Abstract
Behavioral inhibition (BI) is a temperament characterized during early childhood by increased fearfulness to novelty, social reticence to unfamiliar peers, and heightened risk for the development of anxiety. Heightened startle responses to safety cues have been found among behaviorally inhibited adolescents who have an anxiety disorder suggesting that this measure may serve as a biomarker for the development of anxiety amongst this risk population. However, it is unknown if these aberrant startle patterns emerge prior to the manifestation of anxiety in this temperament group. The current study examined potentiated startle in 7-year-old children characterized with BI early in life. High behaviorally inhibited children displayed increased startle magnitude to safety cues, particularly during the first half of the task, and faster startle responses compared to low behaviorally inhibited children. These findings suggest that aberrant startle responses are apparent in behaviorally inhibited children during early childhood prior to the onset of a disorder and may serve as a possible endophenotype for the development of anxiety
Keywords: temperament, risk factors, startle, child, anxiety
Behavioral inhibition (BI) is a temperamental trait characterized in early childhood by a heightened state of vigilance and fear in reaction to novelty or unfamiliar situations (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Kagan, Reznick, Snidman, Gibbons, & Johnson, 1988). Young children who show a stable profile of BI throughout childhood are at an increased risk of developing anxiety disorders in adolescence (Hirshfeld et al., 1992; Schwartz, Snidman, & Kagan,1999). However, not all children who are behaviorally inhibited develop psychopathology and as such it is important to identify possible biomarkers in this population that may serve as an endophenotype to identify those who are at the greatest risk for developing an anxiety disorder. One potential marker is the potentiated startle reflex response.
A number of studies have demonstrated that the potentiated startle response differs between adults with an anxiety disorder and non-affected controls (Grillon, Ameli, Goddard, Woods, & Davis, 1994; Morgan, Grillon, Southwick, Davis, & Charney, 1995), as well as adolescents at risk for developing an anxiety disorder (Grillon, Dierker, & Merikangas, 1998; Merikangas, Avenevoli, Dierker, & Grillon, 1999). These studies suggest that individuals with anxiety or those at risk for anxiety display larger startle responses to cues associated with safety compared to non-anxious individuals (Lissek et al., 2005). One learning mechanism by which safety cues can elicit a conditioned fear response is through stimulus generalization, where fear responses are evoked by a range of stimuli that resemble the original conditioned stimulus (Pearce, 1987). Fear conditioning studies contend that anxiety results from abnormal learning processes such as the inability to inhibit fear responses to non-threatening stimuli (Davis, 2006; Mineka & Zinbarg, 2006). For example, individuals with anxiety have increased startle response to non-threatening stimuli that resemble the threatening stimulus (Lissek et al., 2010), and demonstrate increased startle to cues contextually associated with the threatening stimulus as compared to controls (Grillon & Morgan, 1999).
Abnormal fear potentiated startle responses are also characteristic of individuals with BI. Nine-month-old infants who were previously identified as high in motor reactivity and negative affect to novel auditory and visual stimuli (a precursor to BI) demonstrated increased fear-potentiated startle responses during the approach of a stranger as compared to positively reactive infants (Schmidt & Fox, 1998). Using the fear potentiated startle procedure developed by Grillon and Ameli (1998), it has been demonstrated that adolescents who exhibited a stable history of heightened BI and a lifetime history of an anxiety disorder display a larger potentiated startle to safety cues compared to those high behaviorally inhibited adolescents without a history of anxiety (Reeb-Sutherland et al., 2009). However, other studies found no relations between startle and shyness and/or anxiety. Temperamentally shy children demonstrated no differences in baseline startle at 4 years of age and no differences in startle potentiation during the anticipation of a speech at 7 years of age compared to less shy children (Schmidt et al., 1997; Schmidt, Fox, Schulkins, & Gold, 1999). Using an emotion-modulated startle potentiation procedure, Quevedo and colleagues (2010) found that potentiated startle was unrelated to children's level of anxiety. Given these mixed findings in childhood, it is important to explore whether alternative fear potentiation procedures, specifically procedures that potentiate threat using a discrete and explicit stimulus, may elucidate the early startle patterns of children with BI and its relation with anxiety.
