Abstract
Study Design
A new recombinant adenoviral vector expressing Sox9, a chondrocyte-specific transcription factor, was tested in a chondroblastic cell line and primary human intervertebral disc cells in vitro. Direct infection of intervertebral disc cells then was assessed in a rabbit model.
Objectives
To deliver a potentially therapeutic viral vector expressing Sox9 to degenerative human and rabbit intervertebral discs cells, and to assess the effect of Sox9 expression on Type 2 collagen production.
Summary of the Background Data
The concentration of competent Type 2 collagen, an essential constituent of the healthy nucleus pulposus, declines with intervertebral disc degeneration. Recent studies suggest that Sox9 upregulates Type 2 collagen production. Interventions that augment Type 2 collagen production by intervertebral disc cells may represent a novel therapeutic method for patients with degenerative disc disease.
Methods
Adenoviral delivery vectors expressing Sox9 and green fluorescent protein were constructed using the AdEasy system. The chondroblastic cell line, HTB-94, and cultured human degenerated intervertebral disc cells were infected with the vectors. Reverse transcriptase-polymerase chain reaction and immunohistochemical analyses were performed to document increased Type 2 collagen expression. The AdSox9 virus then was injected directly into the intervertebral discs of three rabbits. After 5 weeks, the injected discs were evaluated histologically.
Results
The AdSox9 virus efficiently transduced HTB-94 cells and degenerated human disc cells. Western blot analysis confirmed increased Sox9 production. Increased Type 2 collagen production was demonstrated in infected HTB-94 and human disc cells using both reverse transcriptase-polymerase chain reaction and immunohistochemical staining. In the rabbit model, cells infected with AdSox9 maintained a chondrocytic phenotype, and the architecture of the nucleus pulposus was preserved over a 5-week study period compared to control discs.
Conclusions
A novel adenoviral vector efficiently increased Sox9 and Type 2 collagen synthesis in cultured chondroblastic cells and human degenerated disc cells. In a rabbit model, sustained Sox9 production preserved the histologic appearance of the nucleus pulposus cells in vivo. These findings suggest a potential role for Sox9 gene therapy in the treatment of human degenerative disc disease.
Keywords: degenerative disc disease, gene therapy, nucleus pulposus, Sox9, type 2 collagen
Human intervertebral disc degeneration is a formidable clinical problem and a leading cause of pain and disability, resulting in significant health care–related costs.1–3 The degenerative process in intervertebral discs is associated with a series of biochemical and morphologic changes that combine to alter the biomechanical properties of the motion segment.4–11 Disc degeneration itself can cause low back pain,12–14 but also may be an early event in the spinal degenerative cascade, ultimately leading to a variety of degenerative pathologies of the spine. Intervertebral discs have limited intrinsic capacity for repair,15,16 so treatments of disc degeneration so far have revolved largely around pain-control measures or spinal surgeries with variable outcomes. An alternative approach might involve biologic therapies, such as genetic modification, that could slow or reverse the disc degenerative process.
The pathogenesis of intervertebral disc degeneration continues to be area of investigation. However, many of the changes that occur in the composition of the extracellular matrix and the phenotypic profile of the disc cells themselves remain unclear. The nucleus pulposus is essential for normal disc biomechanical functioning, and some evidence indicates that disc degeneration may begin in the nucleus pulposus.17–19 As disc degeneration progresses, the distinguishing features of the nucleus pulposus are lost. In the young adult, the nucleus pulposus contains a high percentage of Type 2 collagen synthesized by the disc cells. With aging, synthesis of Type 2 collagen declines, whereas degradation of existing Type 2 collagen gradually increases. Furthermore, Type 1 collagen production within the disc increases, leading to a higher proportion of less compliant Type 1 collagen.4 In a parallel development, the degenerating intervertebral disc cells begin to assume more of a spindle shape, as opposed to chondrocytic morphology, reflecting an underlying change in the functional demands for the cells and the surrounding extracellular matrix. These histologic and biochemical changes also have been documented in several animal models of disc degeneration. 8,21–23 Ultimately, with advanced degeneration, a disorganized fibrous matrix replaces the previously well-organized disc structure.
The decline in Type 2 collagen within the nucleus pulposus features prominently in the pathogenesis of disc degeneration, and reversing this trend may afford an opportunity for modifying early disc degeneration. The regulation of Type 2 collagen synthesis undoubtedly is a complex interplay of local and systemic factors. Recent studies have demonstrated Sox9 to be an essential transcription factor for Type 2 collagen synthesis and also for chondrogenesis.24–28 Therefore, Sox9 has been termed a “master regulator” of the chondrocyte phenotype.29–32 As a member of the Sry-type HMG-box family of genes, Sox9 plays an important role in early embryogenesis, appearing as early as 10 days past coitus (dpc) in the mouse.28 It is expressed in mesenchymal condensations before and during chondrogenesis, and is critical to normal skeletal development. In mouse embryo chimeras, Sox9−/− cells were unable to form cartilage from undifferentiated mesenchymal tissue.33 Mice with heterozygous Sox9+/− died perinatally, and were notable for cartilage-related defects such as cleft palate and extremity deformities.34 In a parallel development, mutations of the human Sox9 gene result in the skeletal dysmorphology syndrome, campomelic dysplasia.35 According to Lefebvre et al,26 Sox9 binds to a potent chondrocyte-specific enhancer region in intron 1 for the Proaα1(II) gene. This interaction upregulates Proα1(II) gene expression, and thereby, Type 2 collagen synthesis. The enhancing capacity of Sox9 on the Proα1(II) gene can be augmented further by the addition of L-Sox5 and Sox6.36
In this study, it was hypothesized that chondroblastic and primary human degenerated intervertebral disc cells can be infected effectively with an adenoviral vector expressing Sox9. These infected cells should demonstrate increased Type 2 collagen synthesis in vitro, as compared with control cells. Moreover, injections of the Sox9-expressing adenoviral vector directly into the nucleus pulposus in a rabbit model of disc degeneration should delay degenerative changes in the intervertebral disc consistent with increased Type 2 collagen synthesis in vivo.
