Abstract
Significance: Hepatic ischemia-reperfusion (IR) injury results from the temporary deprivation of hepatic blood supply and is a common side effect of major liver surgery (i.e., transplantation or resection). IR injury, which in most severe cases culminates in acute liver failure, is particularly pronounced in livers that are affected by non-alcoholic fatty liver disease (NAFLD). In NAFLD, fat-laden hepatocytes are damaged by chronic oxidative/nitrosative stress (ONS), a state that is acutely exacerbated during IR, leading to extensive parenchymal damage. Recent Advances: NAFLD triggers ONS via increased (extra)mitochondrial fatty acid oxidation and activation of the unfolded protein response. ONS is associated with widespread protein and lipid (per)oxidation, which reduces the hepatic antioxidative capacity and shifts the intracellular redox status toward an oxidized state. Moreover, activation of the transcription factor peroxisome proliferator-activated receptor α induces expression of mitochondrial uncoupling protein 2, resulting in depletion of cellular energy (ATP) reserves. The reduction in intracellular antioxidants and ATP in fatty livers consequently gives rise to severe ONS and necrotic cell death during IR. Critical Issues: Despite the fact that ONS mediates both NAFLD and IR injury, the interplay between the two conditions has never been described in detail. An integrative overview of the pathophysiology of NAFLD that renders steatotic hepatocytes more vulnerable to IR injury is therefore presented in the context of ONS. Future Directions: Effective methods should be devised to alleviate ONS and the consequences thereof in NAFLD before surgery in order to improve resilience of fatty livers to IR injury. Antioxid. Redox Signal. 21, 1119–1142.
Introduction
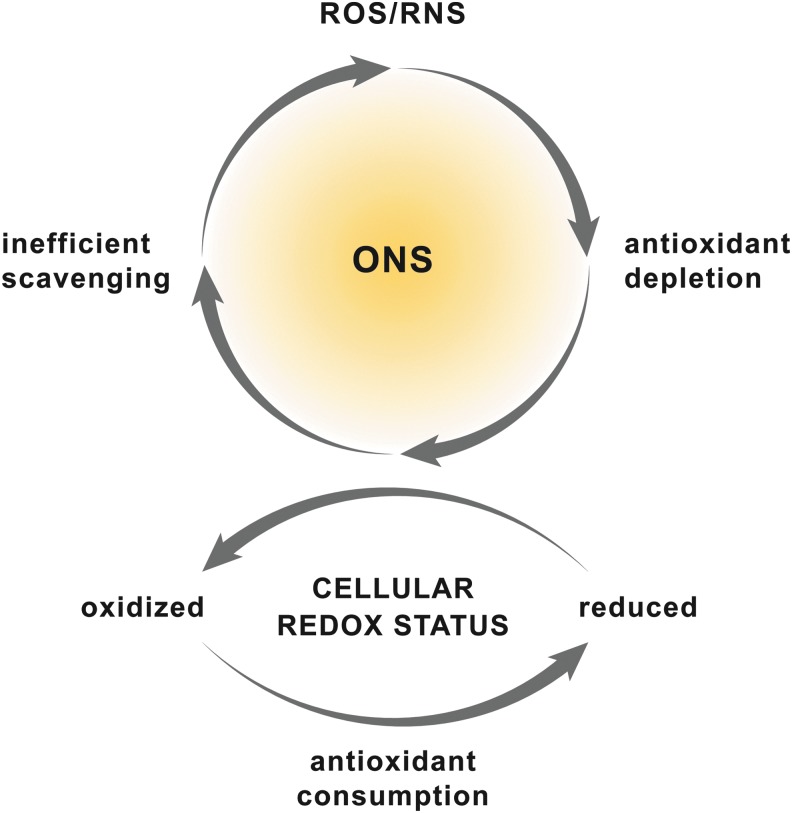
Oxidative/nitrosative stress (ONS) is a state in which the balance between pro- and antioxidants is disrupted and shifted towards the former (73) (Fig. 1). ONS can result from an increased formation of reactive oxygen and nitrogen species (ROS and RNS, respectively) (73), a perturbed cellular redox status (77), or a combination of both. Irrespective of the cause, the propensity for ONS is especially pronounced when endogenous repair mechanisms fail to clear oxidized and nitrated biomolecules (74). Over the years, ONS has been recognized as a key contributor to a variety of pathologies, including those affecting the brain (212), heart (167), vasculature (67), and liver.
FIG. 1.
Etiology of ONS. ONS is the result of a disrupted balance between intracellular levels of ROS/RNS and antioxidants. The production of large amounts of ROS/RNS consumes cellular antioxidants, which leads to inefficient ROS/RNS scavenging and hence ONS (upper panel). Simultaneously, the cellular redox status shifts toward a more oxidized state. Alternatively, disruption of the cellular redox status can induce ONS because of the consequent consumption and depletion of cellular antioxidants (bottom panel). ONS, oxidative/nitrosative stress; RNS, reactive nitrogen species; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The liver is particularly prone to ONS due to its high metabolic rate and because hepatocytes are rich in ROS/RNS-producing mitochondria (section “Mitochondria”), cytochrome P450 (CYP) enzymes (section “Extramitochondrial FA oxidation”), and inducible nitric oxide synthase (iNOS, section “Reactive nitrogen species”) (92). Consequently, many hepatopathologies are associated with ONS, including viral hepatitis (112), alcoholic and non-alcoholic fatty liver disease (NAFLD) (121), cholestasis (33), and ischemia-reperfusion (IR) injury, the inevitable side effect of liver surgery performed under vascular exclusion (warm IR, e.g., liver resection) or liver transplantation (cold IR) (94).
In particular, much attention has been focused on the role of ONS in the interplay between NAFLD and IR, as mounting evidence points to an exacerbation of IR injury in fatty livers (208). Patients with NAFLD who undergo a liver resection have an increased risk of postoperative morbidity and mortality (131, 170). In the transplantation setting, steatotic grafts are commonly considered ineligible for transplantation because of the high risk of primary malfunction (128).
While the ONS-mediated processes that dictate NAFLD (177) and IR injury (205) are well documented, an integrative overview of the role of ROS/RNS in the interplay between these conditions is lacking. Nevertheless, a substantial amount of clinical evidence implies that the development of modalities to preserve fatty livers during IR bears great necessity (208). Consequently, this review provides an in-depth account of how the hepatocellular formation of ROS/RNS and the resulting ONS exacerbate the extent of IR injury in fatty livers.
Although the immune response and the extracellular component of hepatic ONS are critical contributors to both conditions, they will be only briefly addressed because these topics have recently been reviewed in the context of NAFLD (14) and IR injury (206).
Non-alcoholic fatty liver disease
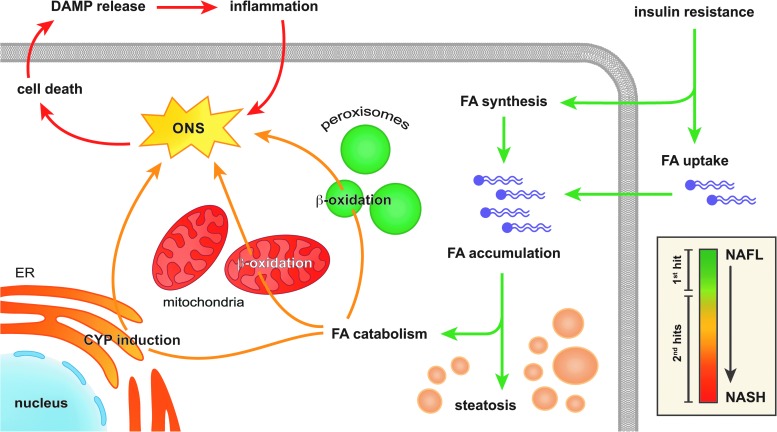
NAFLD is characterized by the presence of vesicular fat in hepatocytes (i.e., steatosis) in the absence of excessive alcohol consumption and constitutes the common denominator in non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), as well as cryptogenic- or NASH cirrhosis (26). At a cellular level, the key feature of NAFLD is the vesicular accumulation of triglycerides (19) (Fig. 2), the ‘first hit’ in the putative two-hit theory (40). From here on, several ‘second hits’ evoke and amplify an inflammatory response that drives the gradual progression from NAFL to NASH (47) (Fig. 2, inset).
FIG. 2.
Pathogenesis of NAFLD. Hepatic insulin resistance increases the uptake as well as the synthesis of FAs by hepatocytes, which results in the accumulation of FAs that are stored as triglycerides and the development of steatosis (green arrows). In addition, FA-catabolizing pathways become hyperactivated to dispose of the excess in FAs (orange arrows) through increased mitochondrial and peroxisomal β-oxidation as well as the induction of CYP enzymes in the ER. Since all of these FA catabolizing pathways produce oxidants, ONS ensues, which eventually induces cell death and the release of DAMPs. Thereafter, DAMPs incite an immune response that further aggravates the ONS and consequently induces cell death, creating a positive feedback loop that eventually leads to pervasive parenchymal inflammation (red arrows). The inset shows how the phenomena mentioned earlier (i.e., FA accumulation, FA catabolism, and inflammation) relate to the pathological stage of NAFLD, ranging from NAFL to NASH (right of scale bar), and the putative two-hit theory (left of scale bar). The scale bar is color matched to the figure arrows. CYP, cytochrome P450; DAMP, damage-associated molecular pattern; ER, endoplasmic reticulum; FA, fatty acid; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The accumulation of fat in NAFLD is a direct result of insulin resistance, which explains the strong correlation between NAFLD and the metabolic syndrome (i.e., obesity, type-2 diabetes, and hyperlipidemia) (9). The causal factors for insulin resistance in metabolic syndrome-associated conditions such as NAFLD are increased levels of peripheral adipose tissue-derived tumor necrosis factor alpha (TNF-α) and hyperlipidemia (217), which impede (hepatic) insulin signaling by inhibiting insulin receptor substrate protein-1 through the activation of c-Jun N-terminal kinase (JNK) (83, 185, 217) (sections “Tumor necrosis factor alpha” and “Unfolded protein response”). Considering that JNK can be activated by ONS (179) and that activated JNK prompts the mitochondrial formation of ROS (207) (section “Tumor necrosis factor alpha”), ONS is both a cause and a consequence of insulin resistance and NAFLD (88, 217). Nevertheless, the finding that selective hepatocellular ablation of JNK actually induced insulin resistance and hepatic steatosis (178) indicates that other pathways might be involved as well.
In the liver, insulin resistance on the one hand increases the uptake of fatty acids (FAs) from the blood and on the other hand augments intracellular FA synthesis (9) (Fig. 2, green arrows). Hepatocytes respond to the consequent excess in intracellular FAs by storing them as triglycerides and by boosting their catabolism through mitochondrial and peroxisomal β-oxidation as well as induction of CYP enzymes in the endoplasmic reticulum (ER) (169) (Fig. 2, yellow arrows). Although the catabolism of FAs via these pathways is instrumental in the removal of fat, it also augments the generation of ROS/RNS, i.e., superoxide anion (O2•−) and its derivatives (154) (section “ROS/RNS and Their Chemical Properties in the Context of NAFLD and IR”). The consequent ONS sensitizes hepatocytes to cell death, which constitutes a key ‘second hit’ in the development of NASH (47) (Fig. 2, red arrows).
The main consequence of ONS-induced cell death is the release of damage-associated molecular patterns (DAMPs), which are endogenous self-antigens that alert the immune system of (impending) cell death once released into the extracellular environment (104). Hepatocyte-derived DAMPs trigger parenchymal inflammation by binding to Toll-like receptor 4 on the surface of Kupffer cells (KCs), the liver's resident macrophages (35). In response, KCs not only release cytokines such as interleukin 1β (IL-1β) (35) and TNF-α (199), but also mobilize NADPH oxidase-2 to form O2•− and upregulate iNOS to generate nitric oxide (•NO) (191, 205). However, genetic ablation of gp91phox (41) or p47phox (136), which prevents the formation of active NADPH oxidase 2, did not affect the extent of lipid peroxidation, hepatic injury, and inflammation in mice that were fed a methionine- and choline-deficient diet (41, 136). An increase in TNF-α mRNA was found mainly in hepatocytes (rather than KCs) of the gp91phox knock-out animals (41). These findings indicate that NASH develops in the absence of NADPH oxidase 2-derived O2•−, yet not through KC-induced extracellular ONS or cytokine production but by a compensatory mechanism that is based on TNF-α signaling by parenchymal cells. Moreover, it is probable that the NADPH oxidase 2-dependent production of O2•− by KCs mainly functions to stimulate cytokine production in these cells through activation of the transcription factor nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) (41) rather than contributing to ONS per se.
The pro-inflammatory state invoked by hepatocellular ONS and consequent DAMP release is further aggravated by the increased levels of gut-derived endotoxins (e.g., lipopolysaccharide) that circulate in obese and insulin-resistant mice (21), which also leads to Toll-like receptor 4-dependent KC activation (139). Guided by these KC-derived molecular cues (e.g., TNF-α), neutrophils accumulate in the liver and increase the state of inflammation and ONS (14). Neutrophils not only co-express NADPH oxidase 2 and iNOS (205), but in addition release the enzyme myeloperoxidase that catalyzes the conversion of hydrogen peroxide (H2O2) to strong oxidants such as hypochlorous acid (HOCl) (60). Accordingly, plasma myeloperoxidase levels are increased in patients who suffer from NASH (173), while myeloperoxidase-deficient mice are less susceptible to develop high-fat diet-induced NASH and corollary liver injury (172). Lastly, extracellular ONS, hepatocyte apoptosis [due to the actions of TNF-α (233), section “Tumor necrosis factor alpha”], and lipopolysaccharide collectively activate stellate cells, which leads to collagen deposition and eventually fibrosis (55). The histological features of these processes, namely leukocyte infiltration, necrosis, and fibrosis (19) are clinically used to distinguish between NAFL and NASH (19).
In sum, TNF-α and activated JNK establish a detrimental self-propagating cycle between the liver, the innate immune system, peripheral adipose tissue, and the gut that entails fat accumulation, insulin resistance, ONS, and inflammation (85). In this complex network of metabolic and inflammatory factors, ONS occupies a central role and contributes to both the development and the progression of NAFLD (9). Of note, many of these factors are highly prevalent in Western societies because of their close association with the metabolic syndrome (52). Furthermore, the ongoing adoption of the Western sedentary and nutritional lifestyle in Asian countries significantly adds to the worldwide population that is at risk for developing NAFLD (46). As a result, the prevalence of NAFLD and NASH is currently estimated at 17–45% and 5–17% (9, 46), respectively, which renders NAFLD the most common liver disease worldwide. The number of fatty livers encountered in the surgical setting is therefore expected to follow this trend.