As reported by Reeb-Sutherland et al. (2009), increased potentiated startle to safety cues may also be used to help distinguish behaviorally inhibited children who develop anxiety from those who do not. However, in that study, startle and psychiatric disorders were measured concurrently making it difficult to determine whether the heightened startle response to safety cues preceded the development of anxiety symptoms. The current study provides a prospective examination of potentiated startle differences in young children characterized with BI prior to onset of heightened anxious symptoms. We explored whether differential startle response patterns can be observed between behaviorally inhibited and non-inhibited children at 7 years of age. We hypothesized that like the findings from Reeb-Sutherland et al. (2009) we would find that high behaviorally inhibited children would demonstrate atypical startle responses only during conditions of safety compared to non inhibited children. In addition, we explored whether startle potentiation and BI were related to 7-year anxiety symptoms.
Methods
Participants
Participants were 181 typically developing children. The sample of children was part of a larger longitudinal study examining the development of emotion and cognition. Children were originally selected for participation at 4 months of age based on their degree of emotional and motor reactivity to novel visual and auditory stimuli (N = 278; Hane, Fox, Henderson, & Marshall, 2008). At 24 and 36 months of age, children returned to the laboratory and participated in a structured observation where children played with novel objects and interacted with unfamiliar adults (Fox et al.,2001; Kagan & Snidman, 1991). Individual differences in BI were assessed based on children's latency to approach and children's proximity to their caregiver. At each age, a single standardized score was computed that summarized each child's degree of BI throughout the tasks (Fox et al., 2001). Children returned to the laboratory at 7 years of age (M = 90.84 months, SD = 2.57, range = 87–99 months) to participate in a variety of social and emotional tasks, including the current potentiated startle procedure and anxiety symptoms assessment.
To provide a measure of BI, a composite score was created by averaging each child's 24- and 36-month BI scores (Pérez-Edgar et al., 2011). Children with a BI score at only one time-point were excluded from the current analysis (N = 47). There was a significant positive correlation between 24- and 36-month BI scores (r(132) = .37, p < .001). In addition, there were no significant differences on any of the independent or dependent measures of interest between children who had a BI score for either one versus both time-points. The categorical grouping of BI was created by calculating the mean of the BI composite score for all participants who had BI data for both time points. Next, participants were split into groups based on this mean score with individuals above the mean classified as high BI and individuals below the mean classified as low BI. The high BI group had a tendency to be older than the low BI group (t(66) = 1.99, p = .051). Other studies examining BI have used alternative categorization procedures, such as top 30% and bottom 30% of the sample to classify high and low BI groups (e.g., Schmidt et al., 1997, 1999). In the current study, we used a more conservative mean split approach in order to preserve the limited sample size.
The current study was approved by the University of Maryland institutional review board. Parents of all children provided written informed consent to participate in the study.
Startle Testing Procedure
The current startle paradigm is similar to that previously described by Reeb-Sutherland et al. (2009). For the current study, the instructions and task were slightly modified to make the paradigm more age-appropriate and to increase child compliance. Prior to data collection, children were fitted with a commercially produced cloth space helmet, two 6-mm miniature EMG electrodes placed under the left eye to record startle activity from the orbicularis oculi muscle, a ground electrode placed on the back of the neck, a cloth collar with attached nylon tubing (3-m long, 3.175-mm internal diameter) aimed at the larynx, and two EAR-3A earplugs. Children were told to pretend to be an astronaut and that they were going on a “space adventure”, and while they were on this adventure they would be hearing sounds (startle probe: 50-ms white noise burst presented binaurally at 105-dB peak sound pressure level and instantaneous rise time), seeing green- or blue-colored screens (12 cm2) or pictures of planets, and sometimes getting a puff of air (peak flow rate of 250 cm3 /s at 700 kPa input pressure) presented to their throat. Children were informed that one of the colors (e.g., blue) indicated that there was a possibility of receiving an unpleasant but not painful puff of air to the neck (threat cue) and that the other color (e.g., green) indicated that there was no possibility of receiving a puff (safety cue). The association between color and threat and safety cues was counter-balanced between children. Prior to starting the experiment, children were given the opportunity to present themselves with a sample puff of air. To maintain the children's attention to the task, they were told that their job as an astronaut was to count the number of planets that they saw and if they reported the correct number of planets at the end of the task they would get a sticker which could later be traded for a prize. At the end of the task, children were rewarded with a sticker regardless of whether or not they counted the correct number of planets.
