SUMMARY
Mononuclear non-haem iron (NHFe) enzymes catalyse a wide variety of oxidative reactions including halogenation, hydroxylation, ring closure, desaturation, and aromatic ring cleavage. These are highly important for mammalian somatic processes such as phenylalanine metabolism, production of neurotransmitters, hypoxic response, and the biosynthesis of natural products.1–3 The key reactive intermediate in the catalytic cycles of these enzymes is an S = 2 FeIV=O species, which has been trapped for a number of NHFe enzymes4–8 including the halogenase SyrB2, the subject of this study. Computational studies to understand the reactivity of the enzymatic NHFe FeIV=O intermediate9–13 are limited in applicability due to the paucity of experimental knowledge regarding its geometric and electronic structures, which determine its reactivity. Synchrotron-based nuclear resonance vibrational spectroscopy (NRVS) is a sensitive and effective method that defines the dependence of the vibrational modes of Fe on the nature of the FeIV=O active site.14–16 Here we present the first NRVS structural characterisation of the reactive FeIV=O intermediate of a NHFe enzyme. This FeIV=O intermediate reacts via an initial H-atom abstraction step, with its subsquent halogenation (native) or hydroxylation (non-native) rebound reactivity being dependent on the substrate.17 A correlation of the experimental NRVS data to electronic structure calculations indicates that the substrate is able to direct the orientation of the FeIV=O intermediate, presenting specific frontier molecular orbitals (FMOs) which can activate the selective halogenation versus hydroxylation reactivity.
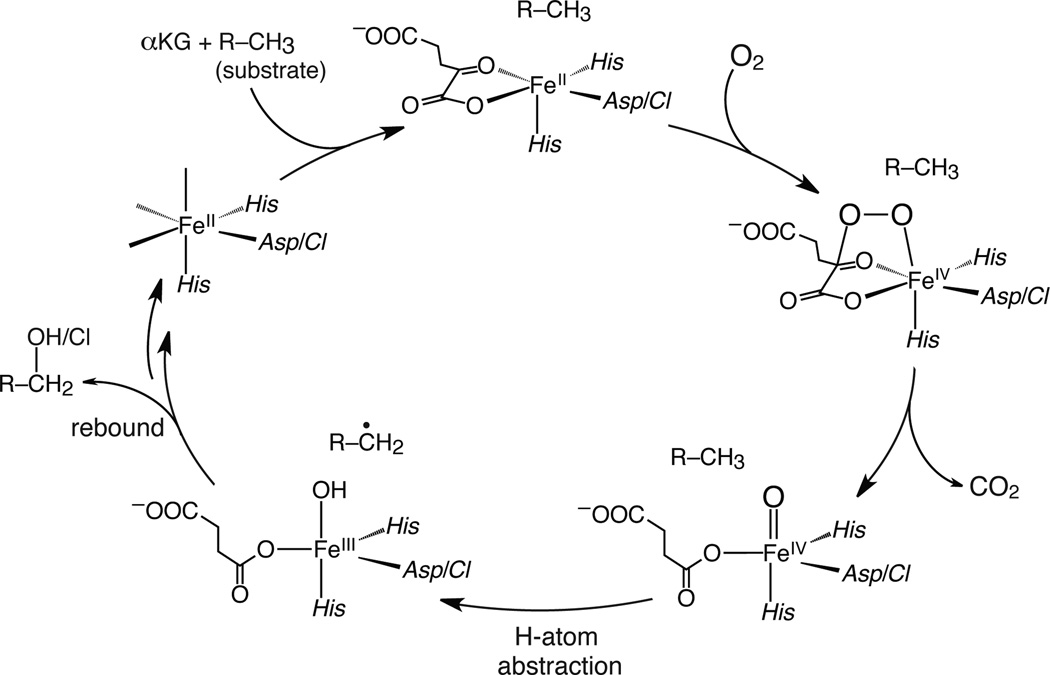
SyrB2, an α-ketoglutarate (αKG)-dependent NHFe enzyme found in Pseudomonas syringae pv. Syringae, halogenates the methyl group of l-Thr using nonribosomal peptide synthetase machinery.18 The FeII active site is ligated by 2 His and 1 halide (Cl−/Br−) (see Supplementary Fig. 1), in contrast to the 2-His/1-carboxylate ‘facial triad’ of other NHFe enzymes.1,2,4,5,8 While their mechanisms of O2 activation leading to the highly-reactive S = 2 FeIV=O intermediate are thought to be similar, there is a notable divergence in their subsequent catalytic cycles (Fig. 1). The FeIV=O species abstracts an H-atom from the substrate to form an FeIII—OH species and a substrate radical; in hydroxylases, the subsequent step is HO• rebound to form a hydroxylated product,1,2 but in SyrB2 the native l-Thr substrate is chlorinated instead, and the 4-Cl-l-Thr product is utilised in the biosynthesis of the phytotoxin syringomycin E.18 Owing to their reactivity, FeIV=O intermediates in enzymes are challenging to trap and characterise. For SyrB2, however, use of the non-native substrate l-cyclopropylglycine (l-Cpg) and the heterologous substrate carrier protein CytC2 has provided a long-lived species at the concentrations required for spectroscopic investigation.17,19
Figure 1.
Catalytic cycle of αKG-dependent NHFe enzymes. αKG and substrate binding induces a 6-coordinate → 5-coordinate conversion (top), providing a site for O2 to bind and form an FeIV—peroxo species that nucleophilically attacks αKG, producing a peroxo-bridged FeIV species (right).24 Decarboxylation of αKG leads to the reactive FeIV=O intermediate (bottom right).
