Abstract
Stem cells, having the potential for self-renewal and multilineage differentiation, are the building blocks for tissue/organ regeneration. Stem cells can be isolated from various sources but are, in general, available in too small numbers to be used directly for clinical purpose without intermediate expansion procedures in vitro. Although this in vitro expansion of undifferentiated stem cells is necessary, stem cells typically diminish their ability to self-renew and proliferate during passaging. Consequently, maintaining the stemness of stem cells has been recognized as a major challenge in stem cell-based research. This review focuses on the latest developments in maintaining the self-renewal ability of stem cells during in vitro expansion by biomaterial strategies. Further, this review highlights what should be the focus for future studies using stem cells for regenerative applications.
Introduction
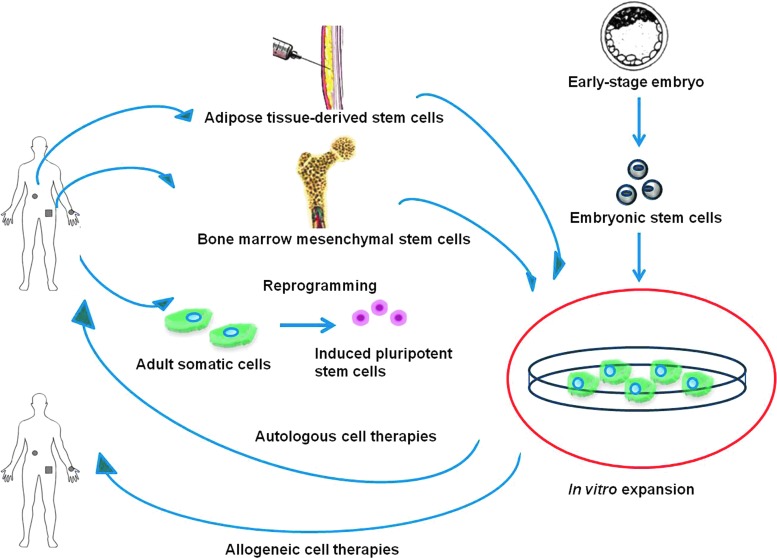
Stem cells, having the ability to self-renew and give rise to multiple cell types,1 are the key factors in both developmental biology and regenerative medicine. In the last decade, an increasing interest in research on stem cells and their clinical applications has become apparent. For therapeutic applications, stem cells are first obtained from either early-stage embryo or adult tissues, expanded in vitro, and transplanted back into patients in order to treat disease or injury (Fig. 1). The most frequently studied stem cells include embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), bone marrow mesenchymal stem cells (BMSCs), and adipose tissue-derived stem cells (ASCs).
FIG. 1.
Schematic diagram of stem cell-based therapies. Stem cells can be obtained from either early-stage embryos or adult tissues, expanded in vitro, and transplanted back to patients. Color images available online at www.liebertpub.com/teb
Human ESCs, derived from the early-stage embryo, are one of the most widely studied cell sources for regenerative medicine (Fig. 2). ESCs exhibit the ability to differentiate into a variety of specialized cell types and, hence, represent a unique opportunity for tissue engineering and regenerative medicine. For example, controlled human ESC differentiation could result in an improved vision for patients with macular degeneration.2 However, their clinical application is mainly limited by their ethical concerns. Recently, human iPSCs, free of ethical and political issues, have been produced by transfection of certain stem cell-related genes into adult somatic cells, and they have gained great interest in regenerative medicine (Fig. 2). These cells are similar to natural pluripotent stem cells in morphology and have the ability to differentiate into a variety of cell types. Nevertheless, one limitation of human iPSCs is that the efficiency of successfully reprogrammed cells has been incredibly low. The low efficiency rate necessitates in vitro expansion before clinical use of iPSCs. Other concerns involve the difficulties in homogeneous cellular differentiation to specific cell types and the in vivo properties of immortal cells such as the tumorigenic fate of teratoma-initiating iPSCs.3
FIG. 2.
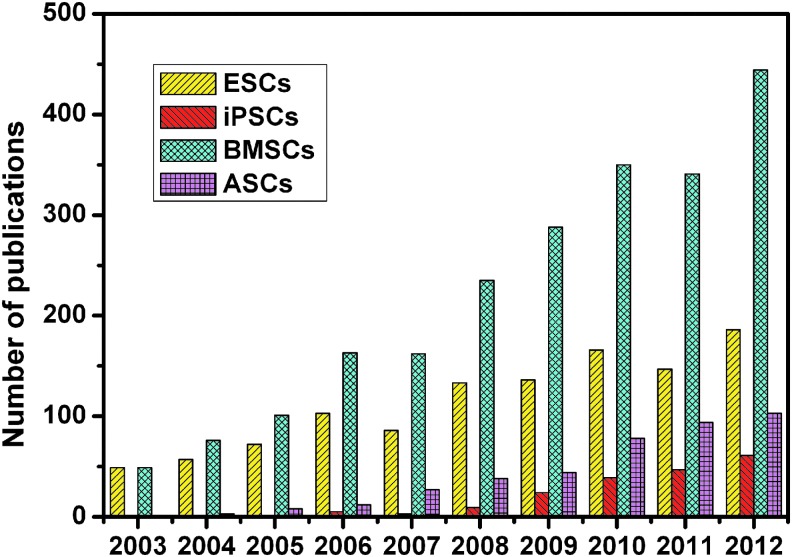
Increasing research in developing new therapies with stem cells in tissue regeneration by using keywords “ESCs, iPSCs, BMSCs or ASCs” and “tissue regeneration” in Web of Science. ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; BMSCs, bone marrow mesenchymal stem cells; ASCs, adipose tissue-derived stem cells. Color images available online at www.liebertpub.com/teb
Mesenchymal stem cells (MSCs) are relatively safe and have been isolated from a variety of tissues, for example, bone marrow,4,5 adipose tissue,6 dental pulp,7 hair follicles,8 dermis,9 heart,10 liver,11 and spleen.12 There has been an increase in adult BMSC research in tissue regeneration (Fig. 2). Transplanted BMSCs can accelerate healing in human cutaneous wounds,13 repair infarcted human myocardium,4 chronic lower extremity wounds,5 and induce the formation of sufficient new bone to enable the reliable placement of dental implants.14 Nevertheless, the bone marrow harvest procedure is complex. ASCs have become one of the most popular stem cell populations in stem cell-based regeneration research (Fig. 2), as adipose tissue can be harvested in larger quantities with less invasive methods. The research to date has tended to focus on their potential for clinical applications. For instance, use of expanded ASCs is a safe and effective treatment for complex perianal fistula6 and depressed scars.15
Besides MSCs, other somatic stem cells play essential roles in regenerative medicine. For instance, the transplantation of peripheral blood stem cell is beneficial for acute myeloid leukemia16; transplantation of neural stem cells (NSCs) can enhance synaptic plasticity, reduce neuronal loss, and improve cognition in animal models of Alzheimer's disease17; hematopoietic stem cell (HSC) transplantation leads to rapid improvement of clinical symptoms and quality of life in interleukin (IL)-10- and IL-10 receptor-deficient patients; and corneal epithelial stem cell therapy using ex vivo expanded autologous cells proves successful in the treatment of unilateral limbal stem cell deficiency.18
Despite promising clinical applications, stem cells are usually found in low numbers, and their response to aging typically diminishes their ability to self-renew and proliferate.19 To be effective for therapeutic applications, large numbers of stem cells are needed. For example, for bone tissue engineering, 160 million cells would be required for 20 cm3 of tissue engineered implants based on using 8 million cells/cm3 scaffold20,21 to gain substantial bone formation in the case of large bone defects. In the case of treating chronic ischemic heart disease by stem cell injection, lack of diffusion of the transplanted cells could also result in low cell delivery efficiency,22 thus high numbers of cells are required. Nevertheless, the fate of stem cells is doomed in the same way: The pluripotency of ESCs is affected by the number of passages23 and mitochondrial dysfunction has been found to occur with prolonged culture of ESCs24; hemangioblasts/blast cells derived from human iPSCs have been shown to exhibit limited growth and expansion capability and early senescence with decreased hematopoietic colony-forming capability25; significant decreases in the proliferation and differentiation potential of murine and human BMSCs were observed during in vitro expansion26–28; and the expression of stemness biomarkers in human ASCs decreased significantly during long-term manipulation, along with the decrease of differentiation ability (adipogenesis, osteogenesis, and neurogenesis).29
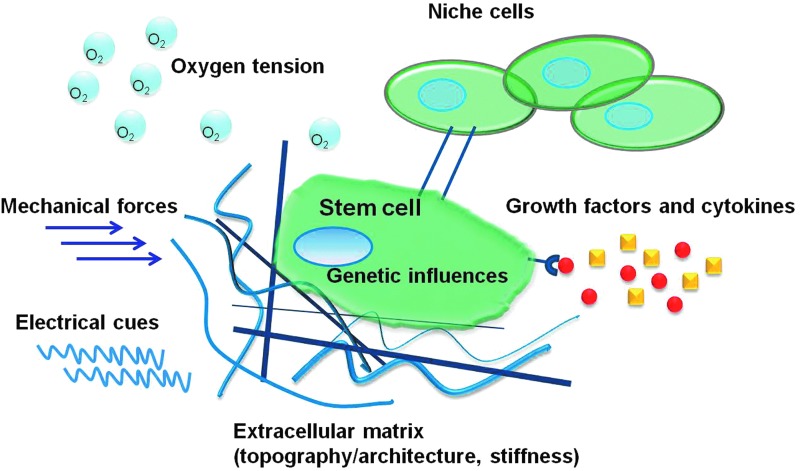
Taken together, the main question is how to maintain the stemness of stem cells during in vitro culture. If this problem can be solved, then a large number of high quality cells could be obtained for clinical purposes. Control of stem cell fate has been well reviewed,30–35 but unfortunately, there is limited research on how to increase stem cell expansion while maintaining their potential. As shown in Figure 3, stem cell fate is regulated by varied factors, including genetic influences, cell–cell communications, growth factors and cytokines, extracellular matrix (ECM; e.g., component contents, topography/architecture), and physiochemical environment (e.g., matrix stiffness, oxygen tension, mechanical forces, and electrical cues). This review mainly focuses on how to maintain the stemness of stem cells by exploiting biomaterial properties.
