Abstract
Background
Increased adiposity at birth may identify infants at high risk of developing obesity. Maternal obesity and hyperglycemia in pregnancy are associated with increased neonatal adiposity; however, features of maternal obesity that contribute to increased neonatal adiposity need further study.
Aims
To measure adiposity in neonates of obese and normal-weight women without gestational diabetes to test the hypothesis that obese women have neonates with increased adiposity compared to neonates of normal-weight women.
Methods
Sixty-one pregnant women, with a normal or obese BMI, and their neonates participated in this cross-sectional study at an academic medical center. Neonatal adiposity, expressed as percent body fat (fat mass/body mass), was measured by air displacement plethysmography and cord blood was assayed for biomarkers.
Results
Adiposity in neonates of obese and normal-weight mothers did not differ. Stratifying mothers by leptin level showed that neo nates born to mothers with higher leptin had significantly higher adiposity (13.2 vs. 11.1%, p = 0.035). In the entire cohort, adiposity positively correlated with cord blood leptin (r = 0.48, p < 0.001) and adiponectin (r = 0.27, p = 0.04) levels.
Conclusion
Obesity in normoglycemic pregnant women was not associated with increased neonatal adiposity. High maternal leptin levels identified neonates with increased adiposity.
Introduction
Childhood obesity is a major public health problem associated with significant comorbidities and is difficult to reverse [1]. Identifying at-risk children may be one cost-effective strategy to target those children needing preventive intervention. Many researchers have proposed that the origins of childhood obesity can be traced to the period of intrauterine development through a process termed fetal programming [2]. Birth weight and its relationship to obesity risk have been extensively studied: both low birth weight and high birth weight neonates appear to have an increased risk of obesity [3]. However, most obese children are the product of a ‘healthy’ pregnancy and had a normal birth weight [4,5]. Therefore, it is imperative to study other factors beyond birth weight that contribute to obesity risk. Increased, adiposity of the newborn, defined by percent body fat (fat mass/body mass), may be associated with obesity risk [6]. However, due to inadequate clinically available techniques to accurately measure adiposity, there are insufficient data to demonstrate that high adiposity at birth is associated with later obesity.
Diabetes in Pregnancy has been associated with increased offspring adiposity [7, 8] and increased rates of childhood [9, 10] and adult obesity [11]. While obesity and insulin resistance are two maternal characteristics that have been associated with increased fetal size [12, 13], the intrauterine environment of obesity without diabetes and its relative contribution to offspring obesity risk has not been clearly delineated. Studies associating maternal prepregnancy obesity with increased neonatal and childhood adiposity are limited and have used imprecise measures such as skinfold thickness [14, 15]. Caliper measurements of skinfolds to quantify adiposity are limited by reproducibility errors [16]. The availability of a new, safe technology of air displacement plethysmography (Pea Pod Infant Body Composition System; Cosmed, Italy) to evaluate infant body composition [17] has facilitated our efforts to study differences in adiposity in neonates of normal-weight and obese women.
The primary objective of this study was to measure adiposity in neonates of healthy obese and normal-weight women with normal glucose tolerance. We hypothesized that women with an obese prepregnancy BMI would have neonates with higher adiposity compared to neonates born to women with a normal prepregnancy BMI. The secondary objective was to characterize biochemical factors other than glucose in obese women that may be associated with adiposity in offspring.
Methods and Procedures
Subjects
Healthy pregnant women with a prepregnancy BMI that was normal (18-25) or obese (>30) were eligible for this study if the result of their 50-gram oral glucose challenge test (GCT) was <130 mg/dl. All pregnant women undergo a GCT between 24 and 28 weeks of gestation. Guidelines for the diagnosis of gestational diabetes mellitus (GDM) during the enrollment period of this study used the two-step process [18] with a 1-hour 50-gram screening test followed by a 100-gram oral glucose tolerance test for those women whose glucose level on the screening test was >140 mg/dl. To minimize the likelihood of missed cases of GDM, we enrolled women with a GCT result of <130 mg/dl. Women carrying singleton pregnancies, aged 18-40 years, were recruited from obstetric practices associated with Northwestern Memorial Hospital. Women were excluded from this study if they had carried more than 3 pregnancies to term, had chronic medical conditions, or delivered their newborn at <37 weeks of gestation. Chronic medical conditions were defined as those requiring daily medications including glucocorticoids, insulin, or antihypertensive therapy.