To habituate the children to the startle probe, they were first presented with eight successive startle probe trials. The first half of the trials was separated by a 10-s inter-stimulus interval (ISI) and the second half by a 20-s ISI. Following habituation, children were reminded which colors were associated with the threat and safety cues. Next, children were randomly presented eight threat, eight safety, and eight intertrial interval (ITI) trials. In addition, seven pictures of planets (500 ms) were also randomly presented. During the safe and threat conditions, children were presented with the safety or threat cue, respectively, followed by a startle probe occurring either 4 or 7 s after cue onset. During half of the threat trials, a puff of air was presented to the neck following the presentation of the startle probe. Threat and safety cues were presented for a total duration of 12 s. During ITI trials, startle probes were delivered randomly without presentation of either threat or safety cues (i.e., children were presented a black screen). The time interval between the onsets of two successive startle probes varied between 17 and 42 s.
Data Collection and Reduction
The raw EMG signal was amplified using a custom bioelectric amplifier (SA Instruments, San Diego, CA) with a gain of 1,000 Hz and filtered online using high- and low-pass filters of 1 and 400 Hz, respectively. The amplified signal was digitized at a sampling rate of 512 Hz using a 16-bit A/D converter (2.5 V input range) and Snap-Master data acquisition software (HEM Data Corporation, Southfield, MI). A 50-μV, 10-Hz signal was input into the channel and recorded for calibration purposes. Raw EMG data were processed and analyzed offline using the EMG Analysis System from James Long Company (Caroga Lake, NY). The signal was digitally filtered offline with high- and low-pass filters of 28 and 250 Hz, respectively. In addition, a digital band-stop filter (50–70 Hz) was used to remove 60-Hz noise. The signal was rectified and smoothed by using a moving average with a 20-ms window. Peak amplitude in relation to the baseline response was determined within a 20- to 120-ms time window following stimulus startle probe onset. The baseline response was defined as the average activity recorded during the 20 ms prior to startle probe onset. To verify that the blink occurred within the 20- to 120- ms time window, EMG responses were visually inspected by a previously trained and reliable coder (B.C.R.-S.). Trials were excluded if the onset of the blink response occurred before the 20-ms window or the blink response was indistinguishable from baseline noise (12% of trials). The low BI group had a tendency to have more noisy trials (M = 4.45, SD = 3.40) than the high BI group (M = 3.20, SD = 2.42; t(66) = 1.76, p = .083). A magnitude of zero was assigned for non-response trials and included in the analysis for participants who did not meet exclusionary criteria as a non-responder (7% of trials).
Children were excluded from the current analyses for the following reasons: non-compliance (low BI, n = 13; high BI, n = 9), excessive movement artifact on 50% or more of the trials in any one condition following habituation (low BI, n = 14; high BI, n = 12), no viewable EMG startle response on more than 70% of the trials in any one condition following habituation (i.e., non-responders; low BI, n = 10; high BI, n = 3), and technical error (N = 4). There were no differences between non-responders and responders on any of the outcome measures. In addition, children who were excluded from the analysis did not significantly differ in terms of gender or BI. Lastly, one participant was determined to be a statistical outlier due to extremely large startle magnitudes and was subsequently excluded from analysis. Table 1 presents demographic statistics for the final sample of 68 participants.
Table 1. Descriptive statistics.
| Low BI | High BI | |
|---|---|---|
| n | 33 (17 females) | 34 (19 females) |
| Age (mo.) | 90.21(2.2) | 91.30 (2.7) |
| BI score | -0.30 (0.2) | 0.42 (0.3) |
| Anxiety (T-score) | 51.90 (3.4) | 52.94 (4.4) |
Measure of Anxiety Symptoms
The Child Behavior Checklist (CBCL/6-18; Achenbach & Rescorla, 2001) was used to assess children's anxiety symptoms. Using a three-point scale ranging from 0 (not true) to 2 (very true), mothers rated statements about how often their child displayed a series of internalizing and externalizing problem behaviors. A DSM-oriented anxiety T-score was derived based on the summation of raw scores on anxiety-related items that are then converted to T-scores based the child's age (American Psychiatric Association, 1994). T-scores of approximately 65 (93rd percentile) to 69 (97th percentiles) and higher indicate borderline and clinical levels of anxiety according to DSM-IV criteria. Anxiety T-scores and raw scores yielded similar results, thus only T-scores are reported.
