Abstract
Significance: The extracellular matrix (ECM) is a dynamic microenvironment that undergoes continuous remodeling, particularly during injury and wound healing. Chronic liver injury of many different etiologies such as viral hepatitis, alcohol abuse, drug-induced liver injury, obesity and insulin resistance, metabolic disorders, and autoimmune disease is characterized by excessive deposition of ECM proteins in response to persistent liver damage. Critical Issues: This review describes the main collagenous and noncollagenous components from the ECM that play a significant role in pathological matrix deposition during liver disease. We define how increased myofibroblasts (MF) from different origins are at the forefront of liver fibrosis and how liver cell-specific regulation of the complex scarring process occurs. Recent Advances: Particular attention is paid to the role of cytokines, growth factors, reactive oxygen species, and newly identified matricellular proteins in the regulation of fibrillar type I collagen, a field to which our laboratory has significantly contributed over the years. We compile data from recent literature on the potential mechanisms driving fibrosis resolution such as MF’ apoptosis, senescence, and reversal to quiescence. Future Directions: We conclude with a brief description of how epigenetics, an evolving field, can regulate the behavior of MF and of how new “omics” tools may advance our understanding of the mechanisms by which the fibrogenic response to liver injury occurs. Antioxid. Redox Signal. 21, 1078–1097.
Extracellular Matrix
Introduction
The extracellular matrix (ECM) represents a noncellular component in tissues and organs that is primarily composed of water, proteins, and proteoglycans. It forms an intricate network that provides a physical scaffold for cells as well as structural support, tensile, compressive strength, and elasticity in all tissues and organs (34). Besides its mechanical and biochemical properties, it helps maintain hydration and homeostasis and by interacting with cell-surface receptors and matrix components it regulates cell differentiation, adhesion, proliferation, migration, and survival (53, 61, 120). The ECM also binds and secretes growth factors and cytokines that drive morphogenesis, cell function, and metabolism (153). Thus, the ECM creates a complex microenvironment that is particularly dynamic in nature and which undergoes continuous remodeling not only during development but also throughout differentiation and wound healing. Accordingly, well-coordinated regulation of ECM remodeling is essential to maintain homeostasis and to prevent disease onset and progression (24).
Collagenous proteins
Collagen is a major fibrillar protein present in the ECM that represents approximately 30% of the total protein content in the body; therefore, it constitutes the principal structural protein in mammalian tissues (34). Thus far, 28 collagens encoded by 49 genes have been identified in vertebrates (44, 120). The members of the collagen superfamily, listed next, are classified based on structure and supramolecular organization. For a summary, please see Table 1.
Table 1.
The Members of the Collagen Family Classified According to Molecular Composition and Tissue Distribution
| Subfamily | Type | Molecular composition | Tissue distribution | References |
|---|---|---|---|---|
| Fibrillar | I | [α1(I)]2, α2(I) | Throughout the body except in the cartilage, bone, skin, tendon, ligaments, cornea | (10, 41, 118) |
| II | [α1(II)]3 | Cartilage, vitreous body, nucleus pulposus | (41, 118) | |
| III | [α1(III)]3 | Blood vessels, intestinal organs, cartilage, skin | (18, 118) | |
| V | [α1(V)]2, α2(V) [α1(V)]3 [α1(V)]2, α4(V) |
Bone, cornea, lung, fetal membranes | (41, 118) | |
| XI | α1(XI), α2(XI), α3(XI) | Articular cartilage, vitreous body | (41, 44, 118) | |
| XXIV | [α1(XXIV)]3 | Bone and cornea | (118, 119) | |
| XXVII | [α1(XXVII)]3 | Cartilage | (118, 119) | |
| FACITs | IX | α1(IX), α2(IX), α3(IX) | Cartilage, vitreous body, cornea | (41, 44, 118) |
| XII | [α1(XII)]3 | Skin, tendon, perichondrium, periosteum, lungs, liver, placenta, vessel walls | (41, 118, 119) | |
| XIV | [α1(XIV)]3 | Skin, tendon, perichondrium, periosteum, lungs, liver, placenta, vessel walls, bone, cornea | (10, 118, 119) | |
| XVI | [α1(XVI)]3 | Hyaline cartilage, skin, basement membranes, tissue junctions | (41, 118) | |
| XIX | [α1(XIX)]3 | Basement membranes, tissue junctions | (44, 118) | |
| XX | [α1(XX)]3 | Corneal epithelium, embryonic skin, sternal cartilage, tendon | (41, 118) | |
| XXI | [α1(XXI)]3 | Basement membranes, tissue junctions, blood vessels | (41, 44, 118) | |
| XXII | [α1(XXII)]3 | Basement membranes, tissue junctions | (44, 118) | |
| Anchoring fibrils | VII | [α1(VII)]3 | Skin, dermal-epidermal junctions, oral mucosa, cervix | (41, 44, 118) |
| Network-forming | IV | [α1(IV)]2, α2(IV) α3(IV), α4(IV), α5(IV) [α5(IV)]2, α6(IV) |
Basement membranes | (41, 44, 118) |
| VI | α1(VI), α2(VI), α3(VI) | Ubiquitous tissue distribution, all connective tissues except bone | (41, 44, 118) | |
| VIII | [α1(VIII)]2, α2(VIII) α1(VIII), [α2(VIII)]2 [α1(VIII)]3 [α2(VIII)]3 |
Cornea, endothelium | (41, 118) | |
| X | [α1(X)]3 | Hypertrophic cartilage | (41, 118) | |
| Transmembrane | XIII | [α1(XIII)]3 | Epidermis, hair follicle, endomysium, intestine, chondrocytes, lungs, liver | (41, 118) |
| XVII | [α1(XVII)]3 | Dermal-epidermal junctions | (41, 118) | |
| XXIII | [α1(XXIII)]3 | Metastatic tumor cells | (118) | |
| XXV | [α1(XXV)]3 | Neurons | (44, 118) | |
| Multiplexins | XV | [α1(XV)]3 | Heart and skeletal muscle, kidney, pancreas | (41, 44, 118, 119) |
| XVIII | [α1(XVIII)]3 | Lung, kidney, brain, smooth muscle, eye, liver | (41, 44, 118, 119) | |
| Other collagens | XXVI | [α1(XXVI)]3 | Testis and ovaries | (44, 118) |
| XXVIII | [α1(XXVIII)]3 | Cartilage, eye, ear, lung | (118) |
FACITs, fibril-associated collagens with interrupted triple helix.
Fibril-forming or fibrillar collagens include type I, II, III, XI collagen and the more recently discovered type XXIV and XXVII collagen. They are the most abundant and widely distributed collagens in the body. Their role is largely mechanical, as they provide tensile strength to both tissues and organs (118).
Fibril-associated collagens with interrupted triple helix (FACITs) constitute the largest collagen subclass, including type IX, XII, XIV, XVI, XIX, XX, XXI, and XXII collagen. FACITs do not form fibrils themselves, but they bind the surface of pre-existing collagen fibrils contributing to fibril enlargement.
Anchoring fibrils, composed largely of type VII collagen, extend from the basal lamina of epithelial cells and attach to the lamina reticularis by wrapping around the reticular fiber type III collagen bundles and constituting the basement membrane (117).
Network-forming collagens contain multiple disruptions in the triple-helical chains, providing flexibility and enabling them to form linear, axial, and lateral associations within protein networks. Type IV collagen is the most important structural component of the basement membrane (41, 75). Type VI collagen is a heterotrimeric molecule that aggregates into filamentous networks and binds multiple matricellular proteins. Type VIII and X collagen are very homologous heterotrimeric short-chain molecules that form hexagonal networks; however, they exhibit different localization (41).
Transmembrane collagens include homotrimeric type XIII, XVII, XXIII, and XXV collagen, most of which are anchored to the plasma membrane. Transmembrane collagen contains several extracellular domains that can be easily detached from the cell surface. They also act as cell surface receptors and soluble extracellular molecules (118, 149).
Multiplexins include type XV and XVIII collagens. They are basement membrane collagens, endostatin precursors, endostatin-XVIII, and endostatin-XV and are secreted by proteolysis (118).
Finally, type XXVI and XXVIII collagen do not properly fit within any of these subgroups, although both of them contain collagenous domains within their structure (44).
Non-collagenous proteins
Among the proteins belonging to the family of non-collagenous proteins from the ECM are fibronectin, tenascins, laminins, elastin, fibrillins, proteoglycans, and matricellular proteins.
Fibronectin is a high-molecular-weight glycoprotein that binds membrane-spanning receptor proteins named integrins and ECM components such as collagen, fibrin, and heparan sulfate proteoglycans (e.g., syndecans). Fibronectin plays a major role in cell adhesion, growth, migration, and differentiation, and it is important for wound healing and embryonic development (104). Altered fibronectin expression, degradation, and organization have been associated with fibrosis and cancer (104, 146, 152).
Tenascins are large oligomeric glycoproteins (10) that under physiological conditions are not highly expressed in the majority of adult tissues; however, they participate in embryonic development, wound healing, tumorigenesis, and inflammation (91). Tenascins frequently reappear around healing wounds and in the stroma of some tumors (17).
Laminins are glycoproteins that are commonly found in basement membranes, which contain domains that are responsible for the interaction with cell surface integrins (124). Laminins condition cell differentiation, migration, and adhesion as well as the phenotype and survival in almost every tissue (138). Defective laminins lead to muscular dystrophy, lethal skin blistering disease, and nephrotic syndrome (159).
Elastic fibers such as elastin and fibrillins are essential ECM components providing the necessary resilience and elastic recoil to tissues. Both of them interact with collagens and proteoglycans, in addition to the ability of fibrillin to bind transforming growth factor-β (TGF-β) (85).
Proteoglycans are highly hydrophilic molecules that hydrate cells and tissues contributing to ECM assembly. They participate in the regulation of numerous signaling cascades via interactions with growth factors such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) (10).
Matricellular proteins are a group of ECM components that control cell function and cell-matrix interactions and signaling (9). They include, among others, thrombospondin-1 and -2 secreted proteins, osteonectin, osteopontin (OPN), and the CCN family (cyr-61 and connective tissue growth factor [CTGF]). This key family of proteins determines cell function via interactions with cell surface receptors, growth factors (VEGF, TGF-β, and platelet-derived growth factor [PDGF]), proteases, and structural ECM proteins (2).
ECM homeostasis
The ECM remodeling is a complex but highly coordinated process resulting from the balance between synthesis, secretion, degradation, and reorganization of its components (86). Synthesis and deposition of ECM constituents occur in response to signals conveyed by cell surface receptors, among which integrins appear to play a major role as demonstrated for the pro-fibrogenic effects of OPN (145).
The most relevant ECM-modifying enzymes are the matrix metalloproteinases (MMPs), a superfamily of zinc-dependent endopeptidases (103). MMPs and other proteinases such as plasmin, thrombin, and cathepsins are synthesized as inactive zymogens requiring proteolytic cleavage of a self-inhibitory pro-domain in order to become activated. MMPs' activity is regulated by specific inhibitors such as the tissue inhibitor of metalloproteinases (TIMPs). These two families of enzymes control ECM homeostasis and remodeling that is essential for normal cellular and organ function (11).