This study was approved by the Northwestern University Institutional Review Board for the conduction of research on human subjects. Participants gave informed, written consent for themselves and their newborns to enroll into this study. This was an observational cross-sectional study consisting of three components: a maternal fasting blood draw between 36 and 38 weeks of gestation, umbilical cord blood collection at the time of delivery, and neonatal anthropometric measurements within 48 h following birth.
Maternal Measurements
Following an overnight fast, participants at 36–38 weeks of gestation presented to the Northwestern University Clinical Research Unit for a blood draw. Maternal height was measured using a wall-mounted stadiometer. The measured height and self-reported prepregnancy weight were used to calculate the prepregnancy BMI. Gestational weight gain was calculated by subtracting the self-reported prepregnancy weight from the weight at the last prenatal visit [15, 19].
Biochemical Assays
Plasma glucose was measured with Synchron CX Delta Systems instrumentation (Beckman Coulter, Inc. Brea, Calif., USA) employing an oxygen rate method using a glucose oxygen electrode and had an interassay coefficient of variation of 2.0–2.3%. Maternal triglycerides were measured by the Triglycerides GPO reagent using a Beckman Coulter Unicel DXC800 analyzer (Beckman Coulter). Concentrations of C-peptide, leptin, and total adiponectin were assayed using radioimmunoassay kits from Millipore Corporation (Billerica, Mass., USA). These samples were stored at −70°C and then run in batches of maternal and cord blood together at a later date. The intra- and interassay coefficients of variation ranged from 2.1 to 4.4% and from 2.9 to 5.9%, respectively. All biochemical assays were performed in duplicate.
Neonatal Anthropometrics
Infant length was obtained with the baby positioned on a hard-surface measuring board. Abdominal circumference was measured at the level of the umbilicus. Measurements were recorded to the nearest 0.1 cm, performed in duplicate, and the results averaged. Air displacement plethysmography with the Pea Pod Infant Body Composition System (Cosmed) was used to measure infant weight and volume. Air displacement testing procedures have been previously described [20]. Briefly, the machine was calibrated according to manufacturer guidelines, the infant was placed naked on the scale, and the weight was measured to the nearest 0.0001 kg. Next, the infant was placed inside the Pea Pod chamber for 2 min for volume measurements. Using pressure-volume equations, body composition, including fat mass and fat-free mass, was calculated to provide percent body fat (adiposity).
Statistical Analysis
The target sample size was 80 mother-infant pairs (40 obese, 40 normal weight) in order to achieve 80% power and detect a 2.3% difference in neonatal adiposity between the 2 groups, assuming a standard deviation of 3.7% and using a two-tailed test with a type 1 error rate of 5% [21]. The study was discontinued prematurely when new criteria for the diagnosis of GDM [22] was instituted at Northwestern Memorial Hospital. Enrollment into the current study depended on normal 1-hour 50-gram GCT results, whereas the new GDM diagnosis criteria require women to undergo a 2-hour fasting oral glucose tolerance test. In the final analysis, the sample size was smaller than anticipated (23 obese, 38 normal weight) but the standard deviation was 3.7% as anticipated. In a post hoc analysis, the power was reduced to 64% to detect a mean difference of 2.3%.
Outcome variables were categorized based on maternal pre-pregnancy BMI and maternal leptin levels. The maternal median leptin level of the entire cohort was used to determine high and low leptin groups. Fisher’s exact tests were used to compare categorical characteristics, and independent-samples t tests were used to compare means. Adjusted analyses were performed using analysis of covariance. Spearman’s correlation rank sum was used to evaluate correlations. p < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS 9.2 software (SAS Institute Inc., Cary, N.C., USA).
Results
Sixty-one women (38 normal weight and 23 obese) enrolled into this study. The maternal demographic and metabolic characteristics are displayed in table 1. Obese women tended to be younger and non-Caucasian and had a lower education level compared to women in the normal-weight group. Obese women had higher levels of glucose on the 1-hour 50-gram GCT compared to normal-weight women, although all subjects had values <130 mg/dl. Fasting blood samples were obtained from 79% of the participants at 36–38 weeks of gestation, and the obese women had higher levels of fasting glucose, C-peptide, and leptin.
Table 1.
