Abstract
Objective
Substantial evidence implicates interstitial collagenases of the MMP family in plaque rupture and fatal thrombosis. Understanding the compensatory mechanisms that may influence the expression of these enzymes and their functions therefore have important clinical implications. This study assessed in mice the unknown relative impact of the two principal collagenases on collagen content and other plaque characteristics.
Approach and Results
apoE−/− mice, MMP-13−/− apoE−/−, MMP-8−/− apoE−/− double knockout (DKO) mice, and MMP-13−/− MMP-8−/− apoE−/− triple knockout (TKO) mice consumed a high-cholesterol diet for 10 and 24 weeks. Both DKO and TKO mice showed comparable atherosclerotic lesion formation compared to apoE−/− controls. Analysis of aortic root sections indicated that lesions of MMP-8/MMP-13-deficient and MMP-13-deficient mice accumulate more fibrillar collagen than apoE−/− controls and MMP-8−/− apoE−/− DKO. We further tested the relative impact of MMPs on plaque collagenolysis using in situ zymography. MMP-13 deletion alone abrogated collagenolytic activity in lesions, indicating a predominant role for MMP-13 in this process. MMP-13 and MMP-13/MMP-8 deficiency did not alter macrophage content, but associated with reduced accumulation of smooth-muscle cells.
Conclusions
These results show that among MMP interstitial collagenases in mice, MMP-13 prevails over MMP-8 in collagen degradation in atheromata. These findings provide a rationale for the identification and selective targeting a predominant collagenase for modulating key aspects of plaque structure considered critical in clinical complications, although they do not translate directly to human lesions, which also contain MMP-1.
Keywords: atherosclerosis, collagen, MMPs
INTRODUCTION
Atherosclerosis remains the major cause of death and premature disability worldwide1. Rupture of plaques with a thin fibrous cap causes most fatal myocardial infarctions2, 3. Because interstitial collagen confers tensile strength upon the fibrous cap, collagenolysis in atheromata participates critically in plaque disruption. Previous work has implicated interstitial collagenases of the matrix metalloproteinase (MMP) family in the regulation of aspects of the structure of atherosclerotic plaques that associate with rupture and fatal thrombosis in humans4–7. These data collectively suggest that therapeutic targeting of interstitial collagenases might reduce characteristics of plaques associated with rupture, and thus limit thrombotic complications.
Yet, broad spectrum inhibitors of MMPs cause unwanted effects that preclude their clinical use,8–11 and pilot clinical studies failed to show beneficial effects of MMP inhibitors8 due to lack of specificity. Understanding the potential redundancy of these enzymes in the regulation of plaque collagen content therefore has not only mechanistic interest, but also important implications for the design of targeted strategies for reducing complications of atherosclerosis. In particular, our prior work showed that mice bearing a mutant form of interstitial collagen that resists breakdown by MMPs accumulate plaque collagen12. Subsequent studies showed that selective inhibition of MMP-13 can also increase the content of plaque collagen6, 13. In addition, these studies presented evidence that MMP-13 predominates as an interstitial collagenase in mouse atheromata. Yet, to date no data directly compare the contribution of combined deficiency of MMP-13 and the other principal interstitial collagenase in mouse lesions, MMP-8, on collagen accumulation and other aspects of plaque structure and cellular composition.4, 14
Mouse atherosclerotic plaques contain two principal interstitial collagenases, whereas human lesions contain three MMPs of this class5, 15. Therefore, the mouse offers a defined approach for testing the redundancy of interstitial collagenase actions during atherogenesis. The present study tested the novel hypothesis that loss of MMP-13 function alone achieves a similar increase in collagen content in experimental atheromata as that caused by deficiency of both MMP-13 and MMP-8. Our study examined both early and established atheromata to assess the role of collagenases in both formation and progression of lesions. The findings furnish new mechanistic insight into the biological roles of interstitial collagenases in experimental atherosclerosis, and support the selective targeting of a single collagenase to influence decisively aspects of plaque structure deemed critical in clinical complications.
MATERIAL AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Characteristics of mice
To examine the relative contribution of MMP-8 and MMP-13 to atherosclerosis, apoE−/−mice, MMP-13−/− apoE−/−, MMP-8−/− apoE−/− double knockout (DKO) mice, and MMP-13−/− MMP-8−/− apoE−/− triple knockout (TKO) mice consumed an atherogenic diet, either for 10 weeks to provoke early lesions (n≥12 per group) or for 24 weeks for advanced atheromata (n≥15). Total cholesterol, and triglycerides did not differ between apoE−/− and MMPs mutant mice after 10 weeks of the atherogenic diet (Table 1). TKO mice, however, had higher body weight (**p≤0.01) than apoE−/− and DKOs mice (**p≤0.01) at this time point. After 24 weeks, TKO mice consistently exhibited increased body weight compared with DKO mice, with higher plasma cholesterol levels compared to controls (**p≤0.01).
Table 1.
