Abstract
The highly dynamic nature of voltage-gated Na+ channel (VGSC) expression and its controlling mechanism(s) are not well understood. In this study, we investigated the possible involvement of nerve growth factor (NGF) in regulating VGSC activity in the strongly metastatic Mat-LyLu cell model of rat prostate cancer (PCa). NGF increased peak VGSC current density in a time- and dose-dependent manner. NGF also shifted voltage to peak and the half-activation voltage to more positive potentials, and produced currents with faster kinetics of activation; sensitivity to the VGSC blocker tetrodotoxin (TTX) was not affected. The NGF-induced increase in peak VGSC current density was suppressed by both the pan-trk antagonist K252a, and the protein kinase A (PKA) inhibitor KT5720. NGF did not affect the Nav1.7 mRNA level, but the total VGSC α-subunit protein level was upregulated. NGF potentiated the cells’ migration in Transwell assays, and this was not affected by TTX. We concluded that NGF upregulated functional VGSC expression in Mat-LyLu cells, with PKA as a signalling intermediate, but enhancement of migration by NGF was independent of VGSC activity.
Keywords: Mat-LyLu, NGF, PKA, Prostate cancer, Voltage-gated Na+ channel
INTRODUCTION
Voltage-gated Na+ channels (VGSCs) are expressed not just in ‘excitable’ tissues (nerve and muscle), but also in a variety of ‘non-excitable’ cells, including lymphocytes (DeCoursey et al., 1985), endothelial cells (Gordienko and Tsukahara, 1994), fibroblasts (Bakhramov et al., 1995), and glial cells (Chiu and Ritchie, 1984). Importantly, VGSC expression is highly dynamic, although the underlying mechanisms are not well understood (Diss et al., 2004). Nerve growth factor (NGF) is a member of the neurotrophin (NT) family of secreted proteins, which are well known for their involvement in neuronal growth promotion, survival, differentiation, plasticity and functional maintenance (Kovalchuk et al., 2004; Lu et al., 2005). Among the functional targets of NGF signalling are VGSCs (Dib-Hajj et al., 1998; Hilborn et al., 1997; Toledo-Aral et al., 1995). NGF may be associated with a variety of downstream signalling intermediates, including protein kinase A (PKA) (D’Arcangelo et al., 1993; Kalman et al., 1990).
VGSC upregulation has been found in human prostate cancer (PCa) in vitro and in vivo, and correlated with metastatic progression (Diss et al., 2005). In vitro, VGSC activity has been shown to potentiate a variety of cell behaviours associated with the metastatic cascade, including morphological development and cellular process extension (Fraser et al., 1999), galvanotaxis (Djamgoz et al., 2001), lateral motility (Fraser et al., 2003), endocytic membrane activity (Krasowska et al., 2004; Mycielska et al., 2003), gene expression including activity-dependent regulation (Brackenbury and Djamgoz, 2006; Mycielska et al., 2005), and invasion (Bennett et al., 2004; Grimes et al., 1995; Laniado et al., 1997; Smith et al., 1998). These findings imply that VGSCs are tonically active in metastatic PCa cells, and enhance metastatic cell behaviour. In fact, functional VGSC expression was considered to be “necessary and sufficient” for potentiation of PCa cell invasiveness (Bennett et al., 2004).
In the strongly metastatic Dunning rat PCa Mat-LyLu cell line, VGSC/Nav1.7 α-subunit mRNA was upregulated over 1000-fold, compared to the isogenic weakly metastatic AT-2 cells (Diss et al., 2001). Although the mechanism responsible for the VGSC upregulation is not yet known, serum concentration was found to modify VGSC current amplitude and kinetics, raising the possibility of modulation of VGSC expression/activity by growth factor(s) (Ding and Djamgoz, 2004). Interestingly, prostate contains one of the highest levels of NGF outside the nervous system (MacGrogan et al., 1992; Murphy et al., 1984). NGF also plays a significant role in proliferation, differentiation and apoptosis in a variety of cancers (Nakagawara, 2001). As regards PCa, it has been shown that anchorage-independent growth of weakly metastatic human LNCaP cells can be stimulated by NGF (Delsite and Djakiew, 1999). Furthermore, NGF secretion has been detected in the strongly metastatic DU145 and PC-3 cell lines, and this enhanced the cells’ invasive capacity in vitro (Djakiew et al., 1993; Geldof et al., 1997).
The available data, taken together, would raise the possibility, therefore, that NGF could be involved in VGSC regulation in PCa cells. In the present study, we investigated this possibility, using the rat Mat-LyLu cell model of metastatic PCa.
MATERIALS AND METHODS
Cell culture and pharmacological treatments
Mat-LyLu cells were cultured as described before (Grimes and Djamgoz, 1998). Prior to addition of any pharmacological agent, including NGF, cells were seeded in normal medium for 24 h, then washed 5 times in serum-free RPMI 1640. For some migration assays, cells were maintained for an additional 24 h in serum-free RPMI 1640 prior to addition of compounds. The following agents were used, added to serum-free RPMI 1640: NGF (1-100 ng/ml; Alomone), K252a (100 nM; Calbiochem), KT5720 (500 nM; Calbiochem), tetrodotoxin (TTX; 1 μM; Alomone). K252a is a general inhibitor of trk (including NGF) receptors (Mallei et al., 2004; Shimazu et al., 2005; Turner et al., 2004). KT5720 has previously been shown to inhibit PKA in a range of cells, including cancer (Ungefroren et al., 1997; Yang et al., 2003; Yoshida et al., 2005). We have previously shown that KT5720 would completely inhibit PKA activity in Mat-LyLu cells (Brackenbury and Djamgoz, 2006). All pharmacological agents used were non-toxic at their working concentrations, as described previously (Fraser et al., 2003).
Electrophysiology
Whole-cell patch clamp recordings were performed on single cells as described previously (Grimes and Djamgoz, 1998). Three voltage-clamp protocols were used (holding potential = −100 mV):
Basic current-voltage (I-V) protocol: Cells were depolarised to test potentials within the range −70 to +70 mV in 5 mV steps. The test pulse duration was 60 ms; the interpulse duration was 2 s.
Steady-state inactivation protocol: Prepulses in the range −130 to −10 mV were applied in 10 mV steps for durations of 1 s. A test pulse of −10 mV was immediately applied for 80 ms. The interpulse duration was 2 s.
TTX protocol: One test pulse of −10 mV (duration 60 ms) was applied while cell was perfused with normal external bath solution. TTX (1 nM - 6 μM) was applied to the cell for 30 s and then the test pulse was repeated. Reversibility of the effect of TTX was tested after returning to normal external bath solution and presenting a final test pulse.