IR injury
Hepatic IR is an iatrogenic condition that results from the temporary withdrawal of blood supply to the liver to deter excessive bleeding during liver resection or allow for transplantation of the organ (159). The pathophysiology of IR injury can be divided into three phases that encompass ROS/RNS production (205). The hyperacute phase marks the first 30 min of reperfusion and is characterized by intracellular ONS (205), which mainly results from excessive mitochondrial formation of O2•− and derivative ROS/RNS (140). This inflicts cell death to a limited extent (70), yet enough to activate KCs, which initiate the second phase (acute phase, 30 min–6 h of reperfusion) (205). During this phase, activated KCs produce and release ROS/RNS as well as pro-inflammatory mediators (e.g., TNF-α) (206). In doing so, KCs incite the most detrimental third phase (chronic phase, >6 h reperfusion) (205), in which leukocytes migrate to the liver (92) and produce large amounts of extracellular ROS/RNS as well as proteases that collectively induce microcirculatory defects and inflict parenchymal necrosis (205).
Clinically, IR injury is characterized by a steep increase in serum transaminases and decreased liver function (66), both resulting from hepatocellular damage, which in most severe cases culminates in acute liver failure (153). Thus, IR injury is a potentially hazardous side effect of life-saving surgical procedures, particularly in fatty livers (208).
ROS/RNS and Their Chemical Properties in the Context of NAFLD and IR
In order to fully appreciate the detrimental effects of ROS/RNS in IR-subjected fatty livers it is important to define the chemical properties of the individual species. Such an appraisal is imperative to assess which ROS/RNS are most likely involved in the resulting hepatopathology and which factors dictate their effects.
Reactive oxygen species
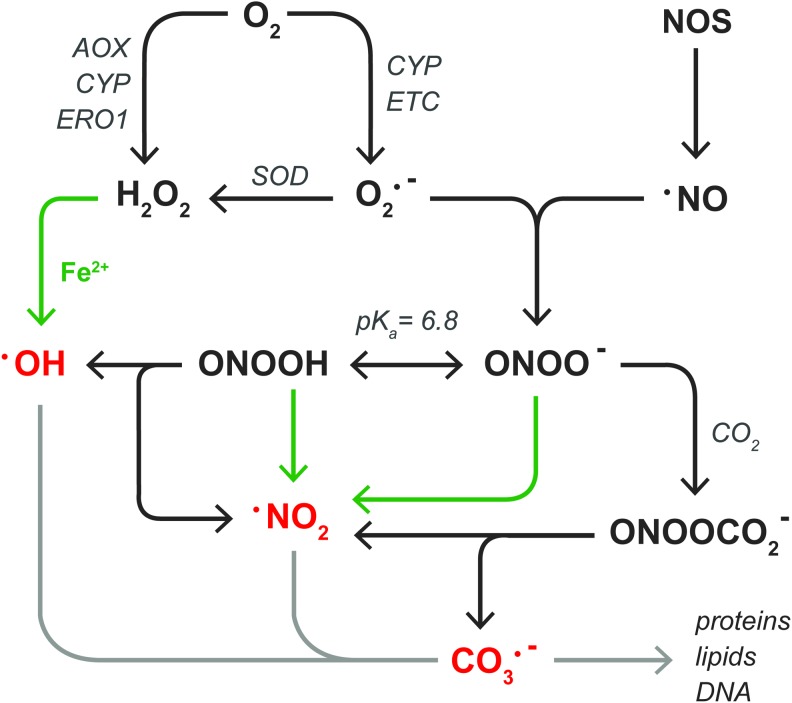
All ROS/RNS are directly or indirectly derived from O2•−, the product of the one-electron reduction of O2 (Fig. 3). The two main sources of O2•− in steatotic hepatocytes are the mitochondrial electron transport chain (ETC) (141) (section “Mitochondria”) and CYP enzymes (227) (section “Other sources of ROS/RNS in NAFLD”). Although NADPH oxidase 4 is moderately expressed in human liver tissue (28), the finding that NADPH oxidase 4-deficient mice are more susceptible to high-fat diet-induced obesity and consequent hepatic steatosis compared with wild-type controls excludes this enzyme as a source of O2•− in NAFLD (117). O2•− has an estimated biological half life (t1/2) of ∼10−6 s (63) and swiftly dismutates into the non-radical oxidant H2O2 in a reaction that is catalyzed by three forms of superoxide dismutase (SOD): mitochondrial manganese-SOD (MnSOD), cytosolic copper-zinc-SOD, and extracellular SOD (230). In hepatocytes, H2O2 is directly formed during the two-electron reduction of O2 by peroxisomal fatty acyl-CoA oxidase (168) (AOX) as well as microsomal CYP enzymes (227) and ER oxidoreductin 1 (68) (ERO1, section “Other sources of ROS/RNS in NAFLD”). Compared with O2•−, H2O2 has a longer lifespan [t1/2≈10−5 s (63)] that, in combination with its membrane-transgressing capacity, enables it to react more distally from its production site (11). H2O2 is scavenged by peroxiredoxins [1–6] (221), glutathione peroxidases [1–4, 7, 8] (84), and catalase (84), but will participate in metal-catalyzed reactions (MCR) in the presence of transition metals such as ferrous iron (Fe2+) (215). Specifically, the reaction between H2O2 and Fe2+, known as the Fenton reaction (49), leads to the formation of highly reactive hydroxyl radicals (•OH) and Fe3+(215). Thereafter, Fe3+ can be reduced to Fe2+ by O2•−, establishing an overall reaction that is known as the Haber-Weiss cycle (215). Due to its reactivity [t1/2≈10−15 s (63)], •OH has a very limited diffusion distance.
FIG. 3.
ROS and RNS relevant to NAFLD and hepatic IR. Superoxide anion (O2•−) is formed in the one-electron reduction of O2 by CYP enzymes or the ETC. Thereafter, different SODs catalyze the dismutation of O2•− into hydrogen peroxide (H2O2). In addition, H2O2 is formed in the two-electron reduction of O2 by AOX, CYP enzymes, or ERO1. H2O2 can react with transition metals such as ferrous iron (Fe2+, green arrows) to form hydroxyl radical (•OH). Alternatively, O2•− reacts with NOS-derived nitric oxide (•NO) to form peroxynitrite anion (ONOO−), which exists in equilibrium with its conjugate acid peroxynitrous acid (ONOOH, pKa=6.8). Both forms of peroxynitrite can react with Fe2+ to generate nitrogen dioxide (•NO2). •NO2 is also formed during the homolytic fission of ONOOH, along with •OH, as well as in the reaction between ONOO- and CO2, also yielding carbonate radical anion (CO3•−). The free radicals •OH, •NO2, and CO3•− are indicated in red to emphasize their high reactivity, which enables them to irreversibly alter the chemical structure of biomolecules (light gray arrow) as described in section “Molecular targets of ROS/RNS in NAFLD and IR.” AOX, fatty acyl-CoA oxidase; ERO1, ER oxidoreductin 1; ETC, electron transport chain; IR, ischemia-reperfusion; NOS, nitric oxide synthase; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The labile iron pool
The role of Fe2+ in ONS deserves close attention, as free Fe2+ associates with amino acids (section “Proteins”) as well as the DNA backbone and, as such, catalyzes redox reactions with H2O2 and peroxynitrite (next section) in a site-specific manner (6, 192). Moreover, Fe2+ directly oxidizes biomolecules in the presence of O2 (89), whereas its non-oxidizing ferric form (Fe3+) is easily reduced to Fe2+ by the antioxidants ascorbic acid and glutathione (GSH) (146). Accordingly, the amount of freely available intracellular iron (i.e., Fe2+ and Fe3+), referred to as the labile iron pool (LIP) (97), is strictly controlled by the storage of iron in ferritin as Fe3+, which prevents its participation in MCR (216). Nevertheless, O2•− has the ability to liberate Fe2+ from iron-sulfur clusters in metalloproteins such as mitochondrial aconitase (142), attesting to the ability of ROS/RNS to augment the LIP. Corroboratively, aconitase activity was significantly hampered in liver mitochondria that were subjected to in vitro anoxia-reoxygenation, an effect that could be partially prevented by decreasing the mitochondrial formation of O2•− (188). Similarly, the administration of a lipid-soluble iron chelator (des ferriexochelin 772SM) (7) and overexpression of the ferritin heavy chain (15) reduced ONS and liver damage, respectively, in rat models of cold IR. Moreover, hepatic iron overload reportedly promotes disease progression in human NAFLD (203), altogether underscoring the significant role of the LIP in ONS. It should be noted that the results of the latter study are to be interpreted with caution, because the histological method that was employed to detect intracellular iron [i.e., Perls' Prussian Blue (115)] involves degradation of ferritin and subsequent selective staining of Fe3+, but not Fe2+(218), which implies that this method does not necessarily provide information on the LIP (97).
The hepatic iron accumulation that is associated with NAFLD could be, at least in part, an effect of ROS formation. In that regard, the expression of transferrin receptor 1, which dictates hepatocellular iron uptake, and ferritin, which determines the amount of free iron (i.e., the LIP), is regulated post-transcriptionally via the binding of iron regulatory proteins to iron-responsive elements in ferritin as well as transferrin receptor 1 mRNA (214). In particular, iron regulatory protein 1 (IRP1) only binds to iron responsive elements following a structural rearrangement that entails the loss of its iron-sulfur cluster and consequent exposure of a cysteine-rich mRNA-binding site. This conformational change, which coincides with activation of the protein, is induced not only by a reduction in cellular iron levels but also by oxidative stress (214). More specifically, exposure of HepG2 cells to persistently low levels of extracellular H2O2 (to mimic a state of chronic inflammation such as NAFLD) activated IRP1 and augmented the LIP (8). Moreover, rats that were fed a high-fat diet showed enhanced IRP1 activity, increased transferritin receptor 1 expression, decreased ferritin levels, and an increase in hepatic total iron content (132). These findings indicate that the inflammatory aspect of NALFD could contribute to hepatocellular iron accumulation as well as augmentation of the LIP and hence ONS. Accordingly, leukocyte influx as well as increased hepatic TNF-α mRNA, malondialdehyde (MDA, a lipid peroxidation end product), and 3-nitrotyrosine (a protein nitration marker) levels were also observed in these high-fat diet-fed rats (132).
Notwithstanding the apparent relationship between inflammation, ONS, and the LIP, the cysteine residues on activated IRP1 are preferential targets for oxidation (section “Proteins”), which ameliorates their mRNA-binding capacity (214). Severe ONS could therefore revoke the effects mentioned earlier inasmuch as the inactivation of IRP1 increases ferritin levels and decreases transferrin receptor 1 expression. The finding that pretreatment with N-acetyl cysteine prevented IRP1 inactivation during early reperfusion in a rat model of warm IR substantiates this notion (195). Consequently, additional studies that investigate iron metabolism and the LIP in NAFLD as well as IR injury are required, as hepatic iron kinetics likely govern the ONS-mediated interplay between both conditions.
Reactive nitrogen species
In addition to ROS, O2•− is a precursor of RNS such as peroxynitrite, the common denominator for peroxynitrite anion (ONOO−) and peroxynitrous acid (194) [ONOOH, pKa=6.8 (165) and t1/2<0.1 s (166)]. Peroxynitrite is formed in the diffusion-controlled reaction between O2•− and •NO [t1/2=1−30 s (90)] (102), which is synthesized by NOS enzymes such as iNOS (61). Its membrane-transgressing ability and long lifetime enable •NO to act on neighboring cells (13), which implies that •NO formed in KCs can diffuse into hepatocytes. iNOS is induced in KCs (211) as well as in hepatocytes (61) in response to inflammatory stimuli such as IL-1β and TNF-α (71), both of which are released during NAFLD (198) and IR (92). Accordingly, hepatic iNOS mRNA and protein levels are increased compared to controls in rodent models of NAFLD (122, 190) and cold IR (82), respectively. Along these lines, iNOS knock-out mice that were fed a high-fructose (i.e., NAFLD-inducing) diet exhibited reduced hepatic TNF-α mRNA, 3-nitrotyrosine, and 4-hydroxynonenal (4-HNE, a lipid peroxidation end product) levels compared with wild-type controls (190). Moreover, the administration of an iNOS inhibitor [N-(1-naphtyl)ethylendiamine dihydrochloride] markedly reduced liver damage, improved regeneration, and increased overall survival in a rat model of cold IR with a concomitant reduction in RNS formation (82).
Although some have claimed that a specific NOS isoform is present in mitochondria, its existence has not yet transcended from dispute (17). Alternatively, hypoxic rat liver mitochondria generate •NO from nitrite (24), although •NO formed under hypoxic conditions was shown to alleviate rather than aggravate mouse liver IR injury in vivo by transiently reducing mitochondrial O2•− formation during early reperfusion (188) (section “Mitochondria”). Moreover, due to the stability and diffusibility of •NO, mitochondrial •NO production is not a prerequisite for mitochondrial RNS formation (164). Instead, the limited diffusion properties of O2•− (2) dictate that the formation of peroxynitrite will predominantly occur in the vicinity of O2•− generation. Since the reaction between O2•− and •NO directly competes with that of O2•− and SODs (20), the generation of peroxynitrite also depends on the availability of SODs. Thus, when SODs become inactivated, as occurs in NAFLD (138, 209) (section “Proteins”), and iNOS is simultaneously induced, which takes place in NAFLD (122) as well as IR (82), the formation of peroxynitrite and derivative radicals (next paragraph) will increase.
Similar to H2O2, peroxynitrite participates in MCR, resulting in the formation of nitrogen dioxide (•NO2) (6). Peroxynitrite is scavenged by peroxiredoxins and glutathione peroxidases (194), but the enzymatic inactivation of ONOO− directly competes with its reactivity toward CO2, which generates •NO2 and a carbonate radical anion (CO3•−) with a ∼35% yield (32, 194). ONOOH, on the other hand, can homolyze into •OH and •NO2 (∼30% yield) (31), but this reaction is slow [k=0.8 s−1] (31, 194) and will therefore predominantly occur in lipophilic compartments (e.g., membranes) where less competing reactions prevail (194). Insofar as the lipid compartment is increased in NAFLD (162), the formation of •OH and •NO2 from ONOOH homolysis might be favored.
The effects of •NO2 [t1/2≈10−6 s (51)] and CO3•− [t1/2≈10−6 s (130)] have only recently received attention as effectors of the cytotoxicity that was initially ascribed to peroxynitrite (12). Both species can directly oxidize biomolecules and are scavenged by ascorbic acid and GSH (12), which is likely to be impaired in NAFLD because of GSH (209) and ascorbic acid depletion (116) as well as in IR due to a transient reduction in GSH levels (103). Furthermore, due to its lipophilicity, •NO2 is an initiator of lipid peroxidation, a process that can be inhibited by α-tocopherol (189) (section, “Lipids”).
In summary, two scenarios can be distinguished in which ROS/RNS are most damaging towards hepatocytes: (i) the combination of H2O2 or peroxynitrite and Fe2+ that induces site-specific, MCR-mediated generation of •OH or •NO2, respectively, and (ii) the formation of peroxynitrite from •NO and O2•−, which gives rise to •NO2, •OH, and/or CO3•− as derivative radicals. Notably, either route applies to NAFLD and IR since both conditions are characterized by excessive formation of O2•− and •NO. Moreover, the formation of peroxynitrite and its derivative radicals is specifically favored in NAFLD due to the depletion of SOD and GSH, respectively. Lastly, experimental evidence suggests a catalytic role for iron in NAFLD and IR, although studies that explicitly address the LIP in both conditions are currently lacking.