Nuclear resonance vibrational spectroscopy (NRVS) utilises 3rd-generation-synchrotron radiation to probe the vibrational sidebands of the 57Fe Mössbauer nuclear-resonant peak at 14.4 keV.20–22 NRVS is a site-selective technique allowing the observation of only normal modes involving Fe motion, which makes it ideal for studying iron-dependent enzymes without interference from protein backbone modes. (SyrB2)FeIV=O can be generated in high purity with both Cl− and Br− ligation of the FeIV=O unit, providing a mass perturbation that aids in the assignment of NRVS peaks and ultimately the structure of the intermediate. The NRVS methodology is coupled with spectroscopically-calibrated density functional theory (DFT) calculations to evaluate specific FMOs responsible for H-atom abstraction that can selectively lead to halogenation or hydroxylation depending on the substrate.
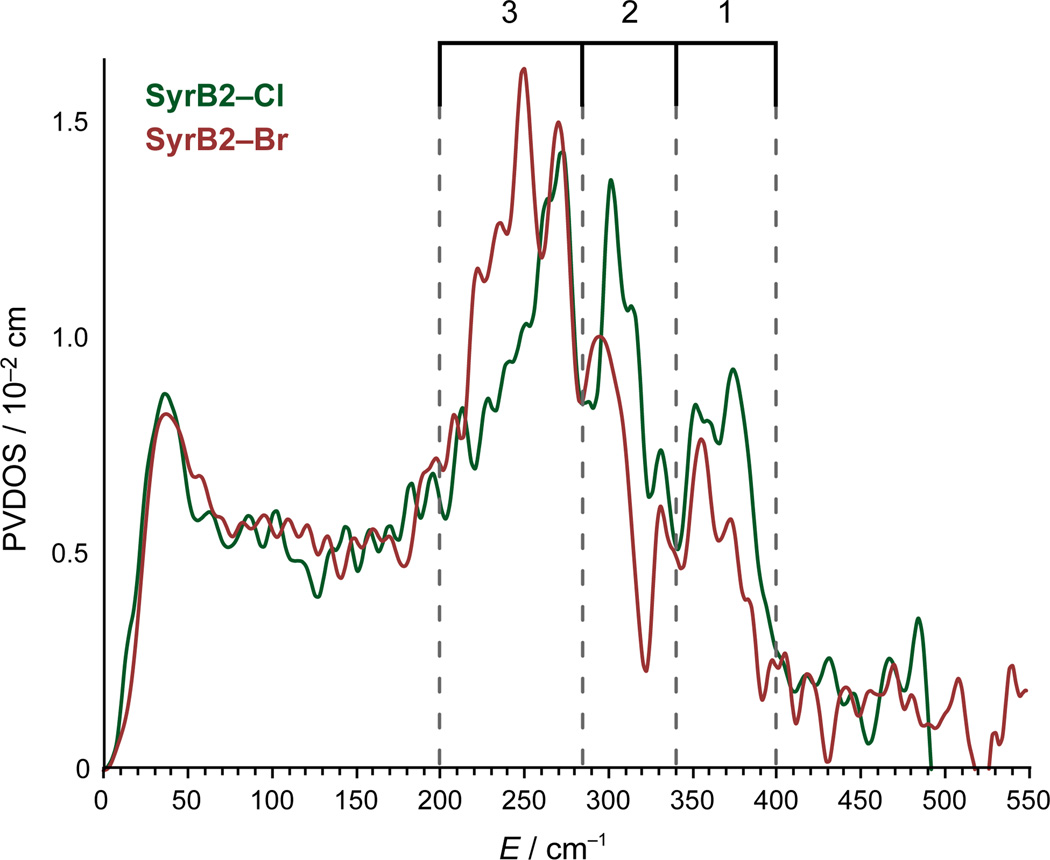
The NRVS partial vibrational density-of-states (PVDOS) spectra of l-Cpg–S–CytC2-bound [(SyrB2)Cl—FeIV=O] (SyrB2–Cl) and [(SyrB2)Br—FeIV=O] (SyrB2–Br) are shown in Fig. 2. For practical considerations (see Methods), data collection was restricted to < 600 cm−1; modes in this region are affected by large (Cl/Br, vide infra) but not small (16O/18O, see Supplementary Fig. 2) mass perturbations. There are three distinct features for each species as indicated by the bracketed energy regions: 1 (340–400 cm−1), 2 (285–340 cm−1) and 3 (200–285 cm−1). For the higher-energy regions 1 and 2, the peaks of SyrB2–Cl are more intense. However, for the low-energy region 3, the peak envelope for SyrB2–Br is considerably more intense and shifted to lower energy.
Figure 2.
NRVS PVDOS spectra of SyrB2–Cl and SyrB2–Br, with regions containing intense features indicated in brackets.
Previous computational studies on the Cl—FeIV=O intermediate of SyrB2 predicted 6-coordinate (6C) structures with the succinate bound as a bidentate ligand to Fe.9–12 The DFT-calculated NRVS spectra of these 6C structures (Supplementary Fig. 3) do not reproduce the splitting pattern and intensity distribution of the experimental data, and can thus be eliminated from consideration.