Recordings were obtained from up to 20 cells per condition, from at least 3 repeat treatments. Data from individual dishes were combined to provide an overall mean and standard error (SEM).
Real-time PCR
Extraction of total RNA, cDNA synthesis and real-time PCR were performed as before (Mycielska et al., 2005). Cytochrome b5 reductase (Cytb5R) gene, shown previously to be unchanged in rat PCa, was the ‘internal’ control gene (Diss et al., 2001). The following primer pairs were used:
Nav1.7: 5′-TTCATGACCTTGAGCAACCC-3′ and 5′-TCTCTTCGAGTTCCTTCCTG-3′; annealing temperature, 60 °C;
Cytb5R: 5′-ACACGCATCCCAAGTTTCCA-3′ and 5′-CATCTCCTCATTCACGAAGC-3′; annealing temperature, 60 °C.
The threshold amplification cycles were determined using the Opticon Monitor 2 software (MJ Research) and then analysed by the 2−ΔΔCT method (Livak and Schmittgen, 2001). The level of Nav1.7 mRNA was compared relative to untreated control cells, for three separate treatments.
Western blotting
Western blots were performed as described previously (Laniado et al., 1997). The primary antibodies used and their dilutions were as follows:
Pan-VGSC antibody (1 μg/ml; Upstate), and
Anti-actinin antibody (1 μl/ml; Sigma).
The secondary antibodies were peroxidase-conjugated swine anti-rabbit, and goat anti-mouse, respectively (Dako). Densitometric analysis was performed using Image- Pro Plus software (Media Cybernetics). Signal intensity was normalised to anti- actinin antibody as a loading control/reference, for at least 3 separate treatments.
Immunocytochemistry and confocal microscopy
Immunocytochemistry was performed as described previously (Chioni et al., 2005). Cells were labelled first with fluorescein isothiocyanate (FITC)-conjugated concanavalin A (Sigma) for 20 min as a plasma membrane marker, and then permeabilised in saponin (0.1 %) for 5 min. Cells were incubated with the pan-VGSC primary antibody for 1 h. The secondary antibody was Alexa567-conjugated goat anti-rabbit IgG (Dako) and mounting was in Vectashield (Vector Laboratories). Samples were viewed using a Leica DM IRBE microscope (with a ×100 objective) with a confocal laser scanner (Leica TCS-NT with Ar/Kr laser).
Digital image analysis
Densitometric analysis was performed using the LCS Lite software (Leica), as follows:
Protein distribution was determined using the “straight line profile” function drawn across the cytoplasm avoiding the nucleus, as described previously (Brackenbury and Djamgoz, 2006). Signal intensity in plasma membrane region, set to cover 1.5 μm inward from the edge of concanavalin A staining, was compared with cytoplasmic signal intensity within the central 30 % of the line profile. Measurements were taken from ≥ 6 cells (randomly chosen) per condition, for three repeat treatments.
Cell surface VGSC expression was determined using the “freeform line profile” function drawn around the cell surface, determined by concanavalin A staining. Measurements were taken from 30 cells per condition, for three repeat treatments.
Internal protein level was assessed in 4.8 μm2 rectangular sections using the “area histogram” function. Measurements were taken from 30 cells per condition, for three repeat treatments.
Migration assay
In order to achieve steady-states, cells were maintained for 24 h in serum-free medium, prior to treatment with NGF (20 ng/ml) and/or TTX (1 μM) for a further 24 h. Cells (1.5 × 105 cells/ml) were then plated onto 12 μm-pore Transwell filters in a 12-well plate, according to the manufacturer’s instructions (Corning), in a 0.1-1 % FBS chemotactic gradient. The number of cells migrating over 7 h was determined using the MTT assay (Grimes et al., 1995). Results were compiled as the mean of five repeats containing at least two platings.
Curve fitting and data analysis
Conductance-voltage relationships and curve fitting were performed as described previously (Ding and Djamgoz, 2004). TTX dose-response data were fitted using Excel (Micosoft) and Origin (OriginLab, Northampton, MA) software to a Langmuir adsoption isotherm:
where IC50 is the concentration of TTX for 50 % VGSC blockage. All quantitative data are presented as means ± standard errors, unless stated otherwise. Statistical significance was determined with Student’s t test, or ANOVA followed by Newman- Keuls post hoc analysis, as appropriate. Results were considered significant at P < 0.05 (*).
RESULTS
NGF increased VGSC functional expression in a dose-dependent manner
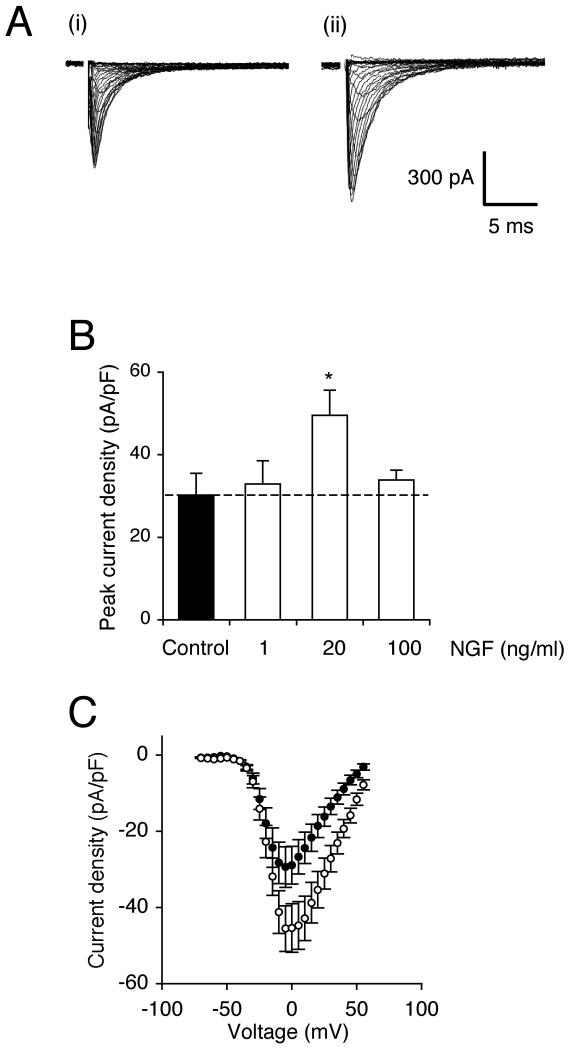
Pre-incubation of Mat-LyLu cells in NGF (20 ng/ml) for 24 h significantly increased peak VGSC current density by 62 %, from −30.8 ± 5.6 pA/pF to −49.9 pA/pF (P < 0.05; n = 20 for each; Figure 1A, B). Lower (1 ng/ml) and higher (100 ng/ml) concentrations of NGF had no effect (Figure 1B). Accordingly, 20 ng/ml NGF was adopted as the working concentration in the remainder of the experiments. Short-term (30 min) incubation with 20 ng/ml NGF had no effect on peak VGSC current density.