Characteristics of KO mice after the indicated period of atherogenic diet
| apoE−/− | apoE−/− MMP13−/− |
apoE−/− MMP8−/− |
apoE−/− MMP13−/− MMP8−/− |
P | |
|---|---|---|---|---|---|
| 10 weeks atherogenic diet | (n=22) | (n=22) | (n=14) | (n=24) | |
| Body weight (g) | 35.8±1.2 | 30.9±0.7 | 35.9±0.9 | 40.9±1.5 | <0.0001 |
| Total Cholesterol (mg/dL) | 568.1±27.1 | 542.2±29.8 | 589.5±47.8 | 520.7±24.83 | ns |
| Triglycerides (mg/dL) | 80.7±10.0 | 68.6±5.0 | 79.8±7.8 | 78.0±10.0 | ns |
| 24 weeks atherogenic diet | (n=28) | (n=27) | (n=15) | (n=31) | |
| Body weight (g) | 33.2±0.6 | 32.3±0.8 | 36.6±1.5 | 41.8±1.4 | <0.0001 |
| Total Cholesterol (mg/dL) | 660.1±92.0 | 844.8±70.5 | 704.6±58.3 | 972.4±88.2 | 0.0028 |
| Triglycerides (mg/dL) | 96.9±10.1 | 115.8±5.6 | 156.7±24.4 | 121.3±7.8 | 0.0201 |
Lack of MMP-8 and MMP-13 activity does not affect the development of atherosclerosis in apoE-deficient mice
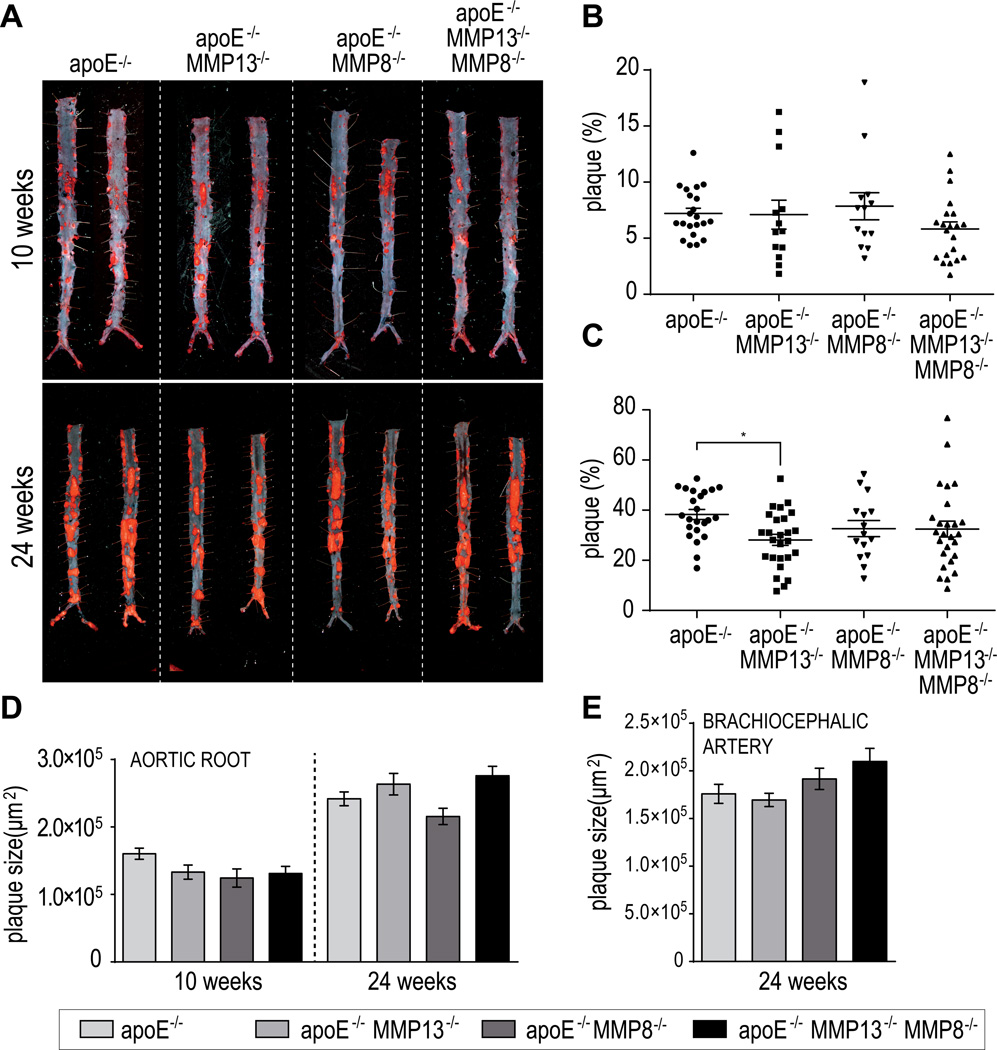
Oil red O en face staining determined the lipid content of atherosclerotic lesions in the descending aorta. Overall, TKO mice exhibited similar plaque size after 10 and 24 weeks on an atherogenic diet, compared with apoE−/− mice and DKO animals (Figures 1A–C). In the aortic root and the brachiocephalic artery, where plaques form earlier, histological analysis of plaque size showed a similar result. Genetic inactivation or pharmacologic inhibition of MMP-13 associated with comparable extent of atheromata compared to apoE-deficient controls (Figures 1D and 1E), in accord with the results observed in our previous studies.6, 13 MMP-8 deletion combined with a lack of MMP-13 had no significant additional effect regarding plaque size, suggesting that MMP-8 and MMP-13 play a minor role in plaque lipid accumulation.
Figure 1.