Materials and Methods
Cell Culture and Chemicals
Human embryonic kidney line HEK 293, human colon cancer line HCT116, and human chondrosarcoma line HTB-94 were purchased from the American Type Culture Collection (Manassas, VA). The HEK 293 and HTB-94 cells were maintained in Dulbecco’s modified Eagle medium (DMEM, Mediatech, Herndon, VA) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FBS, Mediatech) and streptomycin (100 µg/mL final concentration)/ penicillin (100 IU/mL final concentration) (Mediatech) at 37° C in an atmosphere of 5% CO2. The HCT116 cells were maintained in McCoys 5A (Mediatech) supplemented with 10% (v/v) heat-inactivated fetal calf serum and streptomycin (100 µg/mL final concentration)/penicillin (100 IU/mL final concentration) (Mediatech) at 37°C in an atmosphere of 5% CO2. Once the cells had formed a confluent monolayer, they were trypsinized and transferred to a T-25 flask and allowed to expand exponentially until they were approximately 70% to 80% confluent. Unless otherwise indicated, all the chemicals were purchased from Sigma (St. Louis, MO).
Construction and Generation of Recombinant Adenoviral Vector AdSox9
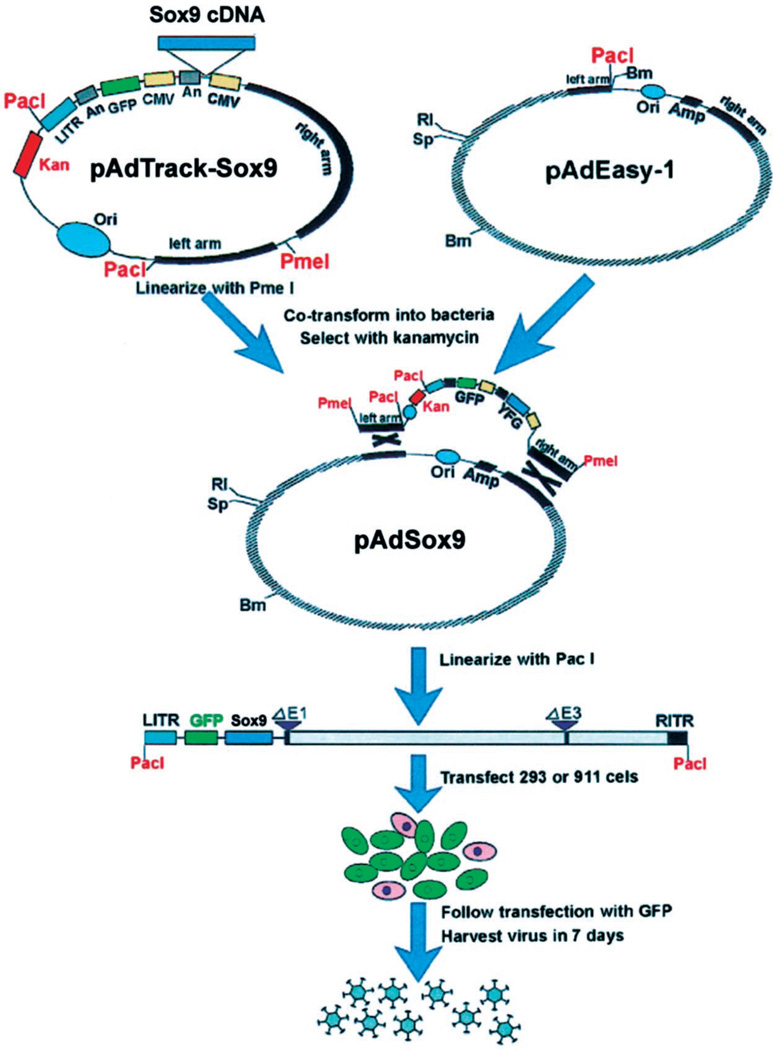
An HA-tagged cDNA coding sequence of human Sox9, pBS-HA-Sox9 vector, was generously provided by Drs. Vincent R. Harley and Sharon McDowall of the University of Melbourne, Australia. The HA-tagged Sox9 cDNA was subcloned into a shuttle vector, pAdTracK-CMV. The resultant pAdTrack-Sox9 was used next to generate adenoviral recombinants through homologous recombination with the adenoviral backbone vector, pAdEasy-1, in BJ5183 bacterial cells (Figure 1). After they had been linearized with Pac I, the adenoviral recombinants were used to produce adenoviruses in HEK 293 packaging cells, resulting in an AdSox9 adenoviral vector that contained a built-in GFP expression cassette. A recombinant adenoviral vector expressing GFP alone also was constructed as a control vector (AdGFP).
Figure 1.
Schematic representation of adenoviral vector AdSox9 construction. An HA-tagged cDNA coding sequence of human Sox9 was subcloned into a shuttle vector, pAdTracK-CMV. Resultant pAdTrack-Sox9 was next used to generate adenoviral recombinants through homologous recombination with the ad-enoviral backbone vector, pAdEasy-1, in BJ5183 bacterial cells. After being linearized with Pac I, the adenoviral recombi-nants were used to produce ad-enoviruses in HEK 293 packaging cells, resulting in an AdSox9 ad-enoviral vector that contained a built-in GFP expression cassette. A recombinant adenoviral vector expressing GFP alone also was constructed as a control vector (AdGFP). For detailed information about AdEasy System, please refer to He et al., Proc Natl Acad Sci (AM) 1998;95:2509, and www.coloncancer.org/adeasy.htm
Isolation and Culture of Human Intervertebral Disc Cells
The use of human disc tissue was approved by the Institutional Review Board of the University of Chicago. After appropriate consent had been obtained, human disc tissue was recovered after discectomy performed in the treatment of patients with degenerative disc pathologies. The specimen (combined nucleus pulposus and anulus fibrosus tissue) was rinsed twice with PBS buffer to remove any residual blood or extraneous material. Remnants of the outer anulus then were identified and removed, leaving the disc tissue. Using a sterile surgical scalpel, the disc tissue was minced carefully and placed in a solution of Type 1 collagenase (0.5 mg/mL in PBS). The sample then was incubated at 37° C for 45 minutes in a shaker. Thereafter, centrifugation was used to separate the collagenase solution from the disassociated cells. After the removal of supernatant, the remaining cellular component was resuspended in Eagle minimal essential medium (EMEM, Mediatech) supplemented with 10% (v/v) heat-inactivated FBS, 1% (v/v) nonessential amino acids (Mediatech), streptomycin (100 µg/mL final concentration)/penicillin (100 IU/mL final concentration) (Mediatech), and 1% (v/v) sodium pyruvate (Mediatech). The resuspended material then was placed in a six-well plate and allowed to incubate overnight at 37° C in an atmosphere of 5% CO2. On the following day, the media was exchanged to remove all components that were not adherent to the cell culture plate. The cells were allowed to grow under the same conditions until they approached confluency. The media was exchanged approximately every 3 to 4 days. Once the cells approached 80% to 90% confluency, they were trypsinized and transferred to either a 25-cm2 cell culture flask in preparation for mRNA isolation or a 48-well plate for immunohistochemistry.