Figure 1. NGF increased peak VGSC current density.
(A) Typical whole-cell VGSC currents elicited by 60 ms depolarising voltage pulses between −70 mV and +70 mV applied from a holding potential of −100 mV: (i) a control cell; (ii) a cell pre-treated with 20 ng/ml NGF for 24 h. (B) Quantitative comparison of peak current densities recorded in control cells and cells pre-treated with 1-100 ng/ml NGF for 24 h. (C) Mean current-voltage relationships for control cells (dark circles) and cells pre-treated with 20 ng/ml NGF for 24 h (light circles). Data are presented as mean ± SEM (n = 20). Significance: (*) P < 0.05; ANOVA with Newman-Keuls.
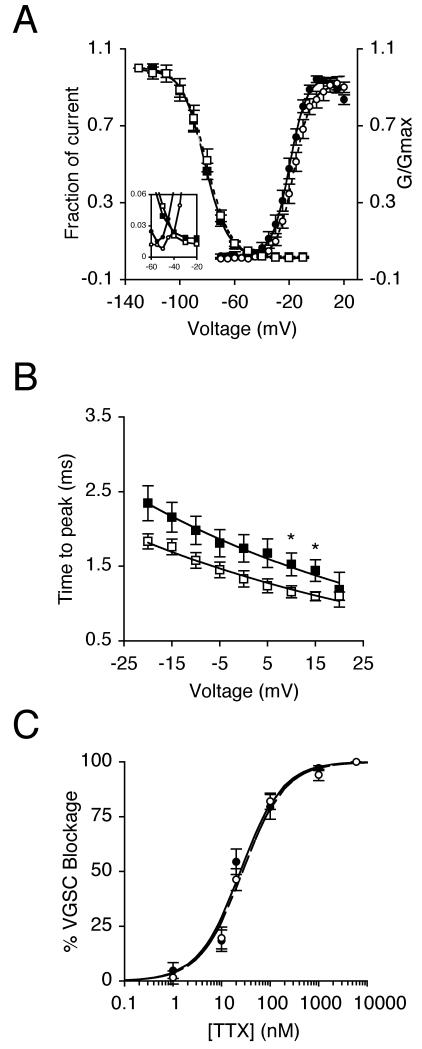
The effect of 24 h incubation with NGF (20 ng/ml) on VGSC characteristics was studied further (Table I). NGF significantly depolarised voltage for current peak (Vp) from −5.0 ± 2.1 mV to 2.3 ± 2.1 mV (P < 0.05; n = 20 for each) and also depolarised the half-activation voltage (V1/2) from −19.7 ± 1.6 mV to −15.3 ± 1.4 mV (P < 0.05; n = 20 for each; Figures 1C, 2A). There was no effect on activation voltage, activation slope factor, or steady-state inactivation (Table I). There was a window current between −55 mV and −10 mV in control cells, and this changed slightly to between −50 mV and −20 mV in NGF-treated cells (Figure 2A inset). The NGF treatment partially reduced the voltage dependency of time to peak (Tp; Figure 2B, Table I). There was no effect on fast (τf) and slow (τs) time constants of inactivation at 10 mV, derived from double-exponential fits (Table I). Similarly, NGF had no effect on the cells’ TTX sensitivity profile (Figure 2C), and the IC50 was unchanged at ~21 nM (Table I).
Table 1. Effect of 24 h treatment with NGF (20 ng/ml) on VGSC characteristics in Mat-LyLu cells.
| Parametera | Control | NGF | P |
|---|---|---|---|
| Ip (pA/pF) | −30.8±5.6 | −49.9±6.2 | 0.03* |
| Va (Mv) | −41.3±1.5 | −40.8±1.2 | 0.77 |
| Vp (mV) | −5.0±2.1 | 2.3±2.1 | 0.02* |
| Activation V1/2 (mV) | −19.7±1.6 | −15.3±1.4 | 0.04* |
| Activation k (mV) | 5.4±0.4 | 5.4±0.3 | 0.99 |
| Inactivation V1/2 (mV) | −84.2±2.9 | −80.6±1.7 | 0.30 |
| Inactivation k (mV) | −7.0±0.5 | −8.0±0.3 | 0.11 |
| Tp at 10 mV(msec) | 1.7±0.2 | 1.2±0.1 | 0.04* |
| Tf at 10 mV(msec) | 0.9±0.1 | 0.8±0.04 | 0.46 |
| Ts at 10 mV(msec) | 4.7±0.4 | 4.8±0.2 | 0.79 |
| IC50 (nM) | 21.4±4.8 | 20.7±5.9 | 0.93 |
Ip, peak current density; Va, activation voltage; Vp, voltage at current peak; V1/2, half-(in)activation voltage; k, slope factor; Tp, time to peak; Tf/s, fast/slow time constant of inactivation. IC50, concentration of TTX at which 50% of VGSC current is inhibited. Data expressed as mean±SEM (n ≥ 6).
P < 0.05; Student’s t-test.
Figure 2. NGF decreased time to peak but did not affect TTX sensitivity.
(A) Mean availability- voltage (squares) and relative conductance (G/Gmax)-voltage relationships (circles) for control cells (dark symbols) and cells pre-treated with 20 ng/ml NGF for 24 h (light symbols). Control (solid lines) and NGF data (dotted lines) are fitted with Boltzmann functions. Inset magnifies a window in which current is activated and not fully inactivated. (B) Dependence of time to peak on membrane voltage for control cells (dark squares), and cells treated with 20 ng/ml NGF for 24 h (light squares). Control (solid line) and NGF data (dashed line) are fitted with single exponential functions. (C) Reduction of VGSC current by TTX for control cells (dark circles), and cells treated with 20 ng/ml NGF for 24 h (light circles). Control (solid line) and NGF data (dashed line) are fitted to Langmuir adsoption isotherms. Data are presented as mean ± SEM (n > 17). Significance: (*) P < 0.05; Student’s t test.
Incubation with K252a (100 nM), a pan-trk receptor inhibitor (Mallei et al., 2004; Shimazu et al., 2005; Turner et al., 2004) for 24 h had no effect on peak VGSC current density (P = 0.19; n = 20 for each). However, co-application of K252a with NGF (20 ng/ml) blocked the increasing effect of NGF on peak current density (P = 0.58 compared with K252a alone; n > 19 for each).