Molecular Targets of ROS/RNS in NAFLD and IR
The biological targets of ROS/RNS are generally divided into lipids, proteins, and DNA. Understanding the reaction mechanisms that underlie their damaging effects is essential to interpret the events observed at a cellular level. Considering that (per)oxidation of proteins and lipids has the most relevant effects with respect to the interplay between NAFLD and IR, the reactions between ROS/RNS and these biomolecules will be addressed next with specific focus on the implications for both conditions.
Lipids
Lipid peroxidation is prevalent in NAFLD, not only due to the abundance of substrate (i.e., FAs) (162) but also because of the nature of the reaction, which is radical chain propagating (226). The susceptibility of an FA to oxidation is proportional to the number of double bonds in its aliphatic tail inasmuch as hydrocarbons that are flanked by two alkenes (i.e., doubly allylic sites) have a ∼20% lower bond dissociation energy compared to those in saturated C–C bonds (226). Accordingly, the polyunsaturated fatty acids (PUFAs, ≥2 double bonds) linoleic acid (LA, ω-6, 1 doubly allylic site), arachidonic acid (AA, ω-6, 3 doubly allylic sites), eicosapentanoic acid (EPA, ω-3, 4 doubly allylic sites), and docosahexanoic acid (DHA, ω-3, 5 doubly allylic sites) are most sensitive to oxidation (226).
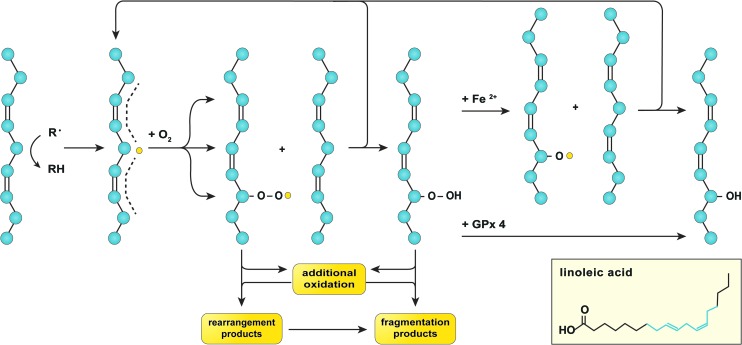
Given their lipophilicity, •OH (149) and •NO2 (160) from MCR and/or ONOOH homolysis generally initiate lipid peroxidation (Fig. 4). The thus formed lipid radical (L•) swiftly reacts with O2 to generate a lipid peroxyl radical (L–OO•) (226) that can undergo intramolecular reactions to yield non-radical cleavage products such as MDA (161). Alternatively, L–OO• can react with another PUFA, thereby propagating the radical chain reaction, undergo MCR (171), or decompose into non-radical end products such as 4-HNE (184) (Fig. 4). Both MDA and 4-HNE are toxic inasmuch as they can form adducts with DNA (125, 158) and proteins (87, 158) (section “Proteins”). Although glutathione peroxidase 4 scavenges L–OOH at the expense of GSH (171), this process is likely compromised in NAFLD and IR because of chronic and acute GSH depletion (103, 209), respectively, which could shift the fate of L–OOH toward MCR and the formation of 4-HNE. Correspondingly, elevated levels of 4-HNE and MDA have been detected in liver samples of NAFLD patients (Table 1), a mouse model of IR (140), as well as rat models of warm (147) and cold (228) fatty liver IR.
FIG. 4.
Lipid peroxidation. Lipid peroxidation is initiated by hydrogen abstraction, mostly from hydrocarbons that are flanked by two alkenes (depicted on the left), that is, those on the aliphatic chains of PUFAs such as linoleic acid (inset, in which the structure highlighted in blue corresponds to the aliphatic structure in the figure). The formed carbon-centered lipid radical (L•, yellow dot) has the ability to relocate three carbon atoms away from the abstraction site (dashed line), where it swiftly reacts with oxygen to form a lipid peroxyl radical (L–OO•). Subsequently, L–OO• can undergo intramolecular modification to form rearrangement products, additional oxidation, or react with a proximal PUFA to generate a lipid hydroperoxide (L–OOH) as well as a new L• (top arrow). L–OOH can undergo additional oxidation or dissociate into fragmentation products. Alternatively, L–OOH can react with GPx 4 to form a lipid hydroxide (L–OH) or undergo ferrous iron (Fe2+)-catalyzed oxidation to form a lipid alkoxyl radical (L–O•), the latter of which has the ability to oxidize another PUFA and generate a new L• (top arrow) as well as a L–OH. All these events lead to membrane destabilization as a result of lipid packing defects, which may have detrimental consequences on cell function and viability. GPx 4, glutathione peroxidase 4; PUFA, polyunsaturated fatty acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 1.
Hepatic Lipid Peroxidation, Protein Oxidation, and Antioxidative Defense Markers in Human Non-Alcoholic Fatty Liver Disease
| Marker | NAFL | NASH | References |
|---|---|---|---|
| Lipid peroxidation | |||
| MDA | NS | + | (4) |
| MDA | + | + | (120) |
| 4-HNE | + | + + | (186) |
| 4-HNE-protein adducts | ND | + | (187) |
| Protein oxidation | |||
| Protein carbonyls | + | NS | (209) |
| 3-Nitrotyrosine | + | + + | (182) |
| 3-Nitrotyrosine | NS | + + | (57) |
| Antioxidants | |||
| GSH | − | − | (209) |
| GSH | − | − | (120) |
| Mitochondrial GSH | ND | − | (187) |
| SOD activity | − | − | (209) |
| Catalase activity | NS | − − | (209) |
| Glutathione peroxidase activity | NS | NS | (209) |
| Total antioxidative capacity measured as μmol uric acid equivalent | − − | − − | (4) |
| Ferritin | + | + | (120) |
NS indicates no significant difference versus control, + indicates a significant increase versus control, + + indicates a significant increase versus control and NAFL, − indicates a significant decrease versus control, and − − indicates a significant decrease versus control and NAFL. ND indicates that no data were obtained in the respective study.
4-HNE, 4-hydroxynonenal; GSH, reduced glutathione; MDA, malondialdehyde; NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis; SOD, superoxide dismutase.
Another contributory factor to the aggravation of IR injury in NAFLD is an increased ω-6/ω-3 FA ratio (4). The disrupted ω-6/ω-3 balance in fatty livers could be in part explained by the reaction rates of LA, AA, EPA, and DHA toward L–OO•, which follow LA<AA<EPA<DHA in a 1.0:3.2:4.0:5.4 ratio (226), indicating that the ω-3 FAs EPA and DHA are more rapidly oxidized than their ω-6 counterparts LA and AA. Since ω-6 FAs are precursors of pro-inflammatory and vasoconstrictive mediators (e.g., thromboxane A2) (42, 109), while ω-3 FAs are metabolized into anti-inflammatory and vasodilatory compounds (e.g., prostaglandin E3) (109), a shift in the ω-6/ω-3 FA ratio toward ω-6 FAs, therefore, augments inflammation (i.e., extracellular ONS) and causes perfusion defects (42). Accordingly, an increased ω-6/ω-3 FA ratio has been shown to exacerbate IR injury in fatty livers, which could be ameliorated by earlier supplementation of ω-3 FAs that corrects the ω-6/ω-3 FA ratio (42, 127). Taken altogether, these data reflect the importance of a proper lipidomic balance in ONS-mediated liver disease and injury.
Consequently, the presence of antioxidants in the lipid compartment is particularly important. The major free radical scavengers in the lipid compartment are α-tocopherol (44) and ubiquinol (also referred to as coenzyme Q10 [UQH2]); the reduced form of ubiquinone (UQ), which is also a component of the ETC (43) (Fig. 5). These antioxidants work in concert to protect membranes against oxidative modification (43), the clinical implications of which are reflected by the beneficial effects of α-tocopherol supplementation on NASH disease activity (determined histologically) found in a randomized controlled trial (183).
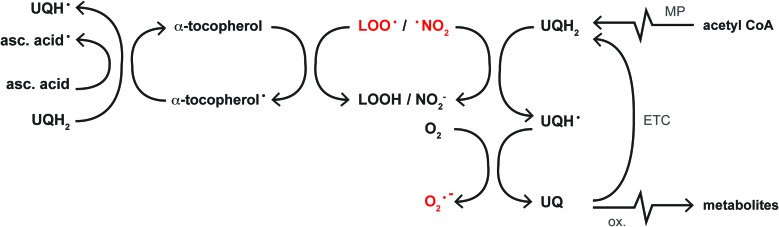
FIG. 5.
Free radical scavenging in the lipid compartment. Initiators of lipid peroxidation such as lipid peroxyl radicals (L–OO•) and nitrogen dioxide (•NO2) are scavenged by α-tocopherol during which an α-tocopherol radical (α-tocopherol•) is formed. The α-tocopherol radical is subsequently reduced by ascorbic acid (asc. acid) or ubiquinol (UQH2, depicted on the left), resulting in the formation of an ascorbic acid (asc. acid•) or ubisemiquinone radical (UQH•), respectively. Alternatively, UQH• is formed during the direct reduction of L–OO• or •NO2 by UQH2 (depicted on the right). UQH• reacts with O2 to form UQ as well as superoxide anion (O2•−). UQ can be regenerated into UQH2 via reduction by the mitochondrial ETC, but UQH2 is also synthesized endogenously from acetyl CoA via the MP (upper right corner). In addition, UQ can undergo (auto-)oxidation (ox.) into inactive metabolites (lower right corner). MP, mevalonate pathway; UQ/UQH2, ubiquinone/ubiquinol, also referred to as coenzyme Q10. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Upon oxidation, the regeneration of α-tocopherol relies on the reducing capacity of ascorbic acid or UQH2 (43), whereas that of UQ depends on the reductive potential of the ETC (126) or de novo synthesis from acetyl CoA via the mevalonate pathway (43). However, hepatic ascorbic acid levels become depleted in rats fed a high-fat diet (116), whereas the decline in UQ(H2) plasma levels in human NAFLD (225) indicates a concomitant reduction in hepatic UQ(H2) content. Inasmuch as UQ is easily (auto-)oxidized into inactive metabolites (38), excessive lipid peroxidation in NAFLD could be responsible for a reduction in intracellular UQ(H2) levels (53), altogether debilitating the antioxidant defense machinery in membranes.
In sum, excessive lipid peroxidation, a disrupted ω-6/ω-3 FA ratio, and a reduced antioxidative capacity contribute to the marked damage in steatotic livers compared with healthy livers after IR (147, 228).
Proteins
Oxidation of proteins irreversibly impairs their structure and function. Generally, cells possess an effective machinery to cope with oxidized proteins, namely the 20S-proteasome (39). However, when the amount of oxidatively modified proteins becomes overwhelming, the extent of protein fragmentation and cross-linking increases (39). This eventually leads to the formation of large protein aggregates, such as the Mallory bodies that are characteristic of NASH (229), which are cytotoxic and resistant to proteolytic digestion (39). The formation of protein aggregates in NAFLD is furthermore promoted by 4-HNE-mediated inhibition of the 20S-proteasome (50).
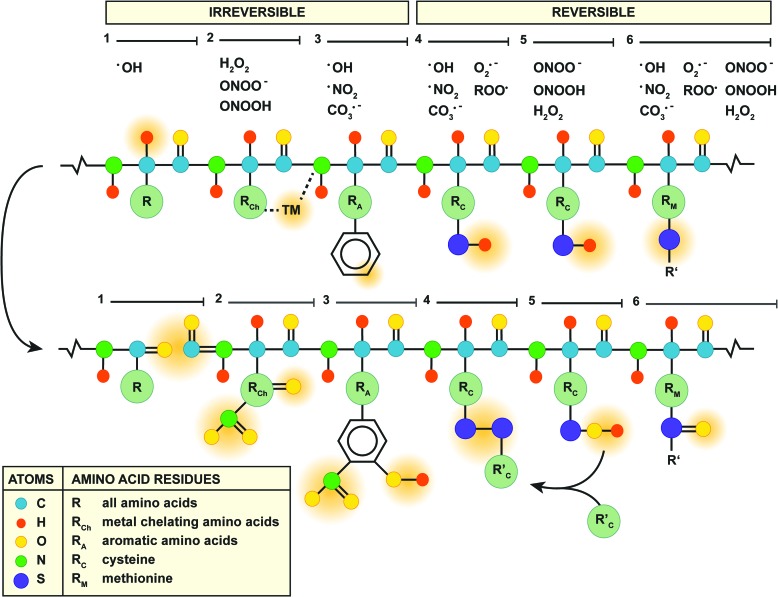
Proteins are irreversibly modified via three mechanisms (Fig. 6, mechanisms 1–3). First, the protein backbone is a target for oxidation by •OH via proton abstraction (59) (Fig. 6, mechanism 1), which eventually results in cleavage of the protein backbone into carbonylated (R=O) fragments (192). Second, MCR takes place at amino acids that chelate transition metals (i.e., lysine, arginine, proline, threonine, histidine, leucine, and cysteine) (192) (Fig. 6, mechanism 2), which, in the presence of H2O2 or peroxynitrite, results in carbonylation (192) or nitration (R–NO2) (6), respectively, of the transition metal-complexed amino acid. Third, the aromatic moieties of tryptophan, tyrosine, phenylalanine, and histidine are preferred targets of peroxynitrite-derived radicals (6) (Fig. 6, mechanism 3), thereby generating hydroxylated (R–OH) or nitrated residues such as 3-nitrotyrosine (6). Of note, 3-nitrotyrosine is a reliable biomarker for the formation of RNS such as peroxynitrite-derived •NO2 in vivo (210).
FIG. 6.
Mechanisms of protein oxidation. Six mechanisms of protein oxidation are depicted (yellow bars and numbers, top and center). The species of ROS/RNS involved in each mechanism are listed above. The top scheme represents the protein backbone with the individual amino acids (see in-figure legend) in which the polygonal/circular forms represent aromatic structures on the respective amino acid residues. The sites of oxidation by ROS/RNS are indicated with orange shading. The bottom scheme shows the structural changes after oxidation, also higlighted in orange. Details on the reaction mechanisms are provided in the text. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Accordingly, elevated levels of 3-nitrotyrosine and protein carbonyls have been measured in liver samples of NAFLD patients (Table 1) as well as in a mouse model of IR (140). The consequences hereof likely contribute to the susceptibility of steatotic hepatocytes to IR injury, especially when these modifications affect antioxidant enzymes. Tyrosine nitration and histidine oxidation, for instance, are key events in the peroxynitrite-induced inactivation of MnSOD (193) and copper-zinc-SOD (5), respectively. The functional loss of these enzymes was found to contribute to the reduced antioxidative capacity in NAFLD livers (209), which is likely to affect cell viability during IR (section “Pathophysiological implications of ROS/RNS in NAFLD and IR”). Moreover, reduced MnSOD activity was observed in two mouse models of NAFLD, despite a significant increase in MnSOD mRNA levels (138), attesting to the fact that post-transcriptional modifications such as oxidation are responsible for the loss of MnSOD activity in NAFLD.
