Summary
Primary central nervous system lymphoma (PCNSL) is an aggressive sub-variant of non-Hodgkin lymphoma (NHL) with morphological similarities to diffuse large B-cell lymphoma (DLBCL). While methotrexate (MTX)-based therapies have improved patient survival, the disease remains incurable in most cases and its pathogenesis is poorly understood. We evaluated 69 cases of PCNSL for the expression of HGAL (also known as GCSAM), LMO2 and BCL6 – genes associated with DLBCL prognosis and pathobiology, and analysed their correlation to survival in 49 PCNSL patients receiving MTX-based therapy. We demonstrate that PCNSL expresses LMO2, HGAL(also known as GCSAM) and BCL6 proteins in 52%, 65% and 56% of tumours, respectively. BCL6 protein expression was associated with longer progression-free survival (, p=0.006) and overall survival (OS, p=0.05), while expression of LMO2 protein was associated with longer OS (p=0.02). Further research is needed to elucidate the function of BCL6 and LMO2 in PCNSL.
Keywords: PCNSL, HGAL, BCL6, LMO2, prognosis
Introduction
Primary central nervous system lymphoma (PCNSL) is an aggressive subtype of non-Hodgkin lymphoma (NHL) that originates in the brain, spinal cord, eyes or leptomeninges (Abrey, et al 2000, Bayraktar, et al 2011). Although PCNSL accounts for only 1-2% of extranodal NHL, its incidence has been rising in recent years, necessitating the development of improved therapies (Olson, et al 2002, Poortmans, et al 2003). The introduction of high dose methotrexate (MTX) - based chemotherapy with or without cranial radiotherapy (RT) has increased the 5-year overall survival (OS) rates to 26-50% (Abrey, et al 2000, Ferreri 2011). While in systemic diffuse large B-cell lymphoma (DLBCL) the addition of rituximab to standard chemotherapy markedly improved patients' OS (Coiffier, et al 2002), the role of rituximab in PCNSL is still controversial and awaits the results of ongoing randomized trials. As a large protein, rituximab shows poor central nervous system (CNS) penetration and evidence supporting the efficacy of rituximab in PCNSL remains low and is based on small nonrandomized studies (Birnbaum, et al 2012, Ferreri 2011, Gregory, et al 2013). Despite improvement in PCNSL survival, there is significant heterogeneity in patient response to therapy and outcome (Abrey, et al 2005). Therefore, it is important to develop novel prognostic biomarkers that can capture the diversity of PCNSL.
The morphological and genetic similarities between DLBCL arising systemically and in the brain have been used to suggest that PCNSL may be a subvariant of DLBCL (Montesinos-Rongen, et al 2008, Rubenstein, et al 2006). Therefore, analysis of the genes known to play a pathogenic or prognostic role in DLBCL may shed further light into PCNSL biology and clinical behaviour.
LIM domain only-2 (LMO2) and human germinal centre-associated lymphoma (HGAL, also known as GCSAM-germinal centre associated, signalling and motility) are expressed in normal germinal centre (GC) B-cells and GC-derived DLBCL and are associated with improved outcome in DLBCL (Alizadeh, et al 2011, Lossos, et al 2003, Lossos, et al 2004, Natkunam, et al 2008). LMO2 protein forms a transcriptional complex that regulates gene expression in DLBCL; its expression is associated with an increased centrosome number (Cubedo, et al 2012). HGAL protein has been implicated in decreasing DLBCL cell motility and enhancing BCR signalling by binding SYK and increasing its kinase activity (Lu, et al 2011, Romero-Camarero, et al 2013). BCL6 is an oncogene that functions as a transcriptional repressor necessary for GC formation (Cattoretti, et al 2005). BCL6 RNA and BCL6 protein expression was shown to predict survival in systemic DLBCL (Lossos, et al 2004, Lossos, et al 2001). In contrast to LMO2 and HGAL, the association between BCL6 protein expression and survival of PCNSL patients was previously examined but led to contradicting results (Braaten, et al 2003, Camilleri-Broet, et al 2006, Chang, et al 2003, Levy, et al 2008, Lin, et al 2006, Momota, et al 2010, Rubenstein, et al 2013). In this study we evaluated the prognostic utility of LMO2, HGAL and BCL6 protein expression to predict improved survival in a large cohort of PCNSL patients treated with MTX-based chemotherapeutic regimens.