Data Analysis
For each condition (habituation, safe, ITI, and threat), startle magnitude and latency of the eight trials were separately averaged into two blocks of four startle responses each (trials 1–4, trials 5–8). To examine startle differences during habituation trials, a repeated-measures ANOVA was conducted with block as the within-subjects factor and BI as the between-subjects factor. Next, to explore startle potentiation across test conditions, a repeated-measures ANOVA was conducted with condition (safe, threat, ITI) and block as the within-subjects factor, and BI as the between-subjects factor. Previous research using similar startle potentiation procedures in adults and adolescents with anxiety have consistently found differences in startle potentiation during the presentation of safety cues (Lissek, 2012; Reeb-Sutherland et al., 2009). Given these previous findings, separate repeated-measures ANOVA for the safe as well as threat and ITI conditions were conducted with block as the within-subjects factor and BI as the between-subjects factor. Follow-up t-tests were performed following significant interaction effects. For magnitude, preliminary analysis was conducted using both the raw startle magnitude (see Table 2) and standardized T-scores (Grillon et al., 1999). Because some results were not similar between the raw and standardized data, likely due to the large variance in startle magnitude between the two BI groups, only analysis on the more conservative standardized T-scores is presented. For the latency analysis, non-response trials were excluded. Thus, sample sizes varied slightly between conditions since some participants only had non-responses for a particular block.
Table 2. Raw startle magnitude and latency.
| Magnitude (μV) | Latency (ms) | |||
|---|---|---|---|---|
|
|
|
|||
| Low BI | High BI | Low BI | High BI | |
| Habituation | 259.05 (241.9) | 330.41 (237.2) | 69.30 (10.7) | 65.87 (7.2) |
| Safe | 135.30 (126.2) | 244.63 (100.0) | 70.68 (7.2) | 63.29 (7.2) |
| Threat | 294.26 (250.5) | 405.95 (261.6) | 67.64 (6.9) | 63.22 (6.9) |
| ITI | 180.23 (177.4) | 259.89 (198.3) | 66.75 (10.5) | 63.12 (6.7) |
The interrelations between 7-year anxiety symptoms, startle potentiation, and BI were explored through independent sample t-tests and bivariate correlations. Next, to better understand the categorical relationships between variables, participants were dichotomized into groups based on the median value of startle amplitude T-scores during safe trials (i.e., high startle, low startle), and on the median value for CBCL anxiety T-scores (i.e., high anxiety, low anxiety). A three-dimensional contingency table was created to analyze the relation among the three categorical variables of interest (i.e., BI, startle amplitude, CBCL anxiety; see Table 3).
Table 3. Three-dimensional contingency table.
| Low Anxiety | High Anxiety | Total | ||
|---|---|---|---|---|
| Low Startle | Low BI | 7 | 11 | 18 |
| High BI | 8 | 7 | 15 | |
| High Startle | Low BI | 5 | 8 | 13 |
| High BI | 7 | 11 | 18 | |
|
| ||||
| Total | 27 | 37 | 64 | |
Results
Startle Habituation
Repeated-measures ANOVA for startle magnitude during the habituation condition revealed a significant effect of block where magnitude was significantly larger during the 1st block than the 2nd block (F(1,62) = 19.77, p < .001, Figure 1, first panel). There were no other significant main or interaction effects. For startle latency, the repeated-measures ANOVA revealed no significant main or interaction effects.
Figure 1.