Contrary to the mechanisms of irreversible protein modification mentioned earlier, oxidation of cysteine and methionine is reversible and, as such, constitutes the main redox mechanism in numerous antioxidant enzymes (192) (Fig. 6, mechanisms 4–6). Oxidation of the cysteine thiol/thiolate (R–SH/R–S−, Fig. 6, mechanism 4) by free radicals, for instance, accounts for the direct antioxidative properties of GSH, in which the formed thiyl radical (R–S•) rapidly reacts with another GSH molecule to form glutathione disulfide (GSSG) (77). Although GSSG can be reduced enzymatically by glutathione reductase (77), GSH stores are gradually depleted during persistent ONS (77), which is a cardinal feature of NAFLD (Table 1). In addition, the lipid peroxidation product 4-HNE can form adducts with cysteine, histidine, and lysine (158) (Table 1), leading to the formation of GSH-HNE conjugates. GSH-HNE conjugate levels were significantly higher in rats fed a high-fat diet compared with standard chow (116), indicating that this process contributes to GSH depletion in NAFLD (116).
Alternatively, H2O2 and peroxynitrite oxidize cysteine thiols via the formation of an intermediate sulfenic acid (100) (R–SOH, Fig. 6, mechanism 5). In particular, the reaction of protein sulfenic acids or nitrosothiols [R–S–NO, from reaction with •NO (54)] with GSH (77), known as S-glutathionylation (R–S–SG), constitutes an important process in the modulation of TNF-α signaling during ONS (157, 174) (section “Tumor necrosis factor alpha”). Moreover, sulfenic acid formation is the working mechanism of H2O2 and peroxynitrite scavenging by peroxiredoxins (221). Peroxiredoxin-4-overexpressing mice that were fed a high-fructose diet showed reduced levels of hepatic 4-HNE, serum MDA, and hepatic TNF-α mRNA levels compared with their wild-type littermates (143). Collectively, these findings highlight the importance of non-radical oxidant-scavenging enzymes in regard to ONS and inflammation in the context of NAFLD.
However, the catalytic site of peroxiredoxins can be inactivated by overoxidation into sulfinic (R–SO2H) and ultimately sulfonic acids (R–SO3H) (100) that reduces their antioxidative capacity (223), although the sulfinic acids on peroxiredoxin 1–4 can still be regenerated by sulfiredoxin (220). Overoxidation and subsequent recuperation of peroxiredoxins was shown to occur in HeLa cells that were incubated with tert-butyl hydroperoxide (an inducer of acute oxidative stress), but complete recovery was slow and depended on both regeneration and de novo synthesis (30). Accordingly, the non-radical oxidant-scavenging capacity of hepatocytes could be affected by overoxidation of peroxiredoxins during IR, particularly under conditions of steatosis due to the prevalent ONS.
Another amino acid prone to oxidation is methionine, which contains a thiol group that can be oxidized into a methionine sulfoxide (R–S=O) by various ROS/RNS (111) (Fig. 6, mechanism 6). Although reversible, methionine sulfoxide formation is not benign per se as the oxidation of methionine-80 in cytochrome c activates its peroxidase activity (197), which constitutes a key event in ONS-induced cell death (96) (section “Mitochondria”). The detrimental effects that emanate from cytochrome c oxidation at methionine-80 underscore the importance of the mitochondrial antioxidative machinery. Not only are mitochondrial antioxidants critical in preventing cell death during IR (140) (section “Mitochondria”), they are also responsible for the detoxification of ETC-derived ROS/RNS and therefore for deterring their diffusion into the cytosol (77). However, the mitochondrial antioxidative defense system consists of a variety of antioxidants, including MnSOD (99), GSH (124), and peroxiredoxin-3 (221), which can be (over)oxidized by ROS/RNS as addressed earlier. Moreover, the mitochondrial antioxidative capacity strongly depends on the cumulative actions of the individual antioxidants (138). For instance, SOD-overexpressing mice that were fed a methionine- and choline-deficient diet were more susceptible to NASH because of mitochondrial GSH depletion and a consequent rise in H2O2 levels, despite a reduction in peroxynitrite formation (138). Thus, the modulation of a single component of the mitochondrial antioxidative defense system can exert inverse and undesirable effects on its overall function.
Conclusively, the ONS in NAFLD likely contributes to attenuation of the overall cellular and particularly mitochondrial antioxidative capacity by (irreversibly) oxidizing critical amino acid residues in antioxidants, which renders steatotic hepatocytes more amenable to IR damage.
Pathophysiological Implications of ROS/RNS in NAFLD and IR
At a cellular level, there are two pivotal sites at which the interplay between NAFLD and IR takes place: the mitochondria and TNF-α signaling, both of which are strongly affected by ONS. In addition, two other sources of ROS/RNS, namely extramitochondrial FA oxidation and the unfolded protein response (UPR), have been described for NAFLD that are not directly influenced by IR but likely contribute to the ONS that sensitizes fatty livers to IR. Therefore, these will also be addressed.
Mitochondria
Under physiological conditions, mitochondria consume more than 90% of cellular O2 in order to generate ATP via oxidative phosphorylation (137). During oxidative phosphorylation, electrons derived from the oxidation of NADH and FADH2 are carried along complex I–IV of the ETC to eventually reduce O2 to H2O (137). The transport of electrons enables the movement of protons from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space, thereby establishing an inward protonmotive force (Δp) that consists of a pH component (i.e., the transmembrane pH gradient, ΔpH) and an electrical component (i.e., the membrane potential, ΔΨ) (105). The Δp-driven return of protons through ATP synthase provides the energy required to phosphorylate ADP to ATP (137). However, a small fraction of the intramitochondrial oxygen is reduced to O2•−, predominantly by complex I, which is favored at a high NADH/NAD+ ratio as well as a large Δp (141).
As mentioned in the introduction, mitochondria constitute an important source of hepatocellular ROS/RNS in NAFLD (154). The basis hereof lies in the large amount of free fatty acids that are transported into the mitochondria to undergo β-oxidation (154). This generates acetyl-CoA, which enters the tricarboxylic acid cycle to reduce NAD+ and FAD (135). Subsequently, as the NADH/NAD+ ratio and Δp increase, so does the production of O2•− (141).
In NAFLD, ROS/RNS formation is further enhanced by the accumulation of nonesterified cholesterol in the mitochondrial membranes, which reduces their fluidity and hinders the transport of GSH into the mitochondrial matrix (118). Moreover, 4-HNE can form adducts with histidine residues in the catalytic center of complex IV, significantly reducing its activity (29). Accordingly, when complex IV is inhibited, so is electron transport in the preceding complexes, leading to an increase in the NADH/NAD+ ratio and hence O2•− formation (141). Although the activity of purified complex IV can be preserved in the presence of 4-HNE when GSH is added (29), the mitochondrial GSH depletion that concurs with NAFLD (118, 187) likely promotes the 4-HNE-dependent inactivation of complex IV. Thus, ONS and mitochondrial GSH depletion further contribute to the generation of (mitochondrial) ROS/RNS in NAFLD.
Mitochondria respond to this metabolic stress by expressing mitochondrial uncoupling protein 2 (UCP2) (145). While UCP2 is usually undetectable in hepatocytes, it is expressed in response to high levels of nonesterified PUFAs via activation of the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α) (95, 145). UCP2 establishes a proton leak over the inner mitochondrial membrane that lowers Δp and hence the formation of O2•− (141), which implies that UCP2 induction alleviates mitochondrial ONS. Along these lines, the degree of proton conductance through UCP2 is directly and proportionally controlled by the amount of O2•− generation in mitochondria via the formation of 4-HNE (142). These findings indicate that UCP2 activity increases parallel to mitochondrial O2•− formation in NAFLD and, as such, aims to prevent excessive ONS. Corroboratively, UCP2 mRNA and protein expression was observed in hepatocytes of steatotic ob/ob mice in contrast to healthy controls, in which UCP2 mRNA was undetectable (27).
Although intended as an antioxidative mechanism, the expression of UCP2 in steatotic hepatocytes affects mitochondrial energy metabolism. Since ETC uncoupling occurs at the expense of Δp, the mitochondrial ATP synthesizing capacity becomes impaired (137), which explains the low basal ATP levels that were measured in ob/ob mice (27). Moreover, basal ATP levels inversely correlate to body mass index in NASH patients (34) as well as in subjects that are clinically unlikely to suffer from NASH (34, 144), indicating that UCP2 expression might already take place in earlier stages of NAFLD.
Although the negative effects of UCP2 expression on ATP generation are usually quiescent (27, 45), they surface when energy demands increase. For instance, NASH patients who were subjected to a fructose (i.e., ATP-depleting) challenge showed significantly a reduced ATP repleting capacity (34). The negative effect of UCP2 on the mitochondrial ATP-generating capacity becomes even more pronounced when the liver is subjected to IR. During ischemia, the lack of O2 forces hepatocytes to switch to anaerobic glycolysis to generate ATP (222). However, due to the limited ATP yield from anaerobic respiration compared with oxidative phosphorylation, cellular ATP/ADP stores gradually become depleted (103) while cessation of ETC activity increases the NADH/NAD+ ratio (103). The high NADH/NAD+ ratio subsequently fuels the acute burst in complex I-driven mitochondrial O2•− formation (141) on reoxygenation (i.e., reperfusion) (22).
In line with the previous findings, transient S-nitrosylation (i.e., inactivation) of complex I during the hyperacute phase of reperfusion, resulting from the intramitochondrial formation of •NO from exogenously administered nitrite during hypoxia, significantly attenuated parenchymal damage in a mouse model of IR (188). This beneficial effect was attributed to a reduction in mitochondrial H2O2 formation and improved ATP synthesis during early reperfusion, conclusions that were based on observations made in nitrite- and vehicle-treated rat liver mitochondria that were subjected to in vitro anoxia-reoxygenation (188). In addition, compared with healthy hepatocytes, steatotic hepatocytes are challenged in neutralizing the high levels of ROS/RNS and in restoring ATP reserves during early reperfusion because of mitochondrial antioxidant depletion and ETC uncoupling (187), which has two major consequences.
First, ATP depletion impairs the function of ion transporters in the plasma membrane, including the Na+/K+ ATPase, which leads to an increase in cytosolic [Na+] during ischemia (23). In response, the Na+/Ca2+ antiporter starts working in reverse, reducing intracellular [Na+] at the expense of a rise in [Ca2+] (23). This Ca2+ freely enters the mitochondria during repolarization (i.e., reperfusion), where it induces mitochondrial permeability transition (MPT) (101, 110). MPT encompasses the formation of membrane pores that enable free movement of small solutes between the mitochondrial matrix and the cytosol (110). During MPT, cytochrome c translocates from the inner mitochondrial membrane to the cytosol, where it activates caspase 9 to initiate apoptosis (150). ATP/ADP depletion (72) and ONS (148) greatly lower the threshold for MPT. Specifically, ROS/RNS oxidize vicinal cysteine residues on the adenine nucleotide translocator, one of the MPT pore components, which augments its sensitivity to Ca2+ (129). Moreover, peroxynitrite-dependent tyrosine nitration was shown to directly promote oligomerization and channel activity of the voltage-dependent anion channel protein, another MPT pore component, in cardiac IR (224). In addition, a complex I-dependent reduction in mitochondrial ROS formation (described earlier) elicited protection against Ca2+-induced MPT in isolated rat liver mitochondria that were subjected to in vitro anoxia-reoxygenation (188), collectively attesting to the role of ROS/RNS in MPT induction.
In addition to MPT induction, ROS/RNS play a pivotal role in the release of cytochrome c by switching on its peroxidase activity (197) (section “Proteins”) and enabling the subsequent peroxidation of cardiolipin (96), a phospholipid with LA side chains that anchors cytochrome c into the inner mitochondrial membrane (96, 150). The formation of cardiolipin-hydroperoxide (CL-OOH) is essential for the release of cytochrome c from mitochondria, because it disrupts the hydrophobic cardiolipin–cytochrome c interaction (150) and destabilizes the lipid bilayer, thereby promoting Bax-dependent permeabilization of the outer mitochondrial membrane (123) (section “Tumor necrosis factor alpha”). Taken altogether, the prevailing ONS in steatotic hepatocytes likely predisposes mitochondria to MPT and cytochrome c release during IR, causing mitochondria to initiate cell death programs.
Second, the cytochrome c-dependent activation of caspase 9 and subsequent apoptosis can only take place when ATP levels are at least 15–20% of physiological baseline (93). When the energy status drops below these levels, hepatocytes are unable to maintain cellular ion homeostasis, which leads to osmotic swelling and ultimately oncotic necrosis (93). In addition, when ATP levels become insufficient during apoptotic signaling, the cell averts to secondary necrosis (93). Given the low basal ATP levels (27, 45) and uncoupling of the ETC (27, 187) in steatotic hepatocytes, the cells are more likely to undergo (secondary) necrosis than healthy cells following MPT (45). This is particularly important in the context of IR injury, as necrotic cells are highly immunogenic (104). As a consequence, KC activation and leukocyte chemotaxis will be more pronounced, giving rise to the extensive tissue damage observed in various mouse models of fatty liver IR (42, 118). In that regard, UCP2-dependent ATP depletion in particular has been correlated to the poor response of NASH-affected rat (187) and mouse (45) livers to IR. However, the role of this phenomenon in the more prevalent NAFL requires more research.
Taken altogether, the metabolic perturbations in NAFLD negatively influence hepatocyte viability during IR through mitochondrial pathways. First, prevalent mitochondrial ONS favors MPT induction and cytochrome c release during IR. Second, since UCP2 expression impairs ATP production, cells are more likely to undergo necrosis than apoptosis following MPT. The consequent exacerbation of DAMP release during reperfusion likely augments the severity of the inflammatory response and corollary parenchymal damage in steatotic versus healthy livers.
Tumor necrosis factor alpha
TNF-α is a pleiotropic cytokine that is released by a plethora of (liver) cell types in response to inflammatory stimuli (206). In addition to its ability to elicit (hepatic) insulin resistance (3), TNF-α is a strong chemoattractant for leukocytes (32) and a potent iNOS inducer (71). Via its pro-inflammatory effects, TNF-α not only mediates the progression of NAFL to NASH (199) but also significantly contributes to IR injury (32, 200). Nevertheless, TNF-α has a dual effect on hepatocytes because it simultaneously activates a multi-branched death signaling cascade as well as a proliferative ‘survival’ pathway (78). In addition, it has recently been described that TNF-α also induces a novel, programmed type of necrosis (204). In fatty livers, the prevailing ONS likely directs hepatocytes toward TNF-α-mediated cell death rather than survival during IR.