In order to generate and evaluate suitable structural candidates, the O2 reaction coordinate taking SyrB2 to its FeIV=O intermediate (Fig. 1) was pursued using DFT calculations. The initial structure was taken from the crystal structure of the SyrB2 FeII active site with the αKG cofactor and Cl− bound (Supplementary Fig. 3)23 and the native substrate l-Thr positioned according to a molecular docking procedure;12 its side-chain was also modified into the non-native substrate l-Cpg to generate a second starting structure. Application of the spectroscopically-calibrated DFT methodology used for a related αKG-dependent mononuclear NHFe enzyme24 resulted in an equivalent O2 reaction coordinate for the FeII active site of SyrB2 (Supplementary Figs. 4 and 5a,b).
This O2 reaction coordinate leads to 1Cpg–Cl (Fig. 3b) and 1Thr–Cl (Supplementary Fig. 5b) with l-Cpg and l-Thr respectively; both are 5-coordinate (5C) trigonal bipyramidal (TBP) FeIV=O structures possessing an axial oxo group and a monodentate succinate. Significantly, in both cases, the Fe—oxo vector is oriented perpendicularly to the target substrate C–H bond, raising interesting implications about π-channel reactivity.25,26
Figure 3.
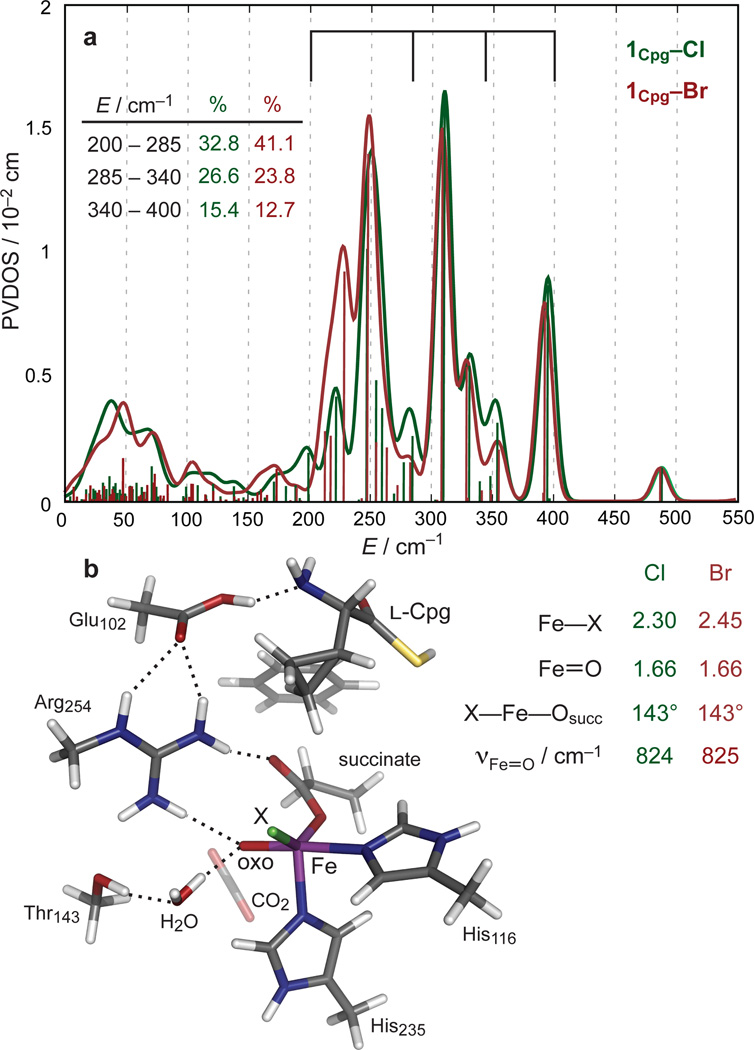
a, DFT-predicted PVDOS NRVS spectra of 5C TBP structural candidate 1Cpg–X for FeIV=O intermediate of SyrB2. Vertical bars represent relative calculated mode-composition factors of vibrational modes, and brackets correspond to energy regions from Fig. 2. (Inset) Peak intensity contributions (from three bracketed regions) to overall PVDOS envelope. b, Structure of 1Cpg–X (left), along with geometric parameters and Fe—oxo stretching frequencies (right).
1Cpg–Cl was evaluated as a structural candidate for the FeIV=O intermediate in the NRVS sample. The geometry-optimised Fe—oxo and Fe—Cl bond lengths (Fig. 3b) of 1Cpg–Cl are in close agreement with the experimental extended X-ray absorption fine structure (EXAFS) values (1.66 Å and 2.31 Å respectively).7 The Br− cognate 1Cpg–Br was generated by replacing Cl− with Br− and reoptimising the structure; its Fe—Br bond length of 2.45 Å (Fig. 3b) agrees well with the EXAFS value of 2.43 Å for the related halogenase CytC3.6 Thus, these 5C TBP intermediates 1Cpg–X (X = Cl/Br) resulting from the O2 reaction coordinate were used for comparison with the experimental NRVS data on the SyrB2 FeIV=O intermediates SyrB2–X.
As seen in Fig. 3, the 5C TBP species 1Cpg–X result in DFT-predicted spectra that reproduce the experimental spectra. First, there are three distinct peaks falling within the energy regions of 200–275 cm−1, 275–340 cm−1 and 340–400 cm−1, matching regions 3, 2, and 1 in Fig. 2. Second, the intensities of the peaks in the two higher-energy regions are greater for 1Cpg–Cl than for 1Cpg–Br, while the intensity of the peak envelope in the lowest-energy region for 1Cpg–Br is greater and shifted to lower energy with respect to that of 1Cpg–Cl, reproducing the spectral intensity distributions of the experimental data (Fig. 2). Other 5C and 6C structures were generated as possible candidates for the FeIV=O species, starting from 1Cpg–X and shifting either the Fe-ligating atoms or the hydrogen-bonding network to the oxo group (Supplementary Fig. 6). From the predicted NRVS spectra of these structures and of the structures generated in previous (computational) studies (Supplementary Fig. 3), all structures except 5C TBP can be eliminated due to their poor agreement with the experimental NRVS data.
