Key Points
During ibrutinib therapy, 1.7% of blood and 2.7% of tissue CLL cells die per day which is 3 and 5 times higher than without treatment.
The fraction of CLL cells that redistribute into the blood during ibrutinib treatment represents 23.3% ± 17% of the tissue disease burden.
Abstract
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib has excellent clinical activity in patients with chronic lymphocytic leukemia (CLL). Characteristically, ibrutinib causes CLL cell redistribution from tissue sites into the peripheral blood during the initial weeks of therapy. To better characterize the dynamics of this redistribution phenomenon, we correlated serial lymphocyte counts with volumetric changes in lymph node and spleen sizes during ibrutinib therapy. Kinetic parameters were estimated by applying a mathematical model to the data. We found that during ibrutinib therapy, 1.7% ± 1.1% of blood CLL cells and 2.7% ± 0.99% of tissue CLL cells die per day. The fraction of the tissue CLL cells that was redistributed into the blood during therapy was estimated to be 23.3% ± 17% of the total tissue disease burden. These data indicate that the reduction of tissue disease burden by ibrutinib is due more to CLL cell death and less to egress from nodal compartments.
Introduction
Bruton tyrosine kinase (BTK) is part of the B-cell receptor (BCR) signaling cascade, which plays a central pathogenic role in chronic lymphocytic leukemia (CLL).1 Ibrutinib is a potent (50% inhibitory concentration, 0.5nM) BTK inhibitor which inactivates BTK through irreversible covalent bonding to Cys-481 in the adenosine triphosphate binding domain of BTK.2 Early-stage clinical trials found ibrutinib to be particularly active in patients with CLL3,4 and mantle cell lymphoma (MCL),5 and the drug recently has been US Food and Drug Administration (FDA)–approved for patients with relapsed CLL and MCL. In CLL, ibrutinib characteristically causes an early redistribution of tissue-resident CLL cells into the peripheral blood, with rapid resolution of enlarged lymph nodes, along with a surge in lymphocytosis. After weeks to months of continuous ibrutinib therapy, normalization of lymphocyte counts and remission is observed in the majority of patients.3,4,6 Although well-documented, a quantitative understanding of the redistribution phenomenon is still lacking, and it is debated whether the degree of tissue shrinkage accounts for the magnitude of the lymphocytosis, or whether tissue cell death plays a significant role.
Study design
Data from 10 previously treated CLL patients who received single-agent ibrutinib at a dose of 420 mg continuously daily on a phase 1/2 clinical trial (PCYC-1102-CA) at MD Anderson Cancer Center between 2010 and 2012 were analyzed (after approval and by the rules of the institutional review board and in accordance with the Declaration of Helsinki). The clinical details of these patients are summarized in Table 1. Ten patients were selected for this analysis in which serial computed tomography (CT) scans were available to quantify changes in volumes of lymph nodes and spleen prior to therapy and at 2 time points during treatment. These volume changes were translated into numbers of affected tissue CLL cells per patients and set into relation with changes in serial blood lymphocyte counts, using average CLL cell volumes and individual blood volumes (supplemental Materials 1-2, available on the Blood Web site).
Table 1.