Western Blotting Analysis
Subconfluent HCT116 cells were infected with AdSox9 or AdGFP. Total cell lysate was collected 24 hours after infection and subjected to 4% to 20% SDS–SPAGE (approximately 10 µg total proteins per lane). After being resolved by electrophoresis, the proteins were transferred to an Immobilon-P membrane (Millipore, Bedford, MA) via electroblotting. The membrane was blocked with 5% nonfat milk in TBST (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.05% Tween-20) at room temperature for 1 hour and probed with a mouse monoclonal anti-HA antibody (12CA5; Roche Molecular Biochemicals, Laval, Canada) for 60 minutes, followed by a 30-minute incubation with an anti-mouse IgG conjugated with horseradish peroxidase (PIERCE, Rockford, IL). The presence of HA-tagged Sox9 protein was visualized using the SuperSignal West Pico chemoluminescent substrate kit (PIERCE).
Total RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction Analysis
Exponentially growing HTB-94 and primary disc cells were infected with AdSox9 and AdGFP. At 48 hours after infection, total RNA was isolated using the RNAgent total RNA Isolation Kit (Promega, Madison, WI). Purified total RNA was used to generate cDNA templates for reverse transcriptase-polymerase chain reaction (RT-PCR). Expression of Type 2 collagen was detected by RT-PCR analysis using the following pair of Type 2 collagen-specific primers: 5'-GCTCGCACCTGCAGAGACCTG-3' and 5'-GTCCACACCGAATT CCTGCTCG-3'.The expected PCR product was approximately 560 bp. The PCR fragments were resolved on a 1% agarose gel. Ethidium bromide staining was performed for visualization of PCR products under ultraviolet light.
Transfection and Luciferase Activity Assay
Subconfluent HTB-94 cells were transfected using LipofectAMINE (Life Technologies, Rockville, MD) with a reporter construct, pGL2–3.774kb, that contained a luciferase reporter gene driven by human Type 2 collagen promoter. This vector was generously provided by Dr. Philippe Galera of the University of Caen, France. At 20 hours after transfection, cells were replated and infected with AdSox9, AdGFP, or no infection (mock). At 36 hours after infection, the cells were lysed for luciferase activity assays by using the Luciferase Assay Kit (Promega). Each assay condition was performed in triplicate.
Immunohistochemical Staining for Type 2 Collagen Production
Subconfluent HTB-94 and primary disc cells were infected with AdSox9 or AdGFP. At 48 hours after infection, cells were fixed and immunostained with a rat anti-Type 2 collagen antibody (provided by Dr. Michael Cremer, University of Tennessee, Memphis, TN). A 1:250 dilution of the anti-Type 2 collagen antibody was used. The control condition consisted of infected cells that were not exposed to the primary antibody, as well as uninfected cells. The cells then were exposed to a 1:1000 dilution of the secondary antibody (antirat antibody conjugated with horseradish peroxidase). Finally, DAB staining was performed using appropriately diluted DAB/ metal concentrate (PIERCE).
Injection of AdSox9 and AdGFP Adenoviral Vectors Into Rabbit Intervertebral Disc Tissues
Appropriate permission for this study was obtained prospectively from the University of Chicago Animal Care and Use Committee. Four skeletally mature New Zealand white rabbits (weight, 2.5 kg) were used. After anesthesia was administered, a transabdominal approach was used to access the anterior aspect of the lumbar intervertebral discs. Three test conditions were studied: 1) control (stab incision without injection), 2) AdGFP (stab incision with injection of mock adenovirus, AdGFP), and 3) AdSox9 (stab incision with injection of AdSox9 virus).
In each animal, six disc levels could be exposed, permitting two discs per condition per animal. At the control disc, a 27-gauge needle puncture through the anterior anulus into the nucleus pulposus was performed. Nuclear material was seen to extrude through the needle track and out of the disc. The authors have found that this method of annulotomy reliably leads to both gross and histologic features of disc degeneration within 6 weeks (Figure 2). In the AdGFP discs, approximately 1 × 109 pfu of AdGFP virus suspended in 10 µL of PBS was injected using a 27-gauge needle placed through the anterior anulus into the nucleus pulposus. In the AdSox9 discs, approximately 1 × 109 pfu of AdSox9 virus was injected using the same technique. The abdominal wound was closed in standard fashion, and no postoperative activity or diet restrictions were imposed on the rabbit.
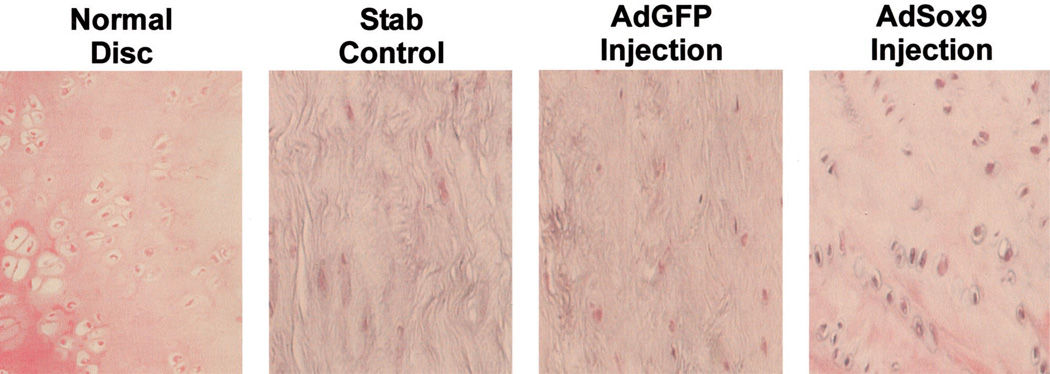
Figure 2.