FIG. 3.
Stem cell fate is regulated by varied factors, including genetic influences, cell–cell communications, growth factors and cytokines, extracellular matrix (e.g., component contents, topography/architecture), and physiochemical environment (e.g., matrix stiffness, oxygen tension, mechanical forces, and electrical cues). Color images available online at www.liebertpub.com/teb
Miscellaneous Approaches for Stem Cell Maintenance
Figure 3 and Table 1 show that stem cell fate is regulated by various factors and accordingly, the stemness of stem cells can be maintained by a variety of approaches, including but not limited to gene transduction, growth factors, and cell–cell interactions.
Table 1.
Miscellaneous Approaches for Stem Cell Maintenance
| Stimuli parameters | Cell types | Observations | References | Limitations |
|---|---|---|---|---|
| Genetic modifications | Human and murine ESCs | The differentiation potential of ESCs can be maintained by the overexpression of Oct4, Sox2, Nanog, Ronin and Zfx | 148–152 | • Lack of the long-term expression of the transgene • Risk to the patients' immune system • Risk of interrupting intrinsic genes complex |
| Human MSCs | Knockdown of p21 enhances proliferation, and osteogenic capacity in human MSCs; MSCs transduced with hTERT can undergo more than 260 population doublings without losing their osteogenic potential | 42,43,153 | ||
| Cell–cell communications | Human ESCs | Various types of feeder cells can support the undifferentiated growth of human ESCs | 65,154 | • Risk of infectious agent contamination • Senescence of feeder cells • Cumbersome, expensive, and time-consuming process |
| Murine HSCs | Endothelial cells can retain their self-renewal and repopulation ability | 63,64 | ||
| Growth factors and cytokines | Human ESCs | Wnt3a and bFGF can retain human ESCs in an undifferentiated state | 55,57,58,155 | • Risk of interrupting the genetic events of normal cells • Risk of malignancy • High production costs |
| Human MSCs | Wnt3a increase proliferation and inhibit osteogenic differentiation, and IL-6 preserves the undifferentiated state of human MSCs | 52,60 | ||
| Murine HSCs | Wnt3a can sustain the self-renewing fate of HSCs with reduced differentiation | 51 | ||
| Biomaterials mimicking ECM components | See Tables 2 and 3 | • Co-operation among many disciplines, including biomaterials, nanotechnology, medicine, biomedical engineering, cell and molecular biology for the development of various forms of advanced biomaterials | ||
| Topography/architecture | Murine ESCs | Nanofibers morphologically mimicking the ECM support the proliferation and self-renewal of murine ESCs | 131–133 | |
| Human MSCs | Nanostructured surface with square lattice symmetry can retain stem-cell phenotype and maintain the growth of human MSCs | 134 | ||
| Matrix stiffness | Murine ESCs and muscle stem cells, human MSCs | Substrates that match the stiffness of their own microenvironments promote their self-renewal ability and maintain their stemness | 139–141 | |
| Oxygen tension | Varied types of cells | Hypoxia maintains undifferentiated states of embryonic, mesenchymal, hematopoietic, and neural stem cell phenotypes | Reviewed in 129 | |
| Mechanical forces | Human ESCs | Biaxial cyclic strain promotes self-renewal and retains pluripotency of human ESC | 142 | |
| Electrical cues | Murine NSCs | Continuous and defined levels of electric current promote NSC proliferation (twofold)138 | 145 | |
| Human MSCs | Pulsed electromagnetic field exposure could enhance cell proliferation; polyelectrolyte hydrogel matrices can support long-term survival and enhance proliferation (1.5-fold) of human MSCs | 146,147 | ||
ESCs, embryonic stem cells; MSCs, mesenchymal stem cells; HSCs, hematopoietic stem cells; bFGF, basic fibroblast growth factor; NSCs, neural stem cells; ECM, extracellular matrix; hTERT, human telomerase; IL-6, interleukin-6.
Transduction of various genes can regulate the fate of tissue-specific adult stem cells and embryonic pluripotent cells.36–41 For example, MSCs transduced with human telomerase can undergo more than 260 population doublings without losing their osteogenic potential, whereas control cells became senescence after 26 population doublings.42,43 Despite promising results, several challenges remain to be overcome. First, genetically modified cells lack the long-term expression of the transgene,44,45 which will significantly limit the clinical and research applications. Second, most current gene delivery strategies are based on viral vectors in order to achieve the stable delivery of genetic information into eukaryotic genomes, which may pose a significant risk to the patients' immune system.46 Third, along with gene delivery strategies, there exists the risk of interrupting intrinsic genes by random insertion of vector sequences.47,48 Moreover, the procedure of gene transduction is relatively complex.
Besides gene transduction, the external signals that control stem cell fate collectively make up the stem cell microenvironment or niche. The niche saves stem cells from depletion, while protecting the host from over-exuberant stem-cell proliferation.49 Therefore, in vitro recapitulation of the in vivo stem cell niche, which constitutes secreted factors, cell–cell interactions and ECM,50 provides a promising approach in reprogramming stem cells for therapeutic purposes.
Growth factors and cytokines hold promise for the maintenance of stem cells due to their ease of application. Wnt proteins, basic fibroblast growth factor (bFGF), transforming growth factor-beta's and their family members, and IL-6 have shown their effects on stem cell maintenance.51–60 However, to maximize their clinical potential, the dosage should be optimized before their clinical administration, because the effects of growth factors on stem cells are dose dependent. For example, low doses of BMP-4 significantly increase the survival of human ASCs and retain their stemness as well as multipotency, whereas high doses increase apoptosis and reduce cell proliferation.61 In another regard, growth factors and cytokines may influence the genetic events of normal cells and, eventually, lead to malignancy.62 Moreover, high production costs of growth factors still exist and safe alternatives to expensive growth factors are desirable for clinical use.
The interactions between stem cells, as well as interactions between stem cells and neighboring differentiated cells, could regulate stem cell fate.63,64 However, there are still several distinct drawbacks of using cell–cell interaction strategies. First, there is a potential of infectious agent contamination when using animal-derived cells for clinical use.65,66 Second, feeder cells used for ESCs also undergo senescence and lose stem cell supportive properties after several passages.67 Third, this process is cumbersome, expensive, and time consuming. Last but not least, the specific mechanisms by which feeder cells support the undifferentiated growth of stem cells has not been defined. This supporting effect is likely not caused by direct cell–cell contact but by certain growth factors secreted by feeder cells. For instance, human ESCs could not be maintained by using human feeder cells lacking the ability to synthesize bFGF, as indicated by the significant decreased expressions of Oct-4 and Nanog in human ESCs cultured in bFGF-knockout group compared with those in the control.65
Due to the limitations of gene transduction, growth factor, and cell–cell interaction approaches, increasing emphasis has been focused on stem cell ECM for stem cell maintenance. Decelluarized ECM from porcine synovium-derived stem cells (SDSCs), a three dimensional (3D) matrix consisting of nanostructured fibers with collagen I as one of the major structural proteins, facilitated SDSCs regaining stem cell phenotypes.68 An increase in cell number from 2.7- to 14.6-fold and an enhanced chondrogenic capacity were observed compared with SDSCs plated on plastic flasks.68 Similar results were observed in human BMSCs and tendon stem cells when the cells were expanded using decellularized ECM from human BMSCs and tendon tissues, respectively.69–71 The ECM that exists as a mix of several different proteins provides instructive cues for cell fate decisions, primarily via the integrin family of cell surface adhesion receptors.72 Those instructive cues can be categorized as biochemical signals (e.g., ECM components) and biophysical cues (e.g., topography and stiffness).30–32,34,35 Thus, biomaterials designed by controlling parameters, including components, material architecture, surface topography, mechanical and electrical properties, may act as an artificial ECM to direct stem cell fate.
