Abstract
The split hand phenomenon refers to predominant wasting of thenar muscles and is an early and specific feature of amyotrophic lateral sclerosis (ALS). A novel split hand index (SI) was developed to quantify the split hand phenomenon, and its diagnostic utility was assessed in ALS patients. The split hand index was derived by dividing the product of the compound muscle action potential (CMAP) amplitude recorded over the abductor pollicis brevis and first dorsal interosseous muscles by the CMAP amplitude recorded over the abductor digiti minimi muscle. In order to assess the diagnostic utility of the split hand index, ALS patients were prospectively assessed and their results were compared to neuromuscular disorder patients. The split hand index was significantly reduced in ALS when compared to neuromuscular disorder patients (P<0.0001). Limb-onset ALS patients exhibited the greatest reduction in the split hand index, and a value of 5.2 or less reliably differentiated ALS from other neuromuscular disorders. Consequently, the split hand index appears to be a novel diagnostic biomarker for ALS, perhaps facilitating an earlier diagnosis.
Keywords: Medicine, Issue 85, Amyotrophic Lateral Sclerosis (ALS), dissociated muscle atrophy, hypothenar muscles, motor neuron disease, split-hand index, thenar muscles
Introduction
The split hand, refers to preferential atrophy of the thenar complex group of intrinsic hand muscles [abductor pollicis brevis (APB) and first dorsal interosseous (FDI)], with relative preservation of hypothenar muscles, and is a specific and early clinical feature of amyotrophic lateral sclerosis (ALS)1-4. The finding that the split hand sign is specific for ALS, suggests a potential role for the split hand sign as a diagnostic biomarker in ALS3.
Quantification of the split hand sign, through the development of a novel neurophysiological biomarker, may further aid in ALS diagnosis. Specifically, the split hand index (SI), which quantifies the split hand phenomenon, is derived by multiplying the compound muscle action potential (CMAP) amplitude recorded over the thenar complex muscles (APB and FDI), and dividing this product by the CMAP amplitude recorded over the hypothenar muscles (namely the abductor digiti minimi, ADM)5.
The diagnosis of ALS relies largely on clinically based criteria encompassing a combination of upper and lower motor neuron signs6. These criteria, however, were deemed insensitive especially in establishing a diagnosis of ALS in the early stages of the disease process7-10. A recent modification of the diagnostic criteria were developed11, and although these criteria appear to increase the diagnostic sensitivity12-16, the increase sensitivity seems restricted to bulbar-onset ALS patients15.
In the absence of a pathognomonic test, the diagnosis of ALS may be significantly delayed8. Ultimately, the institution of neuroprotective therapies and recruitment into clinical trials may be delayed, perhaps beyond the critical therapeutic window period9,17. Consequently, the diagnostic utility of the SI was prospectively assessed in sporadic ALS patients.
Protocol
1. Patient Preparation
Recruit patients prospectively and consecutively. Note: The following protocol is approved by the Sydney West Area Health Service Human Research Ethics Committees. Informed consent was provided by all patients. Patients were recruited prospectively and consecutively from the ALS/neuromuscular clinic at Westmead Hospital.
Determine suitability of ALS patients for testing. Exclude patients who are not diagnosed with ALS or a neuromuscular disorder.
Exclude patients with a coexistent focal neuropathy, median neuropathy at the wrist or ulnar neuropathy at elbow.
Exclude patients with a generalized neuropathy, such as diabetic polyneuropathy.
Extensively investigate and clinically follow-up patients to confirm the diagnosis of ALS or neuromuscular mimic disorders.
Ensure that informed consent is provided by all patients, including ALS and neuromuscular mimic disorder patients, for all the neurophysiological procedures.
2. Clinical Assessment
Determine the clinical staging of all ALS patients by using the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) score18.
Determine the rate of disease progression in all ALS patients according to the previously reported formula; (48-ALSFRS-R)/duration of symptoms)19.