Material demographics
| Normal weight | Obese | P value | |
|---|---|---|---|
| Number | 38 | 23 | |
| Prepregnancy BMI, kg/m2 | 22.0±1.8 | 35.5±4.0 | |
| Age, years | 32.8±5.9 | 30.2±4.5 | 0.06 |
| Parity, % | 0.11 | ||
| 1 | 66 | 43 | |
| 2 or 3 | 34 | 57 | |
| Ethnicity, % | 0.01 | ||
| Caucasian | 71 | 35 | |
| African-American | 11 | 39 | |
| Hispanic | 11 | 22 | |
| Asian | 8 | 4 | |
| Education (college degree), % | 87 | 56 | <0.01 |
| C-section, % | 26 | 48 | 0.10 |
| Gestational weight gaina, lbs | 33.7±9.4 | 29.3±13.0 | 0.16 |
| 1-hour 50-gram GCT, mg/dl | 98.4±14.3 | 108.0±14.3 | 0.01 |
|
| |||
| Fasting sample at 36–38 weeks of gestation | |||
| Number | 33 | 15 | |
| Glucose, mg/dl | 80.9±6.5 | 86.7±8.7 | 0.03 |
| Triglycerides, mg/dl | 192.3±62.7 | 211.1±51.8 | 0.28 |
| C-peptide, ng/ml | 2.4±1.0 | 4.4±2.0 | <0.01 |
| Leptin, ng/ml | 25.0±12.7 | 54.2±14.5 | <0.01 |
| Adiponectin, μg/ml | 10.8±4.3 | 9.6±4.2 | 0.34 |
Data displayed as means ± SD. A multivariate logistic regression analysis was conducted allowing all of the variables that are significant above to come into the model to predict neonatal adiposity. The only significant variable at P < 0.05 in the multivariate analysis was maternal leptin with P = 0.0012.
The mean gestational age at the last prenatal visit was 39.14 weeks for both groups.
The anthropometric characteristics and cord blood hormone levels of the neonates are displayed in table 2. Cord blood was collected from 93% of the participants. The proportion of neonates with adiposity greater than the 90th percentile of the entire sample (> 16% body fat) trended towards significance in the obese group (8% normal weight, 26% obese, p = 0.07). Neonates born to obese women had higher cord blood C-peptide levels compared to neonates born to normal-weight women, and this difference remained after adjusting for gestational weight gain, GCT results, and maternal fasting glucose.
Table 2.
Neonatal anthropometrics and cord blood biomarkers
| Normal weight mothers | Obese mothers | P value | |
|---|---|---|---|
| Number | 38 | 23 | |
| Male gender, % | 34 | 61 | 0.06 |
| Gestational age, weeks | 39.8±1.1 | 39.8±1.0 | 0.97 |
| Length, cm | 50.5±2.0 | 51.1±2.0 | 0.32 |
| Abdominal circumference, cm | 30.3±2.4 | 30.4±2.5 | 0.83 |
| Weight, kg | 3.38±0.47 | 3.6±0.57 | 0.10 |
| Fat mass, kg | 0.40±0.15 | 0.46±0.20 | 0.19 |
| Body fat, % | 11.6±3.3 | 12.5±4.0 | 0.38 |
| Proportion with adiposity greater than the 90th percentile | 3 (7.9%) | 6 (26%) | 0.07 |
|
| |||
| Umbilical cord blood | |||
| Number | 35 | 23 | |
| Glucose, mg/dl | 80.8±21.4 | 82.2±14.9 | 0.78 |
| C-peptide, ng/ml | 0.9±0.3 | 1.2±0.7 | 0.015 |
| Leptin, ng/ml | 9.8±5.9 | 12.3±9.6 | 0.22 |
| Adiponectin, μg/ml | 36.3±6.4 | 38.3±9.8 | 0.33 |
Data are displayed as means ± SD.
The positive correlations between neonatal adiposity and cord blood leptin and adiponectin levels of all participants (leptin: r = 0.48, p < 0.001; adiponectin: r = 0.27, P = 0.04) are displayed in figures la and b, respectively.
Fig. 1.
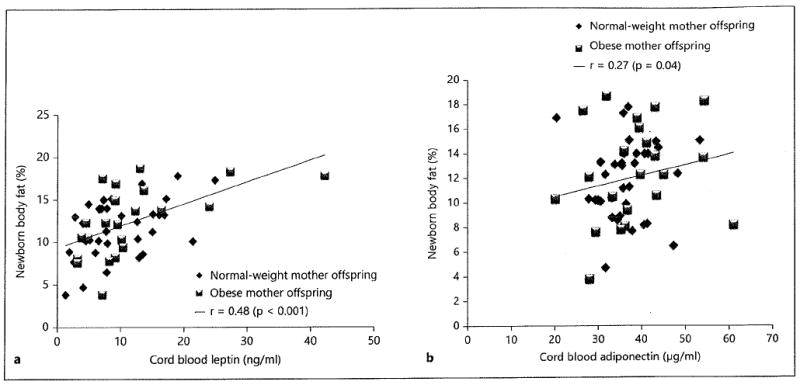
Correlations between newborn percent body fat and cord blood leptin (a) and adiponectin (b).