Patient characteristics and parameter estimates from the model fit
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Average | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | ||||||||||||
| Age, y | 68 | 70 | 81 | 57 | 51 | 75 | 77 | 55 | 67 | 57 | ||
| Sex* | F | M | M | M | M | M | M | M | M | M | ||
| IgVH mutation status† | M | M | M | U | U | U | U | U | M | U | ||
| FISH cytogenetics | 17p− | neg | 11q− | 11q− | 11q− | 11q− | 11q− | 17p− | 11q− | 17p− | ||
| Karyotype (abnormal) | Yes | Yes | No | Yes | Yes | na | Yes | Yes | Yes | Yes | ||
| ZAP70‡ | neg | pos | na | neg | neg | pos | pos | neg | na | pos | ||
| CD38, >30% | No | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | ||
| ALC, K/µL | 39.8 | 10.6 | 138.1 | 19.4 | 26.8 | 21.2 | 0.92 | 29.4 | 52.5 | 7.8 | ||
| Hb, g/dL | 10.8 | 15.2 | 9.6 | 11.2 | 13.7 | 13.5 | 13.8 | 13.2 | 12.2 | 14.8 | ||
| PLT, K/µL | 57 | 141 | 72 | 138 | 115 | 68 | 136 | 79 | 104 | 100 | ||
| Parameter estimates | ||||||||||||
| Blood, mL | 3484 | 5363 | 5088 | 6466 | 5010 | 4714 | 4793 | 6272 | 6350 | 5692 | ||
| d2, d−1§ | 0.002 | 0.022 | 0.014 | 0.016 | 0.018 | 0.014 | 0.010 | 0.011 | 0.047 | 0.018 | 0.017 | 0.011 |
| d1, d−1§ | 0.027 | 0.015 | 0.012 | 0.047 | 0.022 | 0.027 | 0.022 | 0.032 | 0.033 | 0.035 | 0.027 | 0.010 |
| m, d−1§ | 0.0096 | 0.0177 | 0.0146 | 0.0009 | 0.0095 | 0.0061 | 0.0056 | 0.0023 | 0.0088 | 0.0034 | 0.008 | 0.005 |
| α, d−1§ | 0.037 | 0.033 | 0.026 | 0.047 | 0.032 | 0.033 | 0.028 | 0.034 | 0.042 | 0.039 | 0.035 | 0.006 |
| x0, ×109 | 3034 | 3064 | 7044 | 30 209 | 2143 | 4083 | 1294 | 15 452 | 6156 | 7711 | 8019 | 8799 |
| y0, ×109 | 153 | 58 | 674 | 120 | 217 | 73 | 3 | 521 | 358 | 38 | 221 | 226 |
| c, ×109 | 0.4 | 47.5 | 94.7 | 392.1 | 86.3 | 35.7 | 1.0 | 376.4 | 507.8 | 155.9 | 169.8 | 185.4 |
| %|| | 25.9 | 50 | 52.6 | 1.9 | 29.4 | 18.2 | 19.6 | 6.9 | 19.3 | 8.8 | 23.3 | 17.0 |
All values were at baseline values, prior to start of ibrutinib.
ALC, absolute lymphocyte count; FISH, fluorescence in situ hybridization; IgVH, immunoglobulin heavy variable chain; Hb, hemoglobin; na, not available; neg, negative; pos, positive; PLT, platelet; SD, standard deviation; ZAP70, ζ-chain-associated protein kinase 70.
F, female; M, male.
M, mutated; U, unmutated.
Zap-70 by immunohistochemistry.
d−1 stands for “per day.”
Percent (%) indicates percent of pretreatment tissue tumor burden that is redistributed into the blood during treatment.
To characterize the kinetics of the lymphocytosis, a 2-compartment mathematical model, based on work by Messmer and colleagues,7 was fit simultaneously to the data in blood and tissue (supplemental Materials 1-2). Denoting the number of CLL lymphocytes in the tissues and blood by x and y, respectively, the model is given by the following ordinary differential equations, which describe the time evolution of these populations during treatment: dx/dt = −mx − d1(x − c) and dy/dt = mx − d2y.
In the tissue compartment, cells can die with a rate d1, and redistribute into the blood with a rate m. In the blood, CLL cells die with a rate d2. Lymphocyte homing to tissues and cell proliferation can be ignored because these processes are effectively inhibited by ibrutinib. This is evidenced by preclinical data demonstrating that ibrutinib inhibits thymidine incorporation, CLL cell proliferation, and CLL cell migration and homing.8-10 The parameter c is included to phenomenologically account for the observation that the majority of ibrutinib-treated patients do not achieve complete remissions4 (supplemental Materials 2).
