||||
| Overall | 30 | – | 25 | – | 51 | – |
| ER+ | 23 | – | 16 | – | 42 | – |
| ER− | 27 | – | 34 | – | 61 | – |
| CHER-LOB [29] | ||||||
| Overall | 25 | – | 26.3 | – | 46.7 | – |
| ER+ | 25 | – | 22.7 | – | 35.7 | – |
| ER− | 26.6 | – | 35.7 | – | 56.2 | – |
ER estrogen receptor, NeoSphere Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation, NeoALTTO Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization trial, CHER-LOB Chemotherapy Herceptin and Lapatinib in Operable Breast Cancer
Fig. 1.
Design and pCR rate of neoadjuvant studies in HER2-positive breast cancer. Doce docetaxel, Tz trastuzumab, Pz pertuzumab, FEC 5-fluorouracil, epirubicin, and cyclophosphamide, Pacli paclitaxel, L lapatinib, IBC inflammatory breast cancer, LABC locally advanced breast cancer, pCR pathologic complete response
Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation (NeoSphere) was a phase II trial in which patients were randomly assigned to 1 of 4 regimens: trastuzumab and docetaxel (group A); pertuzumab, trastuzumab, and docetaxel (group B); pertuzumab and trastuzumab (group C); and pertuzumab and docetaxel (group D) [27]. The primary endpoint was pathologic complete response (pCR), (defined as no residual cancer in the breast and assessed at surgery after four 3-weekly cycles of therapy). Patients in group B had a significantly better pCR rate than those in group A (45.8 vs. 29 %), and patients in groups A and B both had better pCR rates than patients in groups C (16.8 %) and D (24.0 %). It was interesting that even in the absence of chemotherapy, the combination of trastuzumab and pertuzumab led to pCR in almost 17 % of patients. The advantage of treatment with trastuzumab and pertuzumab over treatment with either agent alone was greatest among women with ER-negative disease.
The NeoAdjuvant Lapatinib and/or Trastuzumab Treatment Optimization (NeoALTTO) trial [28] was a randomized phase III study in which 455 women with invasive, operable HER2-positive breast cancer were randomly assigned to receive trastuzumab, lapatinib, or the combination for 18 weeks along with paclitaxel. In the NeoALTTO trial, before surgery, each group received 3 cycles of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC), followed by 34 weeks of their assigned targeted therapy. The primary endpoint was pCR, (defined as complete disappearance of infiltrating tumor in the breast). Dual anti-HER2 therapy had a 51 % pCR rate versus 30 % with trastuzumab alone (P = 0.001) and 25 % with lapatinib alone (P = 0.13 vs. trastuzumab). As a secondary aim, the total pCR rate (defined as no disease in breast and axillary nodes) was also evaluated; this analysis confirmed the superiority of dual HER2 blockade. As in the NeoSphere study, the benefits of the combined approach were most dramatic in women with ER-negative disease.
Lastly, the Chemotherapy Herceptin and Lapatinib in Operable Breast Cancer (CHER-LOB) study [29] was a randomized phase II trial for patients with operable HER2-positive breast cancer. A total of 121 patients were treated with a chemotherapy backbone consisting of paclitaxel followed by FEC and then patients were randomly assigned to trastuzumab, lapatinib, or the combination of trastuzumab and lapatinib. The primary endpoint was pCR, (defined as no invasive tumor in the breast and axillary lymph nodes). The pCR rates were 25 % (90 % CI 13.1–36.9 %) in arm A with trastuzumab alone, 26.3 % (90 % CI 14–38.1 %) in arm B with lapatinib alone, and 46.7 % (90 % CI 34.4–58.9 %) in arm C with the combination of trastuzumab and lapatinib (exploratory P = 0.019).