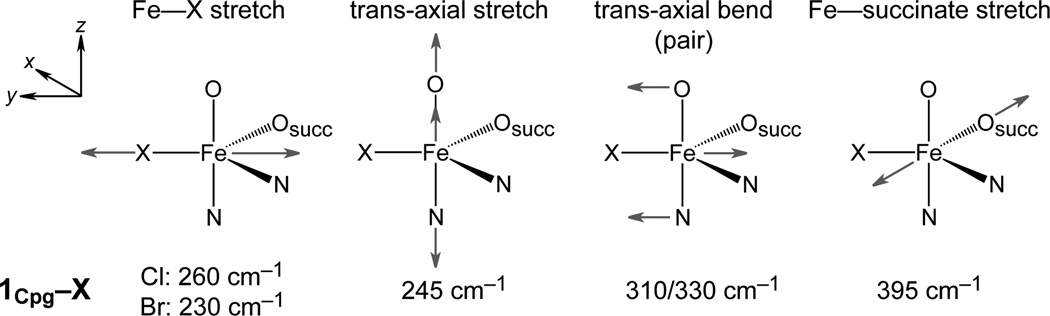
Correlating the DFT-calculated spectra of 5C TBP 1Cpg–X (Fig. 3) with the experimental spectra (Fig. 2), the NRVS peaks can be assigned to four normal modes (Fig. 4): (i) the feature in region 1 (Fig. 2) originates from the Fe—succinate stretch; (ii) the feature in region 2 is composed of a pair of trans-axial bend modes (these would be degenerate in strict TBP symmetry, but are calculated to split in energy because of the wider equatorial X—Fe—succinate angle of 143°);14 the lowest-energy region 3 has a peak envelope calculated to contain (iii) the trans-axial stretch and (iv) the Fe—X stretch, with the Fe—Br stretch being lower in energy by 30 cm−1 and more intense by 1.5 times. The redistribution in intensity is attributed to the mass perturbation of the Br, which has almost no motion in the Fe—X stretching mode and consequently induces greater Fe motion in the mode. This Fe motion is borrowed from higher-energy modes, as analysed in Supplementary Fig. 7. The NRVS peak pattern of SyrB2–X parallels that of a crystallographically-characterised TBP S = 2 FeIV=O model complex (Supplementary Fig. 8),15 further demonstrating the sensitivity of NRVS to geometric structure.
Figure 4.
DFT-predicted normal modes of 5C TBP FeIV=O structures 1Cpg–X, with corresponding frequencies. The Fe—oxo vector defines the z-axis.
Note that two distinct FeIV species are detected by Mössbauer, differing in quadrupole splitting, ΔEQ (see Supplementary Fig. 9) in each FeIV=O intermediate generated.7 A possible explanation for this speciation lies in the hydrogen-bonding interactions with the oxo group: 1Cpg–Cl has two (with Arg254 and H2O), while 1Thr–Cl has one (with H2O). Their predicted NRVS spectra are similar (Supplementary Figs. 6 and 10a), but their calculated ΔEQ’s are different, with that of 1Cpg–Cl (−0.50 mm s−1) being smaller in magnitude than that of 1Thr–Cl (−0.71 mm s−1). Decreasing the number of hydrogen bonds strengthens the Fe—oxo bond, thus increasing the magnitude of (negative) ΔEQ (Supplementary Fig. 10a and Supplementary Table 1). These calculations suggest that variability in hydrogen-bonding interactions with the oxo group results in FeIV speciation, not some structural difference.