Intervertebral disc degeneration induced by needle anulotomy in a rabbit model. A 27-gauge needle puncture through the anterior anulus into the nucleus pulposus was performed. Certain discs then were injected with 10 µL of saline. At 6 weeks after injection, the animals were killed and the lumbar segments retrieved. Macrographic images were prepared from the intact and hemisected specimens. At anulotomy and anulotomy followed by saline-injection of discs, disc space narrowing and osteophyte formation is evident at 6 weeks. Effect of Sox9 on intervertebral disc tissue in rabbits. Recombinant adenoviruses (1 × 109 pfu) were injected into injured rabbit lumbar intervertebral disc. Three test conditions were studied: 1) control (stab incision without injection), 2) AdGFP (stab incision with injection of mock adenovirus, AdGFP), and 3) AdSox9 (stab incision with injection of AdSox9 virus). At 5 weeks after injection, the animals were killed. The lumbar disc tissues were recovered and stained with hematoxylin–eosin. A normal, uninjured disc was included for comparison. The disc that underwent annulotomy without injection of adenovirus as well as the disc that underwent annulotomy with injection of the mock virus AdGFP, demonstrated replacement of the disc with fibrocytic cells and dense extracellular matrix. The disc that received the AdSox9 virus retained the histologic appearance of cartilage.
At 5 weeks after injection, each rabbit was killed, and the lumbar disc tissues were recovered. One half of each disc was embedded in paraffin and used to generate 4-µm-thick sections for hematoxylin & eosin staining. The remaining half was frozen immediately in liquid nitrogen, from which 4-µm sections were made. Each section was fixed and immunohistochemically stained with the same rat anti-Type 2 collagen antibody used for the in vitro study described earlier, at a 1:250 dilution. The negative control condition consisted of sections that were not exposed to the primary antibody. The cells then were exposed to a 1:1000 dilution of the secondary antibody (antirat antibody conjugated with horseradish peroxidase). Finally, DAB staining was performed using appropriately diluted DAB/ metal concentrate (PIERCE).
Results
Generation of a Recombinant Adenovirus AdSox9 That Expresses Sox9 Exogenously
Recombinant adenoviral vectors currently represent one of the most efficient and widely used gene delivery approaches. The authors chose to construct an adenoviral vector that efficiently expressed Sox9. Specifically, the AdSox9 virus was constructed according to the authors’ previously established AdEasy technology (Figure 1). A unique feature of the AdSox9 adenoviral vector was that it also contained a built-in green fluorescent protein (GFP) expression cassette that allows for efficient tracking of gene expression. For a control condition, the authors also constructed a recombinant adenoviral vector that only expressed GFP.
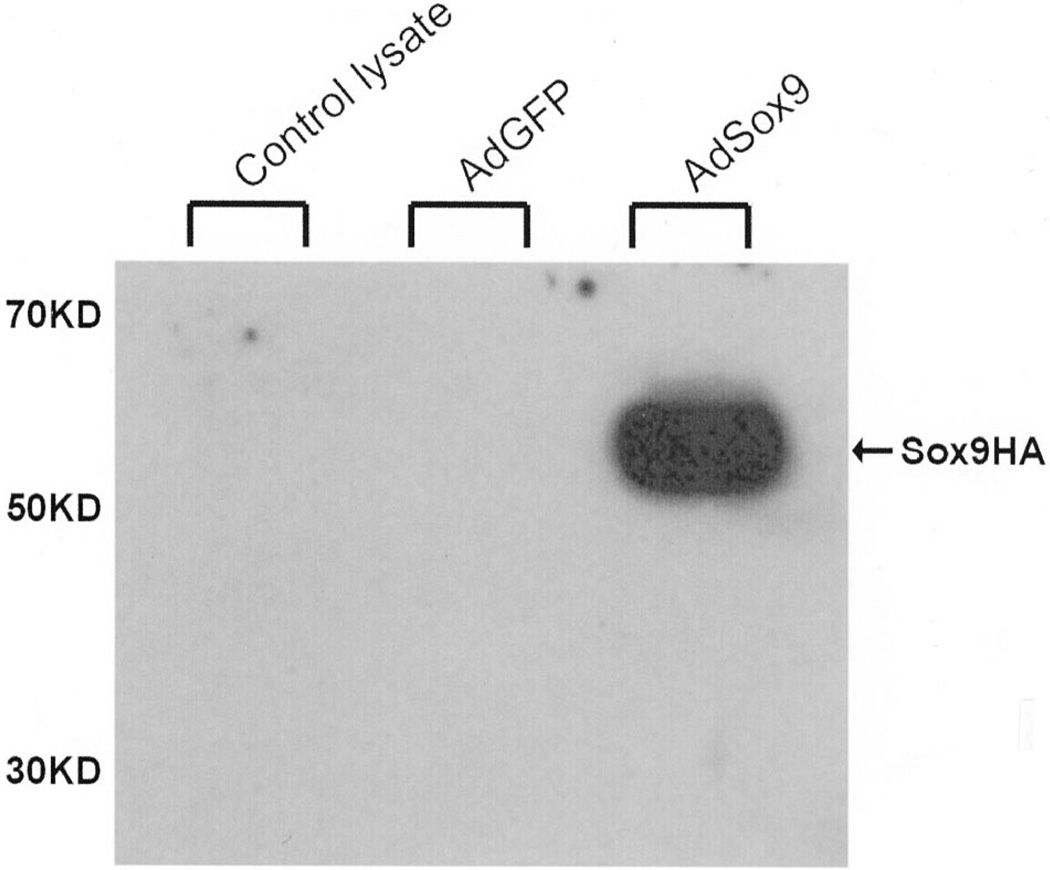
The authors next tested exogenous expression of Sox9 mediated by AdSox9. The Sox9 gene encodes a 509-amino acid protein. To distinguish Sox9 production created by the adenoviral vector from endogenous Sox9, an HA tag was placed on the C-terminus of the Sox9 coding sequence. Thereafter, HCT116 cells were infected with AdSox9 or the control virus AdGFP. At 24 hours after infection, the cells were collected and subjected to Western blotting analysis using an anti-HA antibody. As shown in Figure 3, a marked increase in Sox9–HA protein synthesis was readily detected in the AdSox9-infected cells, whereas the cells infected with AdGFP produced no detectable signal.