Materials and Methods
Patients
A total of 69 specimens from human immunodeficiency virus (HIV)-negative PCNSL patients with DLBCL histology were studied; specimens were contributed from the University of Miami (n=16), Northwestern University (n=9), Memorial Sloan-Kettering Cancer Center (MSKCC) (n=19) and the University of Virginia (n=27). The specimens were selected based on the following criteria: (1) diagnosis of de novo PCNSL with DLBCL histology; (2) availability of tissue obtained at diagnosis before initiation of therapy. For analysis of the association between HGAL, LMO2 and BCL6 expression and patients' outcome, a total of 49 cases were selected among these specimens based on two additional criteria: (1) treatment with curative intent with high dose MTX-based chemotherapeutic regimen; and (2) availability of follow-up and outcome data. Institutional review board approval was obtained from all participating institutions. Information was available regarding the extent and staging of the disease by physical examination, computerized tomography (CT) or magnetic resonance imaging of the brain, CT of the chest, abdomen and pelvis, bone marrow biopsy and lumbar puncture. Age, Karnofsky performance status (KPS), treatment and cerebrospinal fluid/eye involvement (if available) at diagnosis were recorded. Follow-up information was obtained from the patients' medical records and included progression-free survival (PFS) and OS, defined as previously reported (Lossos, et al 2003, Lossos, et al 2001).
Immunohistochemistry
Histological sections were reviewed by two pathologists to confirm the diagnosis based on the World Health Organization classification of hematopoietic tumours (Swerdlow, et al 2008). Antibodies used for immunohistochemical staining of LMO2, HGAL and BCL6 proteins and the staining methods were previously described (Hans, et al 2004, Natkunam, et al 2008, Natkunam, et al 2007a, Natkunam, et al 2007b). As was previously reported by us for these three immunohistochemical biomarkers in systemic DLBCL as well as in an earlier study analysing BCL6, staining in more than 30% of lymphoma cells was a priori assigned a positive score (Hans, et al 2004, Natkunam, et al 2008, Natkunam, et al 2007a). These thresholds were chosen to be consistent with the data reported in systemic DLBCL. LMO2 staining showed a robust and primarily nuclear signal (Natkunam, et al 2007b), whereas HGAL staining was localized to the cytoplasm and membrane (Natkunam, et al 2005). Isolated cytoplasmic LMO2 staining was not considered for scoring. As described previously, the staining intensity of all three markers did not vary among normal and neoplastic lymphoid cells. Two haematopathologists independently scored the slides with an overall concordance rate of 95%. After this initial independent scoring was carried out, the discrepancies were resolved by consensus review over a double-headed microscope to reach agreement regarding a score for each discrepant case, as was reported in our previous studies (Natkunam, et al 2008, Natkunam, et al 2007a).
Statistical Analysis
Correlations between the immunohistochemical biomarkers and associations with clinical parameters were evaluated by Pearson's correlation and presented as asymptotic p-values. Survival curves were estimated using the Kaplan-Meier method and prognostic variables were compared by the log-rank test using the SPSS software (version 21, IBM, New York, NY). Multivariate regression analysis was performed according to the Cox proportional hazards regression model (Cox 1972). OS or PFS were used as the dependent variables to adjust the effects of individual biomarkers expression and clinical variables. A two-tailed P < 0.05 was considered significant.
Results
A total of 69 specimens from patients with PCNSL, with a median age of 66 years (range 27-98) were available. LMO2 protein was expressed in 36 (52%), HGAL in 45 (65%) and BCL6 in 39 (56%) of PCNSL tumours (Figure 1), at frequencies similar to systemic DLBCL (46% for LMO2 (Natkunam, et al 2007b) and 75% for HGAL (Natkunam, et al 2007a)). Twenty-nine cases were positive for both HGAL and LMO2 expression and 22 cases were positive for all three proteins. LMO2 expression correlated with BCL6 (p=0.004) and HGAL (p=0.006) expression, but there was no correlation between the expression of HGAL and BCL6.