Results and discussion
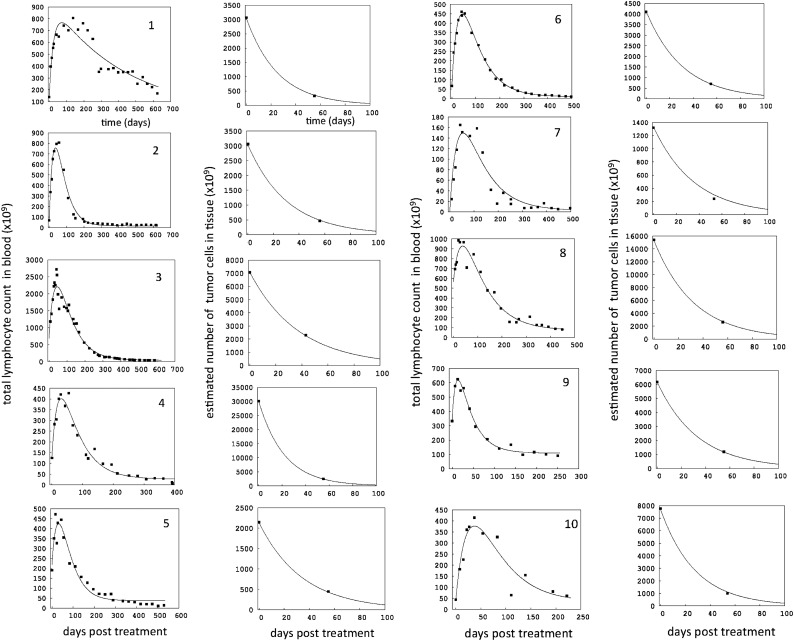
The treatment responses in blood and tissue were consistent with previous patterns3,4 and are shown in Figure 1, demonstrating a good fit of the model to the data. Volumetric tissue changes in a representative patient are displayed in supplemental Figure 1.
Figure 1.
Dynamics of cell populations over time for each of the 10 patients, numbered consecutively. Dots are clinical data, and lines represent the best model fit (see Table 1 for parameters). For each patient, 2 graphs are presented. The left graph depicts the total number of blood lymphocytes over time. Note that these numbers do not represent the standard absolute lymphocyte counts, which are typically presented as the number of cells per microliter of blood. Instead, the number of cells per microliter of blood was multiplied by the blood volume of each patient (supplemental Material 1), to provide numbers that are commensurate with the total number of cells in tissue, which are shown in the right graph for each patient. Only 2 of the 3 tissue volumes were large enough to calculate the number of tissue CLL cells (supplemental Material 1): the measurement before treatment and the first measurement during treatment. Note different scales on the y-axes. Note that the measured initial number of cells in blood and the initial number predicted by the fitted model can differ, which is explained further in supplemental Materials 2.
During ibrutinib therapy, blood CLL cells are estimated to die on average with a rate d2 = 0.017 ± 0.012 days−1 (average ± standard deviation per day). Expressed differently, 1.7% ± 1.1% of the cells die per day, and their average lifespan is about 58 days (supplemental Materials 2). In the tissues, the average death rate of CLL cells was found to be d1= 0.027 ± 0.01 days−1, or 2.7% ± 0.99% of the cells die per day. Thus, they have a shorter lifespan of about 37 days. The average rate at which tissue CLL cells redistribute into the blood is calculated to be m = 0.008 ± 0.005 days−1, and is significantly smaller than the cell death rate in tissue (P = .0002). This translates into 0.8% ± 0.5% of cells redistributing per day. The overall rate of nodal decline (caused by death + redistribution) is thus given by α = d1 + m = 0.035 ± 0.006 (days−1), that is, 3.5% ± 0.6% of cells are lost from tissue per day. This means that the average time until the number of tissue CLL cells has been halved during ibrutinib therapy is 20.3 ± 3.6 days. The parameter estimates for the patients are given in Table 1. This is in line with clinical observation of rapid reduction in lymph node sizes during the first weeks of ibrutinib therapy, and CT assessment of nodal sites at later time points (Figure 1, and Figure 1 from Byrd et al4).
A previous study estimated that in the absence of treatment, ∼0.5% of cells died per day,7 although a single combined rather than a compartment-specific death rate was provided. Hence, ibrutinib therapy increases the tissue and blood cell death rate approximately fivefold and threefold, respectively. We note that although the methodologies used to estimate parameters previously7 and here are different, both are valid techniques and the comparison is instructive. These death rates during ibrutinib therapy may seem high, considering that ibrutinib induced only modest levels of apoptosis in vitro when compared with, for example, cytotoxic agents. Nonetheless, in stromal cell cocultures and suspension cultures, ibrutinib consistently caused apoptosis of ∼10% to 20% of CLL cells over 48 hours,8,9 which is compatible with our patient-based model. We cannot, however, determine whether the different death rates in the compartments are caused by ibrutinib, or whether this is a treatment-independent observation.