Figure 3.
Expression of exogenous Sox9 mediated by the AdSox9 adenoviral vector. Subconfluent HCT116 cells were infected with AdSox9 or AdGFP. Total cell lysate was collected 24 hours after infection and subjected to 4% to 20% SDS–SPAGE (approximately 10 µg total proteins per lane). After being resolved and transferred to an Immobilon-P membrane, the presence of HA-tagged Sox9 protein was probed with a mouse monoclonal anti-HA antibody (12CA5, Roche Molecular Biochemicals) and visualized using the SuperSignal West Pico chemiluminescent substrate kit (PIERCE).
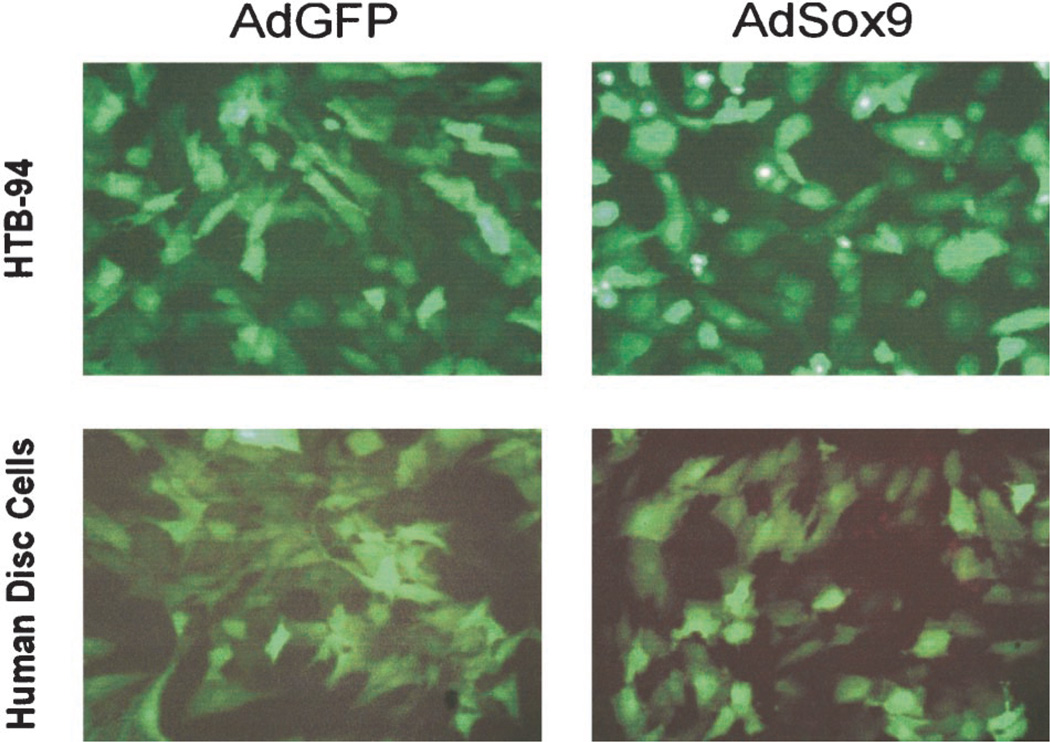
To determine the transduction efficiency of the adenoviral vectors, both AdSox9 and AdGFP were used to infect the chondroblastic line HTB-94 and cultured primary human disc cells. At 24 hours after infection, the cells were examined under a fluorescence microscope for assessment of GFP levels, which should have been coexpressed with Sox9. As demonstrated in Figure 4, comparable infection rates between the AdGFP control condition and the AdSox9 vector were observed. Given that the cells were approximately 70% to 80% confluent at the time of infection, an overall infection efficiency exceeding 60% could be achieved using both adenoviral vectors without any significant adenovirus-related toxicities.
Figure 4.
Efficient transduction of chondrocytes by recombinant adenoviral vectors. Subconfluent HTB-94 and primary human disc cells were infected with AdSox9 or AdGFP. At 24 hours of adenoviral infection, expression of GFP was visualized and recorded using fluorescence microscopy.
Exogenous Sox9 Expression Upregulates the Proα1(II) mRNA Level
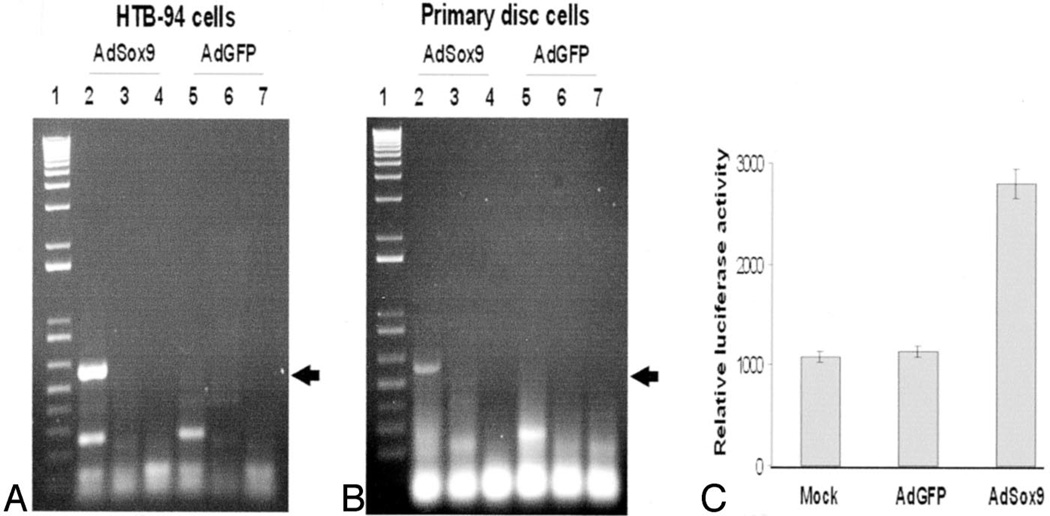
Reverse transcriptase-polymerase chain reaction analysis of Proα1(II) mRNA expression was performed on HTB-94 cells and human disc cells that had been infected with AdSox9 or AdGFP for 48 hours. Expression of Type 2 collagen was detected by RT-PCR analysis using a pair of Type 2 collagen-specific primers. As indicated in Figure 5A and 5B, a strong and specific amplification of the anticipated 560-bp fragment was readily detected in the cells infected with AdSox9. None of the control conditions including the RT control reactions, the internal PCR control reactions, and the cells infected with AdGFP exhibited this band at the corresponding 560-bp region. These results suggest that upregulation of Sox9 led to enhanced expression of Proα1(II) at the mRNA level.
