Abstract
Rapid advances in evidence-based treatment schedules are a hallmark of modern oncology. In rare neoplastic diseases, however, clinical expertise is hard to build and evidence based on randomized trials almost impossible to conduct. Gorham disease is a rare form of lymphatic proliferation accompanied by osteolysis, which usually occurs in young adults. Despite the fact that the clinical course of Gorham disease is often devastating and occasionally fatal, insights into its biological background are sparse and standardized treatment unavailable. Interestingly, recent knowledge on the mechanisms of lymphangiogenesis may help elucidate the pathophysiology of Gorham disease and lead to novel treatment targets. Here, we discuss our current understanding of Gorham disease, discuss established and emerging therapeutic strategies, and attempt to frame a treatment rationale.
Keywords: vascular malformations, molecular biology, tumor biology, lymphangiogenesis, Gorham disease, rare tumors
Introduction
Lymphatic malformations are a rare and heterogeneous group of benign lesions that originate from abnormally formed lymphatic vessels [1]. They can arise in any anatomic location and are present, but not always evident, at birth [2]. Multifocal or diffuse growth of abnormal lymphatic vessels is termed lymphangiomatosis. Gorham disease (also termed Gorham-Stout disease and “vanishing bone disease”) is a form of lymphangiomatosis characterized by diffuse lymphatic vessel proliferation accompanied by progressive osteolysis. It usually presents in young adults without gender or race predilection and can arise in the skull, maxillofacial skeleton, arms, legs, pelvis, and the spine and ribs [3]. Clinical symptoms vary depending on location but usually include pain, swelling and signs of osteolysis on X-ray examinations. In cases of thoracic involvement, the clinical course can be complicated by instability of the thoracic cage and chylothorax, leading to potentially lethal respiratory insufficiency. Gorham disease with thoracic involvement and chylothorax has a reported survival rate of less than 40% [4].
Due to the very low incidence of Gorham disease, current literature is confined to case reports and several small series. As a result, a standard treatment is lacking. A number of reports have described treatment of Gorham disease with surgery (resection of diseased tissue with pleurodesis or thoracic duct ligation in thoracic disease), radiation therapy, and medication (bisphosphonates, thalidomide, and interferon (IFN)-α2b). These reports vary substantially in indication, scheduling, the combinations employed, and the clinical success of the therapies administered. Given its often-devastating clinical course in these young patients, insights into the biology of Gorham disease are vital to develop a comprehensive therapeutic strategy.
It is thought that lymphangioma growth is driven by the proliferation of lymphatic endothelial cells, making them a prime target for therapy. A number of growth factors have been identified that govern the formation, proliferation and function of normal lymphatic vessels [reviewed in [5]], including vascular endothelial cell growth factor (VEGF)-A [6], VEGF-C [7], VEGF-D [8], basic fibroblast growth factor (bFGF) [9] and platelet derived growth factor (PDGF)-BB [10]. In addition, many of these growth factors, including the VEGF family members, signal through the phosphoinositide-3 kinase (PI3K)/Akt pathways, eventually converging on the mammalian target of rapamycin (mTOR) [11]. Three case reports described clinical treatment using targeted antibodies against these lymphangiogenic growth factors. One study administered imitinib mesylate to inhibit PDGFR-β [12], while another combined imatinib mesylate with bevacizumab to also inhibit VEGF [13] and a third successfully used bevacizumab alone [14].
Here, we will review current knowledge of the etiology of Gorham disease. We will discuss treatment options described in the literature, including their presumed mechanism of action and potential for success as a standardized treatment, with a focus on thoracic Gorham disease.
Biological background
Pathological characteristics
Histopathology remains the gold standard for diagnosing Gorham disease. As first described by Gorham and Stout in 1955 [15], the lesions are a diffuse replacement of soft tissue and bone by extremely wide, empty vessels lined with a single layer of endothelium. Loose textured fibrous tissue surrounds the capillary-like vessels, which may extend outwards between adjacent striated muscle fibers. The vascular proliferation can fill the marrow space of bone and the osseous bone can be completely resorbed. The functionality of the proliferative lymphatic vessels remains poorly understood, as does its interconnectedness to the existing lymphatic network. Clinical lymphangiography coupled with radiographic examinations showed that lymphangiectatic tissue was connected with the thoracic duct in a case of thoracic Gorham disease [16]. Though this radiographic discovery led to therapeutic ligation of the thoracic ductwith improvement in the patient's functional status, the befenit was unfortunately transient. The lack of reliable or sustained benefit from thoracic duct ligation in this and other cases of thoracic disease is likely related to the characteritically diffuse involvement of the pleural surface with abnormal lymphatic vessels. New methods to evaluate lymphatic vessel structure and function, including near-infrared imaging, are being developed[17], but their clinical utility in evaluating lymphangiomatosis remains unknown. A number of genetic mutations underlying lymphatic vessel development and its derangement in several human lymphedema syndromes have recently been discovered [5,18]. However, no candidate genes for Gorham disease have been identified [18].