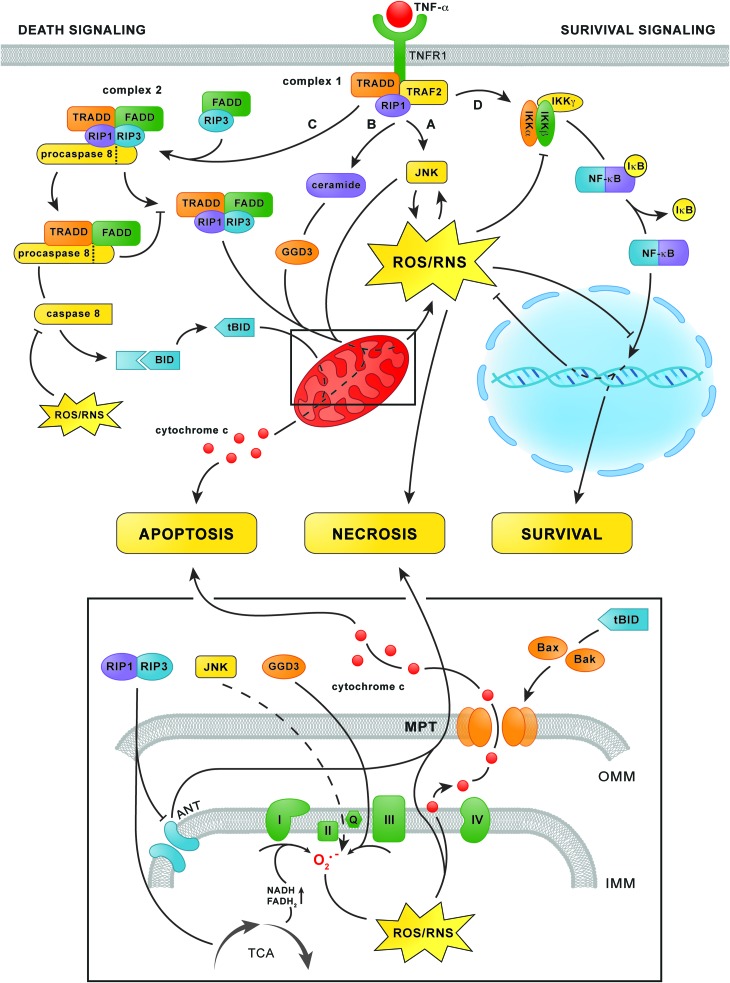
Shortly after binding TNF-α, the cytosolic domain of the TNF-α receptor-1 recruits TNF-α receptor-associated protein with death domain (TRADD), TNF-α receptor-associated factor 2, and receptor-interacting kinase 1 (RIP1) to form the plasma membrane-bound complex 1 (134) (Fig. 7). Complex 1 initiates the survival signaling pathway, which depends on the activation of NF-κB (134), as well as two branches of the trinomial death signaling cascade that are activated by JNK phosphorylation (i.e., activation) (78) and ceramide formation (58). Thereafter, part of complex I dissociates from the cytosolic receptor domain to form complex 2, which mediates the third branch of the death signaling cascade (134). Whether hepatocytes will ultimately proliferate or die following stimulation with TNF-α is strongly influenced by ONS (78).
FIG. 7.
TNF-α signaling in hepatocytes. After TNF-α binding to TNFR1, TRADD, TRAF2, and RIP1 are assembled at the cytosolic domain of TNFR1 to form complex 1 (top center). Complex 1 subsequently initiates two branches of the trinomial death signaling cascade via the phosphorylation of JNK (pathway A) as well as the accumulation of ceramide and the subsequent increase in GGD3 synthesis (pathway B). Both routes increase mitochondrial O2•− formation (bottom panel), although the exact mechanism of JNK-mediated O2•− formation remains elusive (dashed line). The formation of derivative ROS/RNS from O2•− and corollary activation of cytochrome c peroxidase activity subsequently leads to the dissociation of cytochrome c from the IMM (bottom panel), a prerequisite for apoptosis. In addition, sustained JNK-dependent ROS/RNS formation leads to MPT and necrosis. Simultaneously, complex 1 triggers survival signaling via activation of the IKK complex (pathway D, top right), which phosphorylates IκB-α. This enables the dissociation of IκB-α from NF-κB and its translocation to the nucleus to initiate the transcription of anti-apoptotic and antioxidative genes. At a later time point, TRADD and RIP1 dissociate from complex 1 and associate with FADD, procaspase 8, and RIP3 to form complex 2 (upper left corner). Complex 2 mediates the third branch of the death signaling cascade (pathway C) by activating caspase 8, which subsequently truncates Bid into tBid. Thereafter, tBid induces permeabilization of the OMM via the accumulation of Bax and Bak, which enables the release of cytochrome c into the cytosol (bottom panel and top left) and consequent induction of apoptosis. When caspase 8 is inhibited as a result of oxidation, the RIP1/RIP3 complex (top left) fuels mitochondrial ROS/RNS production via upregulation of the TCA cycle and inhibition of the ANT in the IMM (bottom panel), which eventually leads to MPT and necrosis. ANT, adenine nucleotide translocator; FADD, Fas-associated protein with death domain; GGD3, ganglioside GD3; IKK, IκB kinase; IMM, inner mitochondrial membrane; IκB-α, nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor α; JNK, c-Jun N-terminal kinase; MPT, mitochondrial permeability transition; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; OMM, outer mitochondrial membrane; RIP1/3, receptor-interacting kinase 1/3; TCA, tricarboxylic acid; TNF-α, tumor necrosis factor α; TNFR1, TNF-α receptor-1; TRAF2, TNF-α receptor-associated factor 2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The first branch of the death signaling cascade is initiated by activation of the mitogen-activated protein kinase cascade by complex 1, which consists of apoptosis signal-regulating kinase 1 (ASK1), mitogen-activated protein kinase kinase 4/7, and finally phorsphorylation of JNK (78) (Fig. 7, pathway A). Phosphorylated JNK causes ONS by inducing mitochondrial O2•− formation via an unknown mechanism (75, 207) as well as via degradation of ferritin, which augments the LIP (10). Moreover, ROS/RNS sustain JNK phosphorylation by directly activating ASK1 (179). Specifically, oxidation of thioredoxins that constitutively bind to ASK1 enables their dissociation, which enables ASK1 phosphorylation and downstream JNK phosphorylation (179). The existence of this positive feedback loop enables sustained JNK activity, ONS, and ultimately necrosis (98). Accordingly, JNK-dependent ROS/RNS formation and MPT induction were observed in primary hepatocytes in which mitochondrial GSH had been depleted (75), such as fatty hepatocytes (187), as well as in fibroblasts in which NF-κB was selectively inhibited (180, 207)—an ONS-dependent effect that is likely to occur in NAFLD (discussed next). Moreover, selective inhibition of JNK significantly reduced liver damage in rat models of warm (200) and cold IR (201). These findings point to phosphorylated JNK as an important mediator of IR injury, an effect that is likely to be even more prominent in NAFLD because of the prevalent ONS.
In addition, complex 1 initiates the second branch of the death signaling cascade via the activation of acid sphingomyelinase, which hydrolyzes the membrane sphingophospholipid sphingomyelin to form ceramide (58) (Fig. 7, pathway B). The subsequent accumulation of ceramide accelerates the synthesis of ganglioside GD3 in the ER, which enters mitochondria because of their physical continuity with the ER (175), where it promotes O2•− formation at complex III (69). Ceramide accumulation could therefore contribute to ONS in NAFLD when TNF-α is involved, although conclusive evidence is unavailable at this point (152). Nevertheless, inhibition of acid sphingomyelinase by imipramine led to a reduction in parenchymal damage in a mouse model of IR, suggesting that ceramide accumulation contributes to TNF-α-dependent ROS/RNS formation and cell death in IR (119).
At a later time point (>2 h) after TNF-α stimulation, the third branch of the death signaling cascade is activated (134) (Fig. 7, pathway C). Complex 1-derived TRADD and RIP1 bind Fas-associated protein with death domain, procaspase 8, and RIP3, which leads to the formation of complex 2 (134). Complex 2 subsequently releases activated caspase 8, which silences the RIP1/RIP3 complex and cleaves the Bcl-2 protein Bid into tBid (234). Thereafter, tBid induces mitochondrial permeabilization via oligomerization of its Bcl-2 family members Bak and Bax in the outer mitochondrial membrane (56), a process that is facilitated by CL-OOH (123), which enables the release of cytochrome c and the initiation of apoptosis (56). At this point, the three branches of the death signaling cascade converge, as the JNK/ceramide-mediated mitochondrial formation of ROS/RNS is necessary for the formation of CL-OOH and the detachment of cytochrome c from the inner mitochondrial membrane, (i.e., events that are a prerequisite for the initiation of apoptosis) (150) (section “Mitochondria”).
However, when caspase 8 is inactivated in fibroblasts, the RIP1/RIP3 complex triggers MPT-dependent necrosis rather than apoptosis via upregulation of the tricarboxylic acid cycle (231), resulting in an increased NADH/NAD+ ratio and hence, mitochondrial ROS/RNS formation (141), as well as ATP depletion due to inhibition of the adenine nucleotide translocator (196). Considering that the active site of caspase 8 is a cysteine that is susceptible to reversible oxidation and subsequent inactivation by H2O2 in vitro (16), and possibly also peroxynitrite (100), the activity of caspase 8 is probably directly dependent on the cytosolic redox status. Therefore, severely oxidatively/nitrosatively stressed cells could be more likely to undergo necrosis than apoptosis when stimulated by TNF-α because of oxidative caspase 8 inactivation.
As previously mentioned, complex 1 activates a survival pathway parallel to the death signaling cascade (78) (Fig. 7, pathway D). The survival pathway is initiated by activation of the IκB kinase complex, which subsequently phosphorylates nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor α (IκB-α) (62). Phosphorylation of IκB-α enables its dissociation from NF-κB, which thereafter translocates to the nucleus to initiate the transcription of anti-apoptotic and antioxidative proteins such as MnSOD (219), the ferritin heavy chain (156), and cellular FLICE-like inhibitory protein (c-FLIP) (133). When sufficiently activated, the effects of NF-κB impede the death signaling cascade so effectively that, without its inhibition, the transcription of antioxidative (e.g., MnSOD and ferritin) and anti-apoptotic proteins (e.g., c-FLIP) swiftly abrogates the effects of phosphorylated JNK and complex 2, respectively (133).
The activity of NF-κB is, however, inhibited by S-glutathionylation of a specific cysteine on the β-subunit of the IκB kinase complex, which impairs IκB-α phosphorylation (174) and hence its dissociation from NF-κB, as well as a cysteine on the p50 subunit of NF-κB itself, which inhibits NF-κB binding to DNA and subsequent transcription of target genes (157). Accordingly, primary hepatocytes that are incubated with ONS-inducing agents such as glucose oxidase, which generates H2O2, antimycin, which inhibits the ETC at complex III, or diamide, which depletes GSH, undergo either apoptosis or necrosis after stimulation with TNF-α, depending on the extent to which the stressors were applied (76). Inhibition of IκB-α phosphorylation and suppression of NF-κB translocation to the nucleus were proposed as causative factors of the observed effects (76). As mentioned earlier, cytosolic ONS occurs in NAFLD as a direct effect of extramitochondrial FA oxidation (1) (section “Extramitochondrial FA oxidation”) and as an indirect effect of mitochondrial GSH depletion (187), which facilitates efflux of ROS/RNS into the cytosol (77). Thus, the viability of healthy hepatocytes is not affected by TNF-α because of adequate activation and unimpaired NF-κB signaling (180), whereas oxidatively/nitrosatively stressed steatotic hepatocytes that are stimulated with TNF-α have a predilection for apoptosis (233) because of NF-κB inhibition (Fig. 8). Accordingly, apoptosis is a prominent feature of NASH (48). Moreover, when acute intensification of ONS occurs in combination with severe ATP depletion, as for instance during IR (103), additional modulation of the death signaling cascade in the form of caspase 8 inactivation and sustained JNK phosphorylation likely directs steatotic hepatocytes toward necrotic rather than apoptotic cell death following stimulation with TNF-α (Fig. 8). Considering that TNF-α is profusely secreted during IR (200), pervasive parenchymal necrosis is likely to occur in IR-subjected fatty livers.
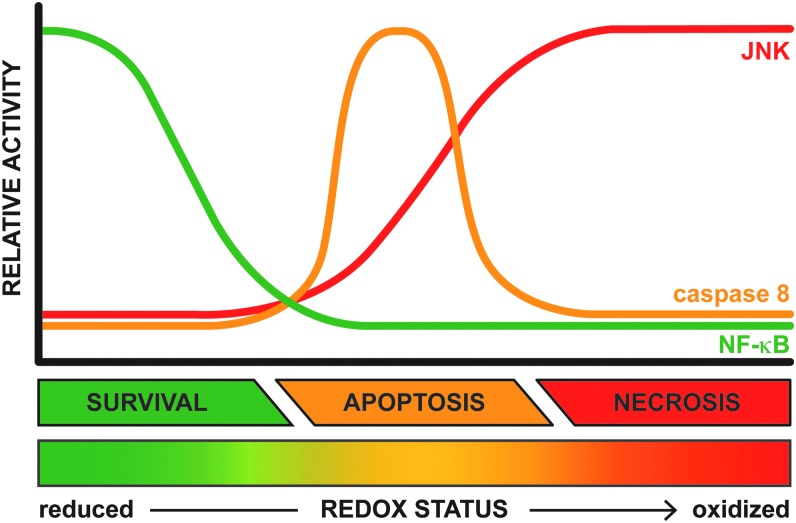
FIG. 8.
Relationship between intracellular redox status and activity of effectors of TNF-α signaling. The outcome of TNF-α signaling in hepatocytes (i.e., proliferation/survival, apoptosis, or necrosis, Fig. 7) is strongly influenced by the intracellular redox status, which can range from highly reduced (in green) to severely oxidized (in red). The graph depicts how the activity of key components of the TNF-α signaling cascade (i.e., JNK, caspase 8, and NF-κB) is influenced by the intracellular redox status. When the intracellular environment is sufficiently reduced, the effects of NF-κB will prevail, resulting in cell proliferation/survival. However, if the cellular redox status shifts toward a more oxidized state, NF-κB signaling is inhibited such that activated JNK and caspase 8 signal transduction pathways can be actualized, resulting in apoptosis. When the intracellular environment becomes severely oxidized, ROS/RNS-dependent necrotic cell death ensues because of the sustained activation of JNK and possibly caspase 8 inactivation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Taken altogether, TNF-α is an important factor in the interplay between NAFLD and IR in which ONS sensitizes hepatocytes to cell death rather than proliferation upon stimulation with TNF-α. TNF-α is a confirmed inducer of apoptosis in NAFLD, whereas the preferred mode of cell death shifts toward necrosis when fatty livers are subjected to severe ONS in combination with high levels of TNF-α and depletion of intracellular energy stores, all of which are cardinal features of IR.