Oil-red-O staining of lesions in abdominal aortas from mice after 10 and 24 weeks on an atherogenic diet (A). Quantification of positive areas after 10 weeks (B) and 24 weeks (C) on western diet. Histological analysis of plaque size on aortic root (D) and brachiocephalic artery sections. Bars represent mean ± SEM. (*p<0.05).
MMP-13 deficiency yields increased more intraplaque collagen accumulation compared to MMP-8-deficient mice
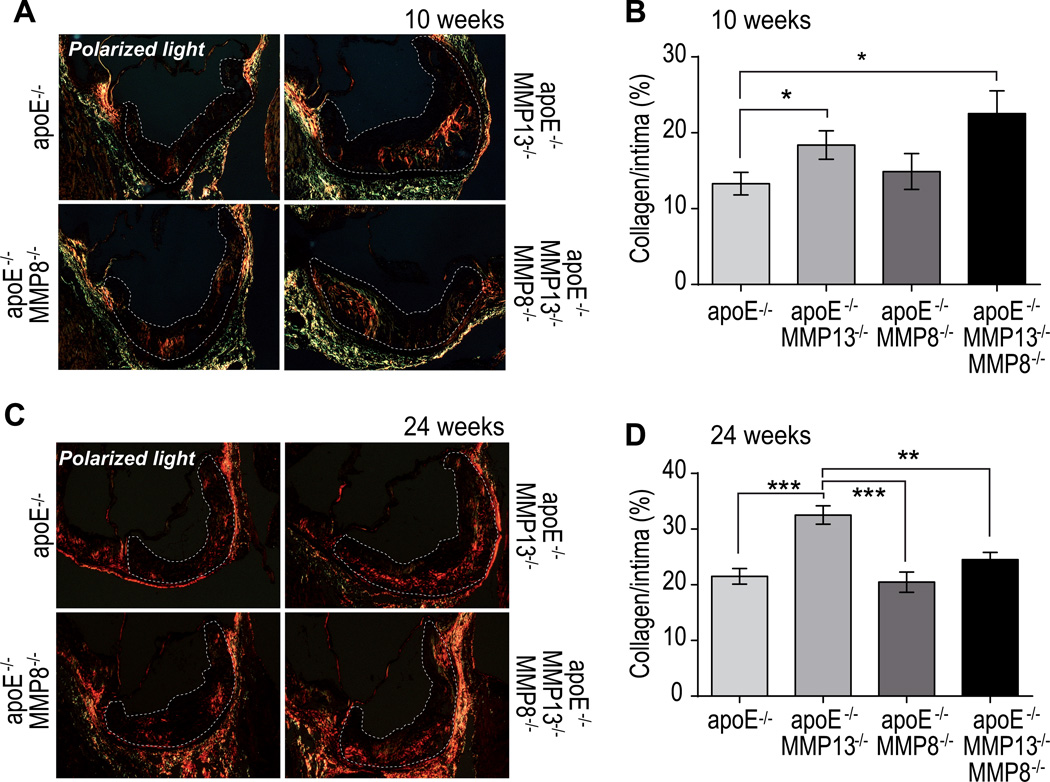
We further investigated collagen content by analyzing aortic root sections stained by picrosirius red under polarized light. After 10 weeks on an atherogenic diet (Figure 2A), the intima of aortic lesions from MMP-13−/− apoE−/− mice accumulated more interstitial collagen than apoE−/− mice, affirming the critical role of MMP-13 in plaque collagenolysis. MMP-8−/− apoE−/− exhibited a slight and statistically insignificant increase in plaque collagen. MMP-13 deletion associated with a 71% increase in collagen accumulation compared to MMP-8 deficient mice, indicating a predominant role of MMP-13 in collagen degradation in murine atheromata. Combined deletion of MMP-8 and MMP-13 in TKO mice did not correlate with any significant additional increase of collagen accumulation compared with DKO mice, further suggesting that MMP-8 contributes little to this process (Figure 2B). Established plaques (Figure 2C), showed a 66.2% increase in the fibrillar collagen content in MMP-13 apoE DKO mice compared to apoE−/− controls (Figure 2D). Strikingly, lesions from TKO mice showed less collagen accumulation compared to those from DKO mice, and did not differ significantly from apoE−/− controls or MMP-8 apoE deficient animals. Analysis of picrosirius red-stained collagen fibers under filtered polarized light allowed us to assess the impact of MMP-13 and MMP-8 on this index of collagen fiber thickness.16–18 This approach revealed an increased proportion of mature, thick collagen fibers in DKO mice (observed under polarized light with a red filter) in early and advanced atherosclerotic lesions, compared with apoE-deficient mice. When associated with MMP-13 deficiency, lack of MMP-8 did not enrich early or advanced atherosclerotic lesions with thick collagen fibrils.
Figure 2.
Picrosirius red staining viewed under linearly polarized light to show fibrillar collagen in plaques after 10 (A) and 24 (C) weeks of atherogenic diet. Quantification of intimal collagen content (B–D). Bars represent mean ± SEM. (*p<0.05, **p<0.01, ***p<0.001).