Bone resorption
An increased number of osteoclasts are often, though not always present in Gorham disease. In addition to increased numbers of osteoclasts, several authors have described increased expression of a variety of hydrolytic enzymes, including acid phosphatase and leucine aminopeptidase in pericvascular mononuclear cells [19,20]. Gorham and Stout speculated that “hyperaemia, changes in local pH, and mechanical causes” might drive osteolysis. Later in vitro work with patients’ cultured tissues suggested a contributary role for interleukin (IL)-6 in bone resorption [21]. Other in vitro work showed that osteoclast precursors found in Gorham tissues displayed increased sensitivity to humoral factors and were capable of secreting cytokines and angiogenic factors, which may function in both a paracrine and autocrine fashion [22,23]. The exact mechanism by which osteoclast activity is upregulated during the expansion of lymphatic endothelial cells remains unknown.
Immunohistochemical markers
The panendothelial marker CD31 (platelet endothelial cell adhesion molecule) is consistently expressed by the proliferating lymphatic endothelial cells in Gorham disease [24]. CD105 is also expressed by the endothelial cells [25], as well as by surrounding bone marrow, macrophages, and fibroblasts. Relatively specific markers for lymphatic endothelial cells are lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), podoplanin, Prox1, and VEGF-receptor-3 (VEGFR-3) [reviewed in [5]]. Though data have only been published for limited number of tumors, most of the CD31-positive vessels Gorham disease co-express LYVE-1 [12,26], a marker for lymphatic endothelial cells that is also expressed in liver and spleen sinusoids [27]. As LYVE-1 positive cells are not present in normal bone [28], the origin of the lymphatic endothelial cells in the bone is likely due to invasion, though transdifferentiation from blood endothelial cells or monocytic cells is also possible. VEGFR-3, the receptor for lymphangiogenic growth factors VEGF-C and –D, is expressed by less than half of the proliferating vessels [29]. In addition, VEGFR-3 is expressed by macrophages in the surrounding tissue [12]. In one case, PDGFR-β was also expressed in most vessels, but VEGF, VEGF-C, and epidermal growth factor (EGF) were not found to stain strongly. These data suggest that Gorham disease has a lymphatic endothelial origin. The lymphatic origin of the disease has made lymphangiogenic growth factors attractive therapeutic targets, though no causal relationship between these targets and tumor growth or invasion has been established.
Serum markers
Recent studies have suggested measurement of several (lymph)angiogenic factors in patients’ serum as markers of clinical disease [12,26,30]. One study found that PDGF-BB was elevated 7-fold in a patient with Gorham disease, using enzyme-linked immunosorbent assays (ELISAs) [12]. In the same patient, fibroblast growth factor (FGF) and the number of circulating endothelial cells, which are mobilized by angiogenic factors, were also elevated, while VEGF, placental growth factor (PlGF), and soluble VEGFR-1 were within normal limits. At the time of testing, this patient had already failed treatment with thalidomide, bisphosphonates, and IFN-α2b. Another report found an increased VEGF level in the serum of a patient with diffuse lymphangiomatosis without evident osteolysis or chylothorax, which subsequently fell after treatment with interferon-α2b. [30]. In a recent report, serum VEGF and VEGF-C levels both fell from slightly above reference range to normal values in one patient upon clinical remission, but levels were not evidently abnormal in another patient at any stage of the disease [26]. These data are consistent with findings from clinical trials using angiogenesis inhibitors that have shown serum biomarkers are not reliable indicators of response [31]. Thus, it is difficult to interpret whether secreted lymphatic growth factors drive the growth of lymphangiomas based on serum levels alone.
Treatment
Surgery
In Gorham disease localized to the maxillofacial region, spine or extremities, bone loss with concomitant functional impairment mandates surgical resection and reconstruction or stabilization procedures. Importantly, surgical reconstruction undertaken in the context of active disease is often unsuccessful, with rapid osteolysis and resorption of bone graft material [32]. It has been strongly recommended that any attempt at surgical reconstruction of a bony defect with biological material be pursued only after the underlying disease has been controlled with one of the non-surgical measures described below [33].
In thoracic Gorham disease, prolonged disease stabilization may be achieved after surgical intervention only [16,34], but successful radical resection of diseased tissue is seldom achieved [4,12]. Progressive respiratory insufficiency may necessitate surgery in specific patients, particularly in cases of pleural effusions and chylothorax. Appropriate interventions include pleurectomy and pleurodesis. Thoracic duct ligation has been successfully applied in some cases, but its contribution to clinical improvement has not been consistently demonstrated [35,36].