In summary, the 24 h NGF treatment upregulated VGSC functional expression. TTX sensitivity was unchanged, consistent the VGSC isoform expression profile remaining the same.
Inhibition of PKA abrogated the effect of NGF on VGSC current enhancement
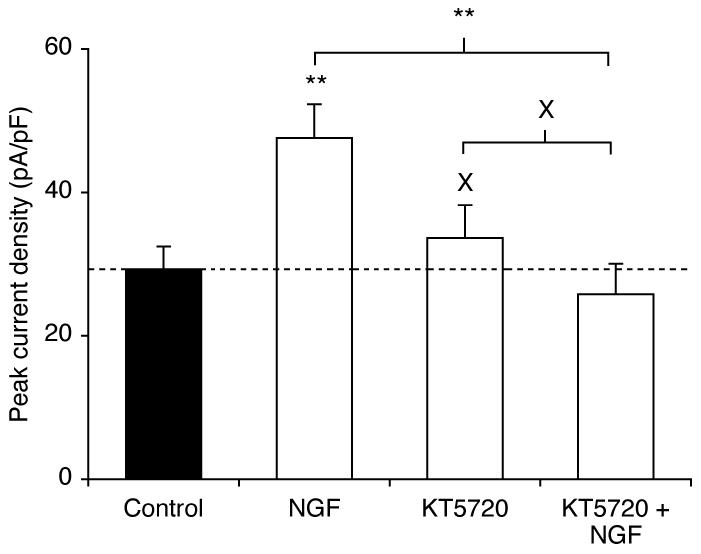
Treatment with KT5720 (500 nM), a PKA inhibitor (Brackenbury and Djamgoz, 2006; Cabell and Audesirk, 1993) for 24 h also had no effect on peak VGSC current density (P = 0.51; n > 19 for each; Figure 3). However, when co-applied with NGF (20 ng/ml), KT5720 blocked the effect of NGF on peak VGSC current density (P = 0.54, cf. KT5720 alone; P < 0.01 cf. NGF alone; n > 19; Figure 3).
Figure 3. The PKA inhibitor KT5720 reversed the potentiating effect of NGF on peak current density.
Peak current densities were recorded after pre-treatment for 24 h in control conditions, or with NGF (20 ng/ml) and/or KT5720 (500 nM). Data are presented as mean and SEM (n > 19). Significance: (X) P > 0.05 cf. control; (*) P < 0.05; ANOVA with Newman-Keuls.
These data are consistent with NGF increasing VGSC functional expression via PKA activity. We next analysed at what level(s) (mRNA, protein, post- translational modification) the VGSC upregulation occurred.
NGF had no effect on Nav1.7 mRNA expression but increased VGSC protein level
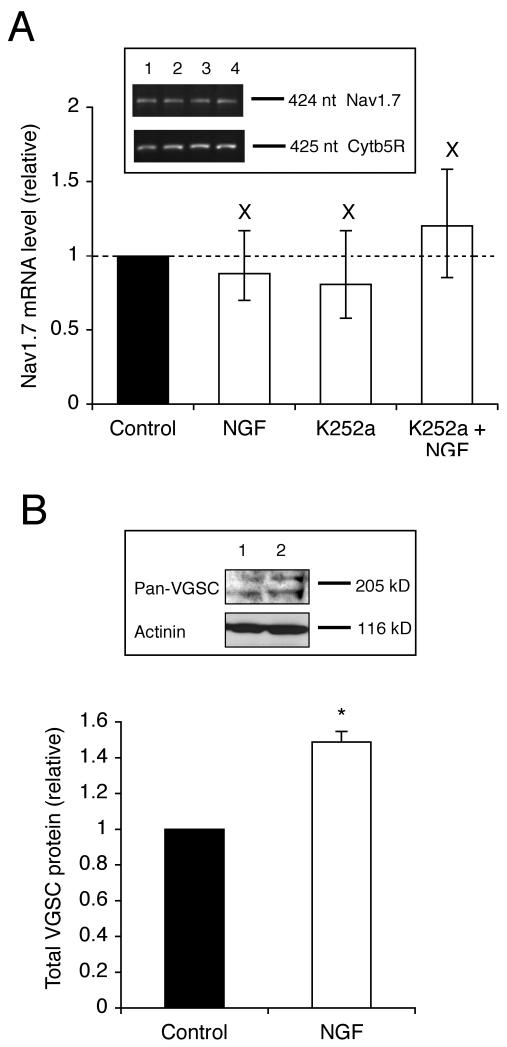
Mat-LyLu cells were treated with NGF and/or K252a for 24 h, after which the mRNA level of Nav1.7, the predominant VGSC isoform expressed in PCa cells (Diss et al., 2001), was assessed by real-time PCR. NGF (20 ng/ml) did not significantly affect the Nav1.7 mRNA level (P = 0.53; n = 3; Figure 4A). Similarly, K252a (100 nM) had no effect, either when applied alone, or when co-applied with NGF (P = 0.56 and 0.30, respectively; n = 3; Figure 4A).
Figure 4. NGF did not affect the Nav1.7 mRNA level, but increased the total VGSC protein level.
(A) Relative Nav1.7 mRNA levels in control cells and cells treated for 24 h with NGF (20 ng/ml), K252a (100 nM), and NGF (20 ng/ml) + K252a (100 nM). The Nav1.7 level was normalised to cytochrome-b5 reductase (Cytb5R) by the 2−ΔΔCt method. Errors are propagated through the 2−ΔΔCt analysis. Inset, typical gel images of PCR products for Nav1.7 and Cytb5R. Lanes: 1, control; 2, pre-treated for 24 h with NGF (20 ng/ml); 3, K252a (100 nM); 4, NGF (20 ng/ml) + K252a (100 nM). (B) Relative total VGSC protein level in control cells and cells treated with NGF (20 ng/ml) for 24 h. The VGSC α-subunit protein level was normalised to the actinin control. Inset, Western blot with 60 μg of total protein per lane from cells treated with or without NGF (20 ng/ml) for 24 h, using a pan-VGSC antibody, and an actinin antibody as a control for loading. Data are presented as mean and SEM (n = 3). Significance: (X) P > 0.05; (*) P < 0.05; (A) ANOVA with Newman-Keuls; (B) Student’s t test.