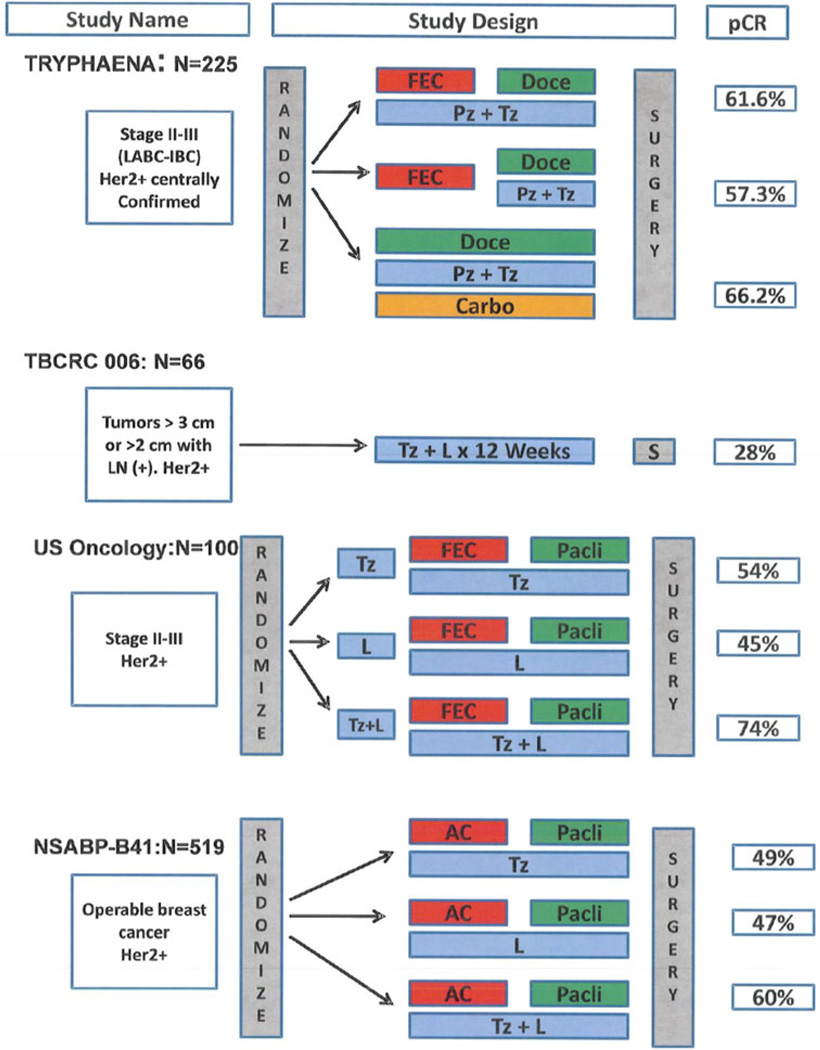
Four additional studies have been presented but not yet published at the time of this review. The clinical design and pCR rate of each study are depicted in Fig. 2.
Fig. 2.
Design and pCR rate of ongoing neoadjuvant studies in HER2-positive breast cancer. IBC inflammatory breast cancer, LABC locally advanced breast cancer, Doce docetaxel, Tz trastuzumab, Pz pertuzumab, FEC 5-fluorouracil, epirubicin, and cyclophosphamide, Pacli paclitaxel, L lapatinib, AC adriamycin and cyclophosphamide, pCR pathologic complete response
Preliminary results of the phase II randomized neoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline-containing or concurrent with an anthracycline-free standard regimen (TRYPHAENA) study were presented at the 2011 San Antonio Breast Cancer Symposium [30]. In this study, 225 women with early HER2-positive breast cancer were randomized to three experimental arms. The primary endpoint of the study was cardiac safety, which was shown to be consistent across all three experimental arms. Concurrent administration of pertuzumab plus trastuzumab with epirubicin resulted in similar cardiac tolerability compared with sequential administration, or the anthracycline-free regimen. Regardless of the chemotherapy chosen, the combination of pertuzumab and trastuzumab resulted in high pCR rates ranging from 57 to 66 %.
The Translational Breast Cancer Research Consortium (TBCRC) protocol 006 is a multicenter phase II single arm study combining lapatinib and trastuzumab for 12 weeks plus letrozole in ER-positive breast cancer (plus goserelin if premenopausal) without the use of chemotherapy. This study was presented at the 2011 ASCO Annual Meeting [31]. A total of 64 evaluable patients were registered with modest adverse events. The overall pCR rate was 28 %, including 21 % for patients with ER-positive breast cancer and 42 % for those with ER-negative disease.