Determine muscle strength in all patients using the Medical Research Council (MRC) rating scale20. Develop a total MRC score comprising upper limb (shoulder abduction; elbow flexion; elbow extension; wrist dorsiflexion; finger abduction; thumb abduction, total score 60) and lower limb (hip flexion; knee extension; ankle dorsiflexion, total score 30) muscle groups. The total MRC score should be 90 if muscle strength is normal.
Determine the site of disease onset, defined as bulbar or limb, in all ALS patients.
3. Neurophysiological Assessment
Undertake motor nerve conduction studies on the median and ulnar nerves with compound muscle action potential (CMAP) responses recorded from the abductor pollicis brevis (APB), first dorsal interosseous (FDI) and abductor digit minimi (ADM) muscles.
Prepare the stimulating sites at the wrist, by cleaning the skin surface with an abrasive gel to reduce skin resistance, followed by application of an alcohol wipe. Set the stimulating current to 20% above the intensity required to produce a maximal CMAP response (supramaximal current).
Ensure that the selected stimulation site exhibits the lowest threshold for stimulation such that volume conduction is avoided.
Prepare recording site over each muscle by cleaning the skin surface with an abrasive gel to reduce skin resistance, followed by application of an alcohol wipe.
Record the size of the compound motor action potential (CMAP) from the APB, ADM, and FDI muscles. Responses to be recorded by 10 mm gold disc electrodes positioned in a belly tendon arrangement over each muscle. Specifically, position the active electrode over the midpoint of the respective muscle ensuring a negative take-off of the CMAP response, while reference electrode to be positioned over the base of thumb (APB and FDI CMAP recordings) and base of digit 5 (for ADM CMAP recordings).
Set the distance between the stimulating cathode and active electrode for APB and ADM compound motor action potential responses to 5 cm and the distance to the FDI at 8 cm.
Position the electrosurgical neutral earth plate on the dorsal aspect of the hand, between stimulating and G1 electrode, with conductive gel to reduce artifact. Prior to application of the neutral earth prepare the site by cleaning the skin surface with an abrasive gel to reduce skin resistance, followed by application of an alcohol wipe.
Ensure the filter settings are between 3 Hz (low-frequency filter) and 10 KHz (high-frequency filter).
Ensure that the sweep speed is set to 20 msec, or 2 msec/division.
Ensure that the sensitivity for recording CMAP responses is set to 5 mV.
Monitor temperature at the site of stimulation throughout the study and ensure that the limb temperature is maintained at 32 °C.
4. Analysis and Interpretation
Measure baseline-to-peak CMAP amplitudes (mV) over the APB, FDI and ADM muscles in ALS patients and the neuromuscular mimic disease controls.
Calculate the split hand index (Figure 1) by multiplying the CMAP amplitude recorded over the APB and FDI muscles, and dividing this product by the CMAP amplitude recorded over the ADM muscle, as follows: SI = APB CMAP * FDI CMAP _________________________ ADM CMAP
Determine the diagnostic utility of the split hand index by comparing the SI values between ALS patients and neuromuscular mimic disorder pathological controls according to the standards for reporting of diagnostic accuracy (STARD) criteria.
Determine optimal diagnostic cutoff values for the split hand index by using the receiver operating characteristic (ROC) curves. Derive ROC curve by plotting the sensitivity (y-axis) and 1-specificty (x-axis) for SI values derived from ALS and neuromuscular disorder patients.
Representative Results
Clinical Phenotype
In total, 44 ALS patients were studied, of which 76% (N=33) were classified as definite or probable and 24% (N=11) as possible ALS according to the Awaji criteria11. The diagnosis of ALS was confirmed in the “possible” cohort after extensive investigations and clinical follow-up for up to 3 years and 53% died during this period. Bulbar-onset disease was evident in 41%, while limb-onset in 59% of ALS patients. At the time of assessment, mean disease duration was 18.9±3.1 months, with a median ALSFRS-R score being 41(32-45), indicating a moderate degree of disability.