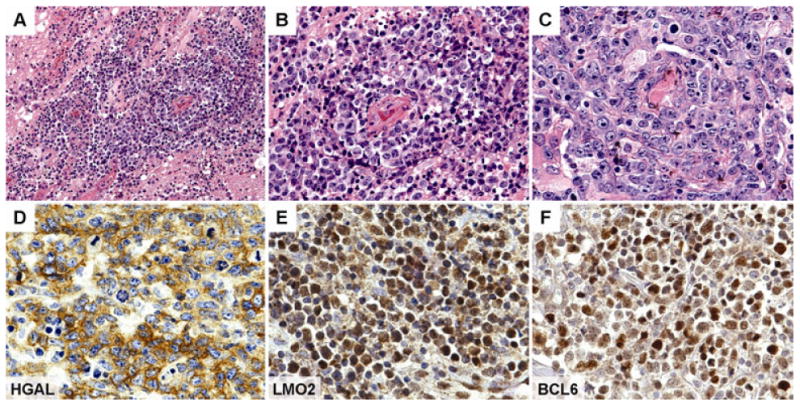
Figure 1. Histological Findings in PCNSL.
Histological sections of a representative central nervous system biopsy shows an atypical lymphoid proliferation infiltrating brain parenchyma in a diffuse pattern (A); the lymphoma cells typically surround vessels and cause angiocentric and angiodestructive lesions (B); higher magnification shows marked cytological atypia of the lymphoma cells with pleomorphic nuclear outlines, prominent nucleoli and associated mitotic figures (C); immunohistochemistry shows that the lymphoma cells are positive for HGAL (D), LMO2 (E) and BCL6 (F). [Original magnification, panel A x200, panel B x400, panels C-F x600].
We evaluated the correlations between HGAL, LMO2 and BCL6 expression with survival in 49 PCNSL patients treated with MTX-base regimens (Table I): high dose MTX alone (n=4), high dose MTX with procarbazine and vincristine (MPV) (n=21) and MPV with cytarabine (n=24). Rituximab was added to chemotherapy in 14 patients and 22 patients received whole brain radiation (4500 Rads). The median follow up of all these patients was 18 months (range 1-122). The median follow up of patients that were alive was 32 months (range 2-119). There was no statistical difference in PFS and OS between patients who did or did not receive rituximab and whole brain radiation (not shown). The previously reported MSKCC prognostic score (Abrey, et al 2006), which subdivides patients into 3 groups based on age and KPS, exhibited borderline statistical correlation with PFS (p=0.05) and OS (p=0.10), most probably due to the small number of patients in each group (not shown).
Table I.
Clinical characteristics and immunohistochemistry results of patients with PCNSL treated with methotrexate-based regimens.
| Case | Age (years) | KPS | Treatment | Rituximab | XRT | LMO2 | HGAL | BCL6 | PFS (Months) | PFS Code | OS (Months) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 90 | MPV+ARA-C | NO | YES | POS | POS | POS | 25.0 | 1 | 33.5 | DEAD |