Since obese women had higher glucose levels compared to normal-weight women, an analysis was conducted comparing neonatal fat mass and percent adiposity between the 2 groups, separately adjusting for maternal GCT results and maternal fasting glucose. Adjusted mean values for fat mass and adiposity were not different between the 2 groups (data not shown).
Due to the finding that maternal leptin was significant after multivariate logistic regression analysis of the maternal factors listed in table 1, the data was stratified by maternal fasting leptin level above and below the median. As displayed in table 3, women in the higher leptin group had, on average, a higher prepregnancy BMI, higher levels of fasting glucose and C-peptide, and a lower adiponectin level. After adjusting for both prepregnancy BMI and gestational weight gain, maternal C-peptide and adiponectin remained significant while glucose became marginally significant (p = 0.055). There were no differences in maternal age, parity, ethnicity, delivery type, maternal education, gestational age, or gender of the newborn between the high- and low-leptin groups. Neonates born to mothers with high leptin had mean adiposity levels 2% higher (13.2 vs. 11.1%) than those of neonates born to mothers with lower leptin. Cord blood leptin and C-eptide levels were higher in the neonates of mothers with high leptin. A multivariate logistic regression analysis was conducted allowing all significant variables in table 3 to come into the model, and the only significant variable was maternal C-peptide (p < 0.001).
Table 3.
Data stratified by maternal leptin level
| Leptin <30.5 ng/ml | Leptin ≥30.5 ng/ml | P value | |
|---|---|---|---|
| Number | 24 | 24 | |
| Prepregnancy BMI, kg/m2 | 23.2±4.7 | 29.8±7.7 | <0.001 |
| Gestational weight gain, lbs | 30.3±6.1 | 35.8±13.9 | 0.08 |
|
| |||
| Maternalfasting sample | |||
| Glucose, mg/dl | 80.0±5.8 | 85.5±28.3 | 0.01 |
| C-peptide, ng/ml | 2.0±0.4 | 4.0±1.8 | <0.001 |
| Leptin, ng/ml | 18.4±7.7 | 49.7±12.8 | |
| Triglycerides, mg/dl | 182.0±65.9 | 214.4±48.7 | 0.06 |
| Adiponectin, μg/ml | 12.0±4.3 | 8.9±3.8 | 0.009 |
|
| |||
| Neonatal anthropometrics | |||
| Male gender, % | 41.7 | 45.8 | 0.78 |
| Length, cm | 50.5±1.6 | 51.0±2.4 | 0.41 |
| Weight, kg | 3.37±0.46 | 3.55±0.64 | 0.29 |
| Fat mass, kg | 0.38±0.15 | 0.48±0.19 | 0.06 |
| Body fat, % | 11.1±3.5 | 13.2±3.2 | 0.035a |
|
| |||
| Cord blood biomarkers | |||
| Glucose, mg/dl | 80.0±25.3 | 83.5±16.4 | 0.60 |
| C-peptide, ng/ml | 0.9±0.3 | 1.2±0.7 | 0.031a |
| Leptin, ng/ml | 7.6±3.9 | 12.5±7.0 | 0.006a |
| Adiponectin, μg/ml | 36.2±5.9 | 38.7±10.4 | 0.34 |
Data are displayed as means ± SD. There were no significant differences in maternal age, parity, ethnicity, delivery type, maternal education, gestational age, or gender of the newborn between the 2 groups.
Adjustment for gestational weight gain did change the significance.
Discussion
The primary outcome of interest was neonatal adiposity; we had hypothesized that neonates of obese women would have more adiposity compared to neonates of normal-weight women. This simple classification based on obese and normal prepregnancy weight in mothers without GDM did not detect a mean difference in neonatal adiposity. The lack of a difference in adiposity in neonates of obese and normal-weight mothers differs from two other previous reports, perhaps due to the different methodology: total body electrical conductivity by Sewell et al. [14] and/or a larger sample size by Hull et al. [23].