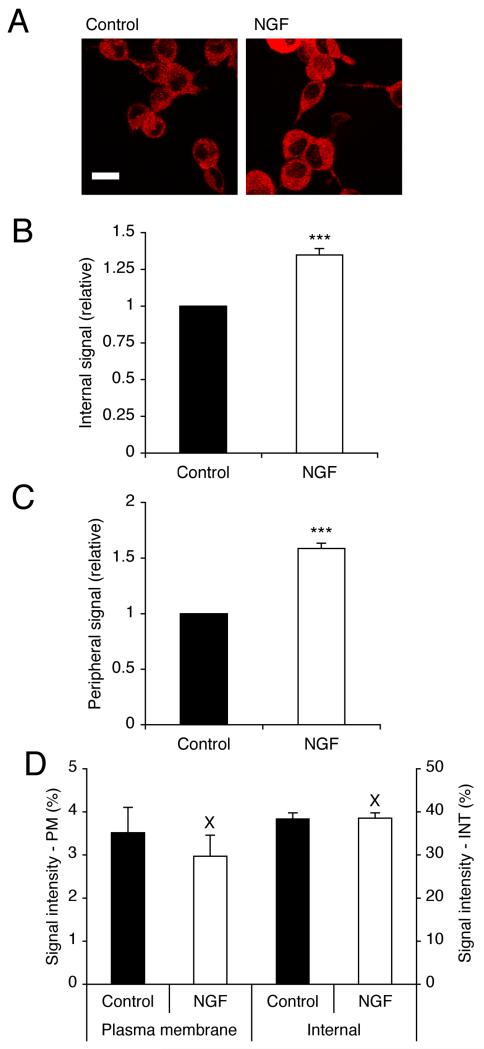
Treatment with NGF for 24 h increased the total VGSC α-subunit protein level, detected by Western blot with a pan-VGSC antibody, by 49 % (P < 0.05; n = 3; Figure 4B). Consistent with this, confocal immunocytochemistry with the pan-VGSC antibody also revealed that NGF increased the VGSC α-subunit protein level (Figure 5A). After treatment with NGF for 24 h, the level of internal and plasma membrane VGSC α-subunit protein levels increased by 35 ± 0.5 % and 59 ± 0.5 %, respectively (P < 0.001 for both; n = 90 cells for each; Figure 5A-C).
Figure 5. NGF increased VGSC immunoreactivity internally and at the plasma membrane.
(A) Typical confocal images of control cells, and cells treated with NGF (20 ng/ml) for 24 h, immunolabelled with pan-VGSC α-subunit antibody. Scale bar, 15 μm. (B) Relative internal VGSC protein level in control cells, and cells treated with NGF (20 ng/ml) for 24 h. (C) Relative peripheral VGSC protein level in control cells, or cells treated with NGF (20 ng/ml) for 24 h. (D) VGSC protein distribution along subcellular cross-sections (%). Left-hand bars, 1.5 μm sections measured inward from edge of concanavalin A staining; Right-hand bars, middle 30 % of cross-section. PM, plasma membrane; INT, internal. Data are presented as mean and SEM (B, C n = 90; D, n = 20). Significance: (X) P > 0.05; (***) P < 0.001; Student’s t test.
The distribution of VGSC immunoreactivity along cellular cross-sections was quantified and two regions were compared: (1) ‘plasma membrane’ and (2) ‘internal’ (Brackenbury and Djamgoz, 2006). The relative level of VGSC protein in both regions was unaffected by the NGF treatment, consistent with there being no change in the trafficking (P = 0.48 and P = 0.93, respectively; n = 20 cells for each; Figure 5D). We concluded that NGF increased VGSC α-subunit protein expression, without affecting the Nav1.7 mRNA level, or the cycling balance of VGSC proteins.
NGF increased migration in vitro
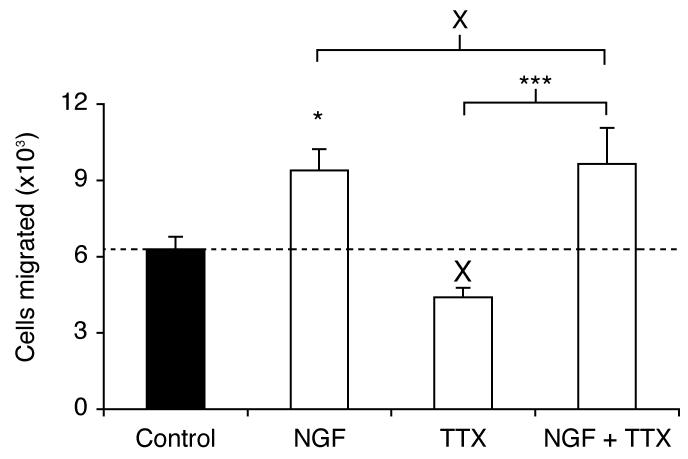
Pre-treatment of Mat-LyLu cells with NGF (20 ng/ml) for 24 h increased migration by 49 % (P < 0.05; n = 12; Figure 6). In contrast, under these conditions, TTX (1 μM) had no effect compared to control (P = 0.134; n = 12). In the presence of TTX, NGF could still increase migration (P < 0.001 cf. TTX alone; n = 12). In fact, there was no difference between the effects of NGF alone, and NGF + TTX (P = 0.83; n = 12). It was concluded that in serum-free growth medium, NGF increased migration, without any VGSC involvement.
Figure 6. NGF increased the relative number of cells migrating through a Transwell chamber over 7 h.
Cells were grown for 24 h in serum-free medium, or with NGF (20 ng/ml), TTX (1 μM), or NGF (20 ng/ml) + TTX (1 μM), prior to the Transwell assay. Data are presented as mean and SEM (n = 12). Significance: (X) P > 0.05, (*) P < 0.05; ANOVA with Newman-Keuls.
DISCUSSION
The main conclusions of this study are as follows: (1) NGF increased total and plasma membrane VGSC protein levels in Mat-LyLu cells, without affecting the level of Nav1.7 mRNA, or the balance of protein cycling. (2) NGF increased VGSC current density, via trk receptor(s). (3) The NGF-induced increase in VGSC current density was dependent on PKA activity. (4) NGF increased migration in vitro, independent of VGSC activity.
Involvement of trk receptors and PKA
Treatment with NGF for 24 h increased VGSC peak current density in a bell-shaped dose-dependent manner, as reported before for growth factors (e.g. Meng et al., 2006; Neal et al., 2003; Wei et al., 2004). Co-application of the pan-trk receptor inhibitor K252a (Mallei et al., 2004; Shimazu et al., 2005) with NGF prevented the NGF- induced increase in VGSC current density, consistent with the NGF effect being mediated by trk receptor activation. TrkA receptors were previously shown to be expressed in various Dunning cell lines and the K252a analogue CEP-751 inhibited their in vivo growth (Dionne et al., 1998).