| 2 | 74 | 90 | MPV+ ARA-C | NO | NO | POS | POS | POS | N/A | N/A | 93.0 | ALIVE |
| 3 | 53 | 80 | MPV | YES | NO | NEG | NEG | NEG | 2.3 | 1 | 13.0 | DEAD |
| 4 | 71 | 90 | MPV+ ARA-C | NO | NO | POS | POS | POS | 20.3 | 1 | 21.0 | DEAD |
| 5 | 69 | 90 | MPV+ ARA-C | NO | NO | NEG | POS | NEG | 7.5 | 1 | 10.0 | DEAD |
| 6 | 29 | 90 | MPV+ ARA-C | YES | YES | POS | POS | POS | N/A | N/A | 19.0 | ALIVE |
| 7 | 73 | 90 | MPV+ ARA-C | YES | NO | POS | NEG | POS | N/A | N/A | 8.0 | ALIVE |
| 8 | 70 | 90 | MPV+ ARA-C | NO | NO | POS | NEG | POS | N/A | N/A | 5.0 | ALIVE |
| 9 | 72 | 70 | MPV | NO | NO | POS | N/A | NEG | 17.0 | 1 | 119.0 | ALIVE |
| 10 | 64 | 80 | MPV | NO | NO | POS | POS | POS | 17.0 | 1 | 18.0 | DEAD |
| 11 | 73 | 80 | MPV+ ARA-C | NO | YES | NEG | NEG | NEG | 21.0 | 1 | 27.0 | DEAD |
| 12 | 73 | 70 | MPV+ ARA-C | NO | YES | NEG | NEG | POS | 12.0 | 1 | 12.0 | DEAD |
| 13 | 71 | 60 | MPV | NO | NO | POS | NEG | NEG | 0.0 | 0 | 3.0 | DEAD |
| 14 | 81 | 70 | MPV | NO | NO | POS | POS | NEG | 18.0 | 1 | 34.0 | DEAD |
| 15 | 76 | 50 | MPV | NO | NO | POS | POS | NEG | 14.0 | 0 | 15.0 | DEAD |
| 16 | 56 | 70 | MPV+ ARA-C | YES | YES | NEG | NEG | NEG | 3.0 | 1 | 9.0 | DEAD |
| 17 | 79 | 70 | MPV | YES | YES | POS | NEG | POS | 37.0 | 1 | 39.0 | DEAD |
| 18 | 76 | 60 | MPV | NO | YES | NEG | NEG | POS | 0.0 | 0 | 9.0 | DEAD |
| 19 | 84 | 60 | MPV | NO | NO | NEG | NEG | POS | 51.0 | 0 | 51.0 | ALIVE |
| 20 | 61 | 70 | MPV+ ARA-C | YES | NO | POS | POS | NEG | 43.0 | 1 | 43.0 | ALIVE |
| 21 | 66 | 80 | MPV+ ARA-C | YES | NO | N/A | POS | POS | 12.0 | 1 | 37.0 | ALIVE |
| 22 | 52 | 80 | MPV | YES | NO | NEG | NEG | POS | 4.0 | 1 | 7.0 | DEAD |
| 23 | 63 | 60 | MPV | YES | NO | POS | N/A | POS | 34.0 | 0 | 34.0 | ALIVE |
| 24 | 40 | 60 | MPV+ ARA-C | YES | NO | POS | POS | POS | 30.0 | 0 | 30.0 | ALIVE |
| 25 | 58 | 70 | MPV+ ARA-C | YES | NO | POS | POS | POS | 27.0 | 0 | 27.0 | ALIVE |
| 26 | 73 | 60 | MPV | YES | YES | POS | POS | POS | 25.0 | 0 | 25.0 | ALIVE |
| 27 | 82 | 60 | MPV | YES | NO | NEG | NEG | NEG | 0.0 | 0 | 2.0 | DEAD |