Biomaterials for Stem Cell Maintenance
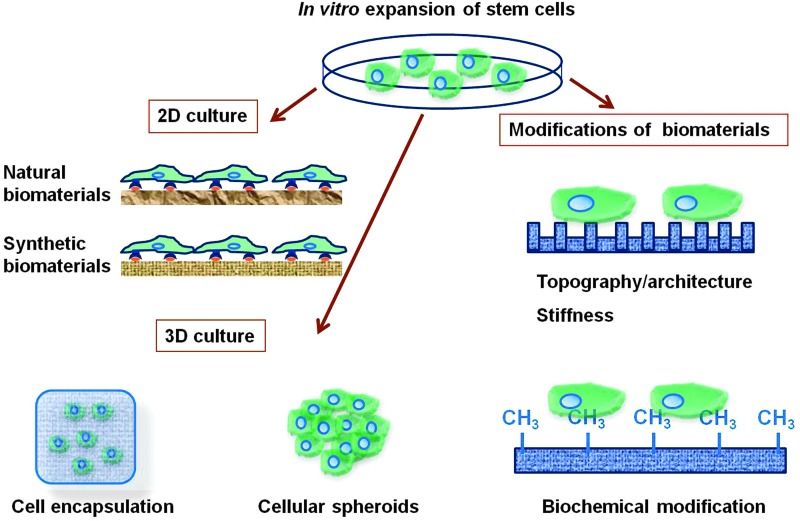
The use of biomaterials, from both natural and synthetic origins, to develop two-dimensional (2D) and 3D cell culture systems for the expansion of pluripotent stem cells (Table 2) as well as adult stem cells (Table 3) is summarized in this section. The modifications of biomaterials in terms of topography/architecture, mechanical properties, and biochemical factors are also described (Fig. 4).
Table 2.
Biomaterials in Pluripotent Stem Cell Expansion
| Biomaterials | Cell types | 2D/3D | Feeder layer | Conditioned medium | Undifferentiated cells can be maintained | References | |
|---|---|---|---|---|---|---|---|
| Natural | Matrigel, vitronectin | Human ESCs and human iPSCs | 2D | No | No | 25 passages | 77 |
| Matrigel, a combination of collagen IV, fibronectin, laminin, and vitronectin | Human ESCs | 2D | No | No | 20 passages | 57 | |
| Matrigel | Human ESCs | 2D | No | No | 9 and 22 passages | 78 | |
| Matrigel, Collagen IV | Human ESCs | 2D | No | Yes | 130 population doublings | 76 | |
| Collagen I | Human ESCs | 2D | No | Yes | 7 passages | 80 | |
| Laminin-111, -332 and -511 | Human ESCs | 2D | No | Yes | 10 passages | 82 | |
| Laminin-511 | Murine ESCs | 2D | No | No | 31 passages | 84 | |
| Human ESCs and iPSCs | 2D | No | No | 20 passages | 85 | ||
| Vitronectin | Human ESCs | 2D | No | No | 7 passages | 87 | |
| Human iPSCs | 2D | No | No | 10 passages | 90 | ||
| Calcium alginate hydrogels | Human ESCs | 3D | No | No | 260 days without passaging in the undifferentiated stage | 112 | |
| Synthetic | PAS | Human ESCs | 2D | No | No | 10 passages | 93 |
| Heparin-binding peptides | Human ESCs | 2D | No | No | 17 passages | 94 | |
| APMAAm | Human ESCs | 2D | No | No | 20 passages | 97 | |
| PMEDSAH | Human ESCs | 2D | No | No | 10 passages | 98 | |
| sIPN | Human ESCs | 3D | No | Yes | 5 days | 104 | |
| HA | Human ESCs | 3D | No | Yes | 20 days | 107 | |
2D, two dimensional; 3D, three dimensional; PAS, peptide-acrylate surfaces; APMAAm, aminopropylmethacrylamide; PMEDSAH, poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide]; sIPN, semi-interpenetrating polymer network; HA, hyaluronic acid.
Table 3.
Biomaterials in Adult Stem Cell Expansion
| Biomaterials | Cell types | 2D/3D | Observations | References | |
|---|---|---|---|---|---|
| Natural | Matrigel | Human MSCs | 2D | Improved cell expansion (1.6-fold) and enhanced neuronal differentiation | 79 |
| Collagen types I and IV together with laminin and fibronectin | Human haematopoietic progenitors | 2D | Increased CD34+ (1.3-fold) and CD41+ (1.2-fold) cell expansions | 81 | |
| Laminin | Murine HSCs | 2D | Amplified HSCs compartment in BMSCs during culture | 86 | |
| Decellularized ECM from human BMSCs | Human BMSCs | 2D | Enhanced proliferation capacity (2 to 4-fold) and increased chondrogenic and osteogenic potentials | 69,70 | |
| ECM from tendon tissue | Human TSCs | 2D | Reduce TSC population doubling time (15%) and preserve the stemness of TSCs | 71 | |
| ECM generated by MSCs from young mice | Aged murine MSCs | 2D | Replication and osteogenesis of aged MSCs can be rescued | 156 | |
| Decelluarized ECM from porcine SDSCs | Porcine SDSCs | 3D | An increase in cell number (2.7 to 14.6-fold) and an enhanced chondrogenic capacity | 68 | |
| Human platelet lysate gel | Human MSCs | 3D | Increased proliferation rates (around twofold) and enhanced colony-forming units outgrowth | 108 | |
| Synthetic | Connecting segment-1 | Human HSCs | 2D | Higher CD34+ cell expansion (sixfold) | 95 |
| Greater expansion of HSCs (2 to 5-fold) and more pluripotent colony-forming units | 96 | ||||
BMSCs, bone marrow mesenchymal stem cells; TSCs, tendon stem cells; SDSCs, synovium-derived stem cells.
FIG. 4.
Biomaterial strategies in vitro expansion of stem cells. 2D and 3D cell culture systems developed by the use of biomaterials, including both natural and synthetic origins, for the expansion of stem cells. The modifications of biomaterials include topography/architecture, mechanical properties, and biochemical factors. 2D, two dimensional; 3D, three dimensional. Color images available online at www.liebertpub.com/teb
Overview of biomaterials
Natural biomaterials
In vitro cell culture is usually carried out on flat substrates, which hold the advantages as a simplified approach to identifying the effect of individual niche components on stem cell fate. The common technique of growing cells on tissue culture polystyrene is gradually being replaced by culturing cells on substrates with a more appropriate composition. Stem cells can sense and respond to ECM molecules through the interaction between integrins on the cell surface and cognate ligand-binding motifs on ECM molecules. The ECM is mainly composed of collagens, laminins, and glycoproteins serving as substrates for a variety of adhesion molecules such as integrins.73 The integrins are a major family of ECM receptors that transmit information from the matrix to cells, thereby playing a key role in the regulation of cell behavior, including cell survival, adhesion, proliferation, and differentiation.74 Designing biomaterials mimicking ECM is a relatively economic and safe approach to regulate the fate of stem cells. Considering that most cells require adhesion to an ECM for survival and growth through ECM–integrin interaction, natural biomaterials consisting of ECM components have been tested for stem cell expansion.
Matrigel
Matrigel is a gelatinous protein mixture and commercially available product comprising several ECM components, such as laminin, type IV collagen, and heparan sulfate proteoglycan.75 Matrigel served as the first feeder-free culture system in which undifferentiated human ESCs could be maintained for 130 population doublings.76 Human ESCs showed successful expression of pluripotency genes and the surface markers of pluripotency proteins during passaging. It should be noted that the stemness of human ESCs can be maintained on Matrigel in mouse embryonic fibroblast conditioned medium, but ESCs on Matrigel without conditioned medium completely differentiated after two passages. Several other chemically defined culture media formulations have been developed to support the undifferentiated proliferation medium of human ESCs on Matrigel substrate in feeder-free conditions.57,77,78 Still, a variety of growth factors and cytokines are required in these defined culture mediums.