The neuromuscular control group comprised 121 patients (mean age 54 years) and included the following diagnosis: entrapment neuropathies-carpal tunnel syndrome (CTS) and ulnar neuropathy at the elbow (UNE), N=62; demyelinating neuropathy (N=9); axonal sensorimotor polyneuropathy (N=36); cervical radiculopathy (11); spino-bulbar muscular atrophy (N=1); Hirayama’s disease (N=1); spinal muscular atrophy (N=1).
Neurophysiological Studies
In ALS patients, there was a significant reduction of CMAP amplitude recorded over the APB (ALS, 3.9±0.5 mV; neuromuscular disorders 8.1± 0.2 mV, P<0.001), FDI (ALS, 4.8±0.7 mV; neuromuscular disorders 10.7±0.3 mV, P<0.001) and ADM (ALS, 6.1±0.5 mV; neuromuscular disorders 9.7±0.2 mV, P<0.001). Importantly, the reduction in CMAP amplitudes was more prominent when recording over the APB (CMAPAPB 3.9±0.5 mV; CMAPADM 6.1±0.5 mV P<0.001) and FDI (CMAPFDI 4.8±0.7 mV; CMAPADM 6.1±0.5 mV P<0.05) muscles then ADM in the ALS cohort.
Of further relevance, the extent of CMAP reduction was more prominent in limb-onset patients when recording over APB (CMAPLIMB-ONSET 3.8±0.4 mV; CMAPBULBAR-ONSET 5.3±0.6 mV P<0.05), FDI (CMAPLIMB-ONSET 4.4±0.7 mV; CMAPBULBAR-ONSET 7.0±0.9 mV P<0.05) and ADM (CMAPLIMB-ONSET 5.8±0.5 mV; CMAPBULBAR-ONSET 7.6±0.6 mV P<0.01) muscles compared to bulbar onset ALS patients. In contrast, the reduction in CMAP amplitudes was most prominent when recording over the APB (8.1±0.2 mV, P<0.0001) and ADM (9.7±0.2 mV, P<0.01) muscles in the neuromuscular cohort (CMAPFDI 10.7±0.3 mV).
Combining the CMAP amplitudes, it was evident that there was a significant reduction of the split hand index in ALS patients compared to neuromuscular disorder patients (ALS 3.5±0.6; neuromuscular disorders 9.1±0.3, P<0.0001, Figure 2A)21. While this reduction in the split hand index was a ubiquitous finding in ALS, it was most pronounced in ALS patients with limb-onset disease (SILIMB-ONSET2.3±0.5; SIBULBAR-ONSET 5.3±1.2, P<0.0001, Figure 2B)21.
Analysis of receiver operating characteristic curves disclosed a good diagnostic accuracy of the split hand index in differentiating ALS from other neuromuscular disorders, as indicated by the fact that the area under curve (AUC) was 0.83 (P<0.0001, Figure 3A)21. An SI value of 5.2 or less, as indicated by dotted black lines (Figure 3A), differentiated ALS from other neuromuscular disorders21. Importantly, the diagnostic utility of the split hand index was greater in ALS patients with limb-onset disease (Figure 3B)21.
In order to assess the diagnostic utility of the split hand index, SI values were compared between those ALS patients meeting the “definite” versus “possible” diagnostic category on the Awaji-Shima criteria11. Importantly, there was a significant reduction of SI in patients classified as “possible” ALS (ALS POSSIBLE SI 5.1±0.8; neuromuscular disorders 9.1±0.3, P<0.01). In addition, of the 11 patients classified as possible ALS, 64% exhibited a reduction in the split hand index value of less than 5.2. Taken together, these findings suggest that an abnormal split hand index may support a diagnosis of ALS in up to 64% of patients not meeting the “definite/probable” Awaji diagnostic criteria.