Figure 5.
Induction of Type 2 collagen expression by exogenous Sox9. A and B, RT-PCR analysis of Type 2 collagen expression. Exponentially growing HTB-94 cells (A) and primary disc cells (B) were infected with AdSox9 and AdGFP. At 48 hours after infection, total RNA was isolated and subjected to reverse transcriptase-PCR reactions. Expression of Type 2 collagen was detected by PR-PCR analysis using a pair of Type 2 collagen-specific primers. The expected PCR product was approximately 560 bp (arrows). Lane 1: 1-kb plus ladder (Life Technologies). Lanes 2 and 5: templates derived from +RT reactions. Lanes 3 and 6: no template control. Lanes 4 and 7: templates derived from −RT reactions. C, Activation of Type 2 collagen promoter-containing reporter. Subconfluent HTB-94 cells were transfected with a reporter construct, pGL2–3.774kb, that contained a luciferase reporter gene driven by human COL2A1 promoter. At 20 hours after transfection, the cells were replated and infected with AdSox9, AdGFP, or no infection (mock). At 48 hours after infection, the cells were lysed and collected for luciferase activity assays using the Luciferase Assay kit (Promega). Each assay condition was performed in triplicate.
To ensure that upregulation of Type 2 collagen by Sox9 was mediated through its interaction with the Proα1(II) promoter, a reporter, pGL2–3.774kb, that contained a luciferase reporter gene driven by the human Proα1(II) promoter was tested. Specifically, HTB-94 cells were first transfected with the reporter construct and then infected with AdGFP (mock virus), AdSox9, or no virus infection. At 36 hours after infection, the cells were lysed and collected for luciferase activity assays. As indicated in Figure 5C, luciferase activity in the cells infected with Sox9 demonstrated a nearly twofold increase, indicating that Sox9 induced Proα1(II) mRNA expression through interaction with its promoter. There was no detectable difference between the uninfected and AdGFP-infected cells. These findings confirm the conclusion that Sox9 can upregulate Proα1(II) mRNA expression.
Immunohistochemical Analysis of Type 2 Collagen Production Induced By Exogenous Sox9
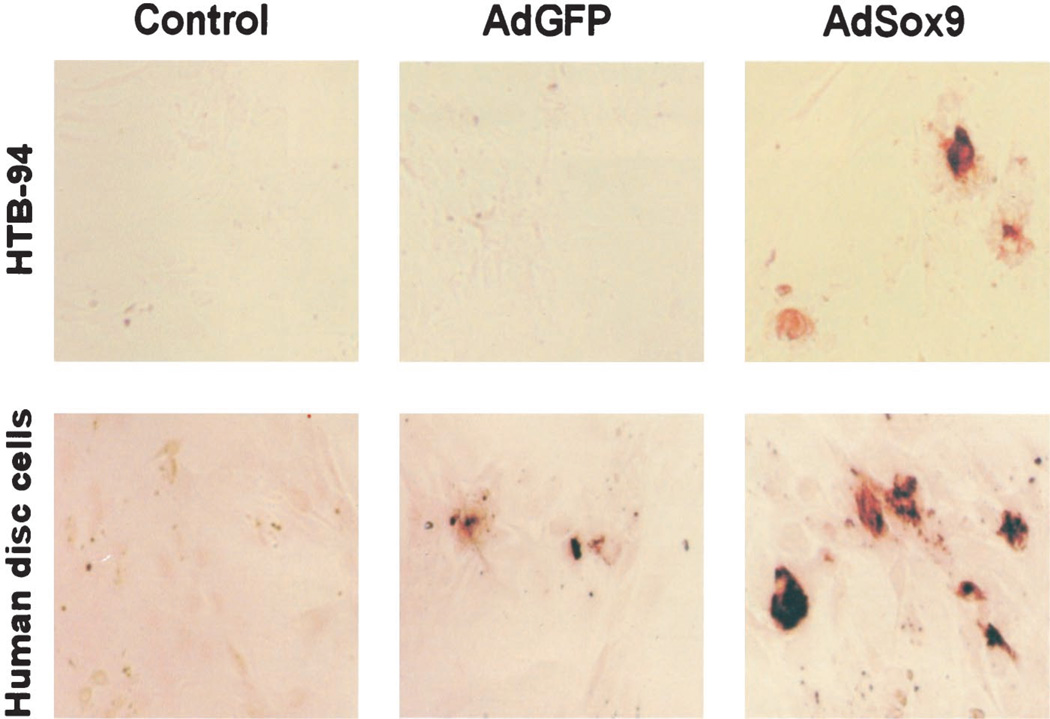
Primary disc cells and HTB-94 cells were infected with AdSox9 or AdGFP. At 48 hours after infection, the cells were fixed and immunohistochemically stained using a rat anti-Type 2 collagen antibody. The control group for both the HTB-94 and human disc cells were infected with Sox9, but were not exposed to the primary antibody during immunohistochemical staining. As illustrated in Figure 6, staining was clearly evident in the AdSox9-infected HTB-94 cells (Figure 6, upper panel), whereas no appreciable staining was detectable in the control or AdGFP-infected cells. In human intervertebral disc cells (Figure 6, lower panel), the addition of exogenous Sox9 markedly increased the production of Type 2 collagen above any basal expression in either of the control conditions. These observations were consistent with the RT-PCR and reporter assay results, indicating that exogenous Sox9 production leads to a significant increase in Type 2 collagen production.
Figure 6.
Immunohistochemical analysis of Type 2 collagen production induced by exogenous Sox9. Subconfluent HTB-94 and primary disc cells were infected with AdSox9 or AdGFP. At 36 hours after infection, the cells were fixed and immunostained with a rat anti-Type 2 collagen antibody (provided by Dr. Michael Cremer, University of Tennessee, Memphis, TN). The control condition represented infected cells immunostained without the primary antibody.