| 28 | 65 | 70 | MPV | YES | NO | NEG | POS | NEG | 1.3 | 1 | 3.6 | DEAD |
| 29 | 45 | 50 | MTX | NO | YES | NEG | POS | NEG | 4.8 | 1 | 4.8 | DEAD |
| 30 | 27 | 40 | MPV | NO | YES | POS | POS | POS | 122.2 | 1 | 122.2 | DEAD |
| 31 | 69 | 60 | MPV | NO | NO | NEG | POS | POS | 5.7 | 1 | 5.7 | DEAD |
| 32 | 69 | N/A | MPV | NO | NO | NEG | POS | NEG | 4.8 | 1 | 4.8 | DEAD |
| 33 | 51 | 60 | MPV+ ARA-C | NO | YES | NEG | POS | NEG | 7.2 | 1 | 11.0 | DEAD |
| 34 | 47 | 50 | MPV | NO | YES | NEG | NEG | NEG | 12.4 | 1 | 12.4 | ALIVE |
| 35 | 44 | 90 | MPV+ ARA-C | NO | YES | NEG | NEG | POS | 116.2 | 0 | 116.5 | ALIVE |
| 36 | 67 | 50 | MPV | NO | NO | NEG | POS | NEG | 34.6 | 1 | 34.6 | DEAD |
| 37 | 66 | 50 | MPV | NO | NO | NEG | NEG | POS | 21.6 | 1 | 59.2 | DEAD |
| 38 | 70 | 60 | MPV+ ARA-C | NO | NO | NEG | NEG | NEG | 18.6 | 1 | 51.0 | DEAD |
| 39 | 80 | 60 | MPV | NO | NO | POS | POS | POS | 0.9 | 1 | 4.7 | DEAD |
| 40 | 46 | 90 | MPV+ ARA-C | NO | YES | POS | POS | POS | 23.8 | 1 | 23.8 | DEAD |
| 41 | 67 | 90 | MTX | NO | YES | NEG | POS | NEG | 5.5 | 1 | 16.0 | DEAD |
| 42 | 74 | 80 | MPV+ ARA-C | NO | NO | NEG | POS | NEG | 0.0 | 0 | 7.5 | DEAD |
| 43 | 64 | 90 | MTX | NO | YES | POS | POS | NEG | 1.4 | 1 | 39.9 | ALIVE |
| 44 | 48 | 90 | MPV+ ARA-C | NO | YES | NEG | POS | POS | 20.3 | 0 | 20.3 | ALIVE |
| 45 | 34 | 60 | MPV+ ARA-C | NO | YES | POS | POS | POS | 1.6 | 0 | 1.6 | ALIVE |
| 46 | 43 | 90 | MPV+ ARA-C | NO | YES | POS | POS | POS | 93.2 | 0 | 93.2 | ALIVE |
| 47 | 27 | 30 | MPV+ ARA-C | NO | YES | POS | POS | POS | 6.4 | 1 | 9.9 | DEAD |
| 48 | 65 | 30 | MTX | NO | YES | POS | POS | POS | 0.0 | 0 | 7.9 | DEAD |
| 49 | 50 | 60 | MPV+ ARA-C | NO | YES | POS | POS | POS | 4.7 | 1 | 4.7 | DEAD |
MPV : High dose methotrexate with procarbazine and vincristine; ARA-C : cytarabine; XRT-brain radiation; N/A : not available
PFS, progression-free survival ; PFS Code : 0- no progression, 1-progression ; OS, overall survival
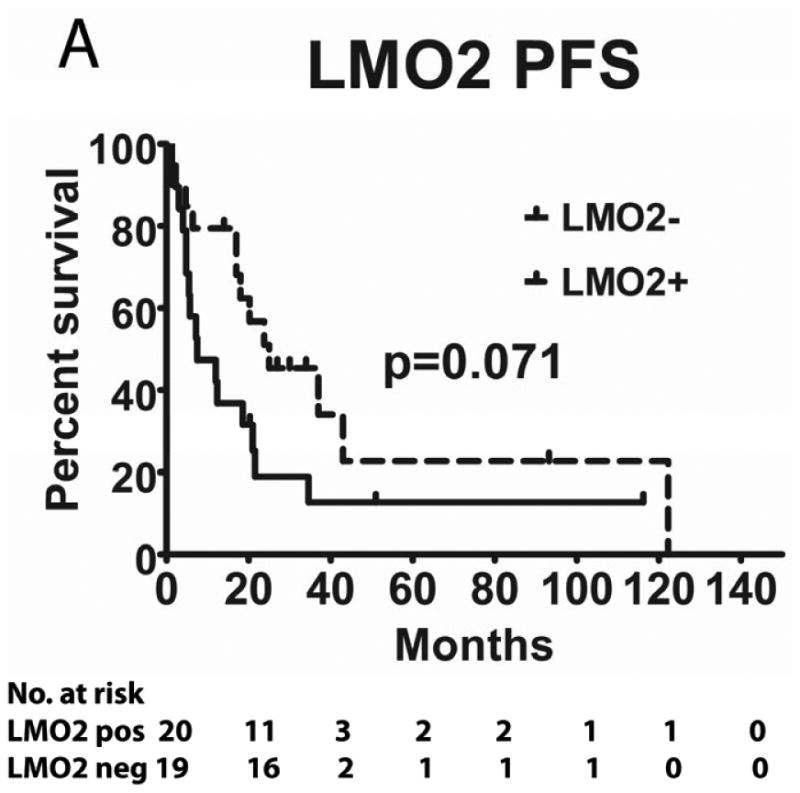