Medical treatment
Interferon-α2b
Interferon-α is an immunomodulatory and antiangiogenic compound that also inhibits production of bFGF [37] and is used in the treatment of hemangiomas [38]. Interferon-α2b monotherapy can stabilize thoracic Gorham disease and be safely combined with bisphosphonates [29,30,39-43]. Interferon has also been utilized as adjuvant therapy after surgical intervention [35,44]. In these reports, disease regression was accompanied by clinical improvement, after which IFN-α2b could be decreased and subsequently discontinued in the course of several months to years.
Though there is growing evidence that interferon-alpha can offer therapeutic benefit in the treatment of Gorham disease, not all patients respond favorably and not all responders enjoy full functional recovery. In a recently published series of 8 pediatric patients, all showed stabilization of osteolytic lesions but only two showed substantial clinical improvement [36]. The persistence of functional impairments in these children likely reflects the severe and irreversible character of the bone and soft tissue injuries caused by the disease prior to the initiation of therapy. Several other authors have also reported failure of interferon-α therapy [12,13,26,45]. While interferon appears to be a promising treatment option for management of Gorham disease, the inclusion of additional pharmacologic therapies may be required to arrest the progression of advanced disease and prevent development of irreparable loss of function.
Bisphosphonates
Bisphosphonates have anti-osteoclastic and pro-osteogenic properties and are widely used in disorders of excessive bone resorption [46]. Their use is well established in treatment of Gorham disease, usually as an adjunct to radiation therapy or interferon-α [reviewed in [3]]. One report described full clinical stabilization of Gorham disease localized to the thorax after bisphosphonate monotherapy [47].
Thalidomide
The synthetic glutamic-acid derivate thalidomide has a broad immunomodulatory effect through blockade of tumor necrosis factor-α. It also acts as an antiangiogenic agent by inhibiting IL-12 and bFGF and is used in the treatment of multiple myeloma [48]. Thalidomide did not lead to sustained clinical improvement in two cases of Gorham disease, either administered together with celecoxib [12] or as monotherapy [49]. In contrast, another report described full disease stabilization by thalidomide in a case of disseminated lymphangiomatosis accompanied by chylothorax, refractory to interferon-α[45].
Targeted therapy
Blockers of angiogenic growth factor receptors are approved for clinical use in cancer and other diseases [50]. These include the VEGF-neutralizing antibody bevacizumab and multitargeted tyrosine kinase inhibitors (TKIs) that block the signaling pathways of several angiogenic growth factors. Imatinib mesylate is a TKI that specifically inhibits the TK domain of abl, c-kit, and PDGFR-β. A report showed that PDGFR-β is expressed in the tissues of a patient with Gorham disease, while its ligand, PDGF-BB, was elevated in the patient's serum. Treatment with imatinib mesylate in this patient, who had previously failed thalidomide and IFN-α2b therapy, was hampered by massive pleural effusions that mandated discontinuation of the treatment [12]. The authors speculated that an inhibitor of vascular permeability, such as bevacizumab, might be added to imatinib mesylate to ameliorate pleural effusions. Another recent report showed disease regression when bevacizumab was added to a regimen of bisphosphonates, IFN-α2b, and imatinib mesylate [13]. In this case, clinical stabilization was sustained under bevacizumab monotherapy. A third report described failure of IFN-α2b –bevacizumab combination therapy, which was salvaged by radiation therapy and surgery [26]. These novel data are the first to provide evidence that therapeutics targeting lymphangiogenic growth factors may provide useful tools to combat Gorham disease.
There has been interest in applying sirolimus, an inhibitor of the mTOR pathway, in the management of a variety of vascular malformations, including lymphangiomatosis [51,52]. Targeting mTOR is attractive as it is downstream in the signaling pathways of both of the primary receptors that stimulate lymphatic growth, VEGFR-2 and VEGFR-3 (Figure 1). In theory, mTOR inhibition should more completely inhibit these signaling pathways when compared to approaches that block only the ligands of these receptors. For instance, bevacizumab, the monoclonal antibody against the VEGF ligand, does not inhibit the binding of VEGF-C to VEGFR-3, thus allowing alternative signaling pathways to continue to drive disease progression. The disadvantage to targeting mTOR is that it is widely activated in many physiological processes and thus potential side effects may not be acceptable. Another potential therapeutic strategy is to target the signaling of both VEGFR-2 and VEGFR-3, using small molecule tyrosine kinase inhibitors. This approach allows signaling from all possible VEGF family ligands to be inhibited, while maintaining some specificity the blood and lymphatic vasculature.