Of potential prognostic relevance, the split hand index significantly correlated with the total MRC score (rho = 0.7, P<0.001) and MRC score from the thenar eminence (rho = 0.8, P<0.001). Of further relevance, the SI exhibited a significant correlation with the rate of disease progression (rho = - 0.4, P<0.05). These findings indicate that SI is associated with disease severity and progression, thereby suggesting a potential role for the SI in ALS prognostication.
 Figure 1. Split hand sign refers to preferential wasting of the thenar complex group of muscles [abductor pollicis brevis (APB) and first dorsal interosseous (FDI)] muscles when compared to the hypothenar [abductor digit minimi (ADM)] muscles. The split hand index is calculated by multiplying the compound muscle action potential (CMAP) amplitude recorded over the APB by that recorded over the FDI and dividing the product by the CMAP amplitude recorded over the ADM. (In part reproduced from Menon and colleagues. Split hand index for the diagnosis of amyotrophic lateral sclerosis. From Menon et al.21
Figure 1. Split hand sign refers to preferential wasting of the thenar complex group of muscles [abductor pollicis brevis (APB) and first dorsal interosseous (FDI)] muscles when compared to the hypothenar [abductor digit minimi (ADM)] muscles. The split hand index is calculated by multiplying the compound muscle action potential (CMAP) amplitude recorded over the APB by that recorded over the FDI and dividing the product by the CMAP amplitude recorded over the ADM. (In part reproduced from Menon and colleagues. Split hand index for the diagnosis of amyotrophic lateral sclerosis. From Menon et al.21
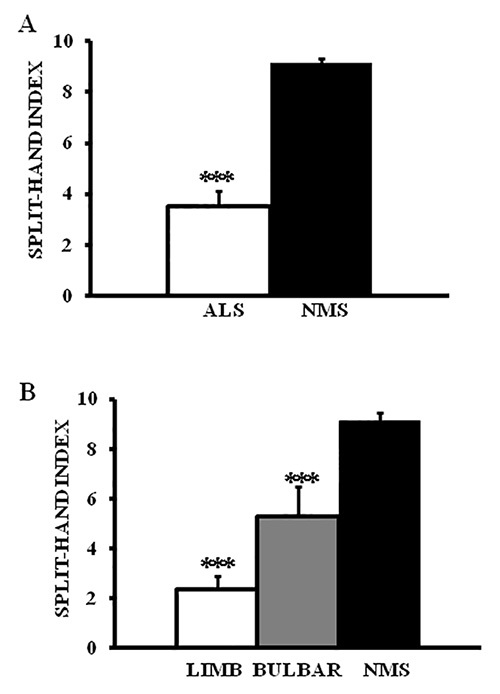 Figure 2. (A) The split hand index (SI) was significantly reduced in amyotrophic lateral sclerosis (ALS) when compared to neuromuscular disorder patients (NMS).(B) The reduction in SI was most prominent in ALS in patients with limb-onset disease. ***P<0.001; ****P<0.0001.
Figure 2. (A) The split hand index (SI) was significantly reduced in amyotrophic lateral sclerosis (ALS) when compared to neuromuscular disorder patients (NMS).(B) The reduction in SI was most prominent in ALS in patients with limb-onset disease. ***P<0.001; ****P<0.0001.