Importantly, in Mat-LyLu cells, the NGF-induced increase in VGSC current density was blocked completely by the PKA inhibitor KT5720 (Figure 3). It has also been reported elsewhere that NGF could increase VGSC functional activity by activating PKA. For example, in PC12 cells, treatment with NGF or activation of PKA (with forskolin or 8-Br-cyclic AMP) for 1-10 days, increased VGSC current density (Bouron et al., 1999; Furukawa et al., 1993), and this could be via activation of PKA (D’Arcangelo et al., 1993; Kalman et al., 1990).
Effects of NGF on VGSC mRNA and protein levels
Treatment for 24 h with NGF did not significantly affect the mRNA level of Nav1.7, the predominant isoform expressed in Mat-LyLu cells (Diss et al., 2001). However, similar treatment increased the total VGSC protein level and membrane current density. These data suggested that NGF induced de novo VGSC protein synthesis, also consistent with ‘short-term’ (30 min) treatment having no effect. The extent of the VGSC protein increase was similar for both the cytoplasm and the plasma membrane. This could be due to the following: (1) The effect of NGF was transcriptional, but included de novo synthesis of mRNA of VGSC isoform(s) other than Nav1.7. For example, NGF has previously been shown to induce Nav1.2 mRNA expression in PC12 cells (D’Arcangelo et al., 1993). (2) The effect of NGF on transcription of Nav1.7 and/or other VGSC isoforms was transient, and occurred prior to the PCR assay at 24 h. (3) The NGF effect was mainly post-transcriptional, upregulating the level of VGSC proteins. Whilst these could include Nav1.7, TTX- resistant VGSCs (Nav1.5, Nav1.8 and Nav1.9) can be ruled out since the IC50 for TTX did not change. Regulation of mRNA and protein levels may be separate and independent (Gu et al., 2006; Martin and Zukin, 2006; Orphanides and Reinberg, 2002; Pfeiffer and Huber, 2006; Ropponen et al., 2001; Schedel et al., 2004; Sola et al., 1999). Furthermore, mRNA localisation/degradation, and translational control processes may be involved (Ben Fredj et al., 2004; St Johnston, 2005; Tiedge et al., 1999). Interestingly, PKA itself has been found to control localised protein synthesis from docked mRNA (Smith et al., 2005). (4) The NGF/PKA-induced upregulation of VGSC protein level was caused by an increase in protein stability, e.g. by reducing ubiquitination (Hino et al., 2005). Further work would be required to evaluate these possibilities, and to confirm these effects in other PCa cell lines, including those of human origin.
NGF, VGSC activity and control of migration
Overall, the data taken together with the published evidence are consistent with NGF increasing VGSC functional expression in Mat-LyLu cells, as follows:
NGF → Trk receptor → PKA activation (Bouron et al., 1999; D’Arcangelo et al., 1993; Kalman et al., 1990) → VGSC α-subunit protein upregulation → increased functional VGSC availability (Wada et al., 2004; Yuhi et al., 1996; Zhou et al., 2000). This scheme does not exclude the possibility that the pro-migratory effect of NGF could occur through PKA.
Pre-treatment of Mat-LyLu cells in serum-free growth medium with NGF for 24 h enhanced migration by ~50 %, in general agreement with the potentiating role of NGF in PCa metastasis (Geldof et al., 1997; Montano and Djamgoz, 2004; Sortino et al., 2000). Interestingly, similar pre-treatment with TTX did not significantly reduce migration in the presence or absence of NGF, suggesting that in serum-free conditions, VGSC activity was not involved in potentiating migration, and that the enhancement by NGF was independent of VGSC activity. However, other ion channels may play a role, e.g. voltage-gated K+ channels (Kim et al., 2004; O’Grady and Lee, 2005). In contrast, we have previously shown that when Mat-LyLu cells were grown in 1 % serum, TTX inhibited migration (Brackenbury and Djamgoz, 2006). Thus, different serum factor(s) may be required for the VGSC-dependent potentiation of migration and/or the NGF-induced upregulation of VGSC activity could enhance other component(s) of the metastatic cascade.
ACNOWLEDGEMENTS
This work was funded by a UK MRC Priority Area (Prostate Cancer) PhD studentship and the Pro Cancer Research Fund (PCRF).
REFERENCES
- Bakhramov A, Boriskin YS, Booth JC, Bolton TB. Activation and deactivation of membrane currents in human fibroblasts following infection with human cytomegalovirus. Biochim Biophys Acta. 1995;1265(2-3):143–151. doi: 10.1016/0167-4889(94)00230-c. [DOI] [PubMed] [Google Scholar]
- Ben Fredj N, Grange J, Sadoul R, Richard S, Goldberg Y, Boyer V. Depolarization-induced translocation of the RNA-binding protein Sam68 to the dendrites of hippocampal neurons. J Cell Sci. 2004;117(Pt 7):1079–1090. doi: 10.1242/jcs.00927. [DOI] [PubMed] [Google Scholar]
- Bennett ES, Smith BA, Harper JM. Voltage-gated Na(+) channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447(6):908–914. doi: 10.1007/s00424-003-1205-x. [DOI] [PubMed] [Google Scholar]
- Bouron A, Becker C, Porzig H. Functional expression of voltage-gated Na+ and Ca2+ channels during neuronal differentiation of PC12 cells with nerve growth factor or forskolin. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(5):370–377. doi: 10.1007/pl00005363. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na(+) channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573(Pt 2):343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabell L, Audesirk G. Effects of selective inhibition of protein kinase C, cyclic AMP-dependent protein kinase, and Ca(2+)-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurons. Int J Dev Neurosci. 1993;11(3):357–368. doi: 10.1016/0736-5748(93)90007-z. [DOI] [PubMed] [Google Scholar]
- Chioni AM, Fraser SP, Pani F, Foran P, Wilkin GP, Diss JK, Djamgoz MB. A novel polyclonal antibody specific for the Na(v)1.5 voltage-gated Na(+) channel ‘neonatal’ splice form. J Neurosci Methods. 2005;147(2):88–98. doi: 10.1016/j.jneumeth.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. On the physiological role of internodal potassium channels and the security of conduction in myelinated nerve fibres. Proc R Soc Lond B Biol Sci. 1984;220(1221):415–422. doi: 10.1098/rspb.1984.0010. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Paradiso K, Shepherd D, Brehm P, Halegoua S, Mandel G. Neuronal growth factor regulation of two different sodium channel types through distinct signal transduction pathways. J Cell Biol. 1993;122(4):915–921. doi: 10.1083/jcb.122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-dependent ion channels in T-lymphocytes. J Neuroimmunol. 1985;10(1):71–95. doi: 10.1016/0165-5728(85)90035-9. [DOI] [PubMed] [Google Scholar]