A US Oncology study [32] presented at the 2011 ASCO Annual Meeting, assessed the neoadjuvant administration of anti-HER2 therapy with trastuzumab, lapatinib, or the combination of both for 2 weeks followed by four cycles of FEC-75 (5-fluorouracil, epirubicin, and cyclophosphamide) then weekly paclitaxel ×12 with the continuation of the same anti-HER2 treatment. The pCR rates were 54, 45, and 74 % for trastuzumab, lapatinib, and the combination of trastuzumab and lapatinib.
Lastly, Robidoux et al. [33] presented the results of NSABP B-41. This was a randomized phase III neoadjuvant clinical trial for patients with HER2-positive breast cancer at least 2 cm in size. A total of 529 patients were randomized to AC (adriamycin plus cyclophosphamide) followed by 4 months of weekly paclitaxel concurrently with trastuzumab (T), lapatinib (L), or the combination of both agents (T + L). The pCR rates in the breast and the lymph nodes were 49.1, 47.4, and 60.4 % for T, L, and T + L, respectively. The authors concluded that combined HER2-targeted therapy produced a numerically higher pCR percentage than single-agent HER2-directed therapy, but the difference was not statistically significant.
Although there were differences between these seven neoadjuvant clinical trials with respect to duration of chemotherapy, use of anthracyclines, choice of taxanes, a window of anti-HER2 therapy without chemotherapy, different definitions of pCR, and sample size per arm, the results of these neoadjuvant trials, taken together, validate the hypothesis that combining 2 HER2-directed agents with non-overlapping mechanisms of action is more effective than use of a single anti-HER2 agent. The finding that patients with ER-negative breast cancer had higher rates of pCR agrees with previously reported findings [34].
Despite the strong evidence of the predictive value of pCR, there is no consensus on the definition of this essential endpoint. A recent meta-analysis of randomized neoadjuvant trials recommended that pCR should be defined as absence of invasive breast cancer in the breast and axillary lymph nodes [35]. Two studies of neoadjuvant therapy for breast cancer, the Taxol Epirubicin Cyclophosphamide Herceptin Neoadjuvant (TECHNO) study [36] and the Neoadjuvant Herceptin (NOAH) study [37], demonstrated that increased pCR rate correlated with increased DFS. Findings from neoadjuvant therapy trials can predict outcomes of trials of adjuvant therapy; indeed, results from the NeoALTTO [28] and GeparQuinto [38] trials, in which lapatinib was not as successful as trastuzumab, anticipated a modification of the large ALTTO adjuvant therapy study, which includes more than 8300 patients (recruitment was just completed) and is assessing treatments similar to those used in the NeoALTTO study, with DFS as the primary endpoint. The lapatinib group in the ALTTO trial was closed after a recommendation from the independent data monitoring committee based on futility.
Combination therapy with an mTOR inhibitor and trastuzumab
Trastuzumab resistance has been associated with activation of the phosphoinositol 3-kinase pathway [39, 40]. Preclinical studies have demonstrated that mTOR inhibition reduces tumor formation/growth in mice with PTEN-deficient tumors and sensitizes response to trastuzumab in mice bearing HER2-overexpressing and PTEN-deficient breast tumor xenografts [40, 41]. Everolimus is a strong oral mTOR inhibitor binding FKBP-12 resulting in an inhibitory complex formation with mTORC1 and inhibition of mTOR kinase activity and reduces the activity of SK61 and 4E-BP1, both involved in protein synthesis [42]. A pooled analysis from two clinical trials conducted in two institutions in patients with HER2-overexpressing metastatic breast cancer who had progressed on trastuzumab-based therapy, the addition of everolimus (Afinitor) produced 15 % partial response and 19 % stable disease lasting 6 months or longer [43].
Preliminary results of a multicenter phase II study in 25 patients with HER2-positive disease that progressed on trastuzumab and paclitaxel were presented at the 2010 ASCO Annual Meeting [44]. Patients were re-treated with trastuzumab plus paclitaxel plus everolimus. Five patients (20 %) and 14 patients (56 %) achieved partial response and stable disease, respectively. The regimen was well tolerated.
Conclusions
Breast cancer is becoming a series of orphan diseases which will make it intensely more complicated to study. There are now targeted therapeutic options available for nearly all breast cancer subtypes characterizing the differing drivers of carcinogenesis. A little over a decade has passed since HER2 became an accepted therapeutic target in standard breast cancer practice. The impact of trastuzumab on the care of women with HER2-positive breast cancer has been profound.