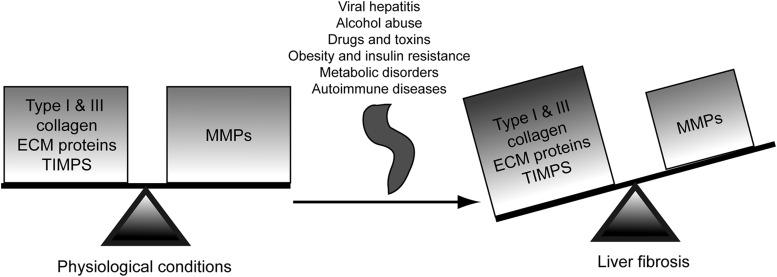
However, in the diseased state, impairment and/or disco-ordination in the activity of these enzymes leads to disruption of the physiological ECM dynamics, loss of the normal tissue architecture, and pathological ECM deposition, all of which contribute to the pathogenesis of fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) (21, 35). As an example, MMP-1 decreases in fibrotic livers whereas MMP-2 increases as fibrosis progresses. Likewise, TIMP-1 and TIMP-2 expression significantly increases in fibrosis, thus inhibiting collagen-degrading MMPs and resulting in a net increase in ECM deposition (82) (Fig. 1).
FIG. 1.
ECM homeostasis. Under physiological conditions, the ECM homeostasis involves a coordinated balance between ECM protein synthesis and degradation. In the diseased state, this equilibrium is perturbed and the composition of the ECM is altered typically by the deposition of fibrillar type I collagen and other proteins, leading to pathological changes in the hepatic architecture. ECM, extracellular matrix; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
Increased myofibroblasts at the forefront of liver fibrosis
Chronic liver disease is characterized by excessive deposition of ECM proteins in response to persistent liver injury. During fibrogenesis, the ECM undergoes continuous changes in quantity as well as in composition, and the amount of ECM proteins in fibrotic livers increases by six-fold compared with that in healthy ones (5). This increase in ECM deposition elicits tissue remodeling, despite which there is substantial scar formation, distortion of the hepatic architecture, and significant impairment of liver function.
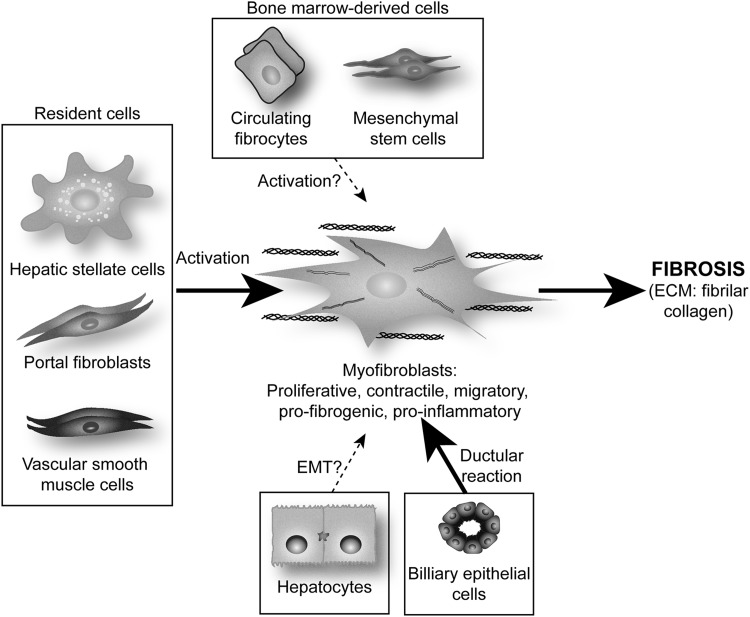
It is now obvious that several cell types participate in the deposition of fibrillar collagen during hepatic fibrogenesis. These pro-fibrogenic cells have diverse origin, but all of them undergo a common process of trans-differentiation and acquisition of a classical myofibroblast (MF)-like phenotype. There are several well-described pro-fibrogenic cells within the liver: resident fibroblasts or MF-like cells mainly represented by hepatic stellate cells (HSC), portal fibroblasts (PF), and vascular smooth muscle cells (101).
In healthy liver, HSC reside in the sinusoidal space of Disse. After chronic injury, they become activated and differentiate into MF-like cells acquiring contractile, pro-inflammatory, and pro-fibrogenic properties (37). Activated HSC migrate and accumulate at the sites of tissue repair, secreting large amounts of ECM, mainly type I collagen and regulating ECM remodeling. Work from our group has dissected how the cross-talk between hepatocytes and HSC (93, 96, 145) and between Kupffer cells (KC) and HSC leads to a fibrogenic response (22, 23, 95, 97–99).
PF are the other main population of liver cells involved in fibrogenesis (6, 60). The portal connective tissue in normal liver contains only quiescent fibroblasts, with no PF, which only appear when a lesion occurs in the portal zone. Derived from small portal vessels, they express markers that are distinct from HSC [e.g., elastin (60)]. Proliferation of biliary cells is often accompanied by an increase in PF, which form onion-like configurations around biliary structures, acquire an MF phenotype, and are, thus, implied in the early deposition of ECM in portal zones (71).
Immunohistochemical studies have localized most of the different collagen-producing cells in cirrhotic rodent and human livers: (i) portal/septal MF are found either in the inner part of the fibrotic septa or within the expanded portal tracts and originate mainly from PF; (ii) activated HSC are largely located in capillarized sinusoids in the space of Disse between the endothelium and the hepatocytes; and (iii) interface MF, essentially situated at the edge between the fibrotic septa and the surrounding parenchyma, originate from activated HSC (40, 101).
The cell populations involved differ according to the pattern of human liver fibrosis: (i) PF in the septa and HSC at the interface between septa and parenchyma in portal diseases such as chronic viral hepatitis and chronic bile duct obstruction and (ii) HSC and second-layer fibroblasts in centrilobular diseases such as alcoholic liver disease (ALD) and nonalcoholic steatohepatitis (NASH) (101).
In addition to endogenous liver precursors, hepatic MF may also originate from bone-marrow-derived cells such as mesenchymal stem cells (MSC) or circulating fibrocytes (73, 121). Studies by Li et al. and Russo et al. demonstrated that MSC are a source of extra-hepatic MF in the damaged liver (83, 121), although MSC-derived MF represent only a small proportion of the total MF that contribute to ECM deposition during liver fibrosis (73). Further studies are required to establish the quantitative relevance of their contribution to liver fibrosis (60, 73, 83, 121).
In the last few years, although still quite controversial, it has been proposed that hepatocytes and biliary epithelial cells could contribute to liver fibrosis by undergoing epithelial-to-mesenchymal transition (EMT) (18). Several in vitro experiments suggest this possibility. Indeed, TGF-β up-regulates mesenchymal genes such as α-smooth muscle actin (α-SMA) and collagen, and down-regulates epithelial genes such as albumin (60, 151). However, lineage tracing in murine models of hepatic fibrosis such as common bile duct ligation (BDL) or an injection of carbon tetrachloride (CCl4) has shown that hepatocytes and biliary epithelial cells do not undergo EMT after liver injury (25), whereas other reports provide in vivo evidence which suggests that EMT occurs during liver injury (102, 160). Additional studies are needed in order to identify the cell type that undergoes EMT, if any, and their specific contribution to ECM deposition during liver injury (60, 151) (Fig. 2).
FIG. 2.
Potential cellular sources of MF in liver injury. MF originate mainly from resident cells (HSC, PF, and vascular smooth muscles cells) via a process of activation and transdifferentiation. Biliary epithelial cells can activate MF due to their active proliferation, the so-called ductular reaction. Although still quite controversial, other progenitors may be recruited from the bone marrow, such as MSC or circulating fibrocytes, and it is also possible that MF could derive from hepatocytes and/or biliary epithelial cells EMT. EMT, epithelial-to-mesenchymal transition; HSC, hepatic stellate cell; MF, myofibroblasts; MSC, mesenchymal stem cells; PF, portal fibroblasts.
Pathological ECM and Liver Disease
Fibrogenesis: the wound-healing response to liver injury
Wound healing or tissue remodeling and repair is a protective mechanism that is activated in response to stress and injury in order to maintain the functional and anatomical integrity of the liver. When injury and the related acute inflammatory reaction cause moderate cell necrosis and ECM damage, tissue repair usually occurs. In response to parenchymal cell loss, hepatocytes restore the physiological liver mass by self-replication regenerating and replacing necrotic or apoptotic cells (59). However, continued injury and deregulation of the normal healing does not repair as effectively; regeneration fails, resulting in liver fibrosis, massive deposition of ECM, scar formation, and, in progressive and persisting injury, hepatic failure (38).
Chronic damage leading to liver fibrosis occurs in response to a variety of insults, such as viral hepatitis, alcohol abuse, obesity, drugs and toxins, insulin resistance, metabolic disorders, venous outflow obstruction, or autoimmune disease. Overall, fibrogenesis is characterized by several key features: (i) immediate damage to the epithelial/endothelial barrier, (ii) continuous hepatocyte injury, (iii) recruitment of inflammatory cells with the subsequent secretion of pro-fibrogenic cytokines and growth factors, (iv) generation of reactive oxygen species (ROS), (v) activation of collagen-producing cells with proliferative, migratory, and contractile features, (vi) matrix signaling to MF, and (vii) marked changes in the composition and quantity of the ECM deposits that distort the normal hepatic architecture by forming fibrotic scars (38).
The distribution of the fibrous material depends on the cause of liver injury. In human chronic viral hepatitis and chronic cholestatic disorders, the fibrotic tissue is initially located around portal tracts, while in ALD, it is located in pericentral and perisinusoidal areas. As fibrosis advances, progression from collagen bands to bridging fibrosis and to frank cirrhosis occurs; the latter is characterized by formation of regenerative nodules of liver parenchyma that are separated by fibrotic septa (42, 47, 67), liver failure, and portal hypertension, which often require liver transplantation. Thus, emerging antifibrotic therapies aim at inhibiting the increase and activation of pro-fibrogenic cells and at preventing ECM deposition, particularly fibrillar type I and III collagen.
Alcoholic liver disease
ALD is a significant clinical problem and a major cause of morbidity and mortality worldwide. The spectrum of disease ranges from fatty liver to alcoholic steatohepatitis (ASH), progressive fibrosis, and HCC (110). In developed countries, ALD is a major cause of end-stage disease that requires transplantation. Current interventions such as nutritional support with vitamin and zinc supplements, steroids combined with N-acetylcysteine for patients with biopsy-proven ASH, and pentoxifyline for patients with contraindications to steroids have proved beneficial for patients with ALD; however, it remains the most important etiology of half of the cirrhosis-related deaths in the United States.
Ethanol exposure causes a state of oxidant stress in hepatocytes, altering the architecture of the endoplasmic reticulum (ER) and the function of the proteasome system. The causative pathological role of both oxidative and nitrosative stress has been extensively demonstrated in ALD as a key factor promoting disease progression.
Many pathways play a key role in how ethanol causes cell injury and induces oxidant stress in the liver. Some of these include redox state changes because of ethanol and acetaldehyde oxidation, generation of acetaldehyde and acetaldehyde adducts, mitochondrial damage, decreased ATP production, membrane damage by the solvent-like actions of ethanol, nutritional interactions, hypoxia, cytokine secretion, KC activation due to an increase in gut-derived lipopolysaccharide (LPS), and an increase in damage-associated molecular patterns such as high mobility group box-1 (HMGB1) that drives both sterile and nonsterile inflammation.
ROS derived from alcohol metabolism in hepatocytes are mainly generated by the mitochondrial respiratory chain, cytochrome P450 isoforms such as cytochrome P450 2E1 (CYP2E1) that metabolizes ethanol per se, xanthine oxidase, and NADPH oxidase (NOX) (16). It is well known that continued alcohol consumption also decreases the antioxidant defense, thus promoting liver injury (15).