- Delsite R, Djakiew D. Characterization of nerve growth factor precursor protein expression by human prostate stromal cells: a role in selective neurotrophin stimulation of prostate epithelial cell growth. Prostate. 1999;41(1):39–48. doi: 10.1002/(sici)1097-0045(19990915)41:1<39::aid-pros6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol. 1998;79(5):2668–2676. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- Ding Y, Djamgoz MB. Serum concentration modifies amplitude and kinetics of voltage-gated Na+ current in the Mat-LyLu cell line of rat prostate cancer. Int J Biochem Cell Biol. 2004;36(7):1249–1260. doi: 10.1016/j.biocel.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Dionne CA, Camoratto AM, Jani JP, Emerson E, Neff N, Vaught JL, Murakata C, Djakiew D, Lamb J, Bova S, George D, Isaacs JT. Cell cycle- independent death of prostate adenocarcinoma is induced by the trk tyrosine kinase inhibitor CEP-751 (KT6587) Clin Cancer Res. 1998;4(8):1887–1898. [PubMed] [Google Scholar]
- Diss JK, Archer SN, Hirano J, Fraser SP, Djamgoz MB. Expression profiles of voltage-gated Na(+) channel alpha-subunit genes in rat and human prostate cancer cell lines. Prostate. 2001;48(3):165–178. doi: 10.1002/pros.1095. [DOI] [PubMed] [Google Scholar]
- Diss JK, Fraser SP, Djamgoz MB. Voltage-gated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J. 2004;33(3):180–193. doi: 10.1007/s00249-004-0389-0. [DOI] [PubMed] [Google Scholar]
- Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8(3):266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- Djakiew D, Pflug BR, Delsite R, Onoda M, Lynch JH, Arand G, Thompson EW. Chemotaxis and chemokinesis of human prostate tumor cell lines in response to human prostate stromal cell secretory proteins containing a nerve growth factor-like protein. Cancer Res. 1993;53(6):1416–1420. [PubMed] [Google Scholar]
- Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, Korohoda W. Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltage gated Na+ channel activity. J Cell Sci. 2001;114(Pt 14):2697–2705. doi: 10.1242/jcs.114.14.2697. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Ding Y, Liu A, Foster CS, Djamgoz MB. Tetrodotoxin suppresses morphological enhancement of the metastatic MAT-LyLu rat prostate cancer cell line. Cell Tissue Res. 1999;295(3):505–512. doi: 10.1007/s004410051256. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Salvador V, Manning EA, Mizal J, Altun S, Raza M, Berridge RJ, Djamgoz MB. Contribution of functional voltage-gated Na(+) channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. lateral motility. J Cell Physiol. 2003;195(3):479–487. doi: 10.1002/jcp.10312. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Onodera H, Kogure K, Akaike N. Time-dependent expression of Na and Ca channels in PC12 cells by nerve growth factor and cAMP. Neurosci Res. 1993;16(2):143–147. doi: 10.1016/0168-0102(93)90081-z. [DOI] [PubMed] [Google Scholar]
- Geldof AA, De Kleijn MA, Rao BR, Newling DW. Nerve growth factor stimulates in vitro invasive capacity of DU145 human prostatic cancer cells. J Cancer Res Clin Oncol. 1997;123(2):107–112. doi: 10.1007/BF01269888. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Tsukahara H. Tetrodotoxin-blockable depolarization-activated Na+ currents in a cultured endothelial cell line derived from rat interlobar arter and human umbilical vein. Pflugers Arch. 1994;428(1):91–93. doi: 10.1007/BF00374756. [DOI] [PubMed] [Google Scholar]
- Grimes JA, Djamgoz MB. Electrophysiological characterization of voltage- gated Na(+) current expressed in the highly metastatic Mat-LyLu cell line of rat prostate cancer. J Cell Physiol. 1998;175(1):50–58. doi: 10.1002/(SICI)1097-4652(199804)175:1<50::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Grimes JA, Fraser SP, Stephens GJ, Downing JE, Laniado ME, Foster CS, Abel PD, Djamgoz MB. Differential expression of voltage-activated Na(+) currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS Lett. 1995;369(2-3):290–294. doi: 10.1016/0014-5793(95)00772-2. [DOI] [PubMed] [Google Scholar]
- Gu X, Lundqvist EN, Coates PJ, Thurfjell N, Wettersand E, Nylander K. Dysregulation of TAp63 mRNA and protein levels in psoriasis. J Invest Dermatol. 2006;126(1):137–141. doi: 10.1038/sj.jid.5700010. [DOI] [PubMed] [Google Scholar]
- Hilborn MD, Rane SG, Pollock JD. EGF in combination with depolarization or cAMP produces morphological but not physiological differentiation in PC12 cells. J Neurosci Res. 1997;47(1):16–26. [PubMed] [Google Scholar]
- Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25(20):9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Wong B, Horvai AE, Cline MJ, O’Lague PH. Nerve growth factor acts through cAMP-dependent protein kinase to increase the number of sodium channels in PC12 cells. Neuron. 1990;4(3):355–366. doi: 10.1016/0896-6273(90)90048-k. [DOI] [PubMed] [Google Scholar]
- Kim Y, Uhm DY, Shin J, Chung S. Modulation of delayed rectifier potassium channel by protein kinase C zeta-containing signaling complex in pheochromocytoma cells. Neuroscience. 2004;125(2):359–368. doi: 10.1016/j.neuroscience.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Curr Opin Neurobiol. 2004;14(5):558–563. doi: 10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Krasowska M, Grzywna ZJ, Mycielska ME, Djamgoz MB. Patterning of endocytic vesicles and its control by voltage-gated Na(+) channel activity in rat prostate cancer cells: fractal analyses. Eur Biophys J. 2004;33(6):535–542. doi: 10.1007/s00249-004-0394-3. [DOI] [PubMed] [Google Scholar]
- Laniado ME, Lalani EN, Fraser SP, Grimes JA, Bhangal G, Djamgoz MB, Abel PD. Expression and functional analysis of voltage-activated Na(+) channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am J Pathol. 1997;150(4):1213–1221. [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- MacGrogan D, Saint-Andre JP, Dicou E. Expression of nerve growth factor and nerve growth factor receptor genes in human tissues and in prostatic adenocarcinoma cell lines. J Neurochem. 1992;59(4):1381–1391. doi: 10.1111/j.1471-4159.1992.tb08451.x. [DOI] [PubMed] [Google Scholar]
- Mallei A, Rabin SJ, Mocchetti I. Autocrine regulation of nerve growth factor expression by Trk receptors. J Neurochem. 2004;90(5):1085–1093. doi: 10.1111/j.1471-4159.2004.02568.x. [DOI] [PubMed] [Google Scholar]