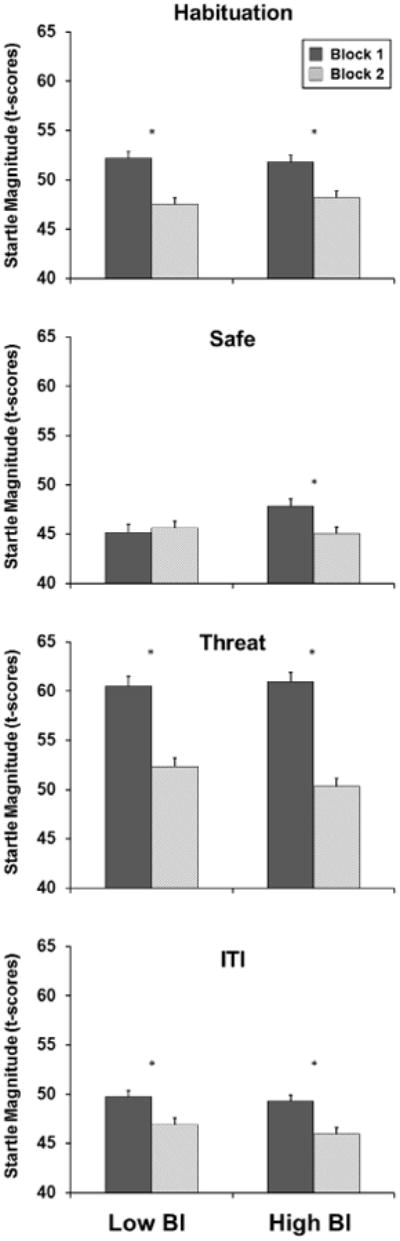
Startle magnitude T-scores across the 4 conditions (habituation, safe, threat, ITI) for low (left) and high (right) behaviorally inhibited children. The block for each condition was created by separately averaging the trials from the 1st half (trials 1-4, dark gray) and 2nd half (trials 5-8, light gray) for each condition. Error bars represent standard error of mean.
Fear Potentiated Startle
The repeated measures ANOVA with BI as the between subjects factor and condition and block as the within-subjects factor revealed a significant condition block interaction (F(2, 132) = 31.87, p < .001) where there was a significant decreasing linear trend across blocks for the threat condition (F(1,67) = 86.66, p < .001) and the ITI condition (F(1,67) = 21.26, p < .001); however, the linear trend was not significant for the safe condition (F(1, 67) = 3.09, p = .08). In addition, there was a significant main effect of condition (F(2, 132) = 167.46, p < .001). Follow-up t-tests revealed that startle magnitude during the threat condition was significantly larger than during the safe condition (t(67) = 15.62, p < .001) and during the ITI condition (t(67) = 12.41, p < .001), and startle magnitude during the ITI condition was significantly larger than during the safe condition (t(67) = 4.49, p < .001). In addition, there was a significant main effect of block, where the first half of trials were significantly larger than second half of trials (F(1,66) = 60.93, p < .001). No other main or interaction effects were significant. Analysis of startle latency revealed a main effect of BI, where the high BI group had significantly shorter latencies than the low BI group (F(1, 63) = 9.23, p < .003). No other main of interaction effects were significant.
Separate analysis of the safe condition showed that there was a significant BI × block interaction effect for magnitude (F(1, 66) = 5.35, p < .024, Figure 1, second panel). Follow-up t-tests revealed that the high BI group had significantly larger startle magnitude compared to the low BI group during the 1st block (t(66) = 2.68, p < .009), however, there were no differences in startle magnitude between groups during the 2nd block (t(66) = .76, p = .45). No significant main effects of BI or block were found. The repeated- measures ANOVA for latency revealed a main effect of BI, where the high BI group had a significantly shorter response latency than the low BI group (F(1, 63) = 7.74, p < .007). No other significant main or interaction effects were found.
A significant main effect of block was found for both the threat (F(1, 66) = 86.20, p < .001, Figure 1, third panel) and the ITI (F(1, 66) = 20.88, p < .001; Figure 1, last panel) conditions where magnitude was significantly larger for the 1st block compared to the 2nd block. No other main or interaction effects were found for threat and ITI magnitude. The repeated-measures ANOVA for latency revealed a significant main effect of BI for the threat condition (F(1, 66) = 6.91, p < .011) and a main effect of BI for the ITI condition (F(1, 66) = 4.35, p = .041), where the high BI group had a shorter latency than the low BI group. No other significant main or interaction effects for threat and ITI latency were obtained.