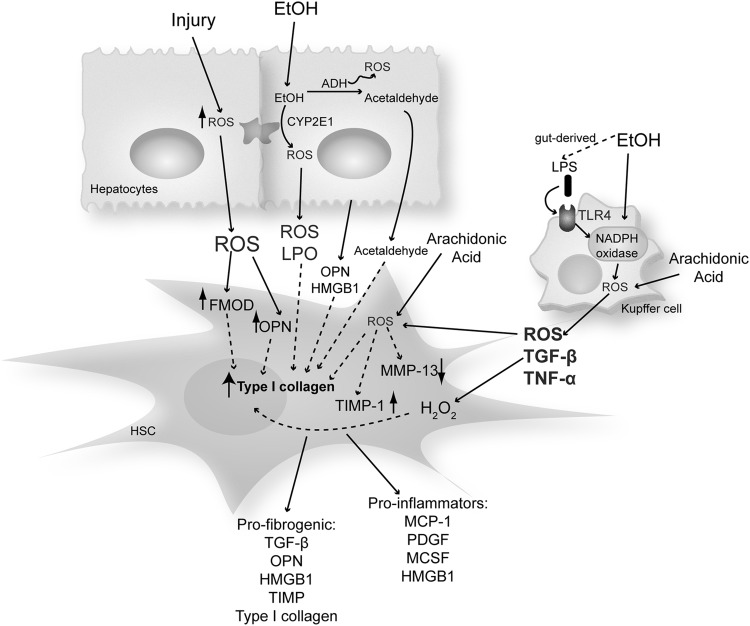
Many of these pathways are not exclusive of one another, and it is likely that several of them contribute to the ability of ethanol to induce a state of oxidant stress (see sections Non-alcoholic steatohepatitis and Other etiologies). Moreover, several of these mechanisms and secreted mediators induced by them drive HSC activation, further magnifying liver injury (22, 23, 93, 95, 97, 100, 145) (Fig. 3).
FIG. 3.
Increased ROS during liver injury mediate the pro-fibrogenic response in HSC. Ethanol metabolism in hepatocytes generates ROS, which trigger HSC activation and type I collagen synthesis. Moreover, arachidonic acid potentiates the hepatocyte-derived effects of ethanol on HSC. Ethanol also promotes the translocation of LPS from the gut lumen into the portal circulation, subsequently activating KC to generate ROS and other factors. This further impacts the pro-fibrogenic behavior of HSC increasing type I collagen and TIMP-1 and decreasing MMP 13 expression. Other ROS-sensitive matricellular proteins such as FMOD, OPN, TGF-β, and HMGB1 derived from either hepatocytes or KC signal to HSC increase type I collagen synthesis. FMOD, fibromodulin; HMGB1, high mobility group box-1; KC, Kupffer cells; LPO, lipid peroxides; LPS, lipopolysaccharide; MCSF, macrophage colony-stimulating factor; OPN, osteopontin; ROS, reactive oxygen species; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; TLR, toll-like receptor.
Nonalcoholic steatohepatitis
NASH represents a progressive form of nonalcoholic fatty liver disease. It is characterized by histological liver damage that is similar to ALD in the absence of significant alcohol consumption. Risk factors for NASH include obesity, type II diabetes, hyperlipidemia, total parenteral nutrition, jejuno-ileal bypass surgery, and the use of certain drugs. Although there are no officially approved drugs for the treatment of NASH, metformin has been shown to improve insulin resistance, liver enzymes, and steatosis, whereas its efficiency in liver injury and fibrosis shows mixed results (74).
Oxidative stress markers and lipid peroxidation products are increased, while the antioxidant defense is reduced in the liver and in the plasma from NASH patients (48, 58, 68). In NASH, there is an imbalance in fat synthesis along with an increase in circulating adipokines, elevated ROS, and inflammation leading to fibrosis. Leptin, the adipocyte-derived hormone, promotes hepatic fibrosis by increasing type I(α1) collagen gene (Col1a1) expression in human and rat HSC (125, 135).
Other etiologies
Hepatitis C is a human infectious disease affecting the liver that is caused by an RNA virus from the Flaviviridae family. The hepatitis C virus (HCV) is transmited primarily by the blood. Acute HCV infection is asymptomatic in 50%–90% of cases. Chronic infection is associated with variable degrees of hepatic inflammation and fibrosis progression, regardless of HCV genotype and viral load. Liver disease progression takes place over several decades. Depending on the presence of cofactors, 10%–40% of patients with chronic HCV infection will develop cirrhosis.
Hemochromatosis, a disease characterized by iron overload in the liver, is a hereditary autosomal recessive syndrome with two known mutations in the HFE gene (C282Y and H63D) that disrupt iron metabolism (122). Patients with hemochromatosis develop cirrhosis, as iron deposition catalyzes Fenton reactions that promote the formation of lipid peroxidation-end products. These effects, exacerbated by alcohol consumption, contribute to the fibrogenic response (80).
Lastly, Wilson's disease is an autosomal recessive disorder in copper metabolism that is characterized by excessive copper deposition in the liver, brain, and other tissues. It is caused by mutations in the ATP7B gene that codes for a copper carrier involved in transferring copper from the trans-Golgi system to apo-ceruloplasmin and the transport of copper from the hepatocyte to the bile. Copper deposition causes mitochondrial dysfunction and oxidative stress, hence promoting scarring in the liver (26).
Liver Cell-Specific ECM Regulation
Hepatic stellate cells
A large majority of the MF present in the damaged liver are HSC derived (37). Quiescent HSC express typical markers of neural cells, including glial fibrillary acidic protein (GFAP), synemin, synaptophysin, nerve growth factor receptor p75, and desmin, in addition to markers that are characteristic of adipocytes, such as peroxisome proliferator-activated receptor gamma (PPARγ) (69, 129). In chronic liver injury, quiescent HSC are exposed to growth factors and pro-fibrogenic cytokines and become activated by acquiring an MF phenotype (37). On activation, HSC proliferate, migrate, and become highly contractile. Moreover, they become pro-inflammatory, as they produce and secrete monocyte chemoattractant protein-1 (MCP-1), PDGF, macrophage colony-stimulating factor (MCSF), and HMGB1, in addition to acquiring pro-fibrogenic properties as they produce pro-fibrogenic factors such as TGF-β, OPN, HMGB1, and TIMP and increase the synthesis and deposition of type I and III collagen and fibronectin (28, 60).
Multiple mechanisms and signaling pathways are involved in HSC activation such as factors released by activated KC (ROS and cytokines) (23, 95), damaged hepatocytes (lipid peroxidation products, ROS, OPN, and HMGB1) (97, 145), and hepatocyte-derived apoptotic bodies (46). Phagocytosis of apoptotic bodies makes HSC more resistant to Fas ligand and tumor necrosis factor-α (TNF-α)-related apoptosis (63), increasing their survival and hence perpetuating the fibrogenic response.
Recently, it was described that autophagy can hydrolyze triglycerides in hepatocytes in a process called lipophagy. Lipophagy has been proposed as significant for HSC activation and, thus, for liver fibrogenesis. Indeed, autophagy stimulates loss of lipid droplets and provides the cellular energy that is necessary for HSC activation (51). Additional studies have shown that autophagy can be induced in activated HSC via oxidative injury and ER stress (52).
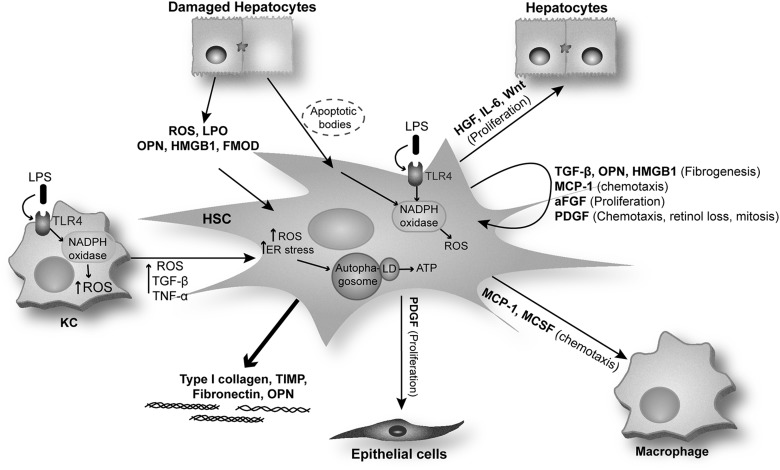
Once activated, HSC secrete growth factors (TGF-β and epidermal growth factor), hepatocyte mitogens (HGF, interleukin 6 [IL-6], Wnt), MCSF, stem cell factor, macrophage chemotactic factors (MCP-1 and TNF-α), as well as autocrine factors, including HSC mitogens (PDGF-BB and aFGF), HSC chemotactic factors (MCP-1, PDGF), and HSC pro-fibrogenic factors (TGF-β, OPN, and HMGB1). Activated HSC also respond to mediators that are secreted by other cells, including endothelin-1, OPN, and HMGB1 amplifying the pro-fibrogenic response (37, 45, 145).
TGF-β is a key inducer of ECM secretion by activated HSC. In addition, leptin and angiotensin II have been shown to exert pro-fibrogenic actions in activated HSC (94). Leptin is an adipogenic hormone that activates the JAK-STAT pathway and induces HSC proliferation while inhibiting HSC apoptosis (126). Activated HSC secrete angiotensin II, which induces fibrogenic effects via activation of NOX. Lastly, blockade of the renin-angiotensin axis attenuates fibrosis in experimental models of chronic liver injury (Fig. 4).
FIG. 4.
HSC activation and factors secreted by activated HSC. After liver injury, quiescent HSC are exposed to factors that are released by activated KC and damaged hepatocytes. HSC are also activated by phagocytosis of hepatocyte-derived apoptotic bodies. Activated HSC synthesize paracrine factors involved in epithelial cell and hepatocyte proliferation as well as in macrophage chemotaxis. Furthermore, they produce autocrine factors that promote HSC activation, fibrogenesis, and chemotaxis. Activated HSC increase the synthesis and deposition of ECM components such as type I collagen, TIMP, fibronectin, and OPN. MCP-1, monocyte chemoattractant protein-1; FGF, fibroblast growth factor; LD, lipid droplets; PDGF, platelet-derived growth factor.
Portal fibroblasts
In healthy liver, PF are located adjacent to bile duct epithelia, where they participate in ECM turnover. PF are the first responders in biliary fibrosis after cholestatic liver injury and are later replaced by perisinusoidal HSC (71). PF, unlike HSC, do not express cytoglobin, desmin, or GFAP and they lack lipid droplets. However, they are characterized by expression of highly specific fibroblast markers such as TE7, elastin, IL-6, fibulin-2, ecto-ATPase nucleoside triphosphate diphosphohydrolase-2, p100, α2-macroglobulin, and neural proteins (60). After liver injury, they also undergo activation, increasing the expression of α-SMA, proliferating, and secreting type I collagen. MF differentiation of PF is induced by growth factors (PDGF-BB), pro-inflammatory cytokines (IL-6 and MCP-1), and pro-fibrogenic proteins (TGF-β2, OPN, and HMGB1) that are secreted mainly by damaged biliary epithelial cells (60).
Kupffer cells
KC are resident macrophages that are characterized by high phagocytic, antigen presentation and pinocytotic capacity. KC release a wide array of soluble mediators, including ROS, cytokines, growth factors, cyclooxygenase, and lipoxygenase metabolites, all of which provide physiologically diverse and pivotal paracrine effects on all other liver cells (90, 141). KC are also central to the liver's homeostatic response to injury, as on degenerative changes in hepatocytes they immediately respond to the insult and release mediators to orchestrate inflammatory and reparative responses (90, 141).
Influx of KC coincides with the appearance of HSC activation markers (64). KC stimulate matrix synthesis, cell proliferation, and release of retinoids by HSC. KC generate ROS, which, in turn, may enhance HSC activation and type I collagen synthesis either by their direct actions on the two type I collagen promoters (Col1a1 and Col1a2) or by inducing pro-fibrogenic cytokines (95). KC also produce nitric oxide, which can counterbalance the effects of ROS by reducing HSC proliferation, contractility, and type I collagen synthesis; however, nitric oxide, when reacting with superoxide radical (O2·−), generates peroxynitrite, which has major profibrogenic effects on HSC (144).