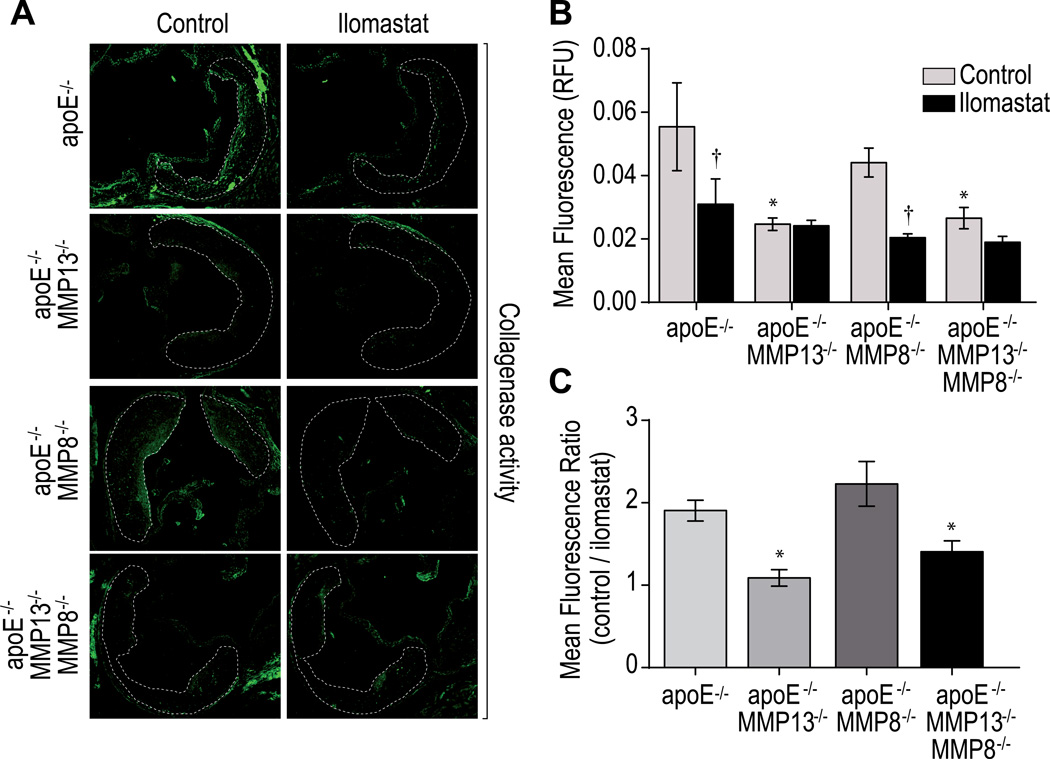
MMP-13 deletion alone abrogates collagenolysis in atherosclerotic lesions
To estimate further the relative contribution of MMP-13 and MMP-8 on collagenolysis in lesions, we performed in situ zymography in the aortic roots of mice after 24 weeks of atherogenic diet. For each group, we compared the mean fluorescence intensity derived from a cleavable collagen-based probe in the presence or absence of a broad-spectrum metalloproteinase inhibitor (ilomastat) as a control (Figure 3). MMP-13 deletion alone in DKO mice completely inhibited collagen degradation in lesions, as did ilomastat treatment (Figure 3A). MMP-8 deficiency did not significantly reduce collagenase activity compared to apoE−/− control. MMP-13 apoE DKO and TKO mice exhibited similar collagenase activity, indicating that compensatory changes in MMP-8 activity do not contribute to plaque collagenolytic capacity in mice with congenital absence of MMP-13 (Figures 3B and 3C).
Figure 3.
In situ zymography for collagenolytic activity in mouse arteries. Representative sections of aortic roots of mutant mice after 24 weeks of atherogenic diet incubated with a fluorescent collagenase-activatable probe in presence or in absence of the broad MMP inhibitor ilomastat (A). Quantification of the mean fluorescence in the intima (B) and the mean fluorescence ratio compared to ilomastat treated samples (C) are represented (n=5). Bars represent mean ± SEM (*p<0.05, **p<0.01, ***p<0.001).