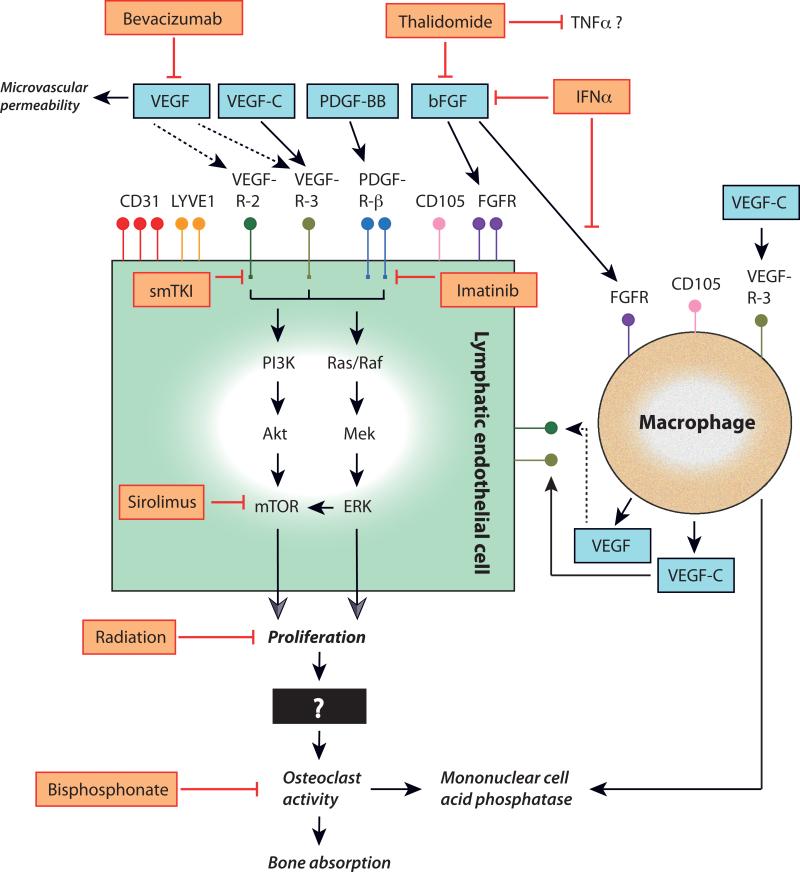
FIGURE 1. Schematic representation of the putative molecular pathways and their clinical inhibitors in Gorham disease.
Lymphatic endothelial cell (LEC) markers shown to be variably expressed in Gorham tissues include cluster of differentiation (CD)-31 protein, lymphatic vessel endothelial hyaluronan receptor (LYVE)-1, and CD105. Growth factors stimulating (black arrows) LEC proliferation and macrophage activation in Gorham disease are shown (blue boxes) and include vascular endothelial growth factor (VEGF; though it may not have a direct effect on LECs in Gorham disease (dashed arrows)), VEGF-C, platelet-derived growth factor (PDGF)-BB, and basic fibroblast growth factor (bFGF). Binding of these ligands to their receptors—VEGF-receptor (R)-2, VEGFR-3, PDGFR-β, and FGFR—will trigger downstream signaling. Clinical inhibitors (orange boxes, red barred lines) of LEC surface receptor proteins and their ligands shown to affect disease progression in Gorham disease include humanized monoclonal antibody bevacizumab, thalidomide and interferon (IFN)-alpha. Imatinib mesylate (imatinib) inhibits signaling through the tyrosine kinase domain of PDGFR-β. Small molecule tyrosine kinase inhibitors (smTKI) that are now in development and in clinical trials may be able to block signaling through the tyrosine kinase domains of activated receptors or their downstream effectors. One promising agent—sirolimus—blocks the mTOR pathway, which is activated through the PI3K/Akt and Ras/Raf signaling pathways. However, there are many other signaling targets that may present potential therapeutic opportunities in Gorham disease. Radiation therapy also halts progression in Gorham disease (in part) by inhibiting LEC proliferation. Bisphosphonates inhibit increased osteoclast activity and the ensuing bone resorption.
Sirolimus has recently been used to successfully treat another lymphatic based disorder, lymphangioleiomyomatosis [53]. Based on this success and the promising activity of this agent in a small case series of children with refractory lymphatic disorders [51], a clinical trial is now underway to prospectively assess the efficacy of sirolimus in children and young adults suffering from lymphangiomatosis.
Radiation therapy
Radiation therapy is effective in halting progression of Gorham disease as described in a large number of case reports and small series [reviewed in [3]]. In one clinical cohort study, radiotherapy achieved disease stabilization in approximately 80% of patients [54]. Dunbar et al. conclude that a moderate dose of 40-45 Gy with conventionally fractionated external beam radiation therapy generally led to a good clinical outcome with few long-term complications [55]. However, in many circumstances these moderate doses cannot be safely delivered due to extensive thoracic involvement and the prohibitive normal tissue tolerances of the lungs and heart. Though doses in the 36-45 Gy range appear to be most consistently effective [54], lower doses in the 16-20 Gy range have also been successfully applied in the management of patients with chylothorax and chylopericardium [56-59].