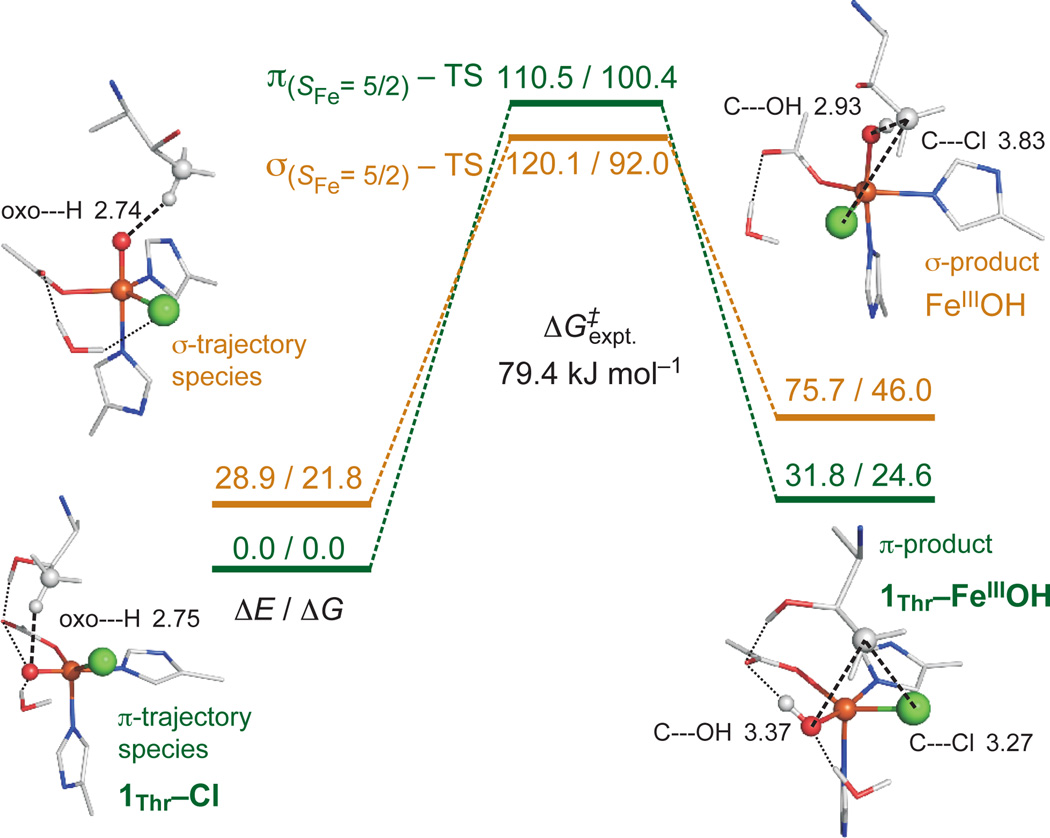
For the native l-Thr substrate, starting from the O2-reaction-coordinate-derived FeIV=O species 1Thr–Cl, having its Fe—oxo vector perpendicular to C–H (Supplementary Figure 5b), the H-atom abstraction reactivity was computationally evaluated (Fig. 5). The target C–H approaches in a π-trajectory, transferring an α-electron into the oxo π-FMO, resulting in an FeIII(S=5/2)—OH first product (1Thr–FeIIIOH). The free-energy barrier ΔG‡ for this π-pathway is +100.4 kJ mol−1, in reasonable agreement with the experimental value of +79.4 kJ mol−1.7 A number of possible explanations have been considered for the subsequent Cl• rebound;17,27 here we show that in this first product, the substrate radical is positioned closer to the Cl than to the OH ligand of FeIII (consideration of their ionic radii places Cl 0.5 Å closer than OH), and OH is also stabilised by hydrogen-bonding to succinate (Fig. 5, right). This conformation disfavours HO• rebound but is well-oriented for Cl• rebound, as observed experimentally with this native substrate. This perpendicular Fe—oxo orientation is, for the 6C structures proposed in previous computational studies,9–12 inaccessible via the O2-activation pathway because a bidentate succinate would block the oxo group from reorienting.
Figure 5.
H-atom abstraction reaction coordinates for π-trajectory (1Thr–Cl, green) and σ-trajectory (orange), with energies (ΔE/ΔG) given in kJ mol−1. Structures of FeIII(S=5/2)—OH products displayed on right (with distances in Å), showing 1Thr–FeIIIOH (with hydrogen-bonding interactions indicated) set up for chlorination and σ-product set up for hydroxylation. For additional structural details, see Supplementary Fig. 11.
Evaluation of the reaction coordinate for O—O cleavage leading to the FeIV=O species (Supplementary Fig. 5) revealed that the positioning of l-Thr is fixed by two hydrogen-bonding interactions (–OH and –NH3+) to Glu102. This configuration results in the perpendicular orientation of the FeIV—oxo vector relative to the substrate C–H bond. However, the alternative substrate l-Nva lacks the –OH group, and thus its –NH3+ group can rotate to form a hydrogen bond with the O—O (peroxy) bridge, leading to a structure with an Fe—oxo vector oriented towards the substrate C–H bond (Supplementary Fig. 5c). An analogous l-Thr orientation was thus generated in order to evaluate its H-atom abstraction trajectory while maintaining the same C–H bond (Fig. 5, left).
For this orientation, the C–H approaches the Fe—oxo unit in a σ-trajectory, transferring an α-electron into the oxo σ-FMO to give an FeIII(S=5/2)—OH product; this σ-pathway has a ΔG‡ of +70.2 kJ mol−1 (Fig. 5). Relative to the π-pathway FeIII—OH product, this FeIII—OH has the substrate radical closer to the OH ligand than the Cl (by 0.5 Å, based on ionic radii), and OH has no hydrogen-bonding partner. The parallel Fe—oxo orientation therefore favours HO• rebound, as is observed experimentally for the non-native substrate l-Nva.17 We also note that the barrier for π-attack is somewhat higher than that for σ-attack, which is consistent with the higher barrier observed experimentally for halogenation relative to hydroxylation (by ~17 kJ mol−1),17 reflecting halogenation selectivity over efficiency.
In summary, we have performed the first NRVS structural characterisation of a NHFe enzyme oxygen intermediate and defined it to be a 5C TBP site with an axial FeIV=O bond. The native-substrate-bound O2 reaction coordinate reproduces this 5C TBP structure and gives an intermediate with its Fe—oxo vector perpendicular to the substrate C–H bond; this Fe—oxo orientation is active in H-atom abstraction, via its π*-FMO. This positions the substrate radical favourably for Cl• rebound, thus defining a selective mechanism in halogenases for chlorination of the native substrate. Alternatively, with a non-native substrate, variation in the O2 reaction coordinate can lead to an intermediate with its Fe—oxo vector parallel to the substrate C–H bond, leading to H-atom abstraction via a σ-pathway and a substrate radical positioned for HO• rebound and resultant hydroxylation.