There was a higher mean cord blood C-peptide level in neonates of obese mothers, consistent with the fetal hyperinsulinemia found in previous studies [12, 24]. The associations between hyperglycemia in pregnancy and increased neonatal adiposity have been well described [8, 21, 25]. However, reports of anthropometrics in newborns of obese mothers with normoglycemia are limited and in these reports [14,23] maternal glucose levels are not documented. Additionally, the findings of this study indicate that a mother’s leptin level makes an important contribution to her newborn’s adiposity.
Higher maternal leptin in late gestation was associated with greater adiposity in newborns, extending the findings of Catalano et al. [12], in which anthropometric estimation (weight, length, and abdominal skinfold thickness with calipers) [26] was used to measure neonatal adiposity. Our results, using the nonbiased technique of air displacement plethysmography, support the finding that increased maternal leptin is associated with increased neonatal adiposity. A high maternal leptin level was also associated with increased cord blood levels of leptin and C-peptide. Elevated cord blood C-peptide, reflecting increased fetal β-cell function, may be a metabolic marker predictive of obesity risk [9]. Increased rates of obesity in children of mothers with diabetes have been related to in utero hyperinsulinemia [27] and increased adiposity at birth [8,28]. While our study population was comprised of nondiabetic mothers, the differences in the fasting biomarker levels (table 3) of mothers with high leptin compared to mothers with low leptin may be associated with increased adiposity in their neonates, similar to the increased adiposity rates demonstrated in studies of offspring of motheis with diabetes [7,8].
Leptin levels in pregnancy correlate to total body fatness [29]; therefore, it is not surprising that mothers with high leptin had a significantly higher BMI. Furthermore, leptin levels increase in pregnancy [29]. Maternal leptin has been shown to correlate with insulin sensitivity during pregnancy, measured by intravenous glucose tolerance tests [30], and thus may serve as an indicator of insulin resistance. In our data, the maternal fasting C-peptide level was the most significant factor in the multivariate analysis of maternal leptin. In women with normal GCT, increased maternal leptin, as an indirect measure of insulin resistance, may be a better predictor of fat babies than maternal obesity alone [31].
The finding that cord blood leptin was positively correlated to neonatal adiposity is consistent with other reports associating cord blood leptin levels to neonatal and childhood adiposity [32,33], suggesting that cord blood leptin serves as an adiposity biomarker in neonates. Cord blood adiponectin was also positively correlated with neonatal adiposity, although not as strongly as cord blood leptin. Multimeric forms of adiponectin may have different biologic activity; however, our adiponectin findings are consistent with others in which both total and high molecular weight adiponectin were measured in cord blood [34].
The results of this study are similar to those found within the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) report on associations with the maternal BMI [35]. In that large multiethnic study of pregnant women, maternal BMI in the obese category (>33.0 at 28 weeks of gestation) were associated with a 16% frequency of ‘high-fat’ babies (defined by neonates with adiposity greater than the 90th percentile of the entire HAPO cohort), compared to a frequency of 8% in the normal-weight BMI category. Similarly, in our sample, the frequency of fat babies (neonates with adiposity greater than the 90th percentile) born to obese women was more than tripled (8% normal weight, 26% obese) compared to normal-weight women and approached statistical significance. It is important to highlight that the majority of babies born to obese mothers in both the HAPO study and this study had ‘normal’ body fat.
One limitation of this study is that the sample size was relatively small. Perhaps with a larger sample size the difference in neonatal adiposity between the obese and normal-weight groups would become significant. However, even if a difference of 1.4% had been found as reported by Sewell et al. [14], the clinical relevance of this small difference for the future risk of obesity is not known. Another limitation is that direct measures of insulin sensitivity were not conducted on participants. Accurate measures of insulin sensitivity using a hyperinsulinemic-euglycemic clamp and/or frequently sampled intravenous glucose tolerance tests are labor and resource intensive. As these tests are invasive and time consuming, notwithstanding the unknown fetal impact during the glucose testing procedure, few studies on pregnant women have been done. Additionally, varied ethnic distribution between the normal and obese cohorts may have influenced the results.
In conclusion, a difference in neonatal adiposity was not found between neonates of prepregnant obese and those of normal weight mothers. Our findings illustrate that other pregnancy factors, such as the maternal leptin level, as an indirect measure of insulin resistance, may influence the adiposity of the newborn. The degree of adiposity of the newborn may be an independent risk factor for childhood obesity [6], and future studies should focus on whether adiposity at birth predicts adiposity in later childhood.