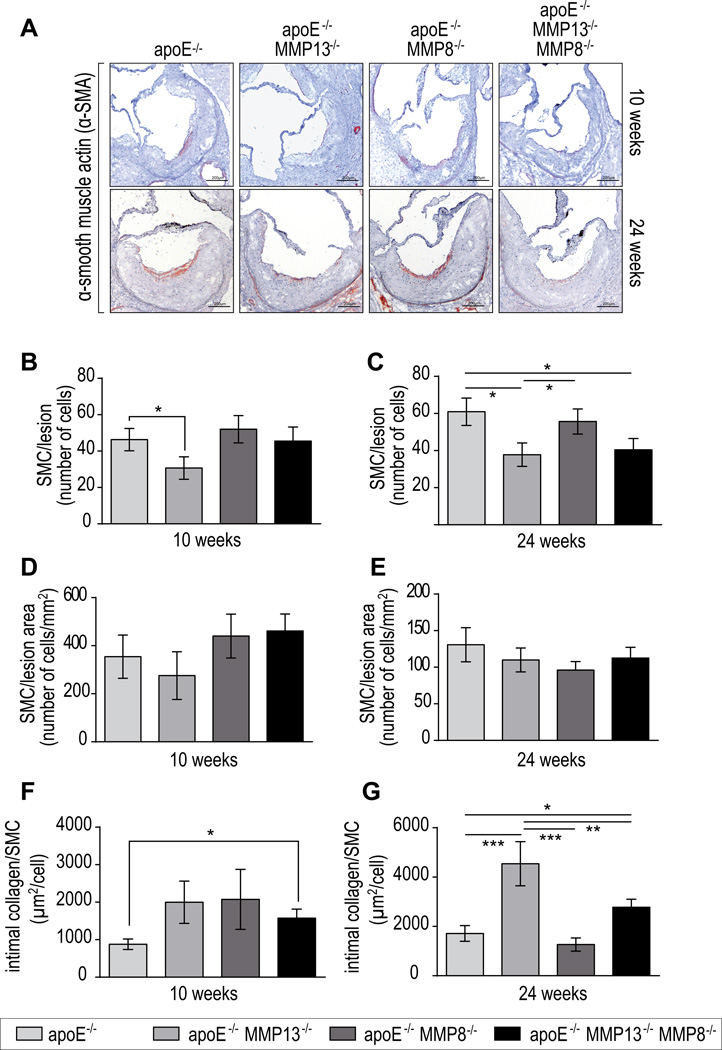
MMP-13 drives the reduction of SMC accumulation on atheromata
SMCs produce most of the interstitial collagen in atherosclerotic lesions. We assessed whether increased collagen content also could result in part from an increase in SMCs in atheromata in the absence of MMP collagenases. Histological analysis showed that SMC accumulation decreased in both MMP-13 apoE DKO and TKO mice, compared with MMP-8−/− apoE−/− animals, which did not show significant difference with the apoE−/− controls. There was a non-significant trend for the early lesions of TKO mice to have fewer SMC (Figure 4B), as previously reported by Fukumoto et al. in atheromata of mice with genetically induced collagenase resistance. In established lesions in mice with MMP-13 or with combined MMP-8/MMP-13 deficiencies SMC content declined significantly (Figure 4C).19 DKO and TKO mice had similar SMC content. The number of SMCs per mm2 of plaque did not vary significantly, but still indicated the trend of the decrease in SMC content in collagenase mutant mice (Figure 4D–E). The ratio between intimal collagen accumulation and SMC number was higher within the DKO mice (2.6 fold compared to apoE−/−) and TKO mice (1.6 fold compared to apoE−/−) in established atheromata (Figure 4F–G). Previous studies reported that MMP-13 collagenase does not impact the de novo collagen expression in lesions.6 Taken together, these results suggest that increased collagen content in MMP-deficient mice results from inhibition of collagen degradation, and not from increased production by intimal cells.
Figure 4.
Representative alpha-smooth mucle cell (SMC) actin staining of aortic root sections of KO mice after 10 and 24 weeks on atherogenic diet (A). Quantification of smooth muscle cells number per lesion and per lesion area after 10 weeks (B,D) and 24 weeks of atherogenic diet (C,E). Intimal collagen content normalized by the number of SMCs in early (F) and advanced lesions (G). Bars represent mean ± SEM. (n≥10 per group, *p<0.05, **p<0.01, ***p<0.001).
Neither MMP-8 nor MMP-13 influences macrophage accumulation
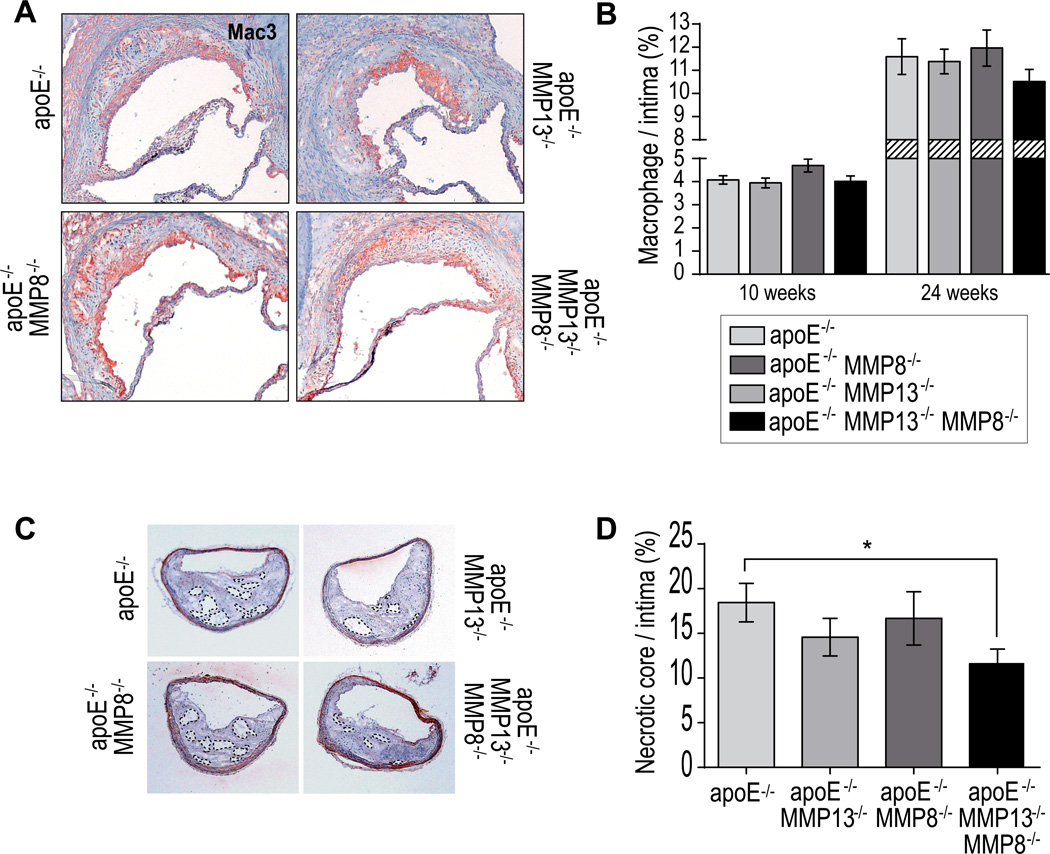
Because macrophages constitute a major source of matrix-degrading proteinases, particularly interstitial collagenases within human atheromata,5 we investigated their presence in atheromatous lesions lacking MMP-8 and/or MMP-13. Macrophage accumulation did not differ between the intima of aortic roots lacking MMP-13, MMP-8, or lacking both MMP-8 and MMP-13, after 10 and 24 weeks on an atherogenic diet (Figures 5A and 5B). These results indicate that MMP collagenases, while affecting SMC accumulation in advanced plaques, do not impede recruitment of inflammatory cells.