Effect of Sox9 on Intervertebral Disc Tissue in a Rabbit Model
The aforementioned data provides evidence that exogenous Sox9 production leads to an increase in Type 2 collagen production in vitro. The authors therefore sought to validate these findings in an in vivo model of disc degeneration that would more closely predict its value in a clinical setting. The New Zealand white rabbit disc stab-incision model of intervertebral disc degeneration has been well characterized and was used in this study (Figure 2). Three test conditions were studied: 1) control (stab incision without injection), 2) AdGFP (stab incision with injection of mock adenovirus, AdGFP), and 3) AdSox9 (stab incision with injection of AdSox9 virus). A total of four rabbits were used. However, one animal died shortly after surgery, leaving three animals for study. At 5 weeks after injection, the rabbits were killed and the intervertebral discs recovered and stained with hematoxylin–eosin. The control discs34 and the AdGFP discs34 were compared with noninjured discs. The central portion of both the control and AdGFP discs was replaced with spindle-shaped fibrocytes, and the extracellular matrix no longer retained the loosely organized appearance of the nucleus pulposus (Figure 2). In contrast, the discs injected with AdSox934 retained a more chondrocytic appearance, with regard to both the cellular morphology and the extracellular matrix (Figure 2). Immunohistochemical staining for Type 2 collagen was performed on frozen sections from each disc. However, significant background staining of Type 2 collagen precluded meaningful comparisons of Type 2 collagen levels between study discs.
Discussion
The data presented in this study corroborate the important role that Sox9 plays in the regulation of Type 2 collagen synthesis. The authors have developed a novel delivery system using an adenoviral vector, and have tested it in several cultured cell lines, including human degenerated intervertebral disc cells. The increased production of Sox9 induced by AdSox9 upregulates Proα1(II) mRNA expression through interaction with the promoter region. This culminates in increased Type 2 collagen production at the cellular level in vitro. Furthermore, in a rabbit intervertebral disc injury model, preliminary data appear to indicate that injection of AdSox9 preserves the chondrocytic appearance of the nucleus pulposus cells, as compared with discs injected with the AdGFP mock virus over a 5-week period.
The current study succeeded in demonstrating transfection of cells from degenerated human intervertebral discs, supporting the potential therapeutic role of this approach in human disc degeneration. Although nonma-nipulated degenerated disc tissue has limited intrinsic ability to synthesize Type 2 collagen,37 Sox9 was able to drive cells from human degenerated discs to produce Type 2 collagen. The significance of the cells maintaining their ability to respond to Sox9, even in the face of disc degeneration, has obvious implications for the biologic treatment of disc degeneration.
Maintaining high concentrations of competent Type 2 collagen in the nucleus pulposus is thought to be important for normal disc function.4,38 Furthermore, the initial increases in Type 2 collagen synthesis seen in response to disc injury suggest a role in disc healing.39 The significance of stimulating Type 2 collagen production for the treatment of degenerative disc disease is not completely understood. Takaishi et al39 demonstrated that during the early stages of disc degeneration in a rabbit model, there is a transient period of increased Proα1(II) mRNA expression approximately 4 weeks after the anulus fibrosus has been incised. This was interpreted as an early attempt at disc repair that was not sustained beyond 4 weeks, and ultimately did not prevent further disc degeneration. Upregulation of Proα1(II) mRNA expression, therefore, appears to be important in the healing process within the nucleus pulposus. In the setting of continued mechanical and nutritional stress, however, the disc cells are unable to sustain this response, and disc degeneration ensues.
With disc degeneration, chondrocytic cells are replaced by fibrocytes synthesizing Type 1 collagen.8 Additionally, an overall decrease in disc cell density with age and degeneration is seen.5,6 In a study of human intervertebral discs, Gruber et al6,40 stated that apoptosis, or programmed cell death, largely accounts for this depopulation over time, and that interventions which delay or halt apoptotic cell death may constitute a means of treating degenerative disc disease. In the current study, Sox9 expression clearly stimulated Type 2 collagen production in cultured human disc cells and a chondrosarcoma cell line, suggesting that Sox9 may help to reduce apoptosis by stimulating chondrogenic factors. In a rabbit disc stab-injury model, Sox9 transduction reduced the histologic transformation of chondroid disc cells into fibrocytes in rabbit discs. The preservation of the chondrocytic appearance of cells within the nucleus pulposus despite previous disruption of the anulus suggests that Sox9 expression also may enhance the capacity of the nucleus pulposus to heal itself after injuries.
Few studies have investigated the role of gene therapy in the treatment of disc degeneration. In 1998, Nishida et al41 demonstrated successful transfer of a lacZ marker gene to rabbit lumbar intervertebral discs using an adenovirus vector. Gene therapy studies from this group have examined the ability of exogenous genes encoding TGF-Tβ1 to stimulate proteoglycan synthesis in rabbit intervertebral discs.42 Growth factors such as TGF-Tβ1 have wide anabolic effects, making them attractive candidates for the stimulation of connective tissue matrix production. However, before considering these growth factors therapeutically, details regarding the methods of action and the wide range of effects on disc tissue must be understood. To the authors’ knowledge, no prior studies have reported gene therapy techniques specifically stimulating production of Type 2 collagen. This may offer an attractive therapeutic approach either alone or in combination with other factors that promote disc healing.
Delivery of gene products to intervertebral disc nucleus pulposus represents a novel method for manipulating the biologic pathways necessary for chondrogenesis. Although a number of techniques for gene therapy have been described,43 adenovirus-mediated gene therapy may provide significant advantages. Adenoviruses have high transfection efficiencies, and can transduce cells independently of cellular proliferation. A limitation of this technique is the decline in transgene expression over time where the immune system is active. In the intervertebral disc, the relatively encapsulated and avascular environment of the nucleus pulposus may, however, limit immune reactivity, permitting prolonged gene expression.
The early in vivo data reported suggest that exogenous Sox9 production may help to preserve the chondrocytic appearance of the disc space in a degenerative milieu. The process of disc degeneration is exceedingly complex, and the upregulation of Type 2 collagen synthesis addresses only a portion of this process. Still, the data in this study suggest that gene therapy using Sox9 may represent a promising new therapeutic intervention in the treatment of degenerative disc disease.
Key Points.
A new recombinant adenoviral vector expressing Sox9, a chondrocyte-specific transcription factor, was used to infect both human degenerative disc cells and rabbit discs in vivo.