To date, there is limited information on the intercellular communication between KC and HSC, as well as on the molecular mechanisms by which KC-derived mediators modulate the fibrogenic response in HSC. Work from our laboratory (see section: ROS and type 1 collagen) demonstrated a novel dual mechanism that is responsible for the increase in type I collagen in HSC co-cultured with KC (95).
Sinusoidal endothelial cells
Liver sinusoidal endothelial cells (SEC) are nonparenchymal cells that are located in hepatic sinusoids. They control the bidirectional exchange of lipoproteins and other nutrients between the blood and the liver cells via their fenestrations (154). The contribution of SEC to the production of fibrillar collagen is still controversial. However, during the early stages of liver fibrosis, SEC lose their fenestrations, decreasing the exchange of metabolites and increasing the secretion of various basement membrane components (type IV collagen, perlecan, entactin, and laminin), all of which contribute to hepatocyte injury. SEC secrete fibronectin and promote the TGF-β-mediated activation of HSC (151). Moreover, SEC contribute to creating a proinflammatory environment by increasing T cells and decreasing hepatic immunotolerance (19).
Cytokines as Regulators of ECM
Transforming growth factor-β
TGF-β can be secreted by biliary epithelial cells, KC, and activated HSC. All isoforms of TGF-β (TGF-β1, TGF-β2, and TGF-β3) are synthesized and secreted as latent precursors, requiring protein processing to generate their active form (27). In its latent state, TGF-β is usually a part of the network of ECM components along with type IV collagen, fibrillin, and fibronectin. Factors determining the secretion of active TGF-β from its latent complex include MMPs, plasminogen activator, plasmin, ανβ6 integrin, and thrombospondins (45). Once activated, TGF-β signals to MF via the serine-threonine kinase TGF-β receptors, TβRI and TβRII.
In quiescent HSC, TGF-β induces the activity of the inhibitory Smad-7, which acts as a negative feedback loop blocking TGF-β-receptor-mediated Smad signaling and terminating the Smad-controlled signal transduction. It has been shown that a Smad-7 response is absent in activated HSC (133), which, along with the TGF-β autocrine loop, mediates the increase in TGF-β during liver fibrogenesis (Fig. 5).
FIG. 5.
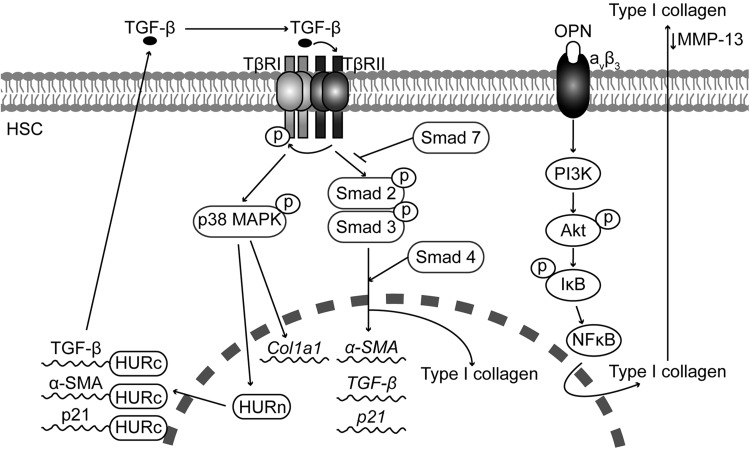
Pro-fibrogenic effects of TGF-β and OPN in activated HSC. TGF-β activates p38 MAPK and Smads in activated HSC. Smads translocate to the nucleus and regulate the expression of pro-fibrogenic genes. TGF-β-activated p38 MAPK increases Col1a1 mRNA expression and the functionality of HuR, which regulates the mRNA stability of anti-proliferative genes such as p21, and the TGF-β-induced expression of α-Sma and Tgf-β. OPN binds integrin aνβ3 and activates the PI3K-Akt-NFκB signaling pathway to up-regulate type I collagen and lower MMP13 protein expression. α-SMA, α-smooth muscle actin; Col1a1, type I(α1) collagen gene; HuR, RNA-binding protein human antigen R; NFκB, nuclear factor kappa B; p38MAPK, p38 mitogen-activated protein kinase.
Several studies have identified Smad-3 as the main inducer of collagen expression in HSC in response to TGF-β (55, 127, 128). Likewise, other studies have demonstrated a role for p38 mitogen-activated protein kinase (p38MAPK) in the transcriptional and translational control of the Col1a1 gene in response to TGF-β (148).
The effect of TGF-β appears to be cell-type specific. For example, TGF-β inhibits HSC proliferation. In the BDL model of liver fibrosis, the anti-proliferative action of TGF-β is mediated by an increase in the interaction between the RNA-binding protein human antigen R (HuR) and p21 mRNA, increasing p21 mRNA stability. These effects of HuR are linked to its cytoplasmic localization, which is controlled by TGF-β via p38MAPK (156). In addition, TGF-β also increases the HuR-mediated stability of mytogenic and pro-fibrogenic genes such as α-SMA and TGF-β mRNA (Fig. 5).
Tumor necrosis factor-α
The contribution of TNF-α to hepatocellular injury and inflammation has been widely studied, whereas the role and the mechanisms involved in the TNF-α-mediated HSC activation and collagen production still remain undefined. In vitro studies have shown that TNF-α inhibits Col1a1 mRNA expression in HSC via p38MAPK, CCAAT enhancer binding protein β (C/EBPβ), and C/EBPδ-dependent mechanisms (56). Likewise, TNF-α exerts its anti-fibrogenic effects by regulating ROS production due to a reduction in GSH levels and an increase in nuclear factor kappa B (NFκB) activity (147). Reactive nitrogen species have been shown to switch on early ECM remodeling in HSC via induction of MMP1 and TNF-α (144). Although TNF-α does not participate in HSC activation by increasing α-SMA or TGF-β expression, it plays an important role in proliferation and in regulating TIMP-1 and MMP-9 expression via the TNF-α receptor 1 (TNFR1). This study also shows that the absence of TNFR1 alone or TNFR1 plus TNFR2 leads to a decrease in Col1a1 mRNA expression, suggesting that in some cases TNF-α could be pro-fibrogenic in the liver (136).
Osteopontin
OPN is a phosphorylated acidic glycoprotein that belongs to the family of matricellular proteins which function as adaptors and modulators of cell-matrix interaction. OPN has important roles as an immune modulator and a mediator of wound repair (77). Plasma OPN concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected as well as in alcoholic individuals. OPN promotes fibrosis progression in patients with NASH and has prognostic value in hepatitis B virus (HBV)-related HCC (111, 132, 157, 162). Likewise, our laboratory has recently demonstrated that HCV-cirrhotic patients have increased co-induction of collagen and cleaved OPN compared with healthy individuals (145).
Several in vivo studies, including our own, have shown that OPN expression increases in CCl4-, BDL-, and thioacetamide (TAA)-induced liver injury and fibrosis (81, 145). Furthermore, Opn−/− mice were less susceptible to CCl4- and TAA-induced liver injury and fibrosis, whereas transgenic mice overexpressing OPN in hepatocytes (OpnHEP Tg) developed spontaneous liver fibrosis and were more vulnerable to CCl4-induced liver injury and fibrosis (145). Hepatocytes, oval cells, billiary epithelial cells, and HSC appear to be the main source of OPN in the liver (145). In vitro studies demonstrate that OPN expression is up-regulated in culture-activated HSC and treatment with OPN induces HSC activation, promotes HSC invasion and migration as well as type I collagen production (81, 145). The pro-fibrogenic effect of OPN is mediated by integrin aνβ3 binding and signaling via the PI3K-pAkt-NFκB pathway (145). Thus, OPN acts as an autocrine and paracrine mediator of the fibrogenic response to liver injury (Fig. 5).
Reactive Oxygen Species
ROS play a crucial role in the pathophysiology of every liver disease. Oxidative stress is a key noxious factor in ALD and NASH. The major sources of ROS in the liver are hepatocytes, KC, and neutrophils. Hepatocytes are implicated in the detoxification of drugs and toxins by the cytochrome P450 enzymes, which require oxygen activation, resulting in ROS generation. KC and neutrophils typically generate ROS via NOX activation in response to pathogens and inflammatory situations. ROS generation is enhanced by alcohol consumption.
ROS and type I collagen
Over the last few years, our laboratory has studied how ROS contribute to type I collagen induction in HSC. Studies using HSC lines transduced to overexpress CYP2E1 as an endogenous generator of ROS indicate that under oxidative stress conditions, Col1a2 mRNA expression is regulated transcriptionally and by mRNA stabilization (98, 99). Likewise, treatment with a polyunsaturated fatty acid such as arachidonic acid along with ethanol to recapitulate events occurring during ALD linked CYP2E1-dependent oxidative stress to induction of cyclooxygenase-2 and the actions of arachidonic acid and ethanol on the activation of the type I collagen gene in HSC (100).
In a follow-up study, to evaluate possible fibrogenic effects of CYP2E1-dependent generation of ROS, a model was developed using co-cultures of HepG2 cells that either express or do not express CYP2E1 with HSC (97). The results from this study suggest that increased translation of the Col1a1 and Col1a2 genes by CYP2E1-derived ROS is responsible for the increase in type I collagen protein which is produced by the CYP2E1-expressing HepG2 cells co-culture (97).
To assess whether fish oil, as a source of polyunsaturated fatty acids from the n-3 series, could synergize with ethanol to promote type I collagen up-regulation in vivo, Col1a2 promoter-βGal transgenic mice were fed a diet that was enriched in fish oil in the presence of ethanol or dextrose and co-cultures of hepatocytes and HSC were established. These studies suggested that fish oil (mainly n-3 polyunsaturated fatty acids) synergizes with ethanol and induces type I collagen, transactivating the Col1a2 promoter through a lipid peroxidation-PKC-PI3K-pAkt-NFκB-driven mechanism in the absence of overt steatosis and inflammation (96).
The impact of KC on the HSC fibrogenic response was examined in an in vitro co-culture model of primary KC and HSCs. Co-culture with KC induced a more activated phenotype and greater proliferation compared with HSC cultured alone. Co-cultured HSC showed elevated phosphorylation of p38, which when inhibited by catalase, anti-IL-6 and siRNA-IL-6 blocked TIMP-1 up-regulation and type I collagen accumulation. Thus, these results unveil a novel dual mechanism that is mediated by hydrogen peroxide (H2O2) and IL-6 by which KC modulate the fibrogenic response in HSC (95). A follow-up study unveiled synergism between arachidonic acid and ethanol to the mechanism by which KC mediate ECM remodeling and suggests that even if chronic ethanol consumption sensitizes HSC to up-regulate anti-fibrogenic signals, their effects are blunted by a second hit such as arachidonic acid (22).
Recent studies suggest that dietary factors such as protein intake can also modulate ROS-mediated collagen synthesis. HSC cultured in histidine-free medium decrease ROS production via the general control nonderepressible 2 (GCN2) and eukaryotic initiation factor 2α (eIF-2α) activation pathway (3) and lower type I collagen synthesis. Furthermore, Gcn2−/− mice showed more collagen deposition and liver injury after acute and chronic CCl4 administration (4). In contrast, leucine regulates type I collagen synthesis in HSC via increased production of ROS from NOX and mitochondria; leading to activation of the PI3K/Akt pathway, which up-regulates the transcription of type I collagen (106, 107). This demonstrates that protein intake could play a role during the progression of liver fibrosis.