For adult stem cells, Matrigel significantly improved cell expansion of MSCs by 1.6-fold through surface modification of tissue-grade polystyrene.79 Nevertheless, Matrigel is derived from mouse tumor cells and may cause pathogen transmission to humans. Growth of stem cells under xeno-free conditions will benefit the later clinical applications.
Collagen
Collagen, the main component of connective tissues, is a classic natural material for tissue engineering and regenerative medicine. Collagen I, which is well defined, a component of several FDA-approved products and widely available, has recently been tested as a potential human ESC growth biomatrix. Collagen I is reported to support the undifferentiated growth of human ESCs for seven passages.80 Human ESCs grown on Collagen I processed a comparable population doubling rate as human ESCs grown on Matrigel. These cells expressed pluripotency proteins and retained a normal karyotype.80 Furthermore, collagen IV has been shown to support the undifferentiated growth of human ESCs, although the cultures on collagen IV did not contain as many undifferentiated colonies as the cultures on Matrigel.76 It should be noted that these culture systems still need the presence of conditioned medium from feeder layers, which is full of growth factors.76,80
For adult stem cells, tissue culture surfaces coated with collagen types I and IV along with laminin and fibronectin increased CD34+ (1.3-fold) and CD41+ (1.2-fold) cell expansions, which provide a better environment for the ex vivo expansion of hematopoietic progenitors.81
Laminin
Laminin, a major component of the ECM, is found in the basal lamina. Recombinant human laminins have been investigated for stem cell culture due to the advantage that they are abundantly available and well-characterized human-origin proteins.82 More than 15 laminin isoforms have been identified83 and human ESCs, commonly expressed abundant integrin α6β1, bind predominantly to laminin-111, -332, and -511.82 Consequently, human ESCs cultured on laminin-111, -332, and -511 substrates can retain an undifferentiated state for 10 passages without feeder layers.82 However, similar as for collagen substrates, these culture systems need the presence of conditioned medium, for which they are feeder free but not xeno free.82 Without conditioned medium or feeder cells, Domogatskaya et al. reported that recombinant human laminin-511 but not -332, -111 alone was sufficient to maintain the self-renewal of mouse ESCs for approximately 31 passages.84 Later, the same research group reported that this xeno-free system could also support the undifferentiated growth of human ESCs and iPSCs for at least 20 passages, indicating great potential for therapeutic purposes.85
In addition, laminin also benefits ex vivo culture of murine HSCs, as it amplified HSCs compartment in BMSCs during culture.86
Vitronectin
Vitronectin, an abundant secreted glycoprotein found in serum and the ECM, was shown to promote human ESC attachment through interaction with αvβ5 integrin.87 Braam et al. reported that recombinant vitronectin was a functional alternative to Matrigel, supporting human ESC sustained self-renewal and pluripotency in defined, nonconditioned medium for at least 1 month (seven passages).87 Later, other groups also reported that vitronectin could support the undifferentiated expansion of human ESCs with a variety of defined medium.88,89 The expanded human ESCs maintained differentiation capacity and expressed pluripotency markers as strongly as the human ESCs cultured on feeder layers.88,89 More recently, human iPSCs successfully maintained their stemness for 10 passages when cultured on vitronectin-coated dishes in xeno-free medium.90
However, the response of MSCs and ESCs is different in fate determination when cultured on vitronectin substrate. For example, contact with vitronectin promotes the osteogenic differentiation of human MSCs.91 Thus, the results obtained from one cell type cannot simply be transferred directly to other types of cells.
Although the results show great potential, natural biomaterials in general present several limitations when used as cell culture systems. First, they are usually not well-defined and difficult to control at the molecular level, and, therefore, may contribute to the variability in results.92 Second, the difficulty in purification and sterilization makes the manipulation more elaborate and complicated. Third, natural biomaterials may invoke immunogenic responses after implantation due to the structure similarity to biological substances. Thus, well-defined synthetic materials with controlled properties may be safer for clinical applications.
Synthetic biomaterials
Widespread clinical applications of stem cells require chemically defined materials that are low in cost, with tunable physical properties and long-term stability. For that reason, defined synthetic biomaterials have been developed to replace the complicated natural materials due to the advantages of ease of scale-up and decreased risks of disease transmission from unknown pathogens. Recently, synthetic peptides and polymers have been developed for stem cell expansion.
Synthetic peptides
ECM molecules are usually quite large and present diverse domains for cell adhesion. Biologically active peptides are organic compounds that serve as a synthetic alternative to ECM proteins. Synthetic peptides hold the advantages of stability, ease of synthesis, and conjugation to materials. Melkoumian et al. reported that human ESCs could be successfully maintained on synthetic peptide-acrylate surfaces (PAS) in chemically defined xeno-free medium for more than 10 passages with similar phenotypic marker expression as cells cultured on Matrigel.93 Here, amine-containing peptides derived from active domains of ECM, including bone sialoprotein, vitronectin, fibronectin, and laminin, were conjugated to PAS to provide a synthetic alternative to complex ECM. At the same time, Klim et al. screened more than 500 unique surfaces based on 18 bioactive peptides by using an array to present bioactive peptides either alone, in different combinations, or at varying surface densities.94 The results showed that the surfaces presenting heparin-binding peptides which recognize cell surface glycans supported the long-term growth of human ESCs and maintained their pluripotency markers for more than three months with a defined medium.94
For adult stem cells, surface immobilization of adhesion peptides on substrate benefits ex vivo expansion of HSCs from umbilical cord blood.95,96 HSCs cultured on synthetic substrates surface-immobilized with peptides containing the connecting segment-1 binding motif (EILDVPST) showed 2- to 5-fold greater expansion of HSCs and more pluripotent colony-forming units than those on fibronectin-grafted and polyamine-grafted dishes, suggesting that the specific interaction between HSCs and connecting segment-1 helps maintain the pluripotency of HSCs during the ex vivo expansion.96
Synthetic polymers
Preadsorption of proteins or peptides to substrates increases cost and limits scalability. Recently, the long-term self-renewal of human ESCs was reported to be successfully maintained on hydrogel interfaces of aminopropylmethacrylamide (APMAAm) for more than 20 passages in chemically defined media without the previous attachment of any biological coatings such as peptides, proteins, or Matrigel™.97 Since the interface was not functionalized with proteins or peptides to promote cell adhesion, it was identified that bovine serum albumin adsorbing from the culture media played a key role in human ESC attachment to APMAAm interfaces.97 Villa-Diaz et al. developed another standardized and fully defined synthetic polymer coating, poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH), which could sustain the undifferentiated growth of human ESCs in commercially available defined medium for 10 passages.98 In this case, six polymer coatings by surface-initiated graft polymerization were synthesized and tested, and only PMEDSAH supported attachment and proliferation of human ESCs. However, the mechanism of this phenomenon is not clear, and the contribution of various physicochemical properties (such as wettability, stiffness, surface topography, and zeta potential) of PMEDSAH still needs to be determined. Those findings demonstrate that chemically defined, synthetic biomaterials can be applied to propagate ESCs. However, no report is available with regard to their effects on the self-renewal of adult stem cells based on our literature survey. Moreover, the mechanisms by which the substrates function remain largely unclear.
Biomaterials for 3D cell culture
The 2D culture systems mentioned earlier present useful methods for stem cell expansion, but there are certain limitations. Cells grown on 2D substrates exhibit an unnatural dorsal/ventral polarized state that is in contrast to the situation in vivo, where cells are completely embedded in a 3D microenvironment. Accordingly, 3D cell culture models, which elicit a more physiological state, provide a promising alternative for monolayer cell culture. Cells grown in a 3D environment display more similarities to in vivo cells in morphology and molecular regulation.99 Compared with the 2D culture, 3D culture provides another dimension for external mechanical inputs and cell adhesion, which affects integrin ligation, cell contraction, and associated intracellular signaling.100,101 Thus, developing 3D culture systems have been evaluated for stem cell maintenance. In 3D cell culture models, cells are embedded in biomaterials, cultured as cellular spheroids, or grown on 3D scaffold materials.102 Biocompatible 3D scaffolds have been well reviewed by Moroni et al. for tissue engineering applications.103 However, no such scaffold has been reported to be utilized as a 3D system for stem cell expansion. This is most likely because the pattern of cell seeding, attachment, and growth on these 3D scaffolds is similar to that seen on 2D surfaces. Thus, this review mainly focuses on cell encapsulation and cellular spheroid methods for the expansion of stem cells.