The AdSox9 virus efficiently infected degenerated human disc cells and significantly increased Type 2 collagen production in infected human disc cells.
In a rabbit model, discs injected with AdSox9 maintained a chondrocytic appearance over a 5-week study period that differed from that of control discs.
These findings suggest a potential role for Sox9 gene therapy in the treatment of human degenerative disc disease, where loss of Type 2 collagen is a typical finding.
Acknowledgments
The authors thank Dr. Vincent R. Harley, University of Melbourne, Australia, Dr. Michael Cremer, University of Tennessee, Memphis, TN, and Dr. Philippe Galera, University of Caen, France, for their generous provision of Sox-9 cDNA, anti-COLIIa antibody, and COLIIa promoter reporters, respectively.
Supported in part by research grants from the Orthopedic Research and Education Foundation and the Schweppe Foundation (TCH).
Footnotes
Device status/drug statement: The submitted manuscript does not contain information about medical devices or drugs.
Conflict of interest: No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 1992;4:226–232. doi: 10.1097/00002281-199204000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22:263–271. [PubMed] [Google Scholar]
- 3.Webster BS, Snook SH. The cost of 1989 workers’ compensation low back pain claims. Spine. 1994;19:1111–1115. doi: 10.1097/00007632-199405001-00001. discussion 1116. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc: Comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Guiot BH, Fessler RG. Molecular biology of degenerative disc disease. Neurosurgery. 2000;47:1034–1040. doi: 10.1097/00006123-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kaapa E, Han X, Holm S, et al. Collagen synthesis and Types I, III, IV, and VI collagens in an animal model of disc degeneration. Spine. 1995;20:59–66. doi: 10.1097/00007632-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Lipson SJ, Muir H. Experimental intervertebral disc degeneration: Morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24:12–21. doi: 10.1002/art.1780240103. [DOI] [PubMed] [Google Scholar]
- 10.Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- 11.Yasuma T, Koh S, Okamura T, et al. Histological changes in aging lumbar intervertebral discs: Their role in protrusions and prolapses. J Bone Joint Surg [Am] 1990;72:220–229. [PubMed] [Google Scholar]
- 12.Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 13.Paajanen H, Erkintalo M, Parkkola R, et al. Age-dependent correlation of low back pain and lumbar disc regeneration. Arch Orthop Trauma Surg. 1997;116:106–107. doi: 10.1007/BF00434112. [DOI] [PubMed] [Google Scholar]
- 14.Salminen JJ, Erkintalo MO, Pentti J, et al. Recurrent low back pain and early disc degeneration in the young. Spine. 1999;24:1316–1221. doi: 10.1097/00007632-199907010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Baer AE, Wang JY, Kraus VB, et al. Collagen gene expression and mechanical properties of intervertebral disc cell–alginate cultures. J Orthop Res. 2001;19:2–10. doi: 10.1016/S0736-0266(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 16.Humzah MD, Soames RW. Human intervertebral disc: Structure and function. Anat Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 17.Adler JH, Schoenbaum M, Silberberg R. Early onset of disk degeneration and spondylosis in sand rats (Psammomys obesus) Vet Pathol. 1983;20:13–22. doi: 10.1177/030098588302000102. [DOI] [PubMed] [Google Scholar]
- 18.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 19.Silberberg R, Aufdermaur M, Adler JH. Degeneration of the intervertebral disks and spondylosis in aging sand rats. Arch Pathol Lab Med. 1979;103:231–235. [PubMed] [Google Scholar]
- 20.Trout JJ, Buckwalter JA, Moore KC, et al. Ultrastructure of the human intervertebral disc: I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- 21.Hutton WC, Toribatake Y, Elmer WA, et al. The effect of compressive force applied to the intervertebral disc in vivo: A study of proteoglycans and collagen. Spine. 1998;23:2524–2537. doi: 10.1097/00007632-199812010-00007. [DOI] [PubMed] [Google Scholar]
- 22.Kaapa E, Holm S, Han X, et al. Collagens in the injured porcine intervertebral disc. J Orthop Res. 1994;12:93–102. doi: 10.1002/jor.1100120112. [DOI] [PubMed] [Google Scholar]
- 23.Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665–676. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 24.Bell DM, Leung KK, Wheatley SC, et al. SOX9 directly regulates the Type 2 collagen gene. Nat Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Zhou X, Lefebvre V, et al. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefebvre V, Huang W, Harley VR, et al. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegner M. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright E, Hargrave MR, Christiansen J, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 29.de Crombrugghe B, Lefebvre V, Behringer RR, et al. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- 30.Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–540. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- 31.Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem. 2000;275:3687–3692. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Q, Eberspaecher H, Lefebvre V, et al. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Bi W, Deng JM, Zhang Z, et al. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 34.Bi W, Huang W, Whitworth DJ, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanai Y, Koopman P. Structural and functional characterization of the mouse Sox9 promoter: Implications for campomelic dysplasia. Hum Mol Genet. 1999;8:691–696. doi: 10.1093/hmg/8.4.691. [DOI] [PubMed] [Google Scholar]
- 36.Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6, and Sox9 are coexpressed in chondrogenesis and cooperatively activate the Type 2 collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poiraudeau S, Monteiro I, Anract P, et al. Phenotypic characteristics of rabbit intervertebral disc cells: Comparison with cartilage cells from the same animals. Spine. 1999;24:837–844. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Mosler S, Rui H, et al. Structural and functional implications of age-related abnormal modifications in collagen II from intervertebral disc. Matrix Biol. 1995;14:643–651. doi: 10.1016/s0945-053x(05)80028-9. [DOI] [PubMed] [Google Scholar]
- 39.Takaishi H, Nemoto O, Shiota M, et al. Type 2 collagen gene expression is transiently upregulated in experimentally induced degeneration of rabbit intervertebral disc. J Orthop Res. 1997;15:528–538. doi: 10.1002/jor.1100150408. [DOI] [PubMed] [Google Scholar]
- 40.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro . Spine. 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Nishida K, Kang JD, Suh JK, et al. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. discussion 2443. [DOI] [PubMed] [Google Scholar]
- 42.Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adeno-virus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Zou X, Bunger C. Gene therapy and spinal disorders. Int Orthop. 2001;25:1–4. doi: 10.1007/s002640000207. [DOI] [PMC free article] [PubMed] [Google Scholar]