Matrix-associated molecules and oxidant stress
Lately, several matrix-associated molecules have been implicated in the ROS-driven HSC fibrogenic response. As described earlier, work from our laboratory identified that OPN, an oxidant stress-sensitive cytokine, plays a major role in liver fibrosis (145).
Recently, we established that fibromodulin (FMOD), an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice (93). Our studies demonstrate that liver samples from patients with cirrhosis had higher levels of Fmod mRNA and protein than controls. Moreover, BDL, CCl4, and TAA treatment increased levels of FMOD protein in wild-type mice. Infection of HSC with an adenovirus that expressed FMOD increased the expression of type I collagen, indicating activation of HSC. Recombinant FMOD promoted proliferation, migration, and invasion of HSC, contributing to their fibrogenic activity. Lastly, co-culture of hepatocytes or SEC with HSC increased ROS in the culture medium, along with type I collagen and FMOD, which was prevented by catalase (93).
Metalloproteinases and oxidant stress
In an in vitro O2·− generating system, Galli et al. evaluated the role of MMP-2 in HSC proliferation and invasiveness (39). Using xanthine and xanthine oxidase as a generator of O2·−, they showed an increase in MMP-2 and in proteins from its activation complex such as MT1-MMP and TIMP2, which provide a negative feedback mechanism, induce HSC proliferation, and increase their invasive potential. The addition of GM6001 (a broad-spectrum MMP inhibitor), OA-Hy (an MMP-2 inhibitor), or vitamin E and superoxide dismutase polyethylene glycol (antioxidants) blocked these effects (39).
Fibrosis Resolution
Apoptosis
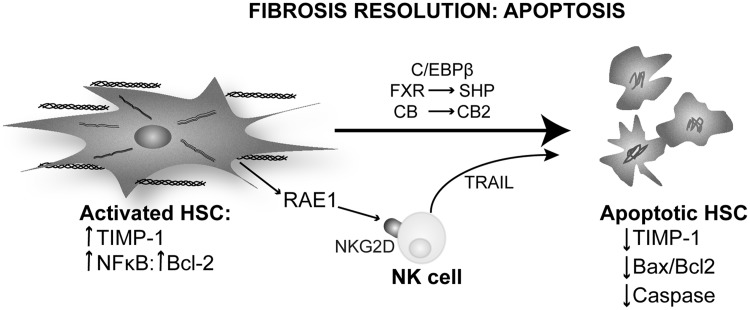
Apoptosis of activated HSC is the best-characterized mechanism of fibrosis resolution. Watson et al. identified NFκB as a key regulator for HSC survival and proliferation by maintaining the expression of the anti-apoptotic molecule Bcl-2 (150). Inhibition of the NFκB pathway increases HSC apoptosis by JNK up-regulation both in humans and in rodents (150).
C/EBPβ has been linked to HSC survival and apoptosis in liver fibrosis. C/EBPβ activates caspase 8-dependent apoptosis, which is inactivated by ribosomal S-6 Kinase (RSK) phosphorylation. Buck and Chojkier demonstrated that the nonphosphorylatable form in C/ebpβ Tg mice protects them from CCl4-induced liver fibrosis by inducing apoptosis of HSC. Treating wild-type mice with an RSK-inhibitory peptide induced HSC apoptosis, prevented liver fibrosis progression, and promoted regression (12).
Farnesoid X receptor (FXR) is a ligand-regulated transcription factor that acts as a sensor for bile acids. Fiorucci et al. demonstrated that an FXR-small heterodimer partner regulatory cascade modulates TIMP-1 and MMPs' expression in HSC and promotes resolution of liver fibrosis (33). This mechanism blocks the anti-apoptotic signaling of TIMP-1 and modulates the sensitivity to other apoptotic signals in HSC. In vivo treatment with an FXR ligand protected against liver fibrosis and helped fibrosis resolution (33).
Cannabinoids and their receptors have been linked to the resolution of liver fibrosis due to their apoptotic effects in MF. The cannabinoid receptors CB1 and CB2 have opposing roles in liver fibrosis. Stimulation of CB2 receptor in HSC promotes growth arrest via the COX2 pathway and apoptosis involving oxidative stress in a cannabinoid concentration-dependent manner (66). CB1 receptor plays a different role, as Cb1−/− exhibit less liver fibrosis than CCl4-injected wild-type mice. HSC isolated from Cb1−/− mice undergo apoptosis in serum-deprived medium, whereas normal HSC maintain their viability. Moreover, pharmacological blockade of the CB1 receptor decreases HSC proliferation and reduces the number of MF in vivo (137). Furthermore, cannabinoids induce HSC apoptosis that is mediated by ER-stress activating ASK1/JNK (84).
Natural killer (NK) cells are a part of the innate immune response and have been long implicated in apoptosis. Radaeva et al. showed that intrahepatic NK contribute to the resolution of liver fibrosis by targeting activated HSC (114). Activated but not quiescent HSC express RAE1, a ligand for the NK cells receptor NKG2D. An interaction of RAE1 with NKG2D triggers a cytotoxic response in NK cells that express TRAIL (death receptor activator) and activates HSC apoptosis (114). Glässner et al. confirmed this finding in human cells. However, there is a loss of NK cell activity with progression of fibrosis in human liver disease (43) (Fig. 6).
FIG. 6.
Mechanisms of fibrosis resolution: apoptosis. Activated HSC could undergo apoptosis as a part of the mechanism of fibrosis resolution. They transition from a survival phenotype that is characterized by enhanced expression of TIMP-1, activation of the Bcl-2/NFκB pathway and phosphorylation of C/EBPβ to an apoptotic phenotype, exhibiting reduction of TIMP-1 expression, and an increase in the Bax/Bcl-2 ratio that leads to activation of caspases. FXR leads to SHP-mediated down-regulation of TIMP. In response to ROS generation and ER stress, cannabinoids stimulate the CB2 receptor, promoting HSC apoptosis. NK cells interact with RAE1 in HSCs via the NKG2D receptor and trigger a TRAIL-mediated apoptotic response in HSC. C/EBPβ, CCAAT enhancer binding protein β; ER, endoplasmic reticulum; FXR, farnesoid X receptor; NK, natural killer; SHP, small heterodimer partner.
Senescence
Cellular senescence is an irreversible form of cell-cycle arrest that is triggered by stress-related events such as DNA damage, chromatin disruption, oxidative stress, and telomere dysfunction. Cells that enter senescence remain viable, and their metabolism is still active as they express senescence-associated secretory phenotype (SASP) but they do not respond to mitogens. Cellular senescence was first described in fibroblasts that experience replicative exhaustion in culture. Senescent fibroblasts are characterized by increased expression of inflammatory cytokines and MMPs as well as by down-regulation of selected ECM proteins (50). They are eventually cleared by NK cells, making senescence a candidate event to study the mechanisms driving fibrosis resolution.
Human HSC undergo senescence in culture after 9–15 passages due to shortening of telomeres. HSC undergoing senescence show a similar phenotype to skin fibroblasts with production of pro-inflammatory cytokines and chemokines, increased MMPs' expression, and reduction in ECM proteins.
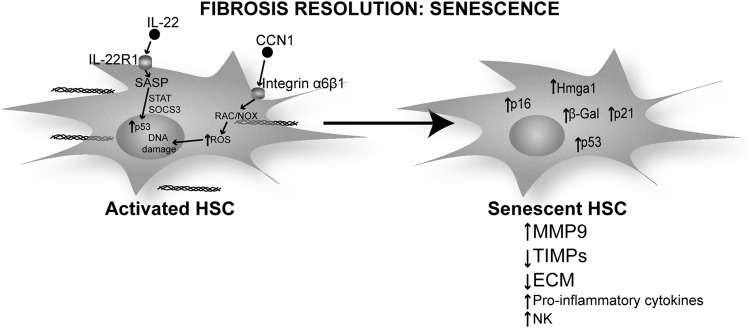
Krizhanovsky et al. described this mechanism in vivo in CCl4-induced liver injury (78). This work identified senescence markers such as β-Gal, p53, p21, p16, and Hmga1 that co-localize with HSC markers such as α-SMA and desmin in fibrotic areas from mouse liver. p53−/− and p16−/− mice lack a senescence program and develop more fibrosis than wild-type mice due to slower resolution on cessation of CCl4 administration. These mice also show as many α-SMA-positive HSC as do transgenic mice expressing a p53 silencing construct under the control of Gfap to target HSC. This suggests that p53 and p16 are key players in regulating HSC proliferation in liver fibrosis and in clearing HSC during the resolution phase (78).
IL-22 induces HSC senescence in vivo and in vitro. Indeed, IL-22 promotes survival in HSC by inducing senescence rather than proliferation as it occurs in epithelial cells. Challenge with IL-22 modulates the expression of SASP by up-regulating pro-inflammatory genes along with MMP-9 and down-regulating TIMPs' expression both in vivo and in vitro. The mechanism involves up-regulation of p53 and p21 (senescence markers) by IL-22 and appears to involve STAT3 and SOCS3, as deletion of these factors abolishes the p53 and p21 response to IL-22 and blocks HSC senescence (76).
A recent study shows that the matricellular protein CCN1, highly expressed in human cirrhotic livers, plays a role in MF senescence after liver injury (70). Ccn1−/− mice develop more liver fibrosis and senescence markers such as β-Gal and p16 when compared with wild-type or Ccn Tg mice. In addition, CCN1 engages integrin α6β1 and activates ROS production via the RAC1-NOX1 pathway. The authors suggest that an increase in ROS could lead to DNA damage and, thus, activate the p53 and p16 pathway to induce senescence (70) (Fig. 7).
FIG. 7.
Mechanisms of fibrosis resolution: senescence. Cellular senescence in HSC is characterized by a change from the proliferative matrix-secreting MF phenotype of activated HSC to a cell-cycle arrested nonproliferative phenotype that secretes pro-inflammatory cytokines, chemokines, and matrix remodeling proteins, reduces ECM protein deposition and TIMPs expression. Senescent HSC preserve markers of HSC activation such as α-SMA and senescence-related markers such as β-Gal, p53, p21, p16, and Hmga1. IL-22 signaling via the IL-22R1 receptor and CCN1 signaling via integrin α6β3 are the mechanisms proposed for the induction of senescence in HSC during the resolution of liver fibrosis. IL, interleukin.
Reversal to quiescence
Although still a controversial issue, experimental evidence suggesting differentiation of MF to a more quiescent phenotype has been proposed in CCl4 or BDL-treated rats 1 year after cessation of the insult (72). This was demonstrated by decreased type I collagen and TIMP-1 expression along with increased active MMPs (36, 105).
Macrophages are important for the reversion of activated HSC to quiescent cells, as macrophages clear several mediators that are essential for HSC activation. A study showed that depletion of macrophages during reversal of fibrosis delays matrix degradation (5). Scar-associated MMP-13-expressing macrophages have been targeted as the responsible subpopulation that is involved in the reversal to quiescence (30, 57). Models of chronic liver injury have also revealed that CCR-2 is indispensable for macrophage recruitment during liver fibrosis and that Ccr2−/− mice elicit sustained TIMP-1 and reduced MMP-13 expression, thus enabling a persistent fibrogenic response (92). During reversal to quiescence, there is a rapid decline in TIMPs' expression, tipping the balance between MMPs and TIMPs and resulting in increased scar degradation (59).
Reversibility of liver fibrosis in humans: assessment and therapy
Regression of liver fibrosis is typically defined as a decrease in total matrix content (57). The majority of patients show a variable degree of reversibility of fibrosis after antiviral treatment (triple therapy with peginterferon alfa, ribavirin, and telaprevir or boceprevir), but there is no complete reversal to quiescence (36). So far, the assessment of regression largely relies on histopathological scoring of liver tissue obtained by needle biopsy, an invasive procedure that only provides a limited and static measurement of fibrosis (14). Over the past years, novel tests have been developed to assess the degree of stiffness noninvansively, for instance, transient elastography (FibroScan, now FDA approved for noninvasive liver diagnosis) and serum biomarkers of fibrosis such as the FibroTest® algorithm (14). Therefore, the availability of novel diagnostic techniques for monitoring the progression of liver disease will facilitate the evaluation of reversal from fibrosis and also of the efficiency of antifibrotic therapies.