Cell encapsulation
Embedding cells in semi-permeable materials is the most common approach for 3D cell culture. A completely synthesized ECM hydrogel composed of a semi-interpenetrating polymer network (sIPN) was prepared from poly(N-isopropylacrylamide-coacrylic acid) [p(NIPAAm-co-AAc)].104 To promote cell adhesion, the polymer network was interpenetrated by polyacrylic acid-graft-Ac-CGGNGEPRGDTYRAY-NH2 [p(AAc)-g-RGD] linear polymer chains, which binds to several integrin receptors, including α1, αv, β1, and αvβ3.105 By varying the polymer components and the density of the network cross-linker, sIPNs with a range of cell-adhesion ligand densities and matrix stiffness could be created.104 Although specific cell–matrix interactions were promoted by the peptide sequences presented in the sIPN, conditioned medium from fibroblast feeder layers was still needed to maintain self-renewal of human ESCs.104 In another study, hyaluronic acid (HA) hydrogel has been tested for controlling human ESC self-renewal. The rationale to choose HA was that the HA content is highly present in undifferentiated cells and decreases at the onset of differentiation.106 It was found that human ESCs cultured in HA maintained their undifferentiated state, preserved their normal karyotype, and maintained their full differentiation capacity as indicated by embryoid body formation for 20 days.107 Afterward, as the cells remodeled the hydrogel, colonies near the surface were released, making it difficult to accurately quantify cell proliferation as well for the long-term maintenance of stem cells.
For the expansion of adult stem cells, Walenda et al. demonstrated a novel 3D matrix fabricated from human platelet lysate (HPL) gel for the expansion of MSCs.108 Since HPL is of human origin, it can be used as a substitute for fetal calf serum without the risk of xenogeneic immune reactions or transmission of bovine pathogens. This gel culture system facilitates a twofold increase in proliferation rate and enhanced colony-forming units outgrowth compared with tissue culture plastic for culture expansion of MSCs.108
Cellular spheroids
Cellular spheroids that often mimic the in vivo situation more closely than a cell encapsulation system provide an alternative 3D culture system.109–111 Cellular spheroids are aggregate cells that grow free of foreign materials.102 Spheroids may have contact with culture materials, but no foreign material is present within spheroids. For example, human ESCs formed spheroids by encapsulation in calcium alginate hydrogels.112 The encapsulated human ESCs retained an undifferentiated state, and their pluripotency in a feeder layer-free and xeno-free environment for approximately 260 days. ESCs increased in size and number without escaping the confines of the hydrogel for the duration of the cultures. Furthermore, these encapsulated ESCs were easily recovered from the hydrogels by using a dissolution buffer.112
For adult stem cells, the methods that have been reported to induce cellular spheroids include spinner flasks and rotating wall vessel bioreactor,113 microwells,114 nonadherent surface,115 hanging drops,116 forced-aggregation technique,117 and micropatterned surfaces.118,119 Moreover, spheroid formation of several cell types has been reported on chitosan films.109,120–123 The gene expression of self-renewal markers (i.e., Oct4, Sox2, and Nanog) in MSCs and ASCs was significantly higher for the cells in spheroids in comparison to the monolayer cells,109,120 indicating that the cells in spheroids experienced a probably de-differentiating and returned to a more primitive state.109
The 3D culture systems provide promising results for the in vitro expansion of stem cells. However, the mechanisms of cellular interaction within these systems are not clear. It is often difficult to clarify the direct effects of the biomaterial itself from the diffusional limitations that are common to all 3D culture. Limitations in the diffusion of soluble factors might account for some of the differences frequently observed between 2D and 3D culture systems.124 For instance, the stem cell maintenance might be caused by the oxygen gradients (owing to the low solubility of oxygen in aqueous media) that could attribute to the higher expression of self-renewal marker genes.125,126 All human nucleated cells can sense oxygen tension and respond to a reduced O2 concentration (hypoxia).127 The conventional in vitro cell culture is performed under 21% oxygen tension.128 On the other hand, stem cell niches are usually located in regions of low oxygen tension.125,126 For example, the oxygen concentrations of mesenchymal, neural, and HSC niches are between 1% and 8%.129 It has been well reviewed by Mohyeldin et al. that hypoxia maintains undifferentiated states of embryonic, mesenchymal, hematopoietic, and NSC phenotypes.129 These studies indicate that optimization of oxygen tension for each type of stem cells is essential for their stemness maintenance during in vitro expansion. Future work is directed to clarify the influence of biophysical and biochemical cues of biomaterials on stem cell fate determination, which may help reduce the complexity and develop advanced biomaterials for stem cell expansion.
Modification of biomaterials for stem cell maintenance
Topography/architecture
The cell/material interface has been shown to exert considerable influence on stem cell function and differentiation.130 Recently, polymeric nanofibers morphologically mimicking the ECM/basement membrane were found to support the proliferation and self-renewal of mouse ESCs.131,132 In addition, Markert et al. screened 504 different types of topographical surface microstructures in order to identify specific topography that can support the expansion of undifferentiated murine ESCs without feeder cells or conditional medium.133 The results of this study indicated that stem cells are affected mainly by the lateral and vertical dimensions of the microstructures, while the distribution of the topographical patterns is less important.133 This finding is of great importance for the design of biomaterials for stem cell maintenance.
For adult stem cells, a nanostructured surface with square lattice symmetry has been identified to retain stem-cell phenotype and to maintain the growth of adult MSCs.134 It is likely that such nanoscale features alter the interaction of integrin receptors within cell adhesions, resulting in changes in intracellular tension. Significant progress has been made in designing biomaterials to enhance stem cell differentiation.33,135 From the opposite point of view, one can take advantages from these studies and use them to fabricate novel biomaterials to inhibit stem cell differentiation and facilitate stem cell maintenance. For example, disordered nanoscale structures can stimulate human MSCs to produce bone mineral in vitro, even in the absence of osteogenic supplements,136 while the ordered square nanostructures can retain their stem-cell phenotype and multipotency134; Thus, the designing of novel biomaterial platforms based on the current knowledge on biomaterials and cell differentiation may enhance the in vitro expansion of stem cells.
Taken together, these studies demonstrate that nanoscale patterning can serve as a powerful tool for the manipulation of stem cells. Since most studies focused on ESCs, more research work on how to maintain the potential of tissue-specific adult stem cells by controlling the topography of their substrates should be explored in future.
Biomechanical factors
Stem cells can “feel” and act in response to the stiffness of the substrates.137 Tissue culture plates, usually used for culturing adherent cells, are much stiffer than the cell microenvironments in vivo. Substrate stiffness has been identified as a vital parameter to determine stem cell fate. For instance, rigid matrices that mimic collagenous bone are osteogenic,138 while soft substrates which match the intrinsic stiffness of ESCs promote self-renewal and pluripotency of ESCs.139 Substrates mimicking muscle stiffness regulate self-renewal, enhance survival, prevent differentiation, and promote stemness of muscle stem cells.140 Polyacrylamide gels that mimic the elasticity of bone marrow can maintain MSCs in a quiescent state.141 Accordingly, it is safe to hypothesize that stem cells cultured on substrates which imitate the stiffness of their own microenvironments may be a promising system to promote their self-renewal ability and maintain their stemness.
In addition to stiffness, there is mounting evidence that external mechanical stimulation plays critical roles in stem cell fate determination.35 For instance, Saha et al.142 applied biaxial cyclic strain to a deformable elastic substratum on which the human ESC colonies were cultured. Results showed that human ESC self-renewal was promoted as measured by an increase in self-renewal gene expression (Oct4 and SSEA-4); the pluripotency of human ESCs was retained as evidenced by their ability to differentiate to cell lineages in all three germ layers.142 Thus, the application of mechanical forces may be useful toward stem cell maintenance for therapeutic applications.