- Martin K, Zukin R. RNA Trafficking and Local Protein Synthesis in Dendrites: An Overview. J Neurosci. 2006;26:7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Zhang Z, Zhang R, Liu X, Wang L, Robin AM, Chopp M. Biphasic effects of exogenous VEGF on VEGF expression of adult neural progenitors. Neurosci Lett. 2006;393(2-3):97–101. doi: 10.1016/j.neulet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Montano X, Djamgoz MB. Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS Lett. 2004;571(1-3):1–8. doi: 10.1016/j.febslet.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Watson AY, Rhodes JA. Biological sources of nerve growth factor. Appl Neurophysiol. 1984;47(1-2):33–42. doi: 10.1159/000101200. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Fraser SP, Szatkowski M, Djamgoz MB. Contribution of functional voltage-gated Na(+) channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: II. Secretory membrane activity. J Cell Physiol. 2003;195(3):461–469. doi: 10.1002/jcp.10265. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Palmer CP, Brackenbury WJ, Djamgoz MB. Expression of Na+- dependent citrate transport in a strongly metastatic human prostate cancer PC- 3M cell line: regulation by voltage-gated Na+ channel activity. J Physiol. 2005;563(Pt 2):393–408. doi: 10.1113/jphysiol.2004.079491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169(2):107–114. doi: 10.1016/s0304-3835(01)00530-4. [DOI] [PubMed] [Google Scholar]
- Neal M, Cunningham J, Lever I, Pezet S, Malcangio M. Mechanism by which brain-derived neurotrophic factor increases dopamine release from the rabbit retina. Invest Ophthalmol Vis Sci. 2003;44(2):791–798. doi: 10.1167/iovs.02-0557. [DOI] [PubMed] [Google Scholar]
- O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol. 2005;37(8):1578–1594. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108(4):439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Huber K. Current Advances in Local Protein Synthesis and Synaptic Plasticity. J Neurosci. 2006;26:7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropponen KM, Kellokoski JK, Pirinen RT, Moisio KI, Eskelinen MJ, Alhava EM, Kosma VM. Expression of transcription factor AP-2 in colorectal adenomas and adenocarcinomas; comparison of immunohistochemistry and in situ hybridisation. J Clin Pathol. 2001;54(7):533–538. doi: 10.1136/jcp.54.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedel J, Distler O, Woenckhaus M, Gay RE, Simmen B, Michel BA, Muller-Ladner U, Gay S. Discrepancy between mRNA and protein expression of tumour suppressor maspin in synovial tissue may contribute to synovial hyperplasia in rheumatoid arthritis. Ann Rheum Dis. 2004;63(10):1205–1211. doi: 10.1136/ard.2003.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu K, Takeda K, Yu ZX, Jiang H, Liu XW, Nelson PG, Guroff G. Multiple acute effects on the membrane potential of PC12 cells produced by nerve growth factor (NGF) J Cell Physiol. 2005;203(3):501–509. doi: 10.1002/jcp.20309. [DOI] [PubMed] [Google Scholar]
- Smith P, Rhodes NP, Shortland AP, Fraser SP, Djamgoz MB, Ke Y, Foster CS. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett. 1998;423(1):19–24. doi: 10.1016/s0014-5793(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45(5):765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Sola B, Salaun V, Ballet JJ, Troussard X. Transcriptional and post- transcriptional mechanisms induce cyclin-D1 over-expression in B-chronic lymphoproliferative disorders. Int J Cancer. 1999;83(2):230–234. doi: 10.1002/(sici)1097-0215(19991008)83:2<230::aid-ijc14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sortino MA, Condorelli F, Vancheri C, Chiarenza A, Bernardini R, Consoli U, Canonico PL. Mitogenic effect of nerve growth factor (NGF) in LNCaP prostate adenocarcinoma cells: role of the high- and low-affinity NGF receptors. Mol Endocrinol. 2000;14(1):124–136. doi: 10.1210/mend.14.1.0402. [DOI] [PubMed] [Google Scholar]
- St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6(5):363–375. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- Tiedge H, Bloom FE, Richter D. RNA, whither goest thou? Science. 1999;283(5399):186–187. doi: 10.1126/science.283.5399.186. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14(3):607–611. doi: 10.1016/0896-6273(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Murray SS, Piccenna LG, Lopes EC, Kilpatrick TJ, Cheema SS. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J Neurosci Res. 2004;78(2):193–199. doi: 10.1002/jnr.20256. [DOI] [PubMed] [Google Scholar]
- Ungefroren H, Cikos T, Krull NB, Kalthoff H. Biglycan gene promoter activity in osteosarcoma cells is regulated by cyclic AMP. Biochem Biophys Res Commun. 1997;235(2):413–417. doi: 10.1006/bbrc.1997.6801. [DOI] [PubMed] [Google Scholar]
- Wada A, Yanagita T, Yokoo H, Kobayashi H. Regulation of cell surface expression of voltage-dependent Nav1.7 sodium channels: mRNA stability and posttranscriptional control in adrenal chromaffin cells. Front Biosci. 2004;9:1954–1966. doi: 10.2741/1314. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen YJ, Li D, Gu R, Wang WH. Dual effect of insulin-like growth factor on the apical 70-pS K channel in the thick ascending limb of rat kidney. Am J Physiol Cell Physiol. 2004;286(6):C1258–1263. doi: 10.1152/ajpcell.00441.2003. [DOI] [PubMed] [Google Scholar]
- Yang BC, Lin HK, Hor WS, Hwang JY, Lin YP, Liu MY, Wang YJ. Mediation of enhanced transcription of the IL-10 gene in T cells, upon contact with human glioma cells, by Fas signaling through a protein kinase A-independent pathway. J Immunol. 2003;171(8):3947–3954. doi: 10.4049/jimmunol.171.8.3947. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kanaoka S, Takai T, Uezato T, Miura N, Kajimura M, Hishida A. EGF rapidly translocates tight junction proteins from the cytoplasm to the cell- cell contact via protein kinase C activation in TMK-1 gastric cancer cells. Exp Cell Res. 2005;309(2):397–409. doi: 10.1016/j.yexcr.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Yuhi T, Wada A, Kobayashi H, Yamamoto R, Yanagita T, Niina H. Up- regulation of functional voltage-dependent sodium channels by cyclic AMP- dependent protein kinase in adrenal medulla. Brain Res. 1996;709(1):37–43. doi: 10.1016/0006-8993(95)01252-4. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yi J, Hu N, George AL, Jr., Murray KT. Activation of protein kinase A modulates trafficking of the human cardiac sodium channel in Xenopus oocytes. Circ Res. 2000;87(1):33–38. doi: 10.1161/01.res.87.1.33. [DOI] [PubMed] [Google Scholar]