METHODS (ONLINE)
Computational Methods
Spin-unrestricted DFT calculations were performed using the Turbomole 6.330 and Gaussian 0931 programs. Turbomole 6.3 was used to perform geometry optimisations and frequency calculations of the structural candidates in Supplementary Fig. 6, with the BP8632–34 exchange-correlation functional and the double-ζ def2-SVP basis set.35 Single-point energies were recomputed using the larger triple-ζ basis set def2-TZVP.35 Turbomole calculations were expedited by expanding the Coulomb integrals in an auxiliary basis set, using the RI-J approximation.36,37 Solvation effects were taken into account by using the Conductor-like Screening Model (COSMO) method38,39 with a dielectric constant εr = 4 as is appropriate for the protein environment (the COSMO radii were set up to: (H) 1.30 Å, (C) 2.00 Å, (N) 1.83 Å, (O) 1.72 Å, (Cl) 2.05 Å, (Br) 2.16 Å, (S) 2.16 Å and (Fe) 2.23 Å). This is referred to as the RI-BP86/def2-SVP(or def2-TZVP)/COSMO approach or level of theory.
Gaussian 09 was used to perform geometry optimisations and frequency calculations of the structural candidates 1Cpg–X in Fig. 3, with the functional/basis set combination BP86/6-311G*.40–43 Solvation effects were taken into account with the Polarized Continuum Model (PCM),44–47 using ε = 4.0. This is referred to as the BP86/6-311G*/PCM approach or level of theory.
NRVS PVDOS spectra were simulated by fitting the DFT-calculated mode composition factor48
for each normal mode n with individual Gaussians of FWHM 15 cm−1, using the gennrvs script.49
Mössbauer isomer shifts and quadrupole spittings were calculated according to published methods.50
The initial structure of the SyrB2 FeII active site used for DFT calculations was taken from its crystal structure (Supplementary Fig. 1 and ref. 23). Inclusion of the substrate (l-Cpg-SH, where the terminal –SH group represents truncation at the thioester linkage to the phosphopantetheine cofactor) was modeled according to ref. 12. Except where stated, the S atom of the substrate was frozen during geometry optimisation. Note that the substrate does indeed fit well in the cavity of the active site (Supplementary Fig. 1d).
The O2-reaction coordinate starting from the SyrB2–Cl FeII active site was pursued analogously to ref. 24, at the RI-BP86/def2-SVP(def2-TZVP)/COSMO level of theory. The complete O2-reaction coordinate for the l-Cpg-bound active site (with either Cl− or Br−) is shown in Supplementary Fig. 4, and Supplementary Fig. 5 shows the final O—O cleavage step, leading to the FeIV=O intermediate, for three versions of the active site containing l-Cpg (inert substrate), l-Thr (native substrate), and l-Nva (non-native substrate).
The H-atom abstraction reaction coordinates of the SyrB2–Cl FeIV=O intermediate were evaluated using the Turbomole 6.3 program.30 1Thr–Cl was optimised at the B3LYP51–53+D2/def2-SVP level (where +D2 stands for the second version of Grimme’s empirical dispersion correction54,55). Thermodynamic corrections to give enthalpic (ΔH) and Gibbs (ΔG) energies were calculated at T = 278.15 K to reproduce experimental conditions.7 Single-point energies were calculated at the B3LYP+D2/def2-TZVP/COSMO(εr=4.0) level. The calculated NRVS spectra of these Thr-bound species (Supplementary Fig. 10) are similar to those of their Cpg-bound counterparts (Supplementary Fig. 6), showing that the substrate does not affect the NRVS spectra because it is not directly coordinated to the Fe centre. Starting with 1Thr–Cl and 2Thr–Cl as the reactant complexes (RCs), each H-atom abstraction reaction was pursued along the oxo---H(l-Thr) coordinate and each transition state (TS) was optimised from the highest-energy structure along the reaction coordinate. An internal reaction coordinate was calculated from each optimised TS (forward) to obtain the product (FeIII—OH + substrate radical) and (backward) to confirm the validity of the RC structure.
Supplementary Material
Acknowledgements
Funding for research was provided by the National Institutes of Health (GM-40392 to E.I.S. and GM-69657 to J.M.B. and C.K.) and the National Science Foundation (MCB-0919027 to E.I.S. and MCB-642058 and CHE-724084 to J.M.B. and C.K.). Work at the Advanced Photon Source is supported by the Department of Energy, Office of Science, Contract DE-AC-02-06CH11357. Synchrotron experiments at SPring-8 were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2010B1569). M.S. thanks the RulÌšek group at the IOCB, Prague, for use of their computational resources.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions S.D.W. and M.S. contributed equally to this work. E.I.S., C.K. and J.M.B. designed the experiments. S.D.W., M.S., M.L.M., L.V.L., Y.K., K.P. and C.B.B. performed the experiments. S.D.W., M.S. and E.I.S. analysed the data and wrote the manuscript. E.E., J.Z., Y.Y., S.K. and M.Seto provided technical assistance at the sychrotron beamlines.
The authors declare no competing financial interests.