Biochemical factors
Some biochemical factors have also been reported for the efficient in vitro expansion of stem cells. For example, short-wave UV/ozone radiation treatment on conventional tissue culture plates yields chemically defined surface with high intensities of several secondary ions (e.g., C2H5O+ and C2H6N+) that are favorable to human ESC colony formation.143 This chemically defined surface generates more than thrice the number of cells than feeder-containing substrates per surface area, which will finally facilitate cell therapeutic applications.143 Another example is that human MSCs maintained a stem cell phenotype when cultured on −CH3-modified glass surfaces, but processed osteogenic differentiation when cultured on −NH2- and −SH-modified glass surfaces.144
Bioelectrical factors
The behavior of in vitro cultured cells could also be influenced by bioelectrical cues through application of electrical or electromagnetic currents/fields to stimulate substrate and/or cell construct. For this purpose, Chang et al.145 developed a biphasic current stimulator chip for stem cell culture. This stimulator chip could generate both positive and negative currents in the same culture chamber. Further experiments indicated that this culture system could double NSC proliferation by applying continuous and defined levels of electric current.145 For human MSCs, pulsed electromagnetic field exposure could enhance cell proliferation during the exponential phase and it possibly resulted from the shortening of the lag phase.146 Recently, Lim et al. developed polyelectrolyte hydrogel-based multifunctional matrices, which provide not only 3D structural support to the embedded cells but also dynamic electrical and mechanical cues to the human MSCs.147 These anionic hydrogels could undergo reversible, anisotropic-bending dynamics in an electric field by changing the concentration of anionic groups within the hydrogel. Thanks to their close resemblance to the native cellular environment in multiple aspects, these new 3D electro-mechanical matrices were shown to support human MSC survival for at least 21 days and to enhance cell proliferation by 1.5-fold.
Conclusions
To facilitate the translation of stem cells from bench to bedside, this article reviews recent developments regarding the diverse properties of biomaterials for stem cell expansion. It should be noted that cell fate is influenced by a variety of factors in combination. Robust, fast, safe, and cost-effective methods using defined biomaterials may reconstruct the in vivo niche of stem cells, which will finally benefit both research and routine clinical purpose. The development of 3D culture systems by using biomaterials recapitulating the in vivo stem cell niche will probably provide better microenvironments for stem cells compared with the traditional 2D monolayer culture systems. New insights should be gained into the modifications of biomaterials such as the topography, stiffness, and surface chemistry. Though progress has been made to maintain the self-renewal ability of stem cells, we are still far from the large-scale expansion of undifferentiated stem cells for therapeutic purpose. In the future, biomaterials should be designed to present a combination of physical and chemical factors to expand stem cells. Moreover, the ultimate function of expanded cells requires an in vivo evaluation to confirm their regenerative capacity, for example, by transplantation of expanded cells into animal models. This needs collaboration among many disciplines, including cell and molecular biology, biomaterials, pharmacology, nanotechnology, and medicine.
Acknowledgment
Xiang-Zhen Yan acknowledges the China Scholarship Council for financial support rendered (No. 2010622061).
Disclosure Statement
No competing financial interests exist.
References
- 1.Potten C.S., and Loeffler M.Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110, 1001, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T., Kawai H., Tian F., Ohta Y., and Abe K.Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant 20, 883, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Yang Z., Zhang F., Ma W., Chen B., Zhou F., Xu Z., et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct-relative artery. Cardiovasc Ther 28, 380, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Ravari H., Hamidi-Almadari D., Salimifar M., Bonakdaran S., Parizadeh M.R., and Koliakos G.Treatment of non-healing wounds with autologous bone marrow cells, platelets, fibrin glue and collagen matrix. Cytotherapy 13, 705, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Olmo D., Herreros D., Pascual I., Pascual J.A., Del-Valle E., Zorrilla J., et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 52, 79, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Masthan K.M., Sankari S.L., Babu N.A., and Gopalakrishnan T.Mystery inside the tooth: the dental pulp stem cells. J Clin Diagn Res 7, 945, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersson M., Frances D., and Niemann C.Lineage tracing of hair follicle stem cells in epidermal whole mounts. Methods Mol Biol 989, 45, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Soma T., Kishimoto J., and Fisher D.Isolation of mesenchymal stem cells from human dermis. Methods Mol Biol 989, 265, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Koudstaal S., Jansen Of Lorkeers S.J., Gaetani R., Gho J.M., van Slochteren F.J., Sluijter J.P., et al. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl Med 2, 434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huch M., Boj S.F., and Clevers H.Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen Med 8, 385, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Faustman D.L., and Davis M.Stem cells in the spleen: therapeutic potential for Sjogren's syndrome, type I diabetes, and other disorders. Int J Biochem Cell Biol 42, 1576, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falanga V., Iwamoto S., Chartier M., Yufit T., Butmarc J., Kouttab N., et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13, 1299, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Rickert D., Sauerbier S., Nagursky H., Menne D., Vissink A., and Raghoebar G.M.Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res 22, 251, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Kim M., Kim I., Lee S.K., Bang S.I., and Lim S.Y.Clinical trial of autologous differentiated adipocytes from stem cells derived from human adipose tissue. Dermatol Surg 37, 750, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Vellenga E., van Putten W., Ossenkoppele G.J., Verdonck L.F., Theobald M., Cornelissen J.J., et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 118, 6037, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Chen W.W., and Blurton-Jones M.Concise review: can stem cells be used to treat or model Alzheimer's disease? Stem Cells 30, 2612, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolli S., Ahmad S., Lako M., and Figueiredo F.Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells 28, 597, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Orford K.W., and Scadden D.T.Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 9, 115, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kruyt M.C., de Bruijn J.D., Yuan H., van Blitterswijk C.A., Verbout A.J., Oner F.C., et al. Optimization of bone tissue engineering in goats: a peroperative seeding method using cryopreserved cells and localized bone formation in calcium phosphate scaffolds. Transplantation 77, 359, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Both S.K., van der Muijsenberg A.J., van Blitterswijk C.A., de Boer J., and de Bruijn J.D.A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng 13, 3, 2007 [DOI] [PubMed] [Google Scholar]
- 22.van der Spoel T.I., Vrijsen K.R., Koudstaal S., Sluijter J.P., Nijsen J.F., de Jong H.W., et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: a study on delivery efficiency. J Cell Mol Med 16, 2768, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.Y., Jia Q., Di K.Q., Gao S.M., Wen X.H., Zhou R.Y., et al. Passage number affects the pluripotency of mouse embryonic stem cells as judged by tetraploid embryo aggregation. Cell Tissue Res 327, 607, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Xie X., Hiona A., Lee A.S., Cao F., Huang M., Li Z., et al. Effects of long-term culture on human embryonic stem cell aging. Stem Cells Dev 20, 127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Q., Lu S.J., Klimanskaya I., Gomes I., Kim D., Chung Y., et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells 28, 704, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Kretlow J.D., Jin Y.Q., Liu W., Zhang W.J., Hong T.H., Zhou G., et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol 9, 60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Surdo J., and Bauer S.R.Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C Methods 18, 877, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonab M.M., Alimoghaddam K., Talebian F., Ghaffari S.H., Ghavamzadeh A., and Nikbin B.Aging of mesenchymal stem cell in vitro. BMC Cell Biol 7, 14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Safwani W.K., Makpol S., Sathapan S., and Chua K.H.The changes of stemness biomarkers expression in human adipose-derived stem cells during long-term manipulation. Biotechnol Appl Biochem 58, 261, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Gilbert P.M., and Blau H.M.Engineering a stem cell house into a home. Stem Cell Res Ther 2, 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund A.W., Yener B., Stegemann J.P., and Plopper G.E.The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev 15, 371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutolf M.P., Gilbert P.M., and Blau H.M.Designing materials to direct stem-cell fate. Nature 462, 433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawson E., Mapili G., Erickson K., Taqvi S., and Roy K.Biomaterials for stem cell differentiation. Adv Drug Deliv Rev 60, 215, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Discher D.E., Mooney D.J., and Zandstra P.W.Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., and Chen C.S.Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Underhill G.H., and Bhatia S.N.High-throughput analysis of signals regulating stem cell fate and function. Curr Opin Chem Biol 11, 357, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukacs R.U., Memarzadeh S., Wu H., and Witte O.N.Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 7, 682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T., Heller E., Beronja S., Oshimori N., Stokes N., and Fuchs E.An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature 485, 104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon K.A., Hiew S.Y., Hadjur S., Veiga-Fernandes H., Menzel U., Price A.J., et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell 1, 338, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Ng A.P., Loughran S.J., Metcalf D., Hyland C.D., de Graaf C.A., Hu Y., et al. Erg is required for self-renewal of hematopoietic stem cells during stress hematopoiesis in mice. Blood 118, 2454, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Loughran S.J., Kruse E.A., Hacking D.F., de Graaf C.A., Hyland C.D., Willson T.A., et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol 9, 810, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Simonsen J.L., Rosada C., Serakinci N., Justesen J., Stenderup K., Rattan S.I., et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol 20, 592, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Shi S., Gronthos S., Chen S., Reddi A., Counter C.M., Robey P.G., et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol 20, 587, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Papanikolaou E., and Anagnou N.P.Major challenges for gene therapy of thalassemia and sickle cell disease. Curr Gene Ther 10, 404, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Duch M., Paludan K., Jorgensen P., and Pedersen F.S.Lack of correlation between basal expression levels and susceptibility to transcriptional shutdown among single-gene murine leukemia virus vector proviruses. J Virol 68, 5596, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claeys Bouuaert C., and Chalmers R.M.Gene therapy vectors: the prospects and potentials of the cut-and-paste transposons. Genetica 138, 473, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Flotte T.R., Afione S.A., and Zeitlin P.L.Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol 11, 517, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Nakai H., Yant S.R., Storm T.A., Fuess S., Meuse L., and Kay M.A.Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol 75, 6969, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scadden D.T.The stem-cell niche as an entity of action. Nature 441, 1075, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Watt F.M., and Hogan B.L.Out of Eden: stem cells and their niches. Science 287, 1427, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Boland G.M., Perkins G., Hall D.J., and Tuan R.S.Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93, 1210, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Kalani M.Y., Cheshier S.H., Cord B.J., Bababeygy S.R., Vogel H., Weissman I.L., et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A 105, 16970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng Y.A., and Nusse R.Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6, 568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi F., Pereira L., Hoffman J.A., Shy B.R., Yuen C.M., Liu D.R., et al. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 13, 762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trowbridge J.J., Guezguez B., Moon R.T., and Bhatia M.Wnt3a activates dormant c-Kit(−) bone marrow-derived cells with short-term multilineage hematopoietic reconstitution capacity. Stem Cells 28, 1379, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24, 185, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Xu R.H., Peck R.M., Li D.S., Feng X., Ludwig T., and Thomson J.A.Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2, 185, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Watabe T., and Miyazono K.Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res 19, 103, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Pricola K.L., Kuhn N.Z., Haleem-Smith H., Song Y., and Tuan R.S.Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem 108, 577, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vicente Lopez M.A., Vazquez Garcia M.N., Entrena A., Olmedillas Lopez S., Garcia-Arranz M., Garcia-Olmo D., et al. Low doses of bone morphogenetic protein 4 increase the survival of human adipose-derived stem cells maintaining their stemness and multipotency. Stem Cells Dev 20, 1011, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Aaronson S.A.Growth factors and cancer. Science 254, 1146, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Butler J.M., Nolan D.J., Vertes E.L., Varnum-Finney B., Kobayashi H., Hooper A.T., et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi H., Butler J.M., O'Donnell R., Kobayashi M., Ding B.S., Bonner B., et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 12, 1046, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park Y., Kim J.H., Lee S.J., Choi I.Y., Park S.J., Lee S.R., et al. Human feeder cells can support the undifferentiated growth of human and mouse embryonic stem cells using their own basic fibroblast growth factors. Stem Cells Dev 20, 1901, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Goncalves R., Lobato da Silva C., Cabral J.M., Zanjani E.D., and Almeida-Porada G.A Stro-1(+) human universal stromal feeder layer to expand/maintain human bone marrow hematopoietic stem/progenitor cells in a serum-free culture system. Exp Hematol 34, 1353, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Kanatsu-Shinohara M., Inoue K., Lee J., Yoshimoto M., Ogonuki N., Miki H., et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001, 2004 [DOI] [PubMed] [Google Scholar]
- 68.He F., Chen X., and Pei M.Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 15, 3809, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Pei M., He F., and Kish V.L.Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17, 3067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin H., Yang G., Tan J., and Tuan R.S.Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials 33, 4480, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Zhang J., Li B., and Wang J.H.The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials 32, 6972, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eshghi S., and Schaffer D.V.Engineering Microenvironments to Control Stem Cell Fate and Function. 2008. In: StemBook [Internet]. Cambridge, MA: Harvard Stem Cell Institute; Available from: http://www.ncbi.nlm.nih.gov/books/NBK27048/ [PubMed] [Google Scholar]
- 73.Griffith L.G., and Swartz M.A.Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7, 211, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Loeser R.F.Integrins and cell signaling in chondrocytes. Biorheology 39, 119, 2002 [PubMed] [Google Scholar]
- 75.Kleinman H.K., McGarvey M.L., Liotta L.A., Robey P.G., Tryggvason K., and Martin G.R.Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188, 1982 [DOI] [PubMed] [Google Scholar]
- 76.Xu C., Inokuma M.S., Denham J., Golds K., Kundu P., Gold J.D., et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19, 971, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8, 424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao S., Chen S., Clark J., Hao E., Beattie G.M., Hayek A., et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A 103, 6907, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian L., and Saltzman W.M.Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials 25, 1331, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Jones M.B., Chu C.H., Pendleton J.C., Betenbaugh M.J., Shiloach J., Baljinnyam B., et al. Proliferation and pluripotency of human embryonic stem cells maintained on type I collagen. Stem Cells Dev 19, 1923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Celebi B., Mantovani D., and Pineault N.Effects of extracellular matrix proteins on the growth of haematopoietic progenitor cells. Biomed Mater 6, 055011, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Miyazaki T., Futaki S., Hasegawa K., Kawasaki M., Sanzen N., Hayashi M., et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem Biophys Res Commun 375, 27, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Miner J.H., and Yurchenco P.D.Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20, 255, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Domogatskaya A., Rodin S., Boutaud A., and Tryggvason K.Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells 26, 2800, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Rodin S., Domogatskaya A., Strom S., Hansson E.M., Chien K.R., Inzunza J., et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 28, 611, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Sagar B.M.M., Rentala S., Gopal P.N.V., Sharma S., and Mukhopadhyay A.Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem Biophys Res Commun 350, 1000, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Braam S.R., Zeinstra L., Litjens S., Ward-van Oostwaard D., van den Brink S., van Laake L., et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 26, 2257, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Prowse A.B., Doran M.R., Cooper-White J.J., Chong F., Munro T.P., Fitzpatrick J., et al. Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials 31, 8281, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Yoon T.M., Chang B., Kim H.T., Jee J.H., Kim D.W., and Hwang D.Y.Human embryonic stem cells (hESCs) cultured under distinctive feeder-free culture conditions display global gene expression patterns similar to hESCs from feeder-dependent culture conditions. Stem Cell Rev 6, 425, 2010 [DOI] [PubMed] [Google Scholar]
- 90.Kim H.T., Lee K.I., Kim D.W., and Hwang D.Y.An ECM-based culture system for the generation and maintenance of xeno-free human iPS cells. Biomaterials 34, 1041, 2013 [DOI] [PubMed] [Google Scholar]
- 91.Salasznyk R.M., Williams W.A., Boskey A., Batorsky A., and Plopper G.E.Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol 2004, 24, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hughes C.S., Postovit L.M., and Lajoie G.A.Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Melkoumian Z., Weber J.L., Weber D.M., Fadeev A.G., Zhou Y., Dolley-Sonneville P., et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol 28, 606, 2010 [DOI] [PubMed] [Google Scholar]