The data described in this review corroborate the notion that combining different anti-HER2-targeted agents in patients with HER2-positive breast cancer can outperform their single-agent counterparts in the neoadjuvant and metastatic setting. This dual blockade represents a promising therapeutic strategy, now reaching clinical practice.
Although this represents an enormous step forward, numerous questions remain to be answered: (a) Who are the patients most likely to benefit with this dual blockade approach? (b) How we can identify these patients? (c) Who are those patients that will not require chemotherapy and will get the benefit of dual targeted therapy? (d) How we can predict the development of resistance in these patients? and (e) Could triplet combination further improve clinical efficacy? Which of the doublets has the highest therapeutic ratio? Is there cross-resistance between the doublets?
The discoveries explained above have tremendous implication in terms of the design of future clinical trials of dual-blocked anti-HER2 treatment of breast cancer and we believe this will translate into continued change in the standard of care.
Acknowledgments
The MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672.
Footnotes
Recommended at the 20th Annual Meeting of the Japanese Breast Cancer Society.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Ricardo H. Alvarez, Email: ralvarez@mdanderson.org, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1155 Herman P Pressler CPB5.3450, Houston, TX 77030, USA; Morgan Welch Inflammatory Breast Cancer Research Program and Clinic, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Gabriel N. Hortobagyi, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1155 Herman P Pressler CPB5.3450, Houston, TX 77030, USA
References
- 1.Osborne CK, et al. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980;46(12 Suppl):2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Fletcher JA. The HER2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16(6):413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Olayioye MA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin DN, et al. Targeting EGFR activity in blood vessels is sufficient to inhibit tumor growth and is accompanied by an increase in VEGFR-2 dependence in tumor endothelial cells. Microvasc Res. 2008;76(1):15–22. doi: 10.1016/j.mvr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15(24):7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart MJ, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 9.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 10.Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11.Geyer CE, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 12.Baselga J, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern H. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med. 2012;4(127):127–138. doi: 10.1126/scitranslmed.3001539. [DOI] [PubMed] [Google Scholar]
- 14.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27(34):5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 15.Franklin MC, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 17.Agus DB, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 18.Scheuer W, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 19.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64(7):2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 20.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 21.Konecny GE, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 22.Cameron DW, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analysis. Breast Cancer Res. 2008;112(3):533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell KL, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 24.Cortes J, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(14):1594–1600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28(7):1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slamon D, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianni L, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 28.Baselga J, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guarneri V, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30(16):1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 30.Schneeweiss A, et al. Neoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline-containing or concurrent wit an anthracycline-free standard regimen: a randomized phase II study (TRYPHAENA) AACR-SABCS. 2011:S5–S6. [Google Scholar]
- 31.Chang JCN, et al. TBCRC 006: a multicenter phase II study of neoadjuvant lapatinib and trastuzumab in patients with HER2-overexpressing breast cancer. J Clin Oncol. 2011;29(suppl) doi: 10.1200/JCO.2012.44.8027. (abstr 505). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes F, et al. Correlation of molecular effects and pathologic complete response to preoperative lapatinib and trastuzumab, separately and combined prior to neoadjuvant breast cancer therapy. J Clin Oncol. 2011;29(suppl) (abstr 506). [Google Scholar]
- 33.Robidoux A, et al. Evaluation of lapatinib as a component of neoadjuvant therapy for HER2+ operable breast cancer: NSABP protocol B-41. J Clin Oncol. 2011;29(suppl) doi: 10.1016/S1470-2045(13)70411-X. (abstr LBA506) [DOI] [PubMed] [Google Scholar]
- 34.Guarneri V, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24(7):1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 35.Cortazar P, et al. Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC) Cancer Res. 2012;72(24 Suppl) (Abstract S1–11). [Google Scholar]
- 36.Untch M, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 37.Gianni L, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 38.Untch M, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline–taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13(2):135–144. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 39.Berns K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 40.Nagata Y, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Lu CH, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13(19):5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 42.Nashan B. Early clinical experience with a novel rapamycin derivative. Ther Drug Monit. 2002;24(1):53–58. doi: 10.1097/00007691-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Morrow PK, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29(23):3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalenc F, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab and taxanes: a multicenter phase II clinical trial. J Clin Oncol. 2010;28(suppl):15s. (abstr 1013). [Google Scholar]