Other sources of ROS/RNS in NAFLD
Extramitochondrial FA oxidation
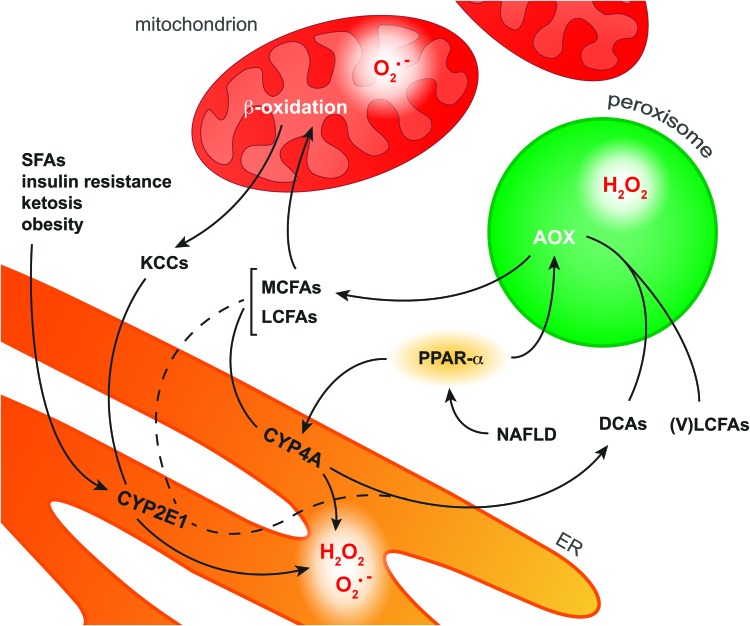
Mitochondria are supported in the oxidation of FAs by peroxisomal β- and microsomal ω-oxidation; pathways that are controlled by PPAR-α (169) (Fig. 9). Peroxisomal β-oxidation is mainly responsible for shortening very long chain FAs that cannot be directly processed by mitochondrial β-oxidation (169). Microsomal ω-oxidation, located in the ER and usually a minor contributor to FA oxidation, involves ω-hydroxylation of medium and long chain FAs (169). This reaction, which is catalyzed by CYP4A enzymes, generates dicarboxylic acids that are further metabolized by peroxisomal β-oxidation (168, 169). In addition, ketone-containing catabolites produced through mitochondrial β-oxidation as well as the FAs AA and lauric acid are processed by another member of the CYP family, namely CYP2E1 (114). In contrast to the other pathways, CYP2E1 is not regulated by PPAR-α but is induced by several other NAFLD-related phenomena such as increased levels of saturated FAs, insulin resistance, ketosis, and obesity (114, 176).
FIG. 9.
Pathways of FA oxidation in hepatocytes. In NAFLD, activation of the transcription factor PPAR-α induces upregulation of the enzymes AOX and CYP4A in peroxisomes and the ER, respectively. CYP4A catalyzes ω-oxidation of MCFAs and LCFAs as a result of which DCAs as well as O2•− and H2O2 are formed. AOX is the first enzyme in the peroxisomal β-oxidation system that converts VLCFAs and ER-derived DCAs into MCFAs, during which H2O2 is formed. MCFAs and LCFAs are transported into the mitochondria to undergo β-oxidation. However, when the mitochondrial β-oxidation system is overwhelmed with substrate, O2•− is formed along with KCCs that diffuse into the cytosol. Cytosolic KCCs are metabolized into glucose by CYP2E1, which is induced in response to various NAFLD-related stimuli such as increased levels of SFAs. In addition, CYP2E1 selectively catalyzes ω-oxidation of the FAs arachidonic acid and lauric acid (dashed line), producing O2•− and H2O2 as byproducts. The ROS produced during these processes can form secondary and tertiary ROS/RNS derivatives (section “ROS/RNS and Their Chemical Properties in the Context of NAFLD and IR”) that are capable of oxidizing biomolecules (section “Molecular Targets of ROS/RNS in NAFLD and IR”). DCA, dicarboxylic acid; KCC, ketone-containing catabolite; (V)LCFA, (very) long-chain fatty acid; MCFA, medium-chain fatty acid; PPAR-α, peroxisome proliferator-activated receptor α; SFA, saturated fatty acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Similar to the mitochondrial pathway, extramitochondrial FA oxidation also generates ROS as byproducts. All CYP enzymes possess a heme center that catalyzes the mono-oxygenation of substrate(s) using O2 and electrons derived from the oxidation of NADPH (227). However, at different stages of the catalytic cycle, oxygen can be prematurely released from the reduced complex, thereby generating O2•− or H2O2 (227). It is likely that peroxynitrite is also formed when iNOS is concomitantly induced (section “ROS/RNS and their chemical properties in the context of NAFLD and IR2”). Insofar as CYP2E1 is the most inefficient or ‘leaky’ CYP isoform, it generally exhibits the largest ROS-generating potential (64). In addition to the microsomal pathway, H2O2 is formed in the first step of peroxisomal β-oxidation, which is catalyzed by the FAD-containing enzyme AOX (168). In this reaction, AOX oxidizes fatty acyl CoA to enoyl CoA, by which the FAD subunit of AOX is reduced to FADH2. To regain catalytic activity, FADH2 is oxidized to FAD in the presence O2, reducing O2 to H2O2 (106).
Although all extramitochondrial pathways constitute a source of ROS and possibly RNS, much attention has been focused on CYP2E1 as a contributor to ONS in NAFLD (176). Not only could the ability of microsomes to oxidize lipids be strongly inhibited by a specific CYP2E1 antiserum (108), but CYP2E1-null mice fed a high-fat diet also showed significantly lower levels of MDA and protein carbonyls as well as reduced JNK phosphorylation compared with wild-type controls (1). The increased activity of CYP2E1 in NAFLD has also been confirmed in patients. CYP2E1 protein levels are increased in NASH-affected livers compared with matched controls (25). In addition, an increased CYP2E1 to total CYP protein ratio was reported for patients with NAFL (209), collectively attesting to the fact that CYP2E1 contributes to the ONS observed in NAFLD (176).
Furthermore, PPAR-α activation by non-esterified PUFAs is expected to contribute to ONS via the induction of CYP4A enzymes and peroxisomal β-oxidation (168) (Fig. 9). However, a PPAR-α agonist (Wy-14,634) actually attenuated fibrosis in mice that were fed a methionine- and choline-deficient diet, despite increased CYP4A expression (91). Moreover, mice nullizygous for AOX, the H2O2-generating enzyme in peroxisomal β-oxidation, spontaneously develop severe steatohepatitis due to the inability to metabolize very long chain FAs, which accumulate, and dicarboxylic acids (80), which damage mitochondria (168). The ability of PPAR-α to increase FA metabolism (169), which prevents dicarboxylic acid-dependent mitochondrial dysfunction as well as the accumulation of peroxidizable PUFAs (168), and to induce UCP2 expression, which reduces mitochondrial ROS/RNS formation (section “Mitochondria”), actually curtails ONS despite the concomitant upregulation of ROS-producing enzymes (91).
Collectively, these findings indicate that peroxisomal β-oxidation and CYP4A are sources of ONS in NAFLD. However, the beneficial effects of inhibiting these ROS-producing pathways are outweighed by the corollary accumulation of harmful substrates (e.g., dicarboxylic acids). Moreover, ample evidence points to PPAR-α-independent CYP2E1 as a significant contributor to cytosolic ONS in NAFLD.
Unfolded protein response
Another potential source of ROS/RNS in NAFLD is the UPR, which is triggered by proteins that are incorrectly assembled and/or processed by the ER, generally resulting from the inability of the ER to deal with perturbations in cellular homeostasis (i.e., ER stress) (86). In NAFLD, ER stress results from elevated levels of saturated FAs (213) and ONS (81), which is underpinned by the causal relationship between obesity and ER stress (151). The ER has three sensors that initiate the UPR: inositol-requiring enzyme 1α, activating transcription factor 6, and double-stranded RNA-dependent protein kinase-like ER kinase (PERK) (232). Of these three, increased phosphorylation of the PERK-regulated transcription factor eukaryotic initiation factor 2 α (eIF2α) has been observed in both NAFL- and NASH-affected patients (163), confirming that the UPR is activated in fatty livers.
The UPR likely contributes to ONS in NAFLD via three routes. First, the redox-active enzymes protein disulfide isomerase (PDI) and ERO1 constitute a source of ROS. PDI and ERO1 regulate protein tertiary structure by catalyzing the formation of intramolecular disulfide bridges (65). In the terminal step of this redox process, the FADH2 subunit on ERO1 reduces O2 to H2O2 and thus regains its oxidizing potential (68). When the ER protein folding demand rises during ER stress, ERO1 and PDI enter a perpetuating cycle of what has been described as ‘futile refolding attempts’ (181), thereby amplifying H2O2 formation (81). Second, ER stress leads to Ca2+ translocation from the ER to the mitochondrial matrix (65), causing increased mitochondrial ROS/RNS formation due to Ca2+-dependent upregulation of the tricarboxylic acid cycle (18), which can induce MPT when the rise in intramitochondrial Ca2+ is sizeable enough (155). Third, inositol-requiring enzyme 1α activates JNK (202), as has been confirmed in NASH-affected human livers (163). Given the fact that activated JNK stimulates mitochondrial ROS formation (207) these findings point not only to ER stress as a source of ROS/RNS in NAFLD but also as a direct contributor to its development, as phosphorylated JNK also induces insulin resistance (3) (section “Non-alcoholic fatty liver disease”).
Normally, PERK concomitantly mobilizes an antioxidant salvage pathway to curtail its own effects (36, 113). In NAFL- and NASH-affected patients, however, there was no increased action of activating transcription factor 4 (163), which is controlled by the PERK-eIF2α signaling axis and usually replenishes the antioxidant reserves, (e.g., by increasing the cellular GSH pool) (113). Considering that the ER typically consumes the newly formed GSH to reduce the disulfide bonds of incorrectly assembled proteins (37), the failure to upscale GSH synthesis not only impacts the cellular antioxidative capacity but also further increases ER stress and ROS production. Although PERK is able to augment the antioxidative capacity via activation of the antioxidant response gene nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (36), which has been confirmed in NAFLD-affected human livers (79), its beneficial effects are likely insufficient to effectively combat the concomitant ONS (79, 107).
In conclusion, the UPR contributes to cytosolic as well as mitochondrial ROS/RNS production, while the concurrent induction of antioxidative defenses either fails or is inadequate in countering ONS.
Concluding Remarks
The exuberant formation of ROS/RNS is a cardinal feature of NAFLD as well as IR and, although the underlying pathological processes differ, several ROS/RNS-mediated effects are common in both conditions. At the molecular level, H2O2 and peroxynitrite are important sources of toxic derivative radicals, particularly in the presence of free Fe2+, which initiate the (per)oxidation of lipids and proteins that leads to depletion of the intracellular antioxidative capacity, functional impairment of biomolecules, and the formation of toxic metabolites.
At a cellular level, ONS sensitizes steatotic hepatocytes to apoptotic and necrotic cell death. UCP2-dependent ATP depletion and ONS render the mitochondria of fatty hepatocytes prone to MPT, which becomes especially relevant during IR. Consequently, DAMP release, KC activation, and leukocyte chemotaxis will be more prolific, prompting severe extracellular ONS and TNF-α release that ultimately culminate in widespread parenchymal necrosis.
ROS/RNS predispose the fatty liver to more severe IR injury via a plethora of damaging effects. Notably, these effects emanate from specific (bio)chemical mechanisms that can serve as targets for interventions that are aimed at reducing ONS-mediated liver injury. Much research so far has been devoted to the role of ONS and mitochondrial uncoupling in either NAFLD or IR, whereas only a few papers address the interplay between both conditions (42, 45, 118, 127, 147, 187). Therefore, more research on this topic is warranted given the increasing prevalence of NAFLD and corollary rise in surgical interventions in patients with fatty livers.
Abbreviations Used
- 4-HNE
4-hydroxynonenal
- Δp
protonmotive force
- AA
arachidonic acid
- ANT
adenine nucleotide translocator
- AOX
fatty acyl-CoA oxidase
- ASK1
apoptosis signal-regulating kinase 1
- c-FLIP
cellular FLICE-like inhibitory protein
- CL-OOH
cardiolipin-hydroperoxide
- CYP
cytochrome P450
- DAMP
damage-associated molecular pattern
- DCA
dicarboxylic acid
- DHA
docosahexanoic acid
- eIF2α
eukaryotic initiation factor 2 α
- EPA
eicosapentanoic acid
- ER
endoplasmic reticulum
- ERO1
ER oxidoreductin 1
- ETC
electron transport chain
- FADD
Fas-associated protein with death domain
- (F)FA
(free) fatty acid
- GGD3
ganglioside GD3
- GSH/GSSG
glutathione/glutathione disulfide
- IKK
IκB kinase
- IL-1β
interleukin 1β
- IMM
inner mitochondrial membrane
- (i)NOS
(inducible) nitric oxide synthase
- IR
ischemia-reperfusion
- IRP1
iron regulatory protein 1
- IκB-α
nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor α
- JNK
c-Jun N-terminal kinase
- KC
Kupffer cell
- KCC
ketone-containing catabolite
- LA
linoleic acid
- LIP
labile iron pool
- MCFA
medium-chain fatty acid
- MCR
metal-catalyzed reaction
- MDA
malondialdehyde
- MnSOD
manganese-superoxide dismutase
- MP
mevalonate pathway
- MPT
mitochondrial permeability transition
- NAFL
non-alcoholic fatty liver
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- OMM
outer mitochondrial membrane
- ONS
oxidative/nitrosative stress
- PDI
protein disulfide isomerase
- PERK
double-stranded RNA-dependent protein kinase-like ER kinase
- PPAR-α
peroxisome proliferator-activated receptor α
- PUFA
polyunsaturated fatty acid
- RIP1/3
receptor-interacting kinase 1/3
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SFA
saturated fatty acid
- SOD
superoxide dismutase
- TCA
tricarboxylic acid
- TNFR1
TNF-α receptor-1
- TNF-α
tumor necrosis factor alpha
- TRADD
TNF-α receptor-associated protein with death domain
- TRAF2
TNF-α receptor-associated factor 2
- UCP2
uncoupling protein 2
- UPR
unfolded protein response
- UQ/UQH2
ubiquinone/ubiquinol, also referred to as coenzyme Q10
- (V)LCFA
(very) long-chain fatty acid
Author Disclosure Statement
R.F.v.G. is supported by a PhD Scholarship from the Academic Medical Center (University of Amsterdam), and M.H. is supported by grants from the Dutch Anti-Cancer Foundation (Stichting Nationaal Fonds Tegen Kanker) in Amsterdam, the Phospholipid Research Center in Heidelberg, the Nijbakker-Morra Foundation in Leiden, and Stichting Technologische Wetenschap (STW). T.M.v.G. and M.J.R. state that no competing financial interests exist.