Acknowledgments
This study was supported by a Diabetes in Pregnancy Program grant to the Feinberg School of Medicine sponsored by Northwestern Memorial Hospital and the Eleanor Wood Prince Grant Initiative, a project of the Women’s Board of Northwestern Memorial Hospital. Services provided by the Northwestern Clinical Research Unit were funded by NUCATS grant UL1RR025741. The authors would like to acknowledge the contribution of Jennifer A. Hoffmann for her assistance in the recruitment of participants and data collection. We thank the mothers who participated in this study and the staff at the Northwestern Clinical Research Unit.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–777. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 4.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 5.Hirschler V, Bugna J, Roque M, Gilligan T, Gonzalez C. Does low birth weight predict obesity lover weight and metabolic syndrome in elementary school children? Arch Med Res. 2008;39:796–802. doi: 10.1016/j.arcmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care. 1999;22:1284–1291. doi: 10.2337/diacare.22.8.1284. [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–1704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- 9.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment – the Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21:B142–B149. [PubMed] [Google Scholar]
- 10.Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987;10:76–80. doi: 10.2337/diacare.10.1.76. [DOI] [PubMed] [Google Scholar]
- 11.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diettreated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464–2470. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Presley L, Minium J, Hauguelde Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahlsson F, Diderholm B, Jonsson B, Norden-Lindberg S, Olsson R, Ewald U, Forslund A, Stridsberg M, Gustafsson J. Insulin resistance, a link between maternal overweight and fetal macrosomia in nondiabetic pregnancies. Horm Res Paediatr. 2010;74:267–274. doi: 10.1159/000295710. [DOI] [PubMed] [Google Scholar]
- 14.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322, el–e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watts K, Naylor LH, Davis EA, Jones TW, Beeson B, Bettenay F, Siafarikas A, Bell L, Ackland T, Green DJ. Do skinfolds accurately assess changes in body fat in obese children and adolescents? Med Sci Sports Exerc. 2006;38:439–444. doi: 10.1249/01.mss.0000191160.07893.2d. [DOI] [PubMed] [Google Scholar]
- 17.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 19.Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern Child Health J. 1998;2:123–126. doi: 10.1023/a:1022996924094. [DOI] [PubMed] [Google Scholar]
- 20.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53:486–492. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 21.HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standards of medical care in diabetes – 2011. Diabetes Care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, Navder K, Yu A, Dorsey K, Gallagher D. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211, el–e7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 25.Vohr BR, McGarvey ST, Coll CG. Effects of maternal diabetes and adiposity on neonatal adiposity and blood pressure. Diabetes Care. 1995;18:467–475. doi: 10.2337/diacare.18.4.467. [DOI] [PubMed] [Google Scholar]
- 26.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173:1176–1181. doi: 10.1016/0002-9378(95)91348-3. [DOI] [PubMed] [Google Scholar]
- 27.Silverman BL, Landsberg L, Metzger BE. Fetal hyperinsulinism in offspring of diabetic mothers: association with the subsequent development of childhood obesity. Ann NY Acad Sci. 1993;699:36–45. doi: 10.1111/j.1749-6632.1993.tb18835.x. [DOI] [PubMed] [Google Scholar]
- 28.Hammami M, Walters JC, Hockman EM, Koo WW. Disproportionate alterations in body composition of large for gestational age neonates. J Pediatr. 2001;138:817–821. doi: 10.1067/mpd.2001.114018. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson B, Lof M, Olausson H, Forsum E. Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br J Nutr. 2010;103:50–57. doi: 10.1017/S0007114509991371. [DOI] [PubMed] [Google Scholar]
- 30.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22:131–138. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 31.Shroff MR, Holzman C, Tian Y, Evans RW, Sikorskii A. Mid-pregnancy maternal leptin levels, birthweight for gestational age and preterm delivery. Clin Endocrinol. 2013;78:607–613. doi: 10.1111/cen.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, et al. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf) 2004;61:88–93. doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 33.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballesteros M, Simon I, Vendrell J, Ceperuelo-Mallafre V, Miralles RM, Albaiges G, Tinahones F, Megia A. Maternal and cord blood adiponectin multimeric forms in gestational diabetes mellitus: a prospective analysis. Diabetes Care. 2011;34:2418–2423. doi: 10.2337/dc11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117:575–584. doi: 10.1111/j.1471-0528.2009.02486.x. [DOI] [PubMed] [Google Scholar]