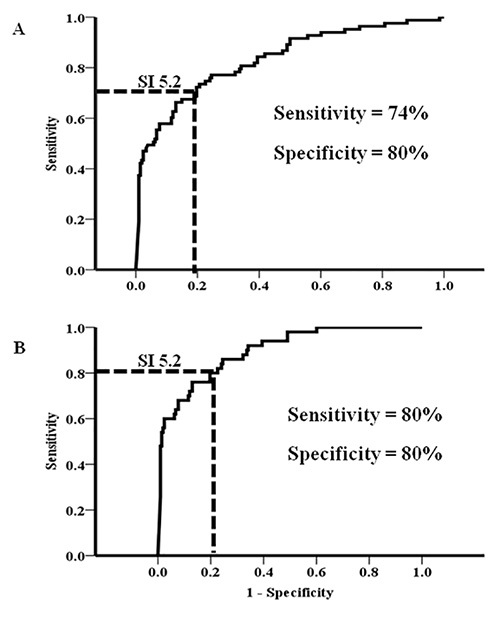 Figure 3. (A) The split hand index (SI) robustly differentiated amyotrophic lateral sclerosis (ALS) from neuromuscular disorder patients (NMS), with an optimal diagnostic cut-off value being 5.2. (B) The diagnostic utility of SI was more prominent in limb-onset ALS patients. (In part reproduced from Menon and colleagues. Split hand index for the diagnosis of amyotrophic lateral sclerosis. From Menon et al.21
Figure 3. (A) The split hand index (SI) robustly differentiated amyotrophic lateral sclerosis (ALS) from neuromuscular disorder patients (NMS), with an optimal diagnostic cut-off value being 5.2. (B) The diagnostic utility of SI was more prominent in limb-onset ALS patients. (In part reproduced from Menon and colleagues. Split hand index for the diagnosis of amyotrophic lateral sclerosis. From Menon et al.21
Discussion
The present study reports on the diagnostic utility of the split hand index in ALS, a novel neurophysiological diagnostic biomarker. The SI reliably distinguished ALS from neuromuscular disorders, with an optimal diagnostic cut-off value of 5.2. The reduction in the SI was most prominent in limb-onset ALS patients. Importantly, a substantial proportion of ALS patients that were classified in the diagnostic “possible” category as per the recently developed diagnostic criteria11, exhibited an abnormal SI. Taken together, findings from the present study suggest that the split hand index may be a diagnostic biomarker of ALS, potentially aiding in an earlier diagnosis of ALS.
Diagnostic criteria, relying on the presence of upper and lower motor neuron dysfunction in multiple regions, have been developed to aid the diagnosis of ALS6,11,22. These criteria, however, have been shown as insensitive and at times limited by patient tolerability due to the need to perform extensive needle EMG sampling7-10,14-16. Consequently, critical diagnostic delay may ensue, perhaps beyond the therapeutic window period.
The split hand index, a simple neurophysiological biomarker, can be readily performed in a clinical setting. Importantly, the present study underscores the diagnostic utility of the split hand index in ALS, particularly in limb-onset disease. Of relevance, the finding that reduction in the split hand index was an early feature in ALS, further underscores its diagnostic utility. Ultimately, the application of SI may lead to recruitment of ALS patients into clinical trials at an earlier stage in the disease process where effectiveness of future neuroprotective agents may be at their peak.
A potential limitation of the split hand index may occur in the setting of coexistent focal pressure mononeuropathies, particularly carpal tunnel syndrome and ulnar neuropathy at the elbow. Clinical, neurophysiological, and radiological correlation should then be utilized to assess for a specific pattern of pathology and alternative techniques need to be implemented so as to establish a diagnosis of ALS23,24. In addition, the SI may be limited in advanced disease, when there may be global atrophy of intrinsic hand muscles. As with focal mononeuropathies, other diagnostic modalities should be utilized to confirm the diagnosis of ALS. In addition, avoiding a contribution from a volume conducted response to the CMAP amplitude is critical prior to conclude the diagnostic value of the SI. Volume conduction may be reduced by appropriate positioning of recording electrodes so as to ensure a CMAP response with negative take-off and appropriate stimulation intensities should be utilized to prevent cross stimulation. If all such potential limitations are addressed, the SI could be utilized as a potential diagnostic aid in ALS.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Funding support from the Motor Neuron Disease Research Institute of Australia (MNDRIA), Sylvia and Charles Viertel Charitable Foundation Clinical Investigator grant, Ramaciotti Foundation and National Health and Medical Research Council of Australia (Project grant number APP1024915) is gratefully acknowledged.