Figure 5.
Representative micrographs (A) and quantification of macrophage (Mac 3) in intima of developing and advanced lesions (B). Bars represent mean ± SEM. Representative sections of brachiocephalic arteries of mutant mice after 24 weeks of atherogenic diet (C). Quantification of necrotic core area (acellular areas within the lesions) (D). Bars represent mean ± SEM. (n≥10 per group, *p<0.05).
Lack of MMP-13 and MMP-8 reduces features associated with plaque rupture
We further assessed features associated with plaque disruption in histological sections of lesions in brachiocephalic arteries, a site of lesions considered prone to rupture in atherosclerotic mice.20 We measured the size of the necrotic core area in the plaques and showed that lack of both MMP-8 and MMP-13 associated with a significant decrease in necrotic core size (Figure 5C–D).
DISCUSSION
Degradation of the extracellular matrix in the plaque’s fibrous cap likely renders a lesion more prone to rupture in humans. Previous data from our group and others suggest that regulation of collagenolysis by MMP collagenases, in conjunction with reduced interstitial collagen synthesis by SMCs in inflamed atheromata generate so called "vulnerable" lesions.12, 21 These studies have not only provided novel mechanistic insight into pathogenesis, but have identified MMPs as an appealing target for clinical intervention to prevent the thrombotic complications of atherosclerosis. Three members of the MMP family, denoted interstitial collagenases (MMP-1, MMP-8, and MMP-13), can cleave triple-helical fibrillar collagen and associate with inflamed plaques in humans.4, 5 These enzymes catalyze the critical initial step in collagen catabolism, yielding fragments that can be degraded by other collagenases or MMPs.22 Initial attempts to inhibit MMPs in clinical trials have consistently failed, due to lack of selectivity of the agents used and associated unwanted actions. In atherosclerosis, therefore, identifying which MMP collagenase(s) plays a major role in this process, and assessing whether lack of function of one interstitial collagenase leads to compensatory changes in other family members has both mechanistic and therapeutic interest.
We previously demonstrated that MMP-13 regulates collagen accumulation in vivo in murine atheromata.6, 13 Mice express another interstitial collagenase, MMP-8, an enzyme also found in human plaques.4 In addition, high serum levels of MMP-8 associate with adverse cardiovascular outcomes.23 Laxton et al. reported that inactivating MMP-8 reduced the extent of atherosclerosis in apoE−/− mice, and increased collagen content in developing atheromata (12 weeks on atherogenic diet).14
In accord with our prior studies, the present results demonstrate that MMP-13 controls the collagen content of both early and established plaques in mice. This study addressed the crucial unresolved issue of the relative contribution of MMP-8 and -13 to these critical aspects of plaques. Concomitant lack of MMP-8 and MMP-13 did not augment collagen accumulation to a greater extent than MMP-13 deficiency alone.
This result indicates a lack of mechanisms that compensate deficiency of MMP-8 or MMP-13 in regard to collagen metabolism in plaques, in contrast to the situation we observed in mouse myocardium with impaired collagenolysis.24 Our in situ zymographic data furnish strong evidence that MMP-13 predominates over MMP-8 as an interstitial collagenase in mouse atheromata. Although MMP-8 shares collagenolytic activity with MMP-13, it does not add to the action of MMP-13 on plaque collagenolysis. This novel finding supports our previous studies, in which genetically- or pharmacologically-driven selective inhibition of MMP-13 did not affect the expression of other potentially collagenolytic enzymes, such as MMP-8, MMP-14, MMP-12, or cathepsin K, in lesions.6, 13 While we can't exclude that MMP-8 plays a role in plaque biology, as shown by Laxton et al14, the present study did not recapitulate the results obtained previously. The present study did not recapitulate the results obtained previously. This discrepancy could result from differences in time points studied or other experimental variations.