The current therapy for reversing liver fibrosis focuses on reducing inflammation and the subsequent liver injury, in addition to targeting HCV replication (29). A number of dietary hepatoprotectants have been used to attenuate or neutralize the upstream inflammatory responses and, thus, HSC activation. Among these, silymarin (found in milk thistle), curcumin (turmeric), resveratrol (a component from red wine), and coffee have been tested (29). Promising results in experimental and clinical research have been given by vitamin E (123), a novel HGF mimetic agent (Refanalin), and caspase inhibitors (29, 155). Of particular interest is the development of PPARγ ligands (also known as glitazones) with the goal to reverse the biochemical and morphological features of HSC activation and, therefore, maintain HSC quiescence (79). Other molecules that are currently being tested in randomized controlled trials include inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase such as statins or their combination with sartans, angiotensin II subtype 1 receptor antagonists, which block HSC proliferation and collagen deposition in patients and in experimental models of liver fibrosis (139). Finally, the development of drugs that are capable of stimulating the degradation of accumulated scar tissue, particularly in patients with advanced disease, is an attractive approach. Indeed, in animal models, collagenolytic MMPs or TIMP-1 scavengers have been delivered systemically via adenoviral vectors and have been proved to stimulate ECM degradation (137).
Epigenetics, an Evolving Field
Epigenetic regulation of HSCs
Epigenetics is a process that alters gene activity without changing the DNA sequence, leading to heritable modifications. These modifications are natural and essential for normal development, differentiation, and tissue-specific gene expression. However, epigenetic abnormalities could occur due to environmental factors such as toxins, drugs, or diseases such as cancer, autoimmune disease, age-related illness, neurological disorders, and ALD (8, 31, 32, 65, 87).
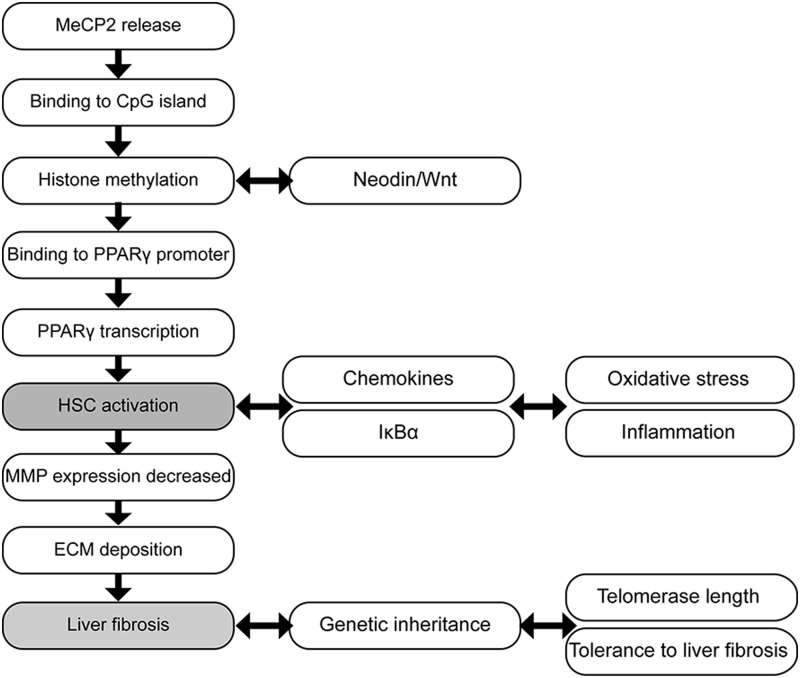
Recent studies have highlighted the regulatory effect of epigenetic modifications on gene expression in HSC and on the risk of developing liver fibrosis (131, 161). Quiescent HSC acquire either an adipogenic or a myogenic phenotype depending on the balance between clusters of genes during their differentiation (142). Under normal conditions, adipogenic genes are dominant and quiescent HSC differentiate into adipogenic cells. When liver injury occurs, the prevalence of myogenic genes such as type I collagen, TIMP-1, or α-SMA promotes the differentiation of adipogenic HSC to myogenic HSC (5, 37). Moreover, aberrant expression of a series of histones and chemokines in activated HSC can aggravate inflammation and generate ROS and that increase HSC differentiation into MF, consequently enhancing liver fibrosis. The degradation of ECM is also affected by epigenetic modulation of matrix-associated enzymes such as MMPs (108).
The study of the methylation status of the promoters from fibrosis-related genes has recently become a hot topic over the past few years. For instance, the methylation status of SP1, a gene related to immunity, is down-regulated after CCl4-induced liver injury (49). Mann and collaborators observed that the offspring of mice exposed to CCl4 developed tolerance to liver injury (88). They hypothesized that genes related to inflammation and differentiation are hypermethylated, whereas most anti-fibrogenetic genes are hypomethylated (Fig. 8).
FIG. 8.
Epigenetic modifications in HSC contribute to the development of liver fibrosis. MeCP2 binds methylated DNA sequences and participates in the regulation of PPARγ mediating the differentiation of HSC and the development of liver fibrosis. These epigenetic modifications are transmitted intergenerationally. MeCP2, methyl-CpG binding protein-2; PPARγ, peroxisome proliferator-activated receptor γ.
Epigenetic regulation of MMPs in HSC alters ECM degradation (113). Impaired histone deacetylation during HSC differentiation leads to increased histone deacytelase-4 and suppression of several MMP genes in quiescent HSC, accordingly silencing MMP activity and contributing to scarring (113). Taking into account the direct interplay between TIMP-3, MMPs, and ECM, methylation of TIMP-1 and TIMP-3 could be considered in the future as a reliable predictor of liver fibrosis (108).
Liver disease and epigenetics
A primary goal for preventing liver fibrosis is to discover accurate methods for early epigenetic diagnosis besides developing noninvasive methods to promote reversibility. In order to do so, we should dissect whether epigenetic alterations occur before proteomic deviations or vice versa. This is of clinical relevance, as epigenetic modifications can regulate HSC genes (131). Preliminary data suggest an association between hypomethylation of the PPARγ gene promoter and mild fibrosis in NAFLD patients (161). Recent studies show that inhibitors of DNA methyltransferase reduce the rate of DNA hypermethylation (143) and that a methyl-deficient diet regulates gene methylation and the development of liver fibrosis (140). Lastly, a recent study demonstrated that the necdin-Wnt pathway could be regulated to mediate anti-adipogenic HSC trans-differentiation via epigenetic repression of PPARγ, which may have therapeutic application. Silencing necdin either with shRNA or with rosmarinic acid and baicalin reverses activated HSC to quiescent cells in a PPARγ-dependent manner by suppressing canonical Wnt signaling (158).
Epigenetic modifications triggered by alcohol appear to play a relevant role in the development and progression of ALD. This has been described in parenchymal and nonparenchymal liver cells and contributes to fat accumulation and inflammation (87). In summary, the regulation of epigenetic alterations in key genes may be of therapeutic potential in the future to reverse liver fibrosis and hopefully, HCC. However, further understanding of the mechanisms of transgenerational inheritance in humans needs to be met.
New Tools to Study ECM
Microarrays
Microarrays have advanced the study of liver fibrosis and cirrhosis in humans and in animal models by identifying differentially expressed genes. A study comparing the expression of 22,000 genes from liver biopsies of HCV-infected patients at different disease stages identified 219 differentially expressed genes (1). Other studies have used DNA or cDNA microarray techniques to investigate the alteration of gene expression in liver fibrosis in in vitro or in vivo models (62, 112, 134). Similar analyses were performed by Mas et al. to identify changes in gene expression in patients with HCV-induced and with alcohol-induced liver cirrhosis (89). This study showed that genes involved in virus and immune responses as well as those regulating apoptosis were increased in HCV-induced cirrhosis whereas genes from the cytochrome P450 superfamily were found to be decreased in alcoholic cirrhosis (89). Lastly, Iizuka et al. demonstrated changes in gene expression according to HCV serotypes (54). Thus, the etiology of liver disease conditions gene signatures. However, the microarray technology has not been widely adopted as a routine clinical test not only due to the cost but also due to the lack of correlation between mRNA and protein expression (119).
Proteomics
Proteomics is a large-scale analysis of protein composition, structure, function, activity, and interaction with other molecules in a given biological system. However, post-translational modifications add a significant layer of complexity to interpret the results from this approach. The ECM components undergo vigorous post-translational modifications conditioning protein function (13). For example, type IV collagen, the major basement membrane component in the liver, undergoes glycosylation, phosphorylation, and ubiquitination, enabling collagen to interact with other ECM proteins and cells. By using ECM cataloguing, Rashid et al. showed that the components of the fibrotic tissue are unique to their localization. They used SDS-PAGE separation and mass spectrometry-based proteomic analysis to dissect the protein–protein interaction network and compare cell type-specific ECM niches between human foreskin fibroblasts and HSC cell lines (115). For example, fibulin-2 is an ECM protein in the protein network in the HSC ECM but is one of the least interacted in the human foreskin fibroblast protein network. This suggests that the composition and organization of the ECM protein network is tissue specific.
A limitation of this study is the lack of direct comparison between quiescent and activated HSC, as this could give a better picture in the changes in HSC in health and disease. Matrigel, a commercial basement membrane matrix, resembles the ECM environment and maintains HSC in quiescence (130). Although Matrigel could be used to reverse HSC activation, it would also add other proteins to the cell-derived ECM and alter the results from the mass spectrometry analysis. Consequently, alternative approaches are needed to study the transition of HSC in disease.
In a pilot study by Poon et al. (109), a new serum proteomic fingerprint associated with liver fibrosis was identified. Using surface-enhanced laser desorption/ionization protein-chip arrays technology, they constructed an artificial network fibrosis index that changes with the stage of liver fibrosis (109).
Although an active effort is in place for the search of noninvasive, cost-effective, and disease- and organ-specific liver fibrosis biomarkers, no reliable parameter is currently available for clinical diagnosis. A limiting factor may be the low reproducibility of the proteomics technology. This pitfall is due to a lack of standardized sample preparation protocol, for example, in sample purification and fractionation before analysis.
Innovation
This review drives from the composition and sources of the extracellular matrix (ECM) in healthy and injured liver to the potential mechanism driving fibrosis resolution such as myofibroblasts' apoptosis, senescence, and reversal to quiescence, thus compiling new advances in the diagnosis and treatment of liver fibrosis. It compiles a general and specific point of view of the role of ECM deposition in an oxidant environment in liver disease.
Future Directions
Liver biopsy is still the gold standard in the assessment of liver disease, but it is an invasive procedure and may cause clinical complications. Variations in diagnosis may also arise from sampling error and inter-observer observation (7, 20, 116). Careful integration of all the existing data from imaging techniques, genomics, transcriptomics, and proteomics analyses will provide a comprehensive view of changes in gene and protein expression, structure, function, and interactions in the disease state. The potential of systems biology for identifying specific and unique patterns of liver disease based on etiology may facilitate the diagnosis, prognosis, and, ultimately, treatment. As a result, increased accuracy in these strategies is required to correlate potential biomarkers with disease.