References
- Weber M, Eisen A, Stewart H, Hirota N. The split hand in ALS has a cortical basis. J. Neurol. Sci. 2000;180:66–70. doi: 10.1016/s0022-510x(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Wilbourn AJ. The “split hand syndrome“. Muscle Nerve. 2000;23 doi: 10.1002/(sici)1097-4598(200001)23:1<138::aid-mus22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, et al. Dissociated small hand muscle atrophy in amyotrophic lateral sclerosis: frequency, extent, and specificity. Muscle Nerve. 2008;37:426–430. doi: 10.1002/mus.20949. [DOI] [PubMed] [Google Scholar]
- Kuwabara S, Mizobuchi K, Ogawara K, Hattori T. Dissociated small hand muscle involvement in amyotrophic lateral sclerosis detected by motor unit number estimates. Muscle Nerve. 1999;22:870–873. doi: 10.1002/(sici)1097-4598(199907)22:7<870::aid-mus9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Menon P, Kiernan MC, Vucic S. Appearance, phenomenology and diagnostic utility of the split hand in amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2011;1:457–462. [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Swash M. Early diagnosis of ALS/MND. J. Neurol. Sci. 1998;160 suppl 1:833–836. doi: 10.1016/s0022-510x(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Chio A. ISIS Survey: an international study on the diagnostic process and its implications in amyotrophic lateral sclerosis. J. Neurol. 1999;246 Suppl 3 doi: 10.1007/BF03161081. [DOI] [PubMed] [Google Scholar]
- Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, et al. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population-based study. Arch. Neurol. 2000;57:1171–1176. doi: 10.1001/archneur.57.8.1171. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, Swash M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph Lateral Scler. 2009;10:53–57. doi: 10.1080/17482960802521126. [DOI] [PubMed] [Google Scholar]
- Boekestein WA, Kleine BU, Hageman G, Schelhaas HJ, Zwarts MJ. Sensitivity and specificity of the 'Awaji' electrodiagnostic criteria for amyotrophic lateral sclerosis: Retrospective comparison of the Awaji and revised El Escorial criteria for ALS. Amyotroph Lateral Scler. 2010;11:497–501. doi: 10.3109/17482961003777462. [DOI] [PubMed] [Google Scholar]
- Douglass CP, Kandler RH, Shaw PJ, McDermott CJ. An evaluation of neurophysiological criteria used in the diagnosis of motor neuron disease. J. Neurol. Neurosurg. Psychiatr. 2010;81:646–649. doi: 10.1136/jnnp.2009.197434. [DOI] [PubMed] [Google Scholar]
- Noto YI, et al. Awaji ALS criteria increase the diagnostic sensitivity in patients with bulbar onset. Clin. Neurophysiol. 2012;123:382–385. doi: 10.1016/j.clinph.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Schrooten M, Smetcoren C, Robberecht W, Van Damme P. Benefit of the Awaji diagnostic algorithm for amyotrophic lateral sclerosis: A prospective study. Ann. Neurol. 2011;70:79–83. doi: 10.1002/ana.22380. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Cudkowicz M. ALS drug development: reflections from the past and a way forward. Neurotherapeutics. 2008;5:516–527. doi: 10.1016/j.nurt.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Kimura F, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66:265–267. doi: 10.1212/01.wnl.0000194316.91908.8a. [DOI] [PubMed] [Google Scholar]
- O'Brien MD. Aid to the examination of the peripheral nervous system. Edinburgh: Saunders Elsevier; 2010. [Google Scholar]
- Menon P, Kiernan MC, Yiannikas C, Stroud J, Vucic S. Split-hand index for the diagnosis of amyotrophic lateral sclerosis. Clin. Neurophysiol. 2013;124:410–416. doi: 10.1016/j.clinph.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cordato DJ, Yiannikas C, Schwartz RS, Shnier RC. Utility of magnetic resonance imaging in diagnosing ulnar neuropathy at the elbow. Clin. Neurophysiol. 2006;117:590–595. doi: 10.1016/j.clinph.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Siao P, Cros D, Vucic S. Practical approach to electromyography. New York: Demos Medical Publishing; 2011. [Google Scholar]