Gorham disease often affects pediatric patients where radiotherapy is reluctantly prescribed due to the potential adverse effects on the growth potential of skeletally immature patients and the long term risk of radiation-induced malignancy. Despite these concerns about the long-term sequelae of radiotherapy delivered to young patients, radiation should be considered in cases where medical therapy has failed to arrest disease progression. In the setting of extensive thoracic disease involving chest wall pleura and lung parenchyma, XRT should be considered earlier in the management of the disease before irreversible respiratory impairment develops. In such circumstances, earlier use of radiation in the lower range of effective doses (16-20 Gy) may be lifesaving and worth the risk of late effects.
Discussion
The growing body of literature, along with the emergence of novel targeted therapies that are able to modulate pathways of lymphangiogenesis, provide clinicians with a small but growing array of treatment options for Gorham disease. Given the rarity of this disorder, it is not likely that a randomized study comparing currently available therapies could be undertaken. While we await the outcome of the prospective study of sirolimus, we will need to continue to utilize agents with known activity in combinations that are tailored to each patient's sites of disease, severity of presentation and identifiable biological features. As we consider how to optimize therapy for this aggressive, heterogeneous disease, several questions need to be answered.
First, what mechanisms drive Gorham disease and what clinical investigations may be useful in that context? The disease process is started by as yet unidentified molecular events. Both aberrant lymphatic vessel formation through sequestration [2] as well as lymphatic endothelial growth factor overexpression may contribute to the ensuing lymphangiomatosis [24]. This leads to excessive proliferation of (LYVE-1- and partly VEGFR-3-positive) lymphatic endothelial cells. These cells can express PDGFR-β, but immunohistochemical staining of other growth factor receptors and their mediators has not been described so far. The expanding, aberrant lymphatic network can destroy adjacent soft tissue and bone through unknown mechanisms, possibly including upregulation of osteoclast proliferation and activity.
VEGF was found elevated in the serum of a patient with diffuse lymphangiomatosis [30], but its mechanistic contribution in Gorham disease remains unclear since its receptors, VEGFR-1 and -2 may not be expressed by the proliferative tissues [23]. Possibly, VEGF is expressed in cells surrounding the proliferating tissues and aggravates local blood microvascular permeability, contributing to the development of pleural effusions in the setting of chest wall disease. Alternatively, VEGF may be differentially expressed among cases of Gorham disease. Both imatinib mesylate and bevacizumab have been used in treatment, but the results are mixed. Based on our own disappointing anecdotal experience with imatinib in the management of a patient with advanced thoracic disease, we would counsel against the inclusion of this agent in the treatment of patients who present with pleural effusions. Given the relatively small but growing body of literature regarding the biology of this disease and the lack of predictive power of serum biomarkers to determine response [31], we argue that immunohistochemical examination of the lymphangioma tissue for the VEGF-, PDGF-, and bFGF-family of ligands and receptors should be undertaken in all newly diagnosed patients. These investigations will not only help us better characterize potential therapeutic targets but may also help us develop a more informed biological rationale for the treatments we employ.
Second, how can we weigh the available options and come to an adequate therapeutic strategy in a particular case? First, surgery may be appropriate in case of localized, resectable disease that is not actively osteolytic– mainly in the cranium and extremities. Surgery also plays an important role when respiratory insufficiency necessitates prompt thoracic surgical intervention. Pharmacologically, bisphosphonates are widely used as an anti-osteolytic adjunct to therapy, but were only once reported to induce disease remission as monotherapy. A number of cases showed that IFN-α2b therapy can induce and sustain disease stabilization. However, failure of this therapy has also been reported. Given the number of cases treated with with IFN-α2b, and bisphosphonates now published, we speculate that combination therapy of IFN-α2b and bisphosphonates should be the first line of medical therapy. Finally, three recent case reports suggested the use of imatinib mesylate and/or bevacizumab [12,26,45]. We speculate that combination therapy of imatinib mesylate and bevacizumab may be appropriate as bevacizumab may decrease possibly worsening vascular edema under imatinib treatment and may have its own anti-lymhpangiogenic effect. Interestingly, imatinib mesylate was shown to also have anti-osteolytic properties [60]. Until we learn more, we propose that targeted antibody therapy is appropriate as second line, when other therapies have failed and targets identified in an individual patient's tissue. Finally, radiotherapy is well established in the treatment of (thoracic) Gorham disease, provided it can be adequately and safely delivered to the target tissue, and the longterm risk of radiation in pediatric patients is justified based on an individual patient's disease-associated risks.
Third, what further insights are needed to advance the treatment of patients with Gorham disease? It remains to be determined what the underlying mechanisms are for initiation and progression of osteolytic activity of the lymphatic endothelial cells. Interestingly, lymphangioleiomyomatosis, an acquired, potentially lethal form of lymphatic vessel hyperplasia, is driven by VEGF-D overexpression resulting from genetic defects in the pericytes of collecting lymphatics [61]. Therefore, the role of other cells in the local microenvironment, such as mononuclear-derived cells, macrophages, and perivascular stromal cells also needs to be elucidated in Gorham disease. Furthermore, lymphangioleiomyomatosis has been effectively treated by inhibiting the mTOR pathway [53]. Potentially, lessons learned from lymphangioleiomyomatosis may be applied in Gorham disease, including in the ongoing prospective trial of sirolimus in lymphangiomatosis patients.