Abbreviations Used
- α-SMA
α-smooth muscle actin
- ALD
alcoholic liver disease
- ASH
alcoholic steatohepatitis
- BDL
bile duct ligation
- C/EBPβ
CCAAT enhancer binding protein β
- Cb1−/−
Cb1 knockout mice
- CCl4
carbon tetrachloride
- Ccn1−/−
Ccn knockout mice
- CCR-2/5
CC-chemokine receptor-2/5
- Ccr2−/−
Ccr2 knockout mice
- Col1a1
type I(α1) collagen gene
- Col1a2
type I(α2) collagen gene
- CTGF
connective tissue growth factor
- CYP2E1
cytochrome P450 2E1
- ECM
extracellular matrix
- eIF-2α
eukaryotic initiation factor 2α
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- FACITs
fibril-associated collagens with interrupted triple helix
- FGF
fibroblast growth factor
- FMOD
fibromodulin
- Fmod−/−
FMOD knockout mice
- FXR
farnesoid X receptor
- GCN2
general control nonderepressible 2
- Gcn2−/−
Gcn2 knockout mice
- GFAP
glial fibrillary acidic protein
- H2O2
hydrogen peroxide
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HGF
hepatocyte growth factor
- HMGB1
high mobility group box-1
- HSC
hepatic stellate cell
- HuR
RNA-binding protein human antigen R
- IL
interleukin
- KC
Kupffer cells
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MCSF
macrophage colony-stimulating factor
- MeCP2
methyl-CpG binding protein-2
- MF
myofibroblasts
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cells
- NASH
nonalcoholic steatohepatitis
- NFκB
nuclear factor kappa B
- NK
natural killer
- NOX
NADPH oxidase
- O2·−
superoxide radical
- OPN
osteopontin
- Opn−/−
OPN knockout mice
- OpnHEP Tg
transgenic mice overexpressing OPN in hepatocytes
- p38MAPK
p38 mitogen-activated protein kinase
- PDGF
platelet-derived growth factor
- PF
portal fibroblasts
- PPARγ
peroxisome proliferator-activated receptor γ
- ROS
reactive oxygen species
- RSK
ribosomal S-6 kinase
- SASP
senescence-associated secretory phenotype
- SEC
sinusoidal endothelial cells
- SHP
small heterodimer partner
- TAA
thioacetamide
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of metalloproteinase
- TLR
toll like receptor
- TNFR1
TNF-α receptor 1
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Acknowledgments
US Public Health Service Grants 5R01 DK069286, 2R56 DK069286, and 3 R56 DK069286-06S1 from the National Institute of Diabetes and Digestive and Kidney Diseases (N. N.); US Public Health Service Grants U01 AA021887-01, 5 P20 AA017067, 5 P20 AA017067-01S1, and 5 P20 AA017067-03S1 from the National Institute on Alcohol Abuse and Alcoholism (N. N.).
References
- 1.Ahmad W, Ijaz B, and Hassan S. Gene expression profiling of HCV genotype 3a initial liver fibrosis and cirrhosis patients using microarray. J Transl Med 10: 41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford AI. and Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone 38: 749–757, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Arriazu E, Perez de Obanos MP, Lopez-Zabalza MJ, Herraiz MT, and Iraburu MJ. Amino acid deprivation decreases intracellular levels of reactive oxygen species in hepatic stellate cells. Cell Physiol Biochem 26: 281–290, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Arriazu E, Ruiz de Galarreta M, Lopez-Zabalza MJ, Leung TM, Nieto N, and Iraburu MJ. GCN2 kinase is a key regulator of fibrogenesis and acute and chronic liver injury induced by carbon tetrachloride in mice. Lab Invest 93: 303–310, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Bataller R. and Brenner DA. Liver fibrosis. J Clin Invest 115: 209–218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, and Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest 87: 292–303, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargere D, and Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38: 1449–1457, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bird A. Perceptions of epigenetics. Nature 447: 396–398, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal 3: 163–165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosman FT. and Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol 200: 423–428, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Brew K. and Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck M. and Chojkier M. A ribosomal S-6 kinase-mediated signal to C/EBP-beta is critical for the development of liver fibrosis. PLoS One 2: e1372, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byron A, Humphries JD, and Humphries MJ. Defining the extracellular matrix using proteomics. Int J Exp Pathol 2013[Epub ahead of print]; DOI: 10.1111/iep.12011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castera L. and Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut 59: 861–866, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Cederbaum AI. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World J Gastroenterol 16: 1366–1376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cederbaum AI. Alcohol metabolism. Clin Liver Dis 16: 667–685, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiquet-Ehrismann R. and Tucker RP. Connective tissues: signalling by tenascins. Int J Biochem Cell Biol 36: 1085–1089, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Choi SS. and Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology 50: 2007–2013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly MK, Bedrosian AS, Malhotra A, Henning JR, Ibrahim J, Vera V, Cieza-Rubio NE, Hassan BU, Pachter HL, Cohen S, Frey AB, and Miller G. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol 185: 2200–2208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox LA, Schlabritz-Loutsevitch N, Hubbard GB, Nijland MJ, McDonald TJ, and Nathanielsz PW. Gene expression profile differences in left and right liver lobes from mid-gestation fetal baboons: a cautionary tale. J Physiol 572: 59–66, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox TR. and Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 4: 165–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cubero FJ. and Nieto N. Ethanol and arachidonic acid synergize to activate Kupffer cells and modulate the fibrogenic response via tumor necrosis factor alpha, reduced glutathione, and transforming growth factor beta-dependent mechanisms. Hepatology 48: 2027–2039, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cubero FJ. and Nieto N. Arachidonic acid stimulates TNFalpha production in Kupffer cells via a reactive oxygen species-pERK1/2-Egr1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 303: G228–G239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley WP, Peters SB, and Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121: 255–264, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Diaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, Russo PA, Rand EB, Furth EE, and Wells RG. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol 39: 102–115, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Dong QY. and Wu ZY. Advance in the pathogenesis and treatment of Wilson disease. Transl Neurodegener 1: 23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dooley S. and ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res 347: 245–256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med 12: 22–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fallowfield JA. Therapeutic targets in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 300: G709–G715, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, and Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 178: 5288–5295, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature 447: 433–440, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ferguson-Smith AC. and Greally JM. Epigenetics: perceptive enzymes. Nature 449: 148–149, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, and Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther 314: 584–595, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Frantz C, Stewart KM, and Weaver VM. The extracellular matrix at a glance. J Cell Sci 123: 4195–4200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol 38Suppl 1: S38–S53, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Friedman SL. Reversibility of hepatic fibrosis and cirrhosis—is it all hype? Nat Clin Pract Gastroenterol Hepatol 4: 236–237, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88: 125–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, Surrenti C, and Casini A. Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology 41: 1074–1084, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 21: 311–335, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Gelse K, Poschl E, and Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55: 1531–1546, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Germani G, Burroughs AK, and Dhillon AP. The relationship between liver disease stage and liver fibrosis: a tangled web. Histopathology 57: 773–784, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Glässner A, Eisenhardt M, Kramer B, Korner C, Coenen M, Sauerbruch T, Spengler U, and Nattermann J. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest 92: 967–977, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Gordon MK. and Hahn RA. Collagens. Cell Tissue Res 339: 247–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gressner AM. and Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 10: 76–99, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guicciardi ME. and Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis 30: 402–410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall A, Germani G, Isgro G, Burroughs AK, and Dhillon AP. Fibrosis distribution in explanted cirrhotic livers. Histopathology 60: 270–277, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, and Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab 89: 4963–4971, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Harder J, Opitz OG, Brabender J, Olschewski M, Blum HE, Nomoto S, and Usadel H. Quantitative promoter methylation analysis of hepatocellular carcinoma, cirrhotic and normal liver. Int J Cancer 122: 2800–2804, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Hayflick L. and Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621, 1961 [DOI] [PubMed] [Google Scholar]
- 51.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, and Friedman SL. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142: 938–946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, Devi LA, and Friedman SL. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol 59: 98–104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iizuka N, Oka M, Yamada-Okabe H, Mori N, Tamesa T, Okada T, Takemoto N, Hashimoto K, Tangoku A, Hamada K, Nakayama H, Miyamoto T, Uchimura S, and Hamamoto Y. Differential gene expression in distinct virologic types of hepatocellular carcinoma: association with liver cirrhosis. Oncogene 22: 3007–3014, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Inagaki Y. and Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut 56: 284–292, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iraburu MJ, Dominguez-Rosales JA, Fontana L, Auster A, Garcia-Trevijano ER, Covarrubias-Pinedo A, Rivas-Estilla AM, Greenwel P, and Rojkind M. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology 31: 1086–1093, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117: 539–548, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irie M, Sohda T, Iwata K, Kunimoto H, Fukunaga A, Kuno S, Yotsumoto K, Sakurai K, Iwashita H, Hirano G, Ueda SI, Yokoyama K, Morihara D, Nishizawa S, Anan A, Takeyama Y, Sakamoto M, Shakado S, and Sakisaka S. Levels of the oxidative stress marker gamma-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J Int Med Res 40: 924–933, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, and Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126: 1795–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Iwaisako K, Brenner DA, and Kisseleva T. What's new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol 27Suppl 2: 65–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jarvelainen H, Sainio A, Koulu M, Wight TN, and Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev 61: 198–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang F, Parsons CJ, and Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol 45: 401–409, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Jiang JX, Mikami K, Venugopal S, Li Y, and Torok NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol 51: 139–148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson SJ, Hines JE, and Burt AD. Macrophage and perisinusoidal cell kinetics in acute liver injury. J Pathol 166: 351–358, 1992 [DOI] [PubMed] [Google Scholar]
- 65.Jones RS. Epigenetics: reversing the ‘irreversible’. Nature 450: 357–359, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, and Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128: 742–755, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Kage M, Shimamatu K, Nakashima E, Kojiro M, Inoue O, and Yano M. Long-term evolution of fibrosis from chronic hepatitis to cirrhosis in patients with hepatitis C: morphometric analysis of repeated biopsies. Hepatology 25: 1028–1031, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ, and Framingham S. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 23: 434–439, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, and Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology 49: 901–910, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Kim KH, Chen CC, Monzon RI, and Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol 33: 2078–2090, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, and Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 83: 163–173, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Kisseleva T. and Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol 22Suppl 1: S73–S78, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, and Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 45: 429–438, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, Uno M, Matsuzawa-Nagata N, Kato K, Ando H, Fujimura A, Hayashi K, Kimura T, Ni Y, Otoda T, Miyamoto K, Zen Y, Nakanuma Y, and Kaneko S. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PLoS One 7: e43056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knupp C. and Squire JM. Molecular packing in network-forming collagens. Adv Protein Chem 70: 375–403, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, and Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 56: 1150–1159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Konno S, Kurokawa M, Uede T, Nishimura M, and Huang SK. Role of osteopontin, a multifunctional protein, in allergy and asthma. Clin Exp Allergy 41: 1360–1366, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, and Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leclercq IA, Sempoux C, Starkel P, and Horsmans Y. Limited therapeutic efficacy of pioglitazone on progression of hepatic fibrosis in rats. Gut 55: 1020–1029, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee M. and Kowdley KV. Alcohol's effect on other chronic liver diseases. Clin Liver Dis 16: 827–837, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Lee SH, Seo GS, Park YN, Yoo TM, and Sohn DH. Effects and regulation of osteopontin in rat hepatic stellate cells. Biochem Pharmacol 68: 2367–2378, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, and Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol 99: 271–279, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, and Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol 50: 1174–1183, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Lim MP, Devi LA, and Rozenfeld R. Cannabidiol causes activated hepatic stellate cell death through a mechanism of endoplasmic reticulum stress-induced apoptosis. Cell Death Dis 2: e170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorena D, Darby IA, Reinhardt DP, Sapin V, Rosenbaum J, and Desmouliere A. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest 84: 203–212, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Lu P, Takai K, Weaver VM, and Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3: pii:a005058, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mandrekar P. Epigenetic regulation in alcoholic liver disease. World J Gastroenterol 17: 2456–2464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, and Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 138: 705–714, 714.e1–e4,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mas VR, Fassnacht R, Archer KJ, and Maluf D. Molecular mechanisms involved in the interaction effects of alcohol and hepatitis C virus in liver cirrhosis. Mol Med 16: 287–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matsuoka M. and Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology 11: 599–605, 1990 [DOI] [PubMed] [Google Scholar]