References
- 1.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, and Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced nonalcoholic steatohepatitis. J Hepatol 57: 860–866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afri M, Gottlieb HE, and Frimer AA. Superoxide organic chemistry within the liposomal bilayer, part II: a correlation between location and chemistry. Free Radic Biol Med 32: 605–618, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Aguirre V, Uchida T, Yenush L, Davis R, and White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, Jalali P, Kandasamy T, Prayitno N, Sherman M, Guindi M, Ma DW, and Heathcote JE. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol 48: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Alvarez B, Demicheli V, Durán R, Trujillo M, Cerveñansky C, Freeman BA, and Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med 37: 813–822, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez B. and Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25: 295–311, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Amersi F, Dulkanchainun T, Nelson SK, Farmer DG, Kato H, Zaky J, Melinek J, Shaw GD, Kupiec-Weglinski JW, and Horwitz LD. A novel iron chelator in combination with a P-selectin antagonist prevents ischemia/reperfusion injury in a rat liver model. Transplantation 71: 112–118, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Andriopoulos B, Hegedüsch S, Mangin J, Riedel HD, Hebling U, Wang J, Pantopoulos K, and Mueller S. Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the iron regulatory protein/iron-responsive element network. J Biol Chem 282: 20301–20308, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221–1231, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Antosiewicz J, Ziolkowski W, Kaczor JJ, and Herman-Antosiewicz A. Tumor necrosis factor-alpha-induced reactive oxygen species formation is mediated by JNK1-dependent ferritin degradation and elevation of labile iron pool. Free Radic Biol Med 43: 265–270, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Antunes F. and Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 475: 121–126, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, and De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med 32: 841–859, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Änggård E. Nitric oxide: mediator, murderer, and medicine. Lancet 343: 1199–1206, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 51: 212–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E, Tyagi S, Otterbein LE, Brouard S, Tobiasch E, Bach FH, Kupiec-Weglinski JW, and Soares MP. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J 17: 1724–1726, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Borutaite V. and Brown GC. Caspases are reversibly inactivated by hydrogen peroxide. FEBS Lett 500: 114–118, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion 3: 187–204, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Brookes PS, Yoon Y, Robotham JL, Anders MW, and Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Brunt EM. Pathology of fatty liver disease. Mol Pathol 20: S40–S48, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Buerk DG, Lamkin-Kennard K, and Jaron D. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med 34: 1488–1503, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, and Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Caraceni P, Ryu HS, van Thiel DH, and Borle AB. Source of oxygen free radicals produced by rat hepatocytes during postanoxic reoxygenation. Biochim Biophys Acta 1268: 249–254, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Carini R, Bellomo G, Dianzani MU, and Albano E. Evidence for a sodium-dependent calcium influx in isolated rat hepatocytes undergoing ATP depletion. Biochem Biophys Res Commun 202: 360–366, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Castello PR, David PS, McClure T, Crook Z, and Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab 3: 277–287, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, and Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 37: 544–550, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, and Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Chavin KD, Yang SQ, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, and Huang CC. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 274: 5692–5700, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Cheng G, Cao Z, Xu X, Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Schenker S, Frosto TA, and Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE). Role of HNE adduct formation with the enzyme subunits. Biochim Biophys Acta 1380: 336–344, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Chevallet M, Wagner E, Luche S, van Dorsselaer A, Leize-Wagner E, and Rabilloud T. Regeneration of peroxiredoxins during recovery after oxidative stress. J Biol Chem 278: 37146–37153, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Coddington JW, Hurst JK, and Lymar SV. Hydroxyl radical formation during peroxynitrous acid decomposition. J Am Chem Soc 121: 2438–2443, 1999 [Google Scholar]
- 32.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr.Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest 85: 1936–1943, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copple BL, Jaeschke H, and Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis 30: 195–204, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, and Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 282: 1659–1664, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, and Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54: 133–144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullinan SB. and Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 279: 20108–20117, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Cuozzo JW. and Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nat Cell Biol 1: 130–135, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Dallner G. and Sindelar PJ. Regulation of ubiquinone metabolism. Free Radic Biol Med 29: 285–294, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie 83: 301–310, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Day CP. and James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 114: 842–845, 1998 [DOI] [PubMed] [Google Scholar]
- 41.dela Peña A, Leclercq IA, Williams J, and Farrell GC. NADPH oxidase is not an essential mediator of oxidative stress or liver injury in murine MCD diet-induced steatohepatitis. J Hepatol 46: 304–313, 2007 [DOI] [PubMed] [Google Scholar]
- 42.El-Badry AM, Jang JH, Elsherbiny A, Contaldo C, Tian Y, Raptis DA, Laczko E, Moritz W, Graf R, and Clavien PA. Chemical composition of hepatic lipids mediates reperfusion injury of the macrosteatotic mouse liver through thromboxane A(2). J Hepatol 55: 1291–1299, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Ernster L. and Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271: 195–204, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Evans HM, Emerson OH, and Emerson GA. The isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E. J Biol Chem 113: 319–332, 1936 [Google Scholar]
- 45.Evans ZP, Ellett JD, Schmidt MG, Schnellmann RG, and Chavin KD. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem 283: 8573–8579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrell GC, Wong VW, and Chitturi S. NAFLD in Asia-as common and important as in the West. Nat Rev Gastroenterol Hepatol 10: 307–318, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Feldstein AE. and Bailey SM. Emerging role of redox dysregulation in alcoholic and nonalcoholic fatty liver disease. Antioxid Redox Signal 15: 421–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, and Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans 65: 899–910, 1894 [Google Scholar]
- 50.Ferrington DA. and Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett 578: 217–223, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Ford E, Hughes MN, and Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic Biol Med 32: 1314–1323, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Ford ES, Giles WH, and Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA 287: 356–359, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Forsmark-Andrée P, Lee L, Dallner G, and Ernster L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic Biol Med 22: 391–400, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Foster MW, McMahon TJ, and Stamler JS. S-nitrosylation in health and disease. Trends Mol Med 9: 160–168, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Perez C, Roy SS, Naghdi S, Lin X, Davies E, and Hajnóczky G. Bid-induced mitochondrial membrane permeabilization waves propagated by local reactive oxygen species (ROS) signaling. Proc Natl Acad Sci U S A 109: 4497–4502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.García-Monzón C, Martín-Pérez E, Iacono OL, Fernández-Bermejo M, Majano PL, Apolinario A, Larrañaga E, and Moreno-Otero R. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol 33: 716–724, 2000 [DOI] [PubMed] [Google Scholar]
- 58.García-Ruiz C, Colell A, Marí M, Morales A, Calvo M, Enrich C, and Fernández-Checa JC. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest 111: 197–208, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrison WM. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem Rev 87: 381–398, 1987 [Google Scholar]
- 60.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, and Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A 98: 11961–11966, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geller DA, Lowenstein CJ, Shapiro RA, Nussler AK, Di Silvio M, Wang SC, Nakayama DK, Simmons RL, Snyder SH, and Billiar TR. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A 90: 3491–3495, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh S. and Karin M. Missing pieces in the NF-κB puzzle. Cell 109: S81–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Giorgio M, Trinei M, Migliaccio E, and Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8: 722–728, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Gorsky LD, Koop DR, and Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem 259: 6812–6817, 1984 [PubMed] [Google Scholar]
- 65.Görlach A, Klappa P, and Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 8: 1391–1418, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Graaf de W, Heger M, Spruijt O, Maas A, de Bruin K, Hoekstra R, Bennink RJ, and van Gulik TM. Quantitative assessment of liver function after ischemia-reperfusion injury and partial hepatectomy in rats. J Surg Res 172: 85–94, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Griendling KK. and FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation 108: 1912–1916, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, and Fass D. Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci U S A 103: 299–304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gudz TI, Tserng KY, and Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem 272: 24154–24158, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Gujral JS, Bucci TJ, Farhood A, and Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology 33: 397–405, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Guo Z, Shao L, Zheng L, Du Q, Li P, John B, and Geller DA. miRNA-939 regulates human inducible nitric oxide synthase posttranscriptional gene expression in human hepatocytes. Proc Natl Acad Sci U S A 109: 5826–5831, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halestrap AP, Woodfield KY, and Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem 272: 3346–5334, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 35: 1147–1150, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Halliwell B. and Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142: 231–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, and Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283: 13565–13577, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han D, Hanawa N, Saberi B, and Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med 41: 627–639, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Han D, Hanawa N, Saberi B, and Kaplowitz N. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol 291: G1–G7, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Han D, Ybanez MD, Ahmadi S, Yeh K, and Kaplowitz N. Redox regulation of tumor necrosis factor signaling. Antioxid Redox Signal 11: 2245–2263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardwick RN, Fisher CD, Canet MJ, Lake AD, and Cherrington NJ. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38: 2293–2301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, Gonzalez FJ, Yeldandi AV, Rao MS, and Reddy JK. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J Biol Chem 274: 19228–19236, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Haynes CM, Titus EA, and Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15: 767–776, 2004 [DOI] [PubMed] [Google Scholar]
- 82.He S, Rehman H, Wright GL, and Zhong Z. Inhibition of inducible nitric oxide synthase prevents mitochondrial damage and improves survival of steatotic partial liver grafts. Transplantation 89: 291–298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, and Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Hochstein P. and Utley H. Hydrogen peroxide detoxication by glutathione peroxidase and catalase in rat liver homogenates. Mol Pharmacol 4: 574–579, 1968 [PubMed] [Google Scholar]
- 85.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Houglum K, Filip M, Witztum JL, and Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest 86: 1991–1998, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Houstis N, Rosen ED, and Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Huang X, Dai J, Fournier J, Ali AM, Zhang Q, and Frenkel K. Ferrous ion autoxidation and its chelation in iron-loaded human liver HepG2 cells. Free Radic Biol Med 32: 84–92, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol 30: 535–560, 1990 [DOI] [PubMed] [Google Scholar]
- 91.Ip E, Farrell G, Hall P, Robertson G, and Leclercq I. Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 39: 1286–1296, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, and Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci 65: 166–176, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Jaeschke H. and Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125: 1246–1257, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Jaeschke H. and Woolbright BL. Current strategies to minimize hepatic ischemia—reperfusion injury by targeting reactive oxygen species. Transplant Rev 26: 103–114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jump DB, Botolin D, Wang Y, Xu J, Christian B, and Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr 135: 2503–2506, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, and Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Kakhlon O. and Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med 33: 1037–1046, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Kamata H, Honda S, Maeda S, Chang L, Hirata H, and Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661, 2005 [DOI] [PubMed] [Google Scholar]
- 99.Kawaguchi T, Noji S, Uda T, Nakashima Y, Takeyasu A, Kawai Y, Takagi H, Tohyama M, and Taniguchi N. A monoclonal antibody against COOH-terminal peptide of human liver manganese superoxide dismutase. J Biol Chem 264: 5762–5767, 1989 [PubMed] [Google Scholar]
- 100.Kettenhofen NJ. and Wood MJ. Formation, reactivity, and detection of protein sulfenic acids. Chem Res Toxicol 23: 1633–1646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JS, Wang JH, and Lemasters JJ. Mitochondrial permeability transition in rat hepatocytes after anoxia/reoxygenation: role of Ca2+-dependent mitochondrial formation of reactive oxygen species. Am J Physiol Gastrointest Liver Physiol 302: G723–G731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kissner R, Nauser T, Bugnon P, Lye PG, and Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol 10: 1285–1292, 1997 [DOI] [PubMed] [Google Scholar]
- 103.Kloek J, Marechal X, Roelofsen J, Houtkooper R, van Kuilenburg A, Kulik W, Bezemer R, Neviere R, van Gulik TM, and Heger M. Cholestasis is associated with hepatic microvascular dysfunction and aberrant energy metabolism before and during ischemia-reperfusion. Antioxid Redox Signal 17: 1109–1123, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Kono H. and Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lambert AJ. and Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382: 511–517, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lazarow PB. and De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A 73: 2043–2046, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leclercq IA. Pro-oxidants or anti-oxidant defenses? Which one to blame in non-alcoholic steatohepatitis pathogenesis? J Gastroenterol Hepatol 27: 1651–1653, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, and Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 105: 1067–1075, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee S, Gura KM, and Puder M. Omega-3 fatty acids and liver disease. Hepatology 45: 841–845, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Lemasters JJ, Theruvath TP, Zhong Z, and Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levine RL, Moskovitz J, and Stadtman ER. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life 50: 301–307, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene 25: 3834–3847, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Lewerenz J. and Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 284: 1106–1115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 77: 517–544, 1997 [DOI] [PubMed] [Google Scholar]
- 115.Lillie RD. and Fullmer HMJA. Histopathologic Technic and Practical Histochemistry. New York: McGraw-Hill, 1976 [Google Scholar]
- 116.Li Q, Tomcik K, Zhang S, Puchowicz MA, and Zhang GF. Dietary regulation of catabolic disposal of 4-hydroxynonenal analogs in rat liver. Free Radic Biol Med 52: 1043–1053, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y, Mouche S, Sajic T, Veyrat-Durebex C, Supale R, Pierroz D, Ferrari S, Negro F, Hasler U, Feraille E, Moll S, Meda P, Deffert C, Montet X, Krause KH, and Szanto I. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int J Obes (Lond) 36: 1503–1513, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Llacuna L, Fernández A, Montfort CV, Matías N, Martínez L, Caballero F, Rimola A, Elena M, Morales A, Fernández-Checa JC, and García-Ruiz C. Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia-reperfusion injury. J Hepatol 54: 1002–1010, 2011 [DOI] [PubMed] [Google Scholar]
- 119.Llacuna L, Marí M, Garcia-Ruiz C, Fernandez-Checa JC, and Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology 44: 561–572, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Malaguarnera L, Madeddu R, Palio E, Arena N, and Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol 42: 585–591, 2005 [DOI] [PubMed] [Google Scholar]
- 121.Mantena SK, King AL, Andringa KK, Eccleston HB, and Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 44: 1259–1272, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, and Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 417: 183–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marí M, Colell A, Morales A, Caballero F, Moles A, Fernández A, Terrones O, Basañez G, Antonsson B, García-Ruiz C, and Fernández-Checa JC. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-κB activation. Gastroenterology 134: 1507–1520, 2008 [DOI] [PubMed] [Google Scholar]