Novel insights will have to come from experiments using individual patients’ tissues. Systematic screening of patients’ tissues for (lymphangiogenic) growth factors may yield novel therapeutic targets. However, substantial advancements in treatment of rare diseases may only come from national and international research networks, such as in lymphangioleiomyomatosis [62,63]. In Gorham disease, at this moment, a solid clinical balance needs to be made for each individual patient taking into account the up-to-date literature as well as the potential for individualized, targeted therapy.
Acknowledgements
This work was supported in part by NIH R00CA137167 and NIH DP2OD008780 (TPP). The authors thank drs. J. Kuilboer, program director Joint Venture UMC Utrecht-Antoni van Leeuwenhoek, for support (JH, IBR).
Footnotes
Conflicts of interest. All authors have no conflicts of interest to disclose.
Contributor Information
Jeroen Hagendoorn, Department of Surgical Oncology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands..
Torunn I. Yock, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, 55 Fruit Street, Boston, MA 02114, United States..
Inne H.M. Borel Rinkes, Department of Surgical Oncology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands..
Timothy P. Padera, Edwin L. Steele Laboratory for Tumor Biology, Massachusetts General Hospital and Harvard Medical School, 55 Fruit Street, Boston, MA 02114, United States..
David H. Ebb, Department of Pediatric Hematology and Oncology, Massachusetts General Hospital and Harvard Medical School, 55 Fruit Street, Boston, MA 02114, United States..
REFERENCES
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tissue Tumors: Mosby Elsevier. 2008 [Google Scholar]
- 2.Blei F. Congenital lymphatic malformations. Annals of the New York Academy of Sciences. 2008;1131:185–194. doi: 10.1196/annals.1413.016. [DOI] [PubMed] [Google Scholar]
- 3.Patel DV. Gorham's disease or massive osteolysis. Clinical medicine & research. 2005;3(2):65–74. doi: 10.3121/cmr.3.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiu K, Kanno R, Suzuki H, et al. Chylothorax associated with massive osteolysis (Gorham's syndrome). The Annals of thoracic surgery. 2002;73(6):1956–1957. doi: 10.1016/s0003-4975(02)03413-6. [DOI] [PubMed] [Google Scholar]
- 5.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. The Journal of experimental medicine. 2002;196(11):1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122(12):3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 8.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubo H, Cao R, Brakenhielm E, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer cell. 2004;6(4):333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Huber S, Bruns CJ, Schmid G, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney international. 2007;71(8):771–777. doi: 10.1038/sj.ki.5002112. [DOI] [PubMed] [Google Scholar]
- 12.Hagendoorn J, Padera TP, Yock TI, et al. Platelet-derived growth factor receptor-beta in Gorham's disease. Nature clinical practice Oncology. 2006;3(12):693–697. doi: 10.1038/ncponc0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunewald TG, Damke L, Maschan M, et al. First report of effective and feasible treatment of multifocal lymphangiomatosis (Gorham-Stout) with bevacizumab in a child. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(8):1733–1734. doi: 10.1093/annonc/mdq331. [DOI] [PubMed] [Google Scholar]
- 14.Aman J, Thunnissen E, Paul MA, et al. Successful treatment of diffuse pulmonary lymphangiomatosis with bevacizumab. Annals of internal medicine. 2012;156(11):839–840. doi: 10.7326/0003-4819-156-11-201206050-00016. [DOI] [PubMed] [Google Scholar]
- 15.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. The Journal of bone and joint surgery American volume. 1955;37-A(5):985–1004. [PubMed] [Google Scholar]
- 16.Chavanis N, Chaffanjon P, Frey G, et al. Chylothorax complicating Gorham's disease. The Annals of thoracic surgery. 2001;72(3):937–939. doi: 10.1016/s0003-4975(00)02417-6. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen JC, Tan IC, Marshall MV, et al. Lymphatic imaging in humans with near-infrared fluorescence. Current opinion in biotechnology. 2009;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. The Journal of cell biology. 2011;193(4):607–618. doi: 10.1083/jcb.201012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heyden G, Kindblom LG, Nielsen JM. Disappearing bone disease. A clinical and histological study. The Journal of bone and joint surgery American volume. 1977;59(1):57–61. [PubMed] [Google Scholar]
- 20.Moller G, Priemel M, Amling M, et al. The Gorham-Stout syndrome (Gorham's massive osteolysis). A report of six cases with histopathological findings. The Journal of bone and joint surgery British volume. 1999;81(3):501–506. doi: 10.1302/0301-620x.81b3.9468. [DOI] [PubMed] [Google Scholar]