Anxiety Problems and Startle Measures
Children's anxiety symptoms T-scores ranged from 50 to 68, with three low BI children and two high BI children scoring in the borderline clinical range or above (see Table 1; note, three participants were excluded due to missing CBCL data). An independent sample t-test was conducted to examine differences in 7-year anxiety symptoms between BI groups, which revealed no group differences (t(63) = .36, p = .72). The bivariate correlation analysis revealed that there were no significant correlations between 7-year anxiety symptoms and any of the startle measures (p's > .40). Similar results were obtained when individual blocks within each condition were examined. Based on the finding that BI groups significantly differed in the 1st block of safe trials, participants were dichotomized into groups according to the median amplitude T-score value during these trials (i.e., low startle, high startle). Accordingly, 19 subjects were classified as high BI and high startle. A chi-square analysis of the three-dimensional contingency table revealed a non-significant relation between BI and anxiety among subjects with high startle (χ2 (1, N = 31) = .07, p < .79), and a non- significant relation among subjects with low startle (χ2 (1, N = 34) = .93, p < .34).
Discussion
The current study expands upon previous work with startle and BI by examining potentiated startle in behaviorally inhibited children during early childhood, prior to the manifestation of anxious symptoms in this at risk group. Children high in BI demonstrated atypical startle patterns only during the safe condition compared to children low in BI. Specifically, high behaviorally inhibited children had a larger startle magnitude during the first half of trials during the safe condition than low behaviorally inhibited children. In addition, high behaviorally inhibited children demonstrated shorter startle latency across all conditions (safe, threat, ITI) compared to low behaviorally inhibited children. However, both BI and startle potentiation to safe cues were not related to 7-year anxiety symptoms. It is important to note that only a small percentage of children had anxiety T-scores in the borderline clinical range or above. Heightened potentiated startle response to safe cues may not discriminate within normal variations of anxious behaviors in temperamentally fearful children but may serve as a biomarker in distinguishing which children with BI go on to develop a disorder. Longitudinal follow-up of this sample will be able to answer this question.
Other at risk samples have also demonstrated abnormal startle response patterns, such as adolescents who have a parental history of an anxiety disorder (Grillon et al., 1998; Merikangas et al., 1999). Similarly, differential startle patterns have been consistently observed between adults with anxiety disorders compared to healthy controls (Grillon et al., 1994; Morgan et al., 1995). It has been suggested that anxious individuals have difficulty distinguishing between threat and safety cues (Britton, Lissek, Grillon, Norcross, & Pine, 2011; Lissek, 2012). Specifically, recent research has demonstrated that anxious individuals have a tendency to overgeneralize conditioned threatening stimuli to stimuli that poses no threat (Lissek et al., 2010). In the current study, our findings suggest that even after high behaviorally inhibited children are verbally informed of the differences between threat and safety cues, they still display increased vigilance during the safe condition. Increased vigilance during the first half of safe trials may be indicative of an overgeneralization of the threatening stimuli (i.e., increased vigilance to any screen color as opposed to just the screen color indicating threat). It appears that only after several exposures to the safe condition that the high behaviorally inhibited children's level of arousal diminishes to that of low behaviorally inhibited children. These differential responses may be partly explained by possible perturbed inhibitory learning mechanisms that are specific to the phenotypic expression of BI (Reeb-Sutherland et al., 2009).
Previous studies examining startle during a baseline period (i.e., Schmidt et al., 1997), or during the anticipation of presenting a speech (i.e., Schmidt et al., 1999), found no differences between high and low temperamentally shy children. In contrast, the current study found differences in BI groups using a fear potentiated startle procedure with an explicit and discrete aversive stimulus that the child was exposed to prior to and during the experimental procedure (i.e., puff of air to the larynx). This procedure has been shown to reliably potentiate startle in both anxious and non-anxious populations, and has been successful in potentiating startle in younger populations (Grillon & Ameli, 1998; Grillon et al., 1999). Taken together, these findings suggest that behaviorally inhibited children are able to successfully regulate during situations with relatively little perceived threat (Schmidt et al., 1997). However, during periods of high perceived threat, as in the current study, behaviorally inhibited children demonstrate atypical startle potentiation as compared to low behaviorally inhibited children.
The current study is one of the first studies to explore the potentiated startle response as a potential endophenotype in anxiety disorders. Given the difficulty in isolating genetic causes of affective disorders such as anxiety, searching for possible endophenotypes may provide useful in understanding the development of psychopathology (Gottesman & Gould, 2003). Abnormalities in potentiated startle are a strong physiological candidate for an anxiety endophenotype given that the biomarker is associated with clinically anxious populations, and is present in offspring of clinically anxious individuals (Grillon et al., 1994, 1998; Merikangas et al., 1999; Morgan et al., 1995). In the current study, we did not find any relation between BI and anxiety in individuals with heightened startle responses. These null findings could be due to limitations in the sensitivity of our measures in detecting small behavioral differences between groups. However, our findings are consistent with the possibility that atypical startle responses precede the behavioral manifestation of anxiety in such risk populations. Future studies should examine whether heightened startle potentiation in at risk samples during childhood predict the development of anxiety in adolescence.