REFERENCES
- 1.Solomon EI, et al. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem. Rev. 2000;100:235–350. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- 2.Costas M, Mehn MP, Jensen MP, Que L., Jr dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 3.Vaillancourt FH, Yeh E, Vosburg DA, Garneau-Tsodikova S, Walsh CT. Nature#x00027;s inventory of halogenation catalysts: oxidative strategies predominate. Chem. Rev. 2006;106:3364–3378. doi: 10.1021/cr050313i. [DOI] [PubMed] [Google Scholar]
- 4.Krebs C, Galoni#x00107; Fujimori D, Walsh CT, Bollinger JM., Jr Non-heme Fe(IV)-oxo intermediates. Acc. Chem. Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eser BE, et al. Direct spectroscopic evidence for a high-spin Fe(IV) intermediate in tyrosine hydroxylase. J. Am Chem. Soc. 2007;129:11334–11335. doi: 10.1021/ja074446s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galoni#x00107; Fujimori D, et al. Spectroscopic evidence for a high-spin Br-Fe(IV)-oxo intermediate in the alpha-ketoglutarate-dependent halogenase CytC3 from Streptomyces. J. Am Chem. Soc. 2007;129:13408–13409. doi: 10.1021/ja076454e. [DOI] [PubMed] [Google Scholar]
- 7.Matthews ML, et al. Substrate-triggered formation and remarkable stability of the C–H bond-cleaving chloroferryl intermediate in the aliphatic halogenase, SyrB2. Biochemistry. 2009;48:4331–4343. doi: 10.1021/bi900109z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panay AJ, Lee M, Krebs C, Bollinger JM, Jr, Fitzpatrick PF. Evidence for a high-spin Fe(IV) species in the catalytic cycle of a bacterial phenylalanine hydroxylase. Biochemistry. 2011;50:1928–1933. doi: 10.1021/bi1019868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandian S, Vincent MA, Hillier IH, Burton NA. Why does the enzyme SyrB2 chlorinate, but does not hydroxylate, saturated hydrocarbons? A density functional theory (DFT) study. Dalton Trans. 2009:6201–6207. doi: 10.1039/b906866j. [DOI] [PubMed] [Google Scholar]
- 10.Kulik HJ, Blasiak LC, Marzari N, Drennan CL. First-principles study of non-heme Fe(II) halogenase SyrB2 reactivity. J. Am Chem. Soc. 2009;131:14426–14433. doi: 10.1021/ja905206k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Visser SP, Latifi R. Carbon dioxide: a waste product in the catalytic cycle of alpha-ketoglutarate dependent halogenases prevents the formation of hydroxylated by-products. J. Phys. Chem. B. 2009;113:12–14. doi: 10.1021/jp8097632. [DOI] [PubMed] [Google Scholar]
- 12.Borowski T, Noack H, Radoń M, Zych K, Siegbahn PEM. Mechanism of selective halogenation by SyrB2: a computational study. J. Am Chem. Soc. 2010;132:12887–12898. doi: 10.1021/ja101877a. [DOI] [PubMed] [Google Scholar]
- 13.Usharani D, Janardanan D, Shaik S. Does the TauD enzyme always hydroxylate alkanes, while an analogous synthetic non-heme reagent always desaturates them? J. Am Chem. Soc. 2012;133:176–179. doi: 10.1021/ja107339h. [DOI] [PubMed] [Google Scholar]
- 14.Bell CB, et al. A combined NRVS and DFT study of FeIV=O model complexes: a diagnostic method for the elucidation of non-heme iron enzyme intermediates. Angew. Chem. Intl. Ed. 2008;47:9071–9074. doi: 10.1002/anie.200803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong SD, et al. Nuclear resonance vibrational spectroscopy on the FeIV=O S = 2 non-heme site in TMG3tren: experimentally calibrated insights into reactivity. Angew. Chem. Intl. Ed. 2011;50:3215–3218. doi: 10.1002/anie.201007692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park K, et al. Nuclear resonance vibrational spectroscopic and computational study of high-valent diiron complexes relevant to enzyme intermediates. Proc. Natl Acad. Sci. USA. 2013;110:6275–6280. doi: 10.1073/pnas.1304238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews ML, et al. Substrate positioning controls the partition between halogenation and hydroxylation in the aliphatic halogenase, SyrB2. Proc. Natl Acad. Sci. USA. 2009;106:17723–17728. doi: 10.1073/pnas.0909649106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaillancourt FH, Yin J, Walsh CT. SyrB2 in syringomycin E biosynthesis is a nonheme FeII α-ketoglutarate- and O2-dependent halogenase. Proc. Natl Acad. Sci. USA. 2005;102:10111–10116. doi: 10.1073/pnas.0504412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs C, et al. Novel approaches for the accumulation of oxygenated intermediates to multi-millimolar concentrations. Coord. Chem. Rev. 2013;257:234–243. doi: 10.1016/j.ccr.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seto M, Yoda Y, Kikuta S, Zhang X, Ando M. Observation of nuclear resonant scattering accompanied by phonon excitation using synchrotron radiation. Phys. Rev. Lett. 1995;74:3828–3831. doi: 10.1103/PhysRevLett.74.3828. [DOI] [PubMed] [Google Scholar]
- 21.Chumakov AI, Sturhahn W. Experimental aspects of inelastic nuclear resonance scattering. Hyperfine Interact. 1999;123–124:781–808. [Google Scholar]
- 22.Sage JT, et al. Nuclear resonance vibrational spectroscopy of a protein active-site mimic. J. Phys.: Condens. Matter. 2001;13:7707–7722. [Google Scholar]
- 23.Blasiak LC, Vaillancourt FH, Walsh CT, Drennan CL. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature. 2006;440:368–371. doi: 10.1038/nature04544. [DOI] [PubMed] [Google Scholar]