- 21.Devlin RD, Bone HG, 3rd, Roodman GD. Interleukin-6: a potential mediator of the massive osteolysis in patients with Gorham-Stout disease. The Journal of clinical endocrinology and metabolism. 1996;81(5):1893–1897. doi: 10.1210/jcem.81.5.8626854. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama T, Sabokbar A, Itonaga I, et al. Cellular and humoral mechanisms of osteoclast formation and bone resorption in Gorham-Stout disease. The Journal of pathology. 2001;195(5):624–630. doi: 10.1002/path.989. [DOI] [PubMed] [Google Scholar]
- 23.Colucci S, Taraboletti G, Primo L, et al. Gorham-Stout syndrome: a monocytemediated cytokine propelled disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(2):207–218. doi: 10.1359/JBMR.051019. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishnan K, Rockson SG. Gorham's disease: an osseous disease of lymphangiogenesis? Annals of the New York Academy of Sciences. 2008;1131:203–205. doi: 10.1196/annals.1413.022. [DOI] [PubMed] [Google Scholar]
- 25.Franchi A, Bertoni F, Bacchini P, et al. CD105/endoglin expression in Gorham disease of bone. Journal of clinical pathology. 2009;62(2):163–167. doi: 10.1136/jcp.2008.060160. [DOI] [PubMed] [Google Scholar]
- 26.Brodszki N, Lansberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta paediatrica. 2011;100(11):1448–1453. doi: 10.1111/j.1651-2227.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 27.Mouta Carreira C, Nasser SM, di Tomaso E, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and downregulation in human liver cancer and cirrhosis. Cancer research. 2001;61(22):8079–8084. [PubMed] [Google Scholar]
- 28.Edwards JR, Williams K, Kindblom LG, et al. Lymphatics and bone. Human pathology. 2008;39(1):49–55. doi: 10.1016/j.humpath.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Timke C, Krause MF, Oppermann HC, et al. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatric blood & cancer. 2007;48(1):108–111. doi: 10.1002/pbc.20461. [DOI] [PubMed] [Google Scholar]
- 30.Dupond JL, Bermont L, Runge M, et al. Plasma VEGF determination in disseminated lymphangiomatosis-Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873–876. doi: 10.1016/j.bone.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nature reviews Clinical oncology. 2009;6(6):327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuriyama DK, McElligott SC, Glaser DW, et al. Treatment of Gorham-Stout disease with zoledronic acid and interferon-alpha: a case report and literature review. Journal of pediatric hematology/oncology. 2010;32(8):579–584. doi: 10.1097/MPH.0b013e3181edb464. [DOI] [PubMed] [Google Scholar]
- 33.Mignogna MD, Fedele S, Lo Russo L, et al. Treatment of Gorham's disease with zoledronic acid. Oral oncology. 2005;41(7):747–750. doi: 10.1016/j.oraloncology.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Hejgaard N, Olsen PR. Massive Gorham osteolysis of the right hemipelvis complicated by chylothorax: report of a case in a 9-year-old boy successfully treated by pleurodesis. Journal of pediatric orthopedics. 1987;7(1):96–99. doi: 10.1097/01241398-198701000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Pfleger A, Schwinger W, Maier A, et al. Gorham-Stout syndrome in a male adolescent-case report and review of the literature. Journal of pediatric hematology/oncology. 2006;28(4):231–233. doi: 10.1097/01.mph.0000203721.83566.e6. [DOI] [PubMed] [Google Scholar]
- 36.Venkatramani R, Ma NS, Pitukcheewanont P, et al. Gorham's disease and diffuse lymphangiomatosis in children and adolescents. Pediatric blood & cancer. 2011;56(4):667–670. doi: 10.1002/pbc.22948. [DOI] [PubMed] [Google Scholar]
- 37.Singh RK, Gutman M, Bucana CD, et al. Interferons alpha and beta downregulate the expression of basic fibroblast growth factor in human carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(10):4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for lifethreatening hemangiomas of infancy. The New England journal of medicine. 1992;326(22):1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 39.Hagberg H, Lamberg K, Astrom G. Alpha-2b interferon and oral clodronate for Gorham's disease. Lancet. 1997350(9094):1822–1823. doi: 10.1016/S0140-6736(05)63639-2. [DOI] [PubMed] [Google Scholar]
- 40.Kose M, Pekcan S, Dogru D, et al. Gorham-Stout Syndrome with chylothorax: successful remission by interferon alpha-2b. Pediatric pulmonology. 2009;44(6):613–615. doi: 10.1002/ppul.20849. [DOI] [PubMed] [Google Scholar]