- 94.Klim J.R., Li L., Wrighton P.J., Piekarczyk M.S., and Kiessling L.L.A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Methods 7, 989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang X.S., Chai C., Zhang Y., Zhuo R.X., Mao H.Q., and Leong K.W.Surface-immobilization of adhesion peptides on substrate for ex vivo expansion of cryopreserved umbilical cord blood CD34+ cells. Biomaterials 27, 2723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen L.Y., Chang Y., Shiao J.S., Ling Q.D., Chen Y.H., Chen D.C., et al. Effect of the surface density of nanosegments immobilized on culture dishes on ex vivo expansion of hematopoietic stem and progenitor cells from umbilical cord blood. Acta Biomater 8, 1749, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Irwin E.F., Gupta R., Dashti D.C., and Healy K.E.Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 32, 6912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villa-Diaz L.G., Nandivada H., Ding J., Nogueira-de-Souza N.C., Krebsbach P.H., O'Shea K.S., et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol 28, 581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elliott N.T., and Yuan F.A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci 100, 59, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Knight B., Laukaitis C., Akhtar N., Hotchin N.A., Edlund M., and Horwitz A.R.Visualizing muscle cell migration in situ. Curr Biol 10, 576, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Roskelley C.D., Desprez P.Y., and Bissell M.J.Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci U S A 91, 12378, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fennema E., Rivron N., Rouwkema J., van Blitterswijk C., and de Boer J.Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 31, 108, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Moroni L., de Wijn J.R., and van Blitterswijk C.A.Integrating novel technologies to fabricate smart scaffolds. J Biomater Sci Polym Ed 19, 543, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Li Y.J., Chung E.H., Rodriguez R.T., Firpo M.T., and Healy K.E.Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A 79, 1, 2006 [DOI] [PubMed] [Google Scholar]
- 105.Rezania A., and Healy K.E.Integrin subunits responsible for adhesion of human osteoblast-like cells to biomimetic peptide surfaces. J Orthop Res 17, 615, 1999 [DOI] [PubMed] [Google Scholar]
- 106.Toole B.P.Hyaluronan in morphogenesis. Semin Cell Dev Biol 12, 79, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Gerecht S., Burdick J.A., Ferreira L.S., Townsend S.A., Langer R., and Vunjak-Novakovic G.Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A 104, 11298, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walenda G., Hemeda H., Schneider R.K., Merkel R., Hoffmann B., and Wagner W.Human platelet lysate gel provides a novel three dimensional-matrix for enhanced culture expansion of mesenchymal stromal cells. Tissue Eng Part C Methods 18, 924, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Cheng N.C., Wang S., and Young T.H.The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 33, 1748, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Yeh Y.C., Lee W.Y., Yu C.L., Hwang S.M., Chung M.F., Hsu L.W., et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 31, 6444, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Kabiri M., Kul B., Lott W.B., Futrega K., Ghanavi P., Upton Z., et al. 3D mesenchymal stem/stromal cell osteogenesis and autocrine signalling. Biochem Biophys Res Commun 419, 142, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Siti-Ismail N., Bishop A.E., Polak J.M., and Mantalaris A.The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 29, 3946, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Frith J.E., Thomson B., and Genever P.G.Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods 16, 735, 2010 [DOI] [PubMed] [Google Scholar]
- 114.Lee W.Y., Chang Y.H., Yeh Y.C., Chen C.H., Lin K.M., Huang C.C., et al. The use of injectable spherically symmetric cell aggregates self-assembled in a thermo-responsive hydrogel for enhanced cell transplantation. Biomaterials 30, 5505, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Tong J.Z., Sarrazin S., Cassio D., Gauthier F., and Alvarez F.Application of spheroid culture to human hepatocytes and maintenance of their differentiation. Biol Cell 81, 77, 1994 [DOI] [PubMed] [Google Scholar]
- 116.Bartosh T.J., Ylostalo J.H., Mohammadipoor A., Bazhanov N., Coble K., Claypool K., et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A 107, 13724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baraniak P.R., and McDevitt T.C.Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res 347, 701, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang W., Itaka K., Ohba S., Nishiyama N., Chung U.I., Yamasaki Y., et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials 30, 2705, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Miyagawa Y., Okita H., Hiroyama M., Sakamoto R., Kobayashi M., Nakajima H., et al. A microfabricated scaffold induces the spheroid formation of human bone marrow-derived mesenchymal progenitor cells and promotes efficient adipogenic differentiation. Tissue Eng Part A 17, 513, 2011 [DOI] [PubMed] [Google Scholar]
- 120.Huang G.S., Dai L.G., Yen B.L., and Hsu S.H.Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 32, 6929, 2011 [DOI] [PubMed] [Google Scholar]
- 121.Lin S.J., Jee S.H., Hsaio W.C., Lee S.J., and Young T.H.Formation of melanocyte spheroids on the chitosan-coated surface. Biomaterials 26, 1413, 2005 [DOI] [PubMed] [Google Scholar]
- 122.Verma P., Verma V., Ray P., and Ray A.R.Formation and characterization of three dimensional human hepatocyte cell line spheroids on chitosan matrix for in vitro tissue engineering applications. In Vitro Cell Dev Biol Anim 43, 328, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Hsu S.H., Huang G.S., and Feng F.Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials 33, 2642, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Baker B.M., and Chen C.S.Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125, 3015, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cipolleschi M.G., Dello Sbarba P., and Olivotto M.The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82, 2031, 1993 [PubMed] [Google Scholar]
- 126.Rehman J.Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berl) 88, 981, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Semenza G.L.HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107, 1, 2001 [DOI] [PubMed] [Google Scholar]
- 128.Szablowska-Gadomska I., Zayat V., and Buzanska L.Influence of low oxygen tensions on expression of pluripotency genes in stem cells. Acta Neurobiol Exp (Wars) 71, 86, 2011 [DOI] [PubMed] [Google Scholar]
- 129.Mohyeldin A., Garzon-Muvdi T., and Quinones-Hinojosa A.Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150, 2010 [DOI] [PubMed] [Google Scholar]
- 130.Lim J.Y., and Donahue H.J.Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng 13, 1879, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Nur E.K.A., Ahmed I., Kamal J., Schindler M., and Meiners S.Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells 24, 426, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Hashemi S.M., Soudi S., Shabani I., Naderi M., and Soleimani M.The promotion of stemness and pluripotency following feeder-free culture of embryonic stem cells on collagen-grafted 3-dimensional nanofibrous scaffold. Biomaterials 32, 7363, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Markert L.D., Lovmand J., Foss M., Lauridsen R.H., Lovmand M., Fuchtbauer E.M., et al. Identification of distinct topographical surface microstructures favoring either undifferentiated expansion or differentiation of murine embryonic stem cells. Stem Cells Dev 18, 1331, 2009 [DOI] [PubMed] [Google Scholar]
- 134.McMurray R.J., Gadegaard N., Tsimbouri P.M., Burgess K.V., McNamara L.E., Tare R., et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 10, 637, 2011 [DOI] [PubMed] [Google Scholar]
- 135.Fu R.H., Wang Y.C., Liu S.P., Huang C.M., Kang Y.H., Tsai C.H., et al. Differentiation of stem cells: strategies for modifying surface biomaterials. Cell Transplant 20, 37, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Dalby M.J., Gadegaard N., Tare R., Andar A., Riehle M.O., Herzyk P., et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6, 997, 2007 [DOI] [PubMed] [Google Scholar]
- 137.Discher D.E., Janmey P., and Wang Y.L.Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Chowdhury F., Li Y., Poh Y.C., Yokohama-Tamaki T., Wang N., and Tanaka T.S.Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One 5, e15655, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gilbert P.M., Havenstrite K.L., Magnusson K.E., Sacco A., Leonardi N.A., Kraft P., et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Winer J.P., Janmey P.A., McCormick M.E., and Funaki M.Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A 15, 147, 2009 [DOI] [PubMed] [Google Scholar]
- 142.Saha S., Ji L., de Pablo J.J., and Palecek S.P.Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol 206, 126, 2006 [DOI] [PubMed] [Google Scholar]
- 143.Saha K., Mei Y., Reisterer C.M., Pyzocha N.K., Yang J., Muffat J., et al. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc Natl Acad Sci U S A 108, 18714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Curran J.M., Chen R., and Hunt J.A.The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 27, 4783, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Chang K.A., Kim J.W., Kim J.A., Lee S.E., Kim S., Suh W.H., et al. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS One 6, e18738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun L.Y., Hsieh D.K., Yu T.C., Chiu H.T., Lu S.F., Luo G.H., et al. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics 30, 251, 2009 [DOI] [PubMed] [Google Scholar]
- 147.Lim H.L., Chuang J.C., Tran T., Aung A., Arya G., and Varghese S.Dynamic electromechanical hydrogel matrices for stem cell culture. Adv Funct Mater 21, 55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643, 2003 [DOI] [PubMed] [Google Scholar]
- 149.Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631, 2003 [DOI] [PubMed] [Google Scholar]
- 150.He S., Nakada D., and Morrison S.J.Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 25, 377, 2009 [DOI] [PubMed] [Google Scholar]
- 151.Dejosez M., Krumenacker J.S., Zitur L.J., Passeri M., Chu L.F., Songyang Z., et al. Ronin is essential for embryogenesis and the pluripotency of mouse embryonic stem cells. Cell 133, 1162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Galan-Caridad J.M., Harel S., Arenzana T.L., Hou Z.E., Doetsch F.K., Mirny L.A., et al. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell 129, 345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yew T.L., Chiu F.Y., Tsai C.C., Chen H.L., Lee W.P., Chen Y.J., et al. Knockdown of p21(Cip1/Waf1) enhances proliferation, the expression of stemness markers, and osteogenic potential in human mesenchymal stem cells. Aging Cell 10, 349, 2011 [DOI] [PubMed] [Google Scholar]
- 154.Eiselleova L., Peterkova I., Neradil J., Slaninova I., Hampl A., and Dvorak P.Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol 52, 353, 2008 [DOI] [PubMed] [Google Scholar]
- 155.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145, 1998 [DOI] [PubMed] [Google Scholar]
- 156.Sun Y., Li W., Lu Z., Chen R., Ling J., Ran Q., et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J 25, 1474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]