There are many contextual and genetic factors that influence the development of anxiety disorders in behaviorally inhibited children (Fox, Hane, & Pine, 2007). For example, the rearing environments of behaviorally inhibited children have been shown to influence developmental pathways that in turn confer risk for internalizing disorders such as anxiety (Degnan, Almas, & Fox, 2010). Behaviorally inhibited children are more likely to display later reticent behaviors when exposed to intrusive and overprotective parenting (Rubin, Burgess, & Hastings, 2002; Rubin, Cheah, & Fox, 2001), as well as when exposed to permissive parenting (Rankin Williams et al., 2009). Conversely, warm and sensitive parenting behaviors lead to more positive socio-emotional outcomes in children who are temperamentally shy (Hane, Cheah, Rubin, & Fox, 2008). Other environmental contexts, such as early childcare placement, affect the continuity of inhibited behaviors of time. It has been demonstrated that highly reactive infants at 4 months of age are less likely to be inhibited at 2 years of age when placed in childcare at least 10 hours per week (Fox et al., 2001). These studies suggest that the risk for anxiety disorders in behaviorally inhibited children is influenced by both environmental and biological factors (Degnan et al., 2010). Although it is unknown how these environmental factors may directly influence the startle response in humans, research from animal studies suggests that the rearing environment can have dramatic effects on the startle reflex response. For example, it has been demonstrated that non-human primates exhibit increased startle reflex responses when raised by peers (Parr, Winslow, & Davis, 2002; Nelson et al., 2009), and when exposed to repeated maternal separation (Sánchez et al., 2005). Atypical startle responses have also been observed in rat pups raised in isolation (Geyer, Wilkinson, Humby, & Robbins, 1993; Varty, Paulus, Braff, & Geyer, 2000). Given these findings in animals, it is possible that overprotective or permissive parenting as well as less time in childcare may lead to the increased startle response to safety cues in some behaviorally inhibited children which, in turn, may lead to the development of anxiety disorders later in life.
The current study is one of only a few studies that have examined potentiated-startle during early childhood. We employed a number of adaptations from traditional potentiated-startle studies, such as using a puff of air as an aversive stimulus as opposed to an electrical shock, and by providing a child-friendly context (i.e., “space adventure”) that enabled children to complete the task. A number of studies have used different procedures to elicit startle responses in young children with and without anxiety either by measuring baseline startle reflex during passive viewing of video clips or by potentiating startle by using valenced slides (Waters, Craske, et al., 2008; Waters, Neumann, Henry, Craske, & Ornitz, 2008) with mixed success (Quevedo et al.,2010). By employing a potentiated-startle procedure that uses an explicitly aversive stimulus as a threat (i.e., puff of air to the larynx), we were able to demonstrate consistent potentiation during the threat condition suggesting that the procedure used in the current study may be ideal for examining potentiated startle in populations of young children.
A number of limitations of the current study should be mentioned. First, we did not perform a psychiatric assessment of the children and so cannot definitively state that none of the children had an anxiety disorder. On the other hand, we did collect CBCL questionnaires from parents and examined the CBCL DSM-derived scale, and as reported, only a small number of children were above that normed threshold. Second, given the concurrent measurement of anxiety symptoms and startle, we were not able to test whether atypical startle potentiation is predictive of the development of anxiety symptoms. Another limitation of the current study is that a large number of children were unable to complete the task or were excluded due to excessive EMG artifact or due to a large number of non-response trials. Although the puff of air to the larynx served as a strong stimulus to potentiate startle, it may have been too aversive for many of the children, therefore, examining other potential stimuli that are less aversive but can produce a reliable potentiated response should be explored (Quevedo et al., 2010).
In summary, the current study was the first to explore individual differences in fear potentiated startle in a prospective sample of young children followed since infancy using a developmentally appropriate paradigm. The results revealed that high behaviorally inhibited children demonstrated aberrant startle differences to safety cues, possibly indicative of an overgeneralization of threatening stimuli.