- 41.Laverdiere C, David M, Dubois J, et al. Improvement of disseminated lymphangiomatosis with recombinant interferon therapy. Pediatric pulmonology. 2000;29(4):321–324. doi: 10.1002/(sici)1099-0496(200004)29:4<321::aid-ppul13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Ozeki M, Funato M, Kanda K, et al. Clinical improvement of diffuse lymphangiomatosis with pegylated interferon alfa-2b therapy: case report and review of the literature. Pediatric hematology and oncology. 2007;24(7):513–524. doi: 10.1080/08880010701533603. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymphhemangiomatosis: a severe case of Gorham-Stout syndrome. Journal of pediatric surgery. 2005;40(3):E47–50. doi: 10.1016/j.jpedsurg.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Yeager ND, Hammond S, Mahan J, et al. Unique diagnostic features and successful management of a patient with disseminated lymphangiomatosis and chylothorax. Journal of pediatric hematology/oncology. 2008;30(1):66–69. doi: 10.1097/MPH.0b013e318159a55a. [DOI] [PubMed] [Google Scholar]
- 45.Pauzner R, Mayan H, Waizman A, et al. Successful thalidomide treatment of persistent chylous pleural effusion in disseminated lymphangiomatosis [corrected]. Annals of internal medicine. 2007;146(1):75–76. doi: 10.7326/0003-4819-146-1-200701020-00022. [DOI] [PubMed] [Google Scholar]
- 46.Russell RG, Xia Z, Dunford JE, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Annals of the New York Academy of Sciences. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 47.Hammer F, Kenn W, Wesselmann U, et al. Gorham-Stout disease--stabilization during bisphosphonate treatment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20(2):350–353. doi: 10.1359/JBMR.041113. [DOI] [PubMed] [Google Scholar]
- 48.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nature reviews Cancer. 2004;4(4):314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 49.Kothari SS, Sharma S, Bhatt K, et al. Recurrent hemorrhagic pericardial effusion in a child due to diffuse lymphangiohemangiomatosis: a case report. Journal of medical case reports. 2010;4:62. doi: 10.1186/1752-1947-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatric blood & cancer. 2011;57(6):1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 52.Reinglas J, Ramphal R, Bromwich M. The successful management of diffuse lymphangiomatosis using sirolimus: a case report. The Laryngoscope. 2011;121(9):1851–1854. doi: 10.1002/lary.21927. [DOI] [PubMed] [Google Scholar]
- 53.McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. The New England journal of medicine. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heyd R, Micke O, Surholt C, et al. Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. International journal of radiation oncology, biology, physics. 2011;81(3):e179–185. doi: 10.1016/j.ijrobp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Dunbar SF, Rosenberg A, Mankin H, et al. Gorham's massive osteolysis: the role of radiation therapy and a review of the literature. International journal of radiation oncology, biology, physics. 1993;26(3):491–497. doi: 10.1016/0360-3016(93)90968-2. [DOI] [PubMed] [Google Scholar]
- 56.Dajee H, Woodhouse R. Lymphangiomatosis of the mediastinum with chylothorax and chylopericardium: role of radiation treatment. The Journal of thoracic and cardiovascular surgery. 1994;108(3):594–595. [PubMed] [Google Scholar]
- 57.Duffy BM, Manon R, Patel RR, et al. A case of Gorham's disease with chylothorax treated curatively with radiation therapy. Clinical medicine & research. 2005;3(2):83–86. doi: 10.3121/cmr.3.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson DW, Klazynski PT, Gordon WH, et al. Mediastinal lymphangioma and chylothorax: the role of radiotherapy. The Annals of thoracic surgery. 1986;41(3):325–328. doi: 10.1016/s0003-4975(10)62780-4. [DOI] [PubMed] [Google Scholar]
- 59.Kandil A, Rostom AY, Mourad WA, et al. Successful control of extensive thoracic lymphangiomatosis by irradiation. Clinical oncology. 1997;9(6):407–411. doi: 10.1016/s0936-6555(97)80140-9. [DOI] [PubMed] [Google Scholar]
- 60.Vandyke K, Fitter S, Dewar AL, et al. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010;115(4):766–774. doi: 10.1182/blood-2009-08-237404. [DOI] [PubMed] [Google Scholar]
- 61.Seyama K, Kumasaka T, Kurihara M, et al. Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphatic research and biology. 2010;8(1):21–31. doi: 10.1089/lrb.2009.0018. [DOI] [PubMed] [Google Scholar]
- 62.Ingelfinger JR, Drazen JM. Patient organizations and research on rare diseases. The New England journal of medicine. 2011;364(17):1670–1671. doi: 10.1056/NEJMe1102290. [DOI] [PubMed] [Google Scholar]
- 63.Nurok M, Eslick I, Carvalho CR, et al. The International LAM Registry: a component of an innovative web-based clinician, researcher, and patientdriven rare disease research platform. Lymphatic research and biology. 2010;8(1):81–87. doi: 10.1089/lrb.2009.0028. [DOI] [PubMed] [Google Scholar]