- 24.Diebold AR, et al. Activation of α-keto acid-dependent dioxygenases: application of an {FeNO}7/{FeO2}8 methodology for characterizing the initial steps of O2 activation. J. Am Chem. Soc. 2011;133:18148–18160. doi: 10.1021/ja202549q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidig ML, et al. Spectroscopic and electronic structure studies of aromatic electrophilic attack and hydrogen-atom abstraction by non-heme iron enzymes. Proc. Natl Acad. Sci. USA. 2006;103:12966–12973. doi: 10.1073/pnas.0605067103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srnec M, Wong SD, England J, Que L, Solomon EI. π-frontier molecular orbitals in S = 2 ferryl species and elucidation of their contributions to reactivity. Proc. Natl Acad. Sci. USA. 2012;109:14326–14331. doi: 10.1073/pnas.1212693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comba P, Wunderlich S. Iron-catalyzed halogenation of alkanes: modeling of nonheme halogenases by experiment and DFT calculations. Chem. Eur. J. 2010;16:7293–7299. doi: 10.1002/chem.201000092. [DOI] [PubMed] [Google Scholar]
- 28.Alp EE, Mooney TM, Toellner T, Sturhahn W. Nuclear resonant scattering beamline at the Advanced Photon Source. Hyperfine Interact. 1994;90:323–334. [Google Scholar]
- 29.Yoda Y, et al. Nuclear resonant scattering beamline at SPring-8. Nucl. Instrum. Methods A. 2001;467–468:715–718. [Google Scholar]
[REFERENCES FOR ONLINE METHODS SECTION]
- 30.Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C. Electronic structure calculations on workstation computers: the program system Turbomole. Chem. Phys. Lett. 1989;162:165–169. [Google Scholar]
- 31.Frisch MJ, et al. Gaussian 09, Revision A.1. Wallingford CT: Gaussian, Inc.; 2009. [Google Scholar]
- 32.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 33.Perdew J. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B. 1986;33:8822–8824. doi: 10.1103/physrevb.33.8822. [DOI] [PubMed] [Google Scholar]
- 34.Vosko SH, Wilk L, Nusair M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys. 2012;58:1200–1211. [Google Scholar]
- 35.Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 36.Eichkorn K, Treutler O, Öhm H, Häser M, Ahlrichs R. Auxiliary basis sets to approximate Coulomb potentials. Chem. Phys. Lett. 1995;240:283–290. [Google Scholar]
- 37.Eichkorn K, Weigend F, Treutler O, Ahlrichs R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theoret. Chim. Acta. 1997;97:119–124. [Google Scholar]
- 38.Klamt A, Schüürmann G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc., Perkin Trans. 1993;20:799–805. [Google Scholar]
- 39.Schäfer A, Klamt A, Sattel D, Lohrenz JCW, Eckert F. COSMO implementation in Turbomole: extension of an efficient quantum chemical code towards liquid systems. Phys. Chem. Chem. Phys. 2000;2:2187–2193. [Google Scholar]
- 40.Wachters A. Gaussian basis set for molecular wave functions containing third-row atoms. J. Chem. Phys. 1970;52:1033–1036. [Google Scholar]
- 41.Hay PJ. Gaussian basis sets for molecular calculations. The representation of 3d orbitals in transition-metal atoms. J. Chem. Phys. 1977;66:4377–4384. [Google Scholar]
- 42.McLean AD, Chandler GS. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980;72:5639–5648. [Google Scholar]
- 43.Krishnan R, Binkley JS, Seeger R, Pople JA. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980;72:650–654. [Google Scholar]
- 44.Mennucci B, Tomasi J. Continuum solvation models: a new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997;106:5151–5158. [Google Scholar]
- 45.Mennucci B, Cancès E, Tomasi J. Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: theoretical bases, computational implementation, and numerical applications. J. Phys. Chem. B. 1997;101:10506–10517. [Google Scholar]
- 46.Cammi R, Mennucci B, Tomasi J. Second-order Møller–Plesset analytical ferivatives for the polarizable continuum model using the relaxed density approach. J. Phys. Chem. A. 1999;103:9100–9108. [Google Scholar]
- 47.Cammi R, Mennucci B, Tomasi J. Fast evaluation of geometries and properties of excited molecules in solution: a Tamm-Dancoff model with application to 4-dimethylamino-enzonitrile. J. Phys. Chem. A. 2000;104:5631–5637. [Google Scholar]
- 48.Leu BM, et al. Quantitative vibrational dynamics of iron in nitrosyl porphyrins. J. Am. Chem. Soc. 2004;126:4211–4227. doi: 10.1021/ja038526h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenderholt A. gennrvs, Pymol script. 2009 available at http://www.stanford.edu/group/solomon/gennrvs/gennrvs.py.txt. [Google Scholar]
- 50.Srnec M, et al. Structural and Spectroscopic properties of the peroxodiferric intermediate of Ricinus communis soluble Δ9 desaturase. Inorg. Chem. 2012;51:2806–2820. doi: 10.1021/ic2018067. [DOI] [PubMed] [Google Scholar]
- 51.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648. [Google Scholar]
- 52.Lee C, Yang W, Parr R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 53.Miehlich B, Savin A, Stoll H, Preuss H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys Lett. 1989;157:200–260. [Google Scholar]
- 54.Grimme S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004;25:1463–1473. doi: 10.1002/jcc.20078. [DOI] [PubMed] [Google Scholar]
- 55.Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006;27:1787–1799. doi: 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.