Acknowledgments
The authors would like to thank the many individuals who contributed to the longitudinal data collection and behavioral coding. We would especially like to thank the parents of the children who participated in our studies. This research was supported by a grant from the National Institutes of Health (HDR3717899) to Nathan A. Fox.
References
- Achenbach TM, Rescorla LA. Manuals for the ASEBA School Age Forms and Profiles. Burlington, VT: Research Center for Children, Youth & Families, University of Vermont; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: The role of threat appraisal and fear learning. Depression and Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. The American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry. 2010;51:497–517. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Hane AA, Pine DS. Plasticity for affective neurocircuitry: How the environment affects gene expression. Current Directions in Psychological Science. 2007;16:1–5. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in pre-pulse inhibition of acoustic startle similar to that in schizophrenia. Biological Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Effects of threat and safety signals on startle during anticipation of aversive shocks, sounds, or airblasts. Journal of Psychophysiology. 1998;12:329–337. [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35:431–439. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear- potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Merikangas KR, Dierker L, Snidman N, Arriaga RI, Kagan J, et al. Nelson C. Startle potentiation by threat of aversive stimuli and darkness in adolescents: A multi-site study. International Journal of Psychophysiology. 1999;32:63–73. doi: 10.1016/s0167-8760(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., III Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hane AA, Cheah C, Rubin KH, Fox NA. The role of maternal behavior in the relation between shyness and social reticence in early childhood and social withdrawal in middle childhood. Social Development. 2008;17:795–811. [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44:1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, et al. Kagan J. Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, John- son MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development. 1988;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: The case for conditioned overgeneralization. Depression and Anxiety. 2012;29:257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. The American Journal of Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Avenevoli S, Dierker L, Grillon C. Vulnerability factors among children at risk for anxiety disorders. Biological Psychiatry. 1999;46:1523–1535. doi: 10.1016/s0006-3223(99)00172-9. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It's not what you thought it was. The American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Morgan CA, III, Grillon C, Southwick SM, Davis M, Charney DS. Fear potentiated startle in post-traumatic stress disorder. Biological Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, et al. Pine DS. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Biological Psychiatry. 2009;66:702–704. doi: 10.1016/j.biopsych.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Davis M. Rearing experience differentially affects somatic and cardiac startle responses in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2002;116:378–386. doi: 10.1037//0735-7044.116.3.378. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb- Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, et al. Fox NA. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Smith T, Donzella B, Schunk E, Gunnar M. The startle response: Developmental effects and a paradigm for children and adults. Developmental Psychobiology. 2010;52:78–89. doi: 10.1002/dev.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin Williams L, Degnan K, Pérez-Edgar K, Henderson H, Rubin K, Pine D, et al. Fox NA. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology. 2009;37:1063–1075. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Pérez-Edgar K, Henderson HA, Lissek S, et al. Fox NA. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin KH, Burgess KB, Hastings PD. Stability and social–behavioral consequences of toddlers' inhibited temperament and parenting behaviors. Child Development. 2002;73:483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Cheah CSL, Fox NA. Emotion regulation, parenting and display of social reticence in preschoolers. Early Education & Development. 2001;12:97–115. [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA. Fear-potentiated startle responses in temperamentally different human infants. Developmental Psychobiology. 1998;32:113–120. doi: 10.1002/(sici)1098-2302(199803)32:2<113::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Varty GB, Paulus MP, Braff DL, Geyer MA. Environmental enrichment and isolation rearing in the rat: Effects on locomotor behavior and startle response plasticity. Biological Psychiatry. 2000;47:864–873. doi: 10.1016/s0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- Waters AM, Craske MG, Bergman RL, Naliboff BD, Negoro H, Ornitz EM. Developmental changes in startle reactivity in school-age children at risk for and with actual anxiety disorder. International Journal of Psychophysiology. 2008;70:158–164. doi: 10.1016/j.ijpsycho.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, Ornitz EM. Baseline and affective startle modulation by angry and neutral faces in 4–8-year-old anxious and non-anxious children. Biological Psychology. 2008;78:10–19. doi: 10.1016/j.biopsycho.2007.12.005. [DOI] [PubMed] [Google Scholar]