- 124.Marí M, Morales A, Colell A, García-Ruiz C, and Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 11: 2685–2700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marnett LJ. Lipid peroxidation—DNA damage by malondialdehyde. Mutat Res 424: 83–95, 1999 [DOI] [PubMed] [Google Scholar]
- 126.Maroz A, Anderson RF, Smith RA, and Murphy MP. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radic Biol Med 46: 105–109, 2009 [DOI] [PubMed] [Google Scholar]
- 127.Marsman HA, Heger M, Kloek JJ, Nienhuis SL, ten Kate FJ, and van Gulik TM. Omega-3 fatty acids reduce hepatic steatosis and consequently attenuate ischemia-reperfusion injury following partial hepatectomy in rats. Digest Liver Dis 43: 984–990, 2011 [DOI] [PubMed] [Google Scholar]
- 128.McCormack L, Dutkowski P, El-Badry AM, and Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol 54: 1055–1062, 2011 [DOI] [PubMed] [Google Scholar]
- 129.McStay GP, Clarke SJ, and Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J 367: 541–548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Medinas DB, Cerchiaro G, Trindade DF, and Augusto O. The carbonate radical and related oxidants derived from bicarbonate buffer. IUBMB Life 59: 255–262, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Meijer de VE, Kalish BT, Puder M, and Ijzermans JN. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 97: 1331–1339, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Meli R, Mattace Raso G, Irace C, Simeoli R, di Pascale A, Paciello O, Pagano TB, Calignano A, Colonna A, and Santamaria R. High fat diet induces liver steatosis and early dysregulation of iron metabolism in rats. PLoS One 8: e66570, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Micheau O, Lens S, Gaide O, Alevizopoulos K, and Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 21: 5299–5305, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Micheau O. and Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114: 181–190, 2003 [DOI] [PubMed] [Google Scholar]
- 135.Miele L, Grieco A, Armuzzi A, Candelli M, Forgione A, Gasbarrini A, and Gasbarrini G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol 98: 2335–2336, 2003 [DOI] [PubMed] [Google Scholar]
- 136.Minicis de S, Seki E, Paik YH, Osterreicher CH, Kodama Y, Kluwe J, Torozzi L, Miyai K, Benedetti A, Schwabe RF, and Brenner DA. Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology 52: 1420–1430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148, 1961 [DOI] [PubMed] [Google Scholar]
- 138.Montfort CV, Matias N, Fernandez A, Fucho R, de Rosa LCL, Martinez-Chantar ML, Mato JM, Machida K, Tsukamoto H, Murphy MP, Mansouri A, Kaplowitz N, Garcia-Ruiz C, and Fernandez-Checa JC. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scavenging in steatohepatitis. J Hepatol 57: 852–859, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moschen AR, Kaser S, and Tilg H. Non-alcoholic steatohepatitis: a microbiota-driven disease. Trends Endocrinol Metab 24: 537–545 [DOI] [PubMed] [Google Scholar]
- 140.Mukhopadhyay P, Horváth B, Zsengellėr Z, Bátkai S, Cao Z, Kechrid M, Holovac E, Erdėlyi K, Tanchian G, Liaudet L, Stillman IE, Joseph J, Kalyanaraman B, and Pacher P. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia–reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med 53: 1123–1138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy MP, Echtay KS, Blaikie FH, Asin-Cayuela J, Cochemé HM, Green K, Buckingham JA, Taylor ER, Hurrell F, Hughes G, Miwa S, Cooper CE, Svistunenko DA, Smith RAJ, and Brand MD. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation. J Biol Chem 278: 48534–48545, 2003 [DOI] [PubMed] [Google Scholar]
- 143.Nabeshima A, Yamada S, Guo X, Tanimoto A, Wang KY, Shimajiri S, Kimura S, Tasaki T, Noguchi H, Kitada S, Watanabe T, Fujii J, Kohno K, and Sasaguri Y. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid Redox Signal 19: 1983–1998, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nair SP, Chacko V, Arnold C, and Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol 98: 466–470, 2003 [DOI] [PubMed] [Google Scholar]
- 145.Nakatani T, Tsuboyama-Kasaoka N, Takahashi M, Miura S, and Ezaki O. Mechanism for peroxisome proliferator-activated receptor-alpha activator-induced up-regulation of UCP2 mRNA in rodent hepatocytes. J Biol Chem 277: 9562–9569, 2002 [DOI] [PubMed] [Google Scholar]
- 146.Nappi AJ. and Vass E. Comparative studies of enhanced iron-mediated production of hydroxyl radical by glutathione, cysteine, ascorbic acid, and selected catechols. Biochim Biophys Acta 1336: 295–302, 1997 [DOI] [PubMed] [Google Scholar]
- 147.Nardo B, Caraceni P, Pasini P, Domenicali M, Catena F, Cavallari G, Santoni B, Maiolini E, Grattagliano I, Vendemiale G, Trevisani F, Roda A, Bernardi M, and Cavallari A. Increased generation of reactive oxygen species in isolated rat fatty liver during postischemic reoxygenation. Transplantation 71: 1816–1820, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Nieminen AL, Byrne AM, Herman B, and Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol 272: C1286–C1294, 1997 [DOI] [PubMed] [Google Scholar]
- 149.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47: 469–484, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, and Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A 99: 1259–1263, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, and Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 152.Pagadala M, Kasumov T, McCullough AJ, Zein NN, and Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab 23: 365–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paugam-Burtz C, Janny S, de Lefosse D, Dahmani S, Dondero F, Mantz J, and Belghiti J. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg 249: 124–128, 2009 [DOI] [PubMed] [Google Scholar]
- 154.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 22: S20–S27, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Petrosillo G, Ruggiero FM, Pistolese M, and Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem 279: 53103–53108, 2004 [DOI] [PubMed] [Google Scholar]
- 156.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, de Smaele E, Cong R, and Beaumont C. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 119: 529–542, 2004 [DOI] [PubMed] [Google Scholar]
- 157.Pineda-Molina E, Klatt P, Vázquez J, Marina A, García de Lacoba M, Pérez-Sala D, and Lamas S. Glutathionylation of the p50 Subunit of NF-κB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40: 14134–14142, 2001 [DOI] [PubMed] [Google Scholar]
- 158.Poli G, Schaur RJ, Siems WG, and Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev 28: 569–631, 2007 [DOI] [PubMed] [Google Scholar]
- 159.Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 48: 541–549, 1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pryor WA, Lightsey JW, and Church DF. Reaction of nitrogen dioxide with alkenes and polyunsaturated fatty acids: addition and hydrogen-abstraction mechanisms. J Am Chem Soc 104: 6685–6692, 1982 [Google Scholar]
- 161.Pryor W, Stanley J, and Blair E. Autoxidation of polyunsaturated fatty acids: II. A suggested mechanism for the formation of TBA-reactive materials from prostaglandin-like endoperoxides. Lipids 11: 370–379, 1976 [DOI] [PubMed] [Google Scholar]
- 162.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, and Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46: 1081–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 163.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, and Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008 [DOI] [PubMed] [Google Scholar]
- 164.Radi R, Cassina A, Hodara R, Quijano C, and Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33: 1451–1464, 2002 [DOI] [PubMed] [Google Scholar]
- 165.Radi R, Denicola A, Alvarez B, Ferrer-Sueta G, and Rubbo H. The biological chemistry of peroxynitrite. In: Nitric Oxide, edited by Ignarro LJ. San Diego, CA: Academic Press, 2000, pp. 57–82 [Google Scholar]
- 166.Radi R, Peluffo G, Alvarez MN, Naviliat M, and Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med 30: 463–488, 2001 [DOI] [PubMed] [Google Scholar]
- 167.Raedschelders K, Ansley DM, and Chen DD. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther 133: 230–255, 2012 [DOI] [PubMed] [Google Scholar]
- 168.Reddy JK. and Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr 21: 193–230, 2001 [DOI] [PubMed] [Google Scholar]
- 169.Reddy JK. and Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 290: G852–G858, 2006 [DOI] [PubMed] [Google Scholar]
- 170.Reddy SK, Marsh JW, Varley PR, Mock BK, Chopra KB, Geller DA, and Tsung A. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case–control study. Hepatology 56: 2221–2230, 2012 [DOI] [PubMed] [Google Scholar]
- 171.Reis A. and Spickett CM. Chemistry of phospholipid oxidation. Biochim Biophys Acta 1818: 2374–2387, 2012 [DOI] [PubMed] [Google Scholar]
- 172.Rensen SS, Bieghs V, Xanthoulea S, Arfianti E, Bakker JA, Shiri-Sverdlov R, Hofker MH, Greve JW, and Buurman WA. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS One 7: e52411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rensen SS, Slaats Y, Nijhuis J, Jans A, Bieghs V, Driessen A, Malle E, Greve JW, and Buurman WA. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol 175: 1473–1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, and Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A 103: 13086–13091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, and Testi R. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J 14: 2047–2054, 2000 [DOI] [PubMed] [Google Scholar]
- 176.Robertson G, Leclercq I, and Farrell GC. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol 281: G1135–G1139, 2001 [DOI] [PubMed] [Google Scholar]
- 177.Rolo AP, Teodoro JS, and Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52: 59–69, 2012 [DOI] [PubMed] [Google Scholar]
- 178.Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, and Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab 10: 491–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, and Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, and Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22: 3898–3909, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Santos CXC, Tanaka LY, Wosniak J, Jr, and Laurindo FRM. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 182.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, and Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 183.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, and Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schneider C, Tallman KA, Porter NA, and Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. J Biol Chem 276: 20831–20838, 2001 [DOI] [PubMed] [Google Scholar]
- 185.Seki E, Brenner DA, and Karin M. A Liver full of JNK: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143: 307–320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seki S, Kitada T, and Sakaguchi H. Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res 33: 132–134, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, and Altomare E. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 57: 957–965, 2008 [DOI] [PubMed] [Google Scholar]
- 188.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, and Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh RJ, Goss SPA, Joseph J, and Kalyanaraman B. Nitration of γ-tocopherol and oxidation of α-tocopherol by copper-zinc superoxide dismutase/H2O2/NO2−: role of nitrogen dioxide free radical. Proc Natl Acad Sci U S A 95: 12912–12917, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Spruss A, Kanuri G, Uebel K, Bischoff SC, and Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid Redox Signal 14: 2121–2135, 2011 [DOI] [PubMed] [Google Scholar]
- 191.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, and Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50: 1094–1104, 2009 [DOI] [PubMed] [Google Scholar]
- 192.Stadtman ER. and Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 193.Surmeli NB, Litterman NK, Miller AF, and Groves JT. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J Am Chem Soc 132: 17174–17185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Szabó C, Ischiropoulos H, and Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007 [DOI] [PubMed] [Google Scholar]
- 195.Tacchini T, Recalcati R, Bernelli-Zazzera BZ, and Cairo C. Induction of ferritin synthesis in ischemic-reperfused rat liver: analysis of the molecular mechanisms. Gastroenterology 113: 946–953, 1997 [DOI] [PubMed] [Google Scholar]
- 196.Temkin V, Huang Q, Liu H, Osada H, and Pope RM. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol 26: 2215–2225, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thariat J, Collin F, Marchetti C, Ahmed-Adrar NS, Vitrac H, Jore D, and Gardes-Albert M. Marked difference in cytochrome c oxidation mediated by HO(*) and/or O(2)(*−) free radicals in vitro. Biochimie 90: 1442–1451, 2008 [DOI] [PubMed] [Google Scholar]
- 198.Tilg H. and Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 343: 1467–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 199.Tomita K. Tumour necrosis factor α signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 55: 415–424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Uehara T, Bennett B, Sakata ST, Satoh Y, Bilter GK, Westwick JK, and Brenner DA. JNK mediates hepatic ischemia reperfusion injury. J Hepatol 42: 850–859, 2005 [DOI] [PubMed] [Google Scholar]
- 201.Uehara T, Xi Peng X, Bennett B, Satoh Y, Friedman G, Currin R, Brenner DA, and Lemasters J. c-Jun N-terminal kinase mediates hepatic injury after rat liver transplantation. Transplantation 78: 324–332, 2004 [DOI] [PubMed] [Google Scholar]
- 202.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, and Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 203.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G, and Fargion S. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 138: 905–912, 2010 [DOI] [PubMed] [Google Scholar]
- 204.Vandenabeele P, Galluzzi L, van den Berghe T, and Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11: 700–714, 2010 [DOI] [PubMed] [Google Scholar]
- 205.van Golen RF, van Gulik TM, and Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med 52: 1382–1402, 2012 [DOI] [PubMed] [Google Scholar]
- 206.van Golen RF, van Gulik TM, and Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev 23: 69–84, 2012 [DOI] [PubMed] [Google Scholar]
- 207.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, and Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev 18: 2905–2915, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Veteläinen R, van Vliet A, Gouma DJ, and van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg 245: 20–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, Varela N, Contreras J, Lazarte R, and Csendes A. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci 106: 261–268, 2004 [DOI] [PubMed] [Google Scholar]
- 210.van der Vliet A, Eiserich JP, Kaur H, Cross CE, and Halliwell B. Nitrotyrosine as biomarker for reactive nitrogen species. In: Methods in Enzymology, Volume 269, edited by Packer L. San Diego, CA: Academic Press, 1996, pp. 175–184 [DOI] [PubMed] [Google Scholar]
- 211.Vollmar B. and Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89: 1269–1339, 2009 [DOI] [PubMed] [Google Scholar]
- 212.von Bernhardi R. and Eugenín J. Alzheimer's disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid Redox Signal 16: 974–1031, 2012 [DOI] [PubMed] [Google Scholar]
- 213.Wang D, Wei Y, and Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147: 943–951, 2006 [DOI] [PubMed] [Google Scholar]
- 214.Wang J. and Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 434: 365–381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wardman P. and Candeias LP. Fenton chemistry: an introduction. Radiat Res 145: 523–531, 1996 [PubMed] [Google Scholar]
- 216.Welch KD, Davis TZ, Eden MEV, and Aust SD. Deleterious iron-mediated oxidation of biomolecules. Free Radic Biol Med 32: 577–583, 2002 [DOI] [PubMed] [Google Scholar]
- 217.Wellen KE. and Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wiberg E, Holleman AF, and Wiberg N. (Eds). Holleman-Wiberg's Inorganic Chemistry. San Diego, CA: Academic Press, 2001, p. 1444 [Google Scholar]
- 219.Wong GH, Elwell JH, Oberley LW, and Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell 58: 923–931, 1989 [DOI] [PubMed] [Google Scholar]
- 220.Woo HA, Jeong W, Chang TS, Park KJ, Park SJ, Yang JS, and Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J Biol Chem 280: 3125–3128, 2005 [DOI] [PubMed] [Google Scholar]
- 221.Wood ZA, Schroder E, Robin Harris J, and Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40, 2003 [DOI] [PubMed] [Google Scholar]
- 222.Xia ZF, Horton JW, Zhao PY, Babcock EE, Sherry AD, and Malloy CR. Effects of ischemia on intracellular sodium and phosphates in the in vivo rat liver. J Appl Physiol 81: 1395–1403, 1996 [DOI] [PubMed] [Google Scholar]
- 223.Yang KS, Kang SW, Woo HA, Hwang SC, Chae HZ, Kim K, and Rhee SG. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J Biol Chem 277: 38029–38036, 2002 [DOI] [PubMed] [Google Scholar]
- 224.Yang M, Camara AK, Wakim BT, Zhou Y, Gadicherla AK, Kwok WM, and Stowe DF. Tyrosine nitration of voltage-dependent anion channels in cardiac ischemia-reperfusion: reduction by peroxynitrite scavenging. Biochim Biophys Acta 1817: 2049–2059, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, Musabak U, Erbil MK, Karaeren N, and Dagalp K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 100: 850–855, 2005 [DOI] [PubMed] [Google Scholar]
- 226.Yin H, Xu L, and Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 111: 5944–5972, 2011 [DOI] [PubMed] [Google Scholar]
- 227.Zangar RC, Davydov DR, and Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199: 316–331, 2004 [DOI] [PubMed] [Google Scholar]
- 228.Zaouali MA, Bardag-Gorce F, Carbonell T, Oliva J, Pantazi E, Bejaoui M, Abdennebi HB, Rimola A, and Roselló-Catafau J. Proteasome inhibitors protect the steatotic and non-steatotic liver graft against cold ischemia reperfusion injury. Exp Mol Pathol 94: 352–359, 2013 [DOI] [PubMed] [Google Scholar]
- 229.Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, and Omary MB. From Mallory to Mallory–Denk bodies: what, how and why? Exp Cell Res 313: 2033–2049, 2007 [DOI] [PubMed] [Google Scholar]
- 230.Zelko IN, Mariani TJ, and Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33: 337–349, 2002 [DOI] [PubMed] [Google Scholar]
- 231.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, and Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336, 2009 [DOI] [PubMed] [Google Scholar]
- 232.Zhang K. and Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang W, Kudo H, Kawai K, Fujisaka S, Usui I, Sugiyama T, Tsukada K, Chen N, and Takahara T. Tumor necrosis factor-α accelerates apoptosis of steatotic hepatocytes from a murine model of non-alcoholic fatty liver disease. Biochem Biophys Res Commun 391: 1731–1736, 2010 [DOI] [PubMed] [Google Scholar]
- 234.Zhao Y, Ding WX, Qian T, Watkins S, Lemasters JJ, and Yin XM. Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology 125: 854–867, 2003 [DOI] [PubMed] [Google Scholar]