The present study also demonstrates a broader impact of MMP collagenases on atherosclerosis biology. Our results show that MMP-13, or MMP-13/MMP-8 deficiency associate with reduced lesional SMCs, even if it does not alter plaque size significantly. Increased intimal collagen content and lack of collagenolytic capacity could impede SMC migration within the plaque, as reflected by the reduced number of intimal SMCs observed in DKO and TKO mice. This situation resembles that we previously observed in collagenase-resistant mice that harbor a mutation in the substrate rather than the proteases.19
Beyond their roles in extracellular matrix remodeling, MMPs could cleave other substrates. The differences in the lipid profile and body weight in the TKO observed here indicate that such non-canonical substrates might include factors that regulate metabolism. This possibility provides a potentially fruitful field for further investigation, beyond the scope of the present study that focused on effects of interstitial collagenases on the extracellular matrix.
MMP-13 and MMP-8 can cleave various membrane-associated and extracellular proteins, in addition to collagen fibers, that could account for the reduced plaque progression in KO mice. MMP-8 plays a more important role in the cleavage of important molecular mediators, such as macrophage inflammatory protein-1 alpha (MIP-1α), CXCL-9, CXCL-10 or angiotensin I.25–29 Indeed, previously reported results showing decreased atherosclerosis in MMP-8−/− apoE−/− mice most likely relate to the modulation of angiotensin production and its impact on blood pressure and vascular inflammation.14 MMP-13 can also affect inflammatory processes by cleaving chemokines (MCP-1, MCP-2) and adhesion molecules (ICAM-1).27, 30 While the effect of MMP-13 and MMP-8 on plaque development reported in this study could result in part from regulation of various inflammatory mediators, the similar macrophage content in mice lacking MMP collagenases suggests that MMP-8 and MMP-13 activities do not alter the recruitment, proliferation, and/or apoptosis of inflammatory cells.
This work probed the mechanism of the regulation of collagen structure in atherosclerotic plaques, and did not aim to model the human disease. As mice do not express MMP-1, a collagenase expressed in human plaques, the results do not translate directly to human disease. Although MMP-1 colocalizes with MMP-13 in the shoulder regions of inflamed human atheromatous plaques and that specific MMP-1 haplotypes associate with plaque burden, its direct role in atherosclerosis remains unclear.7, 31–33 Macrophage-specific transgenic mice expressing human MMP-1 had less advanced atherosclerosis, but overexpression of human tissue inhibitor of metalloproteinase-1 (TIMP-1, an inhibitor of collagenases and other MMPs) showed a tendency to reduce atherosclerotic lesions in apoE−/− mice.34
Beyond the novel mechanistic insight into the redundancy and roles of collagenases in the context of atherosclerosis, these results have therapeutic implications. Broad spectrum MMP inhibition has proven intolerable in clinical studies. Our present findings indicate that selective inhibition of a single member of the MMP interstitial collagenase family can alter fundamental aspects of plaques limiting potential unwanted actions of indiscriminate MMP inhibition. The weight gain in the MMP-13/MMP-8 mice observed here provides an example of an unpredicted and unwanted consequence of broader interference with MMP function. Thus, this study provides new insights on the respective importance of MMP collagenases in murine atheromata. Identification of the predominant MMP collagenase in human lesions could therefore lead to the basis for modulating key aspects of plaque structure considered critical in clinical complications.
Supplementary Material
SIGNIFICANCE.
Atherosclerosis remains the major cause of death and premature disability worldwide. Rupture of plaques with a thin fibrous cap causes most fatal myocardial infarctions. Substantial evidence implicates interstitial collagenases of the MMP family in plaque rupture and fatal thrombosis by degrading the collagen that confers the tensile strength upon the fibrous cap. Our study shows in vivo that among MMP interstitial collagenases, MMP-13 predominates over MMP-8 in collagen degradation in murine atheromata. Because broad spectrum inhibition of MMPs is not translatable into the clinic, due to detrimental side effects, our findings provide the proof of concept that MMP collagenases have differential impact on collagenolysis in atherosclerotic plaques, and that selective targeting of single collagenase could modulate key aspects of plaque structure.
ACKNOWLEDGMENTS
None
Sources of funding
This work was in part supported by grants from the National Heart, Lung and Blood Institute (R01 HL080472 to Dr. Libby), and from the Donald W. Reynolds Foundation (to Dr. Libby).