- 91.Midwood KS. and Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal 3: 287–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Renia L, Pol S, Mallet V, and Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174: 1766–1775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mormone E, Lu Y, Ge X, Fiel MI, and Nieto N. Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice. Gastroenterology 142: 612–621 e5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Munshi MK, Uddin MN, and Glaser SS. The role of the renin-angiotensin system in liver fibrosis. Exp Biol Med (Maywood) 236: 557–566, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology 44: 1487–1501, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Nieto N. Ethanol and fish oil induce NFkappaB transactivation of the collagen alpha2(I) promoter through lipid peroxidation-driven activation of the PKC-PI3K-Akt pathway. Hepatology 45: 1433–1445, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Nieto N, Friedman SL, and Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem 277: 9853–9864, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Nieto N, Friedman SL, and Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology 35: 62–73, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Nieto N, Friedman SL, Greenwel P, and Cederbaum AI. CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology 30: 987–996, 1999 [DOI] [PubMed] [Google Scholar]
- 100.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, and Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem 275: 20136–20145, 2000 [DOI] [PubMed] [Google Scholar]
- 101.Novo E, di Bonzo LV, Cannito S, Colombatto S, and Parola M. Hepatic myofibroblasts: a heterogeneous population of multifunctional cells in liver fibrogenesis. Int J Biochem Cell Biol 41: 2089–2093, 2009 [DOI] [PubMed] [Google Scholar]
- 102.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, and Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 118: 3331–3342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Page-McCaw A, Ewald AJ, and Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8: 221–233, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pankov R. and Yamada KM. Fibronectin at a glance. J Cell Sci 115: 3861–3863, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Pellicoro A, Ramachandran P, and Iredale JP. Reversibility of liver fibrosis. Fibrogenesis Tissue Repair 5Suppl 1: S26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perez de Obanos MP, Lopez-Zabalza MJ, Arriazu E, Modol T, Prieto J, Herraiz MT, and Iraburu MJ. Reactive oxygen species (ROS) mediate the effects of leucine on translation regulation and type I collagen production in hepatic stellate cells. Biochim Biophys Acta 1773: 1681–1688, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Perez de Obanos MP, Lopez Zabalza MJ, Prieto J, Herraiz MT, and Iraburu MJ. Leucine stimulates procollagen alpha1(I) translation on hepatic stellate cells through ERK and PI3K/Akt/mTOR activation. J Cell Physiol 209: 580–586, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Perugorria MJ, Wilson CL, Zeybel M, Walsh M, Amin S, Robinson S, White SA, Burt AD, Oakley F, Tsukamoto H, Mann DA, and Mann J. Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology 56: 1129–1139, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poon TC, Hui AY, Chan HL, Ang IL, Chow SM, Wong N, and Sung JJ. Prediction of liver fibrosis and cirrhosis in chronic hepatitis B infection by serum proteomic fingerprinting: a pilot study. Clin Chem 51: 328–335, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, and Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol 34: 730–739, 2001 [DOI] [PubMed] [Google Scholar]
- 111.Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, Moles A, Mann DA, Bobola N, Sharrocks AD, Thomson BJ, Zaitoun AM, Irving WL, Guha IN, Hanley NA, and Hanley KP. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology 56: 1108–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiang H, Lin Y, Zhang X, Zeng X, Shi J, Chen YX, Yang MF, Han ZG, and Xie WF. Differential expression genes analyzed by cDNA array in the regulation of rat hepatic fibrogenesis. Liver Int 26: 1126–1137, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Qin L. and Han YP. Epigenetic repression of matrix metalloproteinases in myofibroblastic hepatic stellate cells through histone deacetylases 4: implication in tissue fibrosis. Am J Pathol 177: 1915–1928, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, and Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 130: 435–452, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Rashid ST, Humphries JD, Byron A, Dhar A, Askari JA, Selley JN, Knight D, Goldin RD, Thursz M, and Humphries MJ. Proteomic analysis of extracellular matrix from the hepatic stellate cell line LX-2 identifies CYR61 and Wnt-5a as novel constituents of fibrotic liver. J Proteome Res 11: 4052–4064, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T, and Group LS. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128: 1898–1906, 2005 [DOI] [PubMed] [Google Scholar]
- 117.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol 3: a004978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ricard-Blum S. and Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 53: 430–442, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Rogers S, Girolami M, Kolch W, Waters KM, Liu T, Thrall B, and Wiley HS. Investigating the correspondence between transcriptomic and proteomic expression profiles using coupled cluster models. Bioinformatics 24: 2894–2900, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rozario T. and DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol 341: 126–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, and Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130: 1807–1821, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Santos PC, Krieger JE, and Pereira AC. Molecular diagnostic and pathogenesis of hereditary hemochromatosis. Int J Mol Sci 13: 1497–1511, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, and Nash CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sasaki T, Fassler R, and Hohenester E. Laminin: the crux of basement membrane assembly. J Cell Biol 164: 959–963, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saxena NK, Ikeda K, Rockey DC, Friedman SL, and Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 35: 762–771, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, and Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J 18: 1612–1614, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, and Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology 34: 89–100, 2001 [DOI] [PubMed] [Google Scholar]
- 128.Schwabe RF, Bataller R, and Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol 285: G949–G958, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Senoo H, Kojima N, and Sato M. Vitamin A-storing cells (stellate cells). Vitam Horm 75: 131–159, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Shimada H. and Rajagopalan LE. Employment of gene expression profiling to identify transcriptional regulators of hepatic stellate cells. Fibrogenesis Tissue Repair 5Suppl 1: S12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 6: 838–842, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, and Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology 53: 106–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, and Inoue K. Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology 35: 49–61, 2002 [DOI] [PubMed] [Google Scholar]
- 134.Takahara Y, Takahashi M, Wagatsuma H, Yokoya F, Zhang QW, Yamaguchi M, Aburatani H, and Kawada N. Gene expression profiles of hepatic cell-type specific marker genes in progression of liver fibrosis. World J Gastroenterol 12: 6473–6499, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang M, Potter JJ, and Mezey E. Activation of the human alpha1(I) collagen promoter by leptin is not mediated by transforming growth factor beta responsive elements. Biochem Biophys Res Commun 312: 629–633, 2003 [DOI] [PubMed] [Google Scholar]
- 136.Tarrats N, Moles A, Morales A, Garcia-Ruiz C, Fernandez-Checa JC, and Mari M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology 54: 319–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, and Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12: 671–676, 2006 [DOI] [PubMed] [Google Scholar]
- 138.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, and Martin GR. Laminin—a glycoprotein from basement membranes. J Biol Chem 254: 9933–9937, 1979 [PubMed] [Google Scholar]
- 139.Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, Vogt A, Dienes HP, Lammert F, Reichen J, Heller J, and Sauerbruch T. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol 53: 702–712, 2010 [DOI] [PubMed] [Google Scholar]
- 140.Tryndyak VP, Han T, Muskhelishvili L, Fuscoe JC, Ross SA, Beland FA, and Pogribny IP. Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Mol Nutr Food Res 55: 411–418, 2011 [DOI] [PubMed] [Google Scholar]
- 141.Tsukamoto H. Redox regulation of cytokine expression in Kupffer cells. Antioxid Redox Signal 4: 741–748, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Tsukamoto H. Epigenetic mechanism of stellate cell trans-differentiation. J Hepatol 46: 352–353, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Um TH, Kim H, Oh BK, Kim MS, Kim KS, Jung G, and Park YN. Aberrant CpG island hypermethylation in dysplastic nodules and early HCC of hepatitis B virus-related human multistep hepatocarcinogenesis. J Hepatol 54: 939–947, 2011 [DOI] [PubMed] [Google Scholar]
- 144.Urtasun R, Cubero FJ, Vera M, and Nieto N. Reactive nitrogen species switch on early extracellular matrix remodeling via induction of MMP1 and TNFalpha. Gastroenterology 136: 1410–1422, e1–e4,2009 [DOI] [PubMed] [Google Scholar]
- 145.Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, Ge X, Fiel MI, and Nieto N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology 55: 594–608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vakonakis I, Staunton D, Rooney LM, and Campbell ID. Interdomain association in fibronectin: insight into cryptic sites and fibrillogenesis. EMBO J 26: 2575–2583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Varela-Rey M, Fontan-Gabas L, Blanco P, Lopez-Zabalza MJ, and Iraburu MJ. Glutathione depletion is involved in the inhibition of procollagen alpha1(I) mRNA levels caused by TNF-alpha on hepatic stellate cells. Cytokine 37: 212–217, 2007 [DOI] [PubMed] [Google Scholar]
- 148.Varela-Rey M, Montiel-Duarte C, Oses-Prieto JA, Lopez-Zabalza MJ, Jaffrezou JP, Rojkind M, and Iraburu MJ. p38 MAPK mediates the regulation of alpha1(I) procollagen mRNA levels by TNF-alpha and TGF-beta in a cell line of rat hepatic stellate cells(1). FEBS Lett 528: 133–138, 2002 [DOI] [PubMed] [Google Scholar]
- 149.Veit G, Zimina EP, Franzke CW, Kutsch S, Siebolds U, Gordon MK, Bruckner-Tuderman L, and Koch M. Shedding of collagen XXIII is mediated by furin and depends on the plasma membrane microenvironment. J Biol Chem 282: 27424–27435, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Watson MR, Wallace K, Gieling RG, Manas DM, Jaffray E, Hay RT, Mann DA, and Oakley F. NF-kappaB is a critical regulator of the survival of rodent and human hepatic myofibroblasts. J Hepatol 48: 589–597, 2008 [DOI] [PubMed] [Google Scholar]
- 151.Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis 12: 759–768, viii, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.White ES, Baralle FE, and Muro AF. New insights into form and function of fibronectin splice variants. J Pathol 216: 1–14, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wight TN. and Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol 301: G950–G955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wisse E, Braet F, Luo D, De Zanger R, Jans D, Crabbe E, and Vermoesen A. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol 24: 100–111, 1996 [DOI] [PubMed] [Google Scholar]
- 155.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS, Guy CD, Pollard J, Charlton P, and Diehl AM. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology 50: 1421–1430, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Woodhoo A, Iruarrizaga-Lejarreta M, Beraza N, Garcia-Rodriguez JL, Embade N, Fernandez-Ramos D, Martinez-Lopez N, Gutierrez-De Juan V, Arteta B, Caballeria J, Lu SC, Mato JM, Varela-Rey M, and Martinez-Chantar ML. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology 56: 1870–1882, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xie H, Song J, Du R, Liu K, Wang J, Tang H, Bai F, Liang J, Lin T, Liu J, and Fan D. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig Liver Dis 39: 167–172, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Yang MD, Chiang YM, Higashiyama R, Asahina K, Mann DA, Mann J, Wang CC, and Tsukamoto H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology 55: 1271–1281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yurchenco PD. and Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15: 1277–1294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, and Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282: 23337–23347, 2007 [DOI] [PubMed] [Google Scholar]
- 161.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, Oakley F, Burt AD, Wilson CL, Anstee QM, Barter MJ, Masson S, Elsharkawy AM, Mann DA, and Mann J. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med 18: 1369–1377, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Xie H, Wang J, Han Y, Liu Z, and Fan D. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int J Clin Pract 62: 1056–1062, 2008 [DOI] [PubMed] [Google Scholar]