Having obtained these parameters (Table 1), we can calculate the percentage of the total tissue CLL cells before treatment that have redistributed into the blood during the lymphocytosis phase of ibrutinib therapy. Significant redistribution originating from the pretherapy tumor load occurs not only during the initial rise of the lymphocyte levels, but also during the subsequent decline until the dynamics start to stabilize. This phase can be defined mathematically, given by the characteristic time: Tc = 1/d2 + 1/(d1 + m) (supplemental Material 2). On average, the percentage of the tissue CLL cell population that was redistributed into the blood (Table 1) was thus found to be 23.3% ± 17%. Note that there is a fair amount of variation in this percentage among patients, with numbers ranging from 1.9% to 52.6%. These numbers suggest that reduction of tissue disease burden is largely caused by CLL cell death. This is also supported by supplemental Figure 2, showing a significant positive correlation between the rate of nodal decline and the death rate of tissue cells, although no correlation was found between the rate of nodal decline and the rate of CLL cell redistribution.
CLL cell redistribution appears to be a class effect of kinase inhibitors interfering with the BCR and chemokine receptor signaling pathways.11 Similar clinical effects have been reported for the spleen tyrosine kinase inhibitor fostamatinib (R406/R788)12 and the phosphatidylinositol 3-kinase δ inhibitor idelalisib (GS-1101).13,14 CLL cell redistribution was even observed in the early treatment approaches using glucocorticoids, and it was thought that the amount of tissue shrinkage was not reflected in the degree of lymphocytosis, pointing to a role for lympholysis.15 Our calculations suggest that ibrutinib causes a significant amount of cell death in tissue (a larger death rate than in blood), and that a relatively small fraction of the tissue cell burden redistributes to the blood. We likely overestimated the fraction of total tissue cells that redistributed. Our volumetric analysis did not include the bone marrow, which would increase tissue cell burden and lower the estimated fraction. Our study provides a framework to quantitatively examine treatment efficacy of other tyrosine kinase inhibitors or ibrutinib combination therapy in CLL in different disease compartments.
Acknowledgments
The work was supported by a Cancer Prevention and Research Institute of Texas (CPRIT) grant (J.A.B.), a Leukemia & Lymphoma Society Scholar Award in Clinical Research (J.A.B.), and MD Anderson’s Moon Shot Program in CLL.
Appendix: abbreviations and parameter explanations
α = overall nodal decline rate, that is, the rate at which cells disappear from the tissue due to redistribution + death, that is, α = m + d1
c = parameter that determines the (relatively low) level to which the number of CLL cells converges in the long-term during treatment. It is not important for the analysis, but makes for a better fit once cell numbers stabilize
d1 = death rate of CLL cells in tissue
d2 = death rate of CLL cells in blood
m = rate of redistribution of tissue cells to blood
Percent = % of pretreatment tissue tumor burden that is redistributed into the blood during treatment
x0 = total body number of CLL cells in tissue
y0 = total body number of CLL cells in blood
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.W., N.G., N.L.K., O.B., and D.J. devised and performed the experiments, analyzed the data, and designed the figures; M.J.K., W.G.W., H.K., and S.O. provided patient samples and reviewed the manuscript; and J.A.B. designed the research, supervised the study, analyzed the data, and wrote the paper with D.W.
Conflict-of-interest diclosure: D.J. is an employee of Pharmacyclics. J.A.B. and S.O. have received research funding from Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Jan A. Burger, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.
References
- 1.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34(12):592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma. 2013;54(11):2385–2391. doi: 10.3109/10428194.2013.777837. [DOI] [PubMed] [Google Scholar]
- 3.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messmer BT, Messmer D, Allen SL, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115(3):755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 11.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121(9):1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw RK, Boggs DR, Silberman HR, Frei E. A study of prednisone therapy in chronic lymphocytic leukemia. Blood. 1961;17(2):182–195. [Google Scholar]