ABBREVIATIONS
- MMP
Matrix and metalloproteinase
- TKO
Triple knock-out
- DKO
Double knock-out
- SMC
Smooth muscle cell
Footnotes
Disclosures
None
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nat med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 4.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: A novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 5.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and-3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi JO, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 7.Libby P. Collagenases and cracks in the plaque. J Clin Invest. 2013;123:3201–3203. doi: 10.1172/JCI67526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (midas) pilot trial. Arterioscler thromb vasc biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 9.Drummond AH, Beckett P, Brown PD, Bone EA, Davidson AH, Galloway WA, Gearing AJ, Huxley P, Laber D, McCourt M, Whittaker M, Wood LM, Wright A. Preclinical and clinical studies of mmp inhibitors in cancer. Annals of the New York Academy of Sciences. 1999;878:228–235. doi: 10.1111/j.1749-6632.1999.tb07688.x. [DOI] [PubMed] [Google Scholar]
- 10.Krzeski P, Buckland-Wright C, Balint G, Cline GA, Stoner K, Lyon R, Beary J, Aronstein WS, Spector TD. Development of musculoskeletal toxicity without clear benefit after administration of pg-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: A randomized, 12-month, double-blind, placebo-controlled study. Arthritis res ther. 2007;9:R109. doi: 10.1186/ar2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina JR, Reid JM, Erlichman C, Sloan JA, Furth A, Safgren SL, Lathia CD, Alberts SR. A phase i and pharmacokinetic study of the selective, non-peptidic inhibitor of matrix metalloproteinase bay 12-9566 in combination with etoposide and carboplatin. Anticancer drugs. 2005;16:997–1002. doi: 10.1097/01.cad.0000176504.86551.5c. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110:1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 13.Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, Sukhova GK, Aikawa E, Aikawa M, Libby P. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler thromb vasc biol. 2011;31:2464–2472. doi: 10.1161/ATVBAHA.111.231563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laxton RC, Hu Y, Duchene J, et al. A role of matrix metalloproteinase-8 in atherosclerosis. Circ res. 2009;105:921–929. doi: 10.1161/CIRCRESAHA.109.200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orbe J, Fernandez L, Rodriguez JA, Rabago G, Belzunce M, Monasterio A, Roncal C, Paramo JA. Different expression of mmps/timp-1 in human atherosclerotic lesions. Relation to plaque features and vascular bed. Atherosclerosis. 2003;170:269–276. doi: 10.1016/s0021-9150(03)00251-x. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: A potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- 17.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 18.Sweat F, Puchtler H, Rosenthal SI. Sirius red f3ba as a stain for connective tissue. Arch Pathol. 1964;78:69–72. [PubMed] [Google Scholar]
- 19.Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110:1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 20.Johnson J, Carson K, Williams H, Karanam S, Newby A, Angelini G, George S, Jackson C. Plaque rupture after short periods of fat feeding in the apolipoprotein e-knockout mouse: Model characterization and effects of pravastatin treatment. Circulation. 2005;111:1422–1430. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- 21.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J Intern Med. 2008;263:517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuomainen AM, Nyyssonen K, Laukkanen JA, Tervahartiala T, Tuomainen TP, Salonen JT, Sorsa T, Pussinen PJ. Serum matrix metalloproteinase-8 concentrations are associated with cardiovascular outcome in men. Arterioscler thromb vasc biol. 2007;27:2722–2728. doi: 10.1161/ATVBAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 24.Lindsey ML, Yoshioka J, MacGillivray C, Muangman S, Gannon J, Verghese A, Aikawa M, Libby P, Krane SM, Lee RT. Effect of a cleavage-resistant collagen mutation on left ventricular remodeling. Circ Res. 2003;93:238–245. doi: 10.1161/01.RES.0000085580.45279.60. [DOI] [PubMed] [Google Scholar]
- 25.Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice. J Immunol. 2010;184:1575–1588. doi: 10.4049/jimmunol.0900290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Lint P, Libert C. Matrix metalloproteinase-8: Cleavage can be decisive. Cytokine growth factor rev. 2006;17:217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 27.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates cc chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 28.Van den Steen PE, Husson SJ, Proost P, Van Damme J, Opdenakker G. Carboxyterminal cleavage of the chemokines mig and ip-10 by gelatinase b and neutrophil collagenase. Biochem Biophys Res Commun. 2003;310:889–896. doi: 10.1016/j.bbrc.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 29.Diekmann O, Tschesche H. Degradation of kinins, angiotensins and substance p by polymorphonuclear matrix metalloproteinases mmp 8 and mmp 9. Braz J Med Biol Res. 1994;27:1865–1876. [PubMed] [Google Scholar]
- 30.Tarin C, Gomez M, Calvo E, Lopez JA, Zaragoza C. Endothelial nitric oxide deficiency reduces mmp-13-mediated cleavage of icam-1 in vascular endothelium: A role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:27–32. doi: 10.1161/ATVBAHA.108.169623. [DOI] [PubMed] [Google Scholar]
- 31.Jguirim-Souissi I, Jelassi A, Slimani A, Addad F, Hassine M, Hamda KB, Najah M, Maatouk F, Rouis M, Slimane MN. Matrix metalloproteinase-1 and matrix metalloproteinase-12 gene polymorphisms and the outcome of coronary artery disease. Coron Artery Dis. 2011;22:388–393. doi: 10.1097/MCA.0b013e3283478d40. [DOI] [PubMed] [Google Scholar]
- 32.Lehrke M, Greif M, Broedl UC, Lebherz C, Laubender RP, Becker A, von Ziegler F, Tittus J, Reiser M, Becker C, Goke B, Steinbeck G, Leber AW, Parhofer KG. Mmp-1 serum levels predict coronary atherosclerosis in humans. Cardiovasc Diabetol. 2009;8:50. doi: 10.1186/1475-2840-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djuric T, Stojkovic L, Zivkovic M, Koncar I, Stankovic A, Djordjevic A, Alavantic D. Matrix metalloproteinase-1 promoter genotypes and haplotypes are associated with carotid plaque presence. Clin Biochem. 2012;45:1353–1356. doi: 10.1016/j.clinbiochem.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Lemaitre V, O'Byrne TK, Borczuk AC, Okada Y, Tall AR, D'Armiento J. Apoe knockout mice expressing human matrix metalloproteinase-1 in macrophages have less advanced atherosclerosis. J Clin Invest. 2001;107:1227–1234. doi: 10.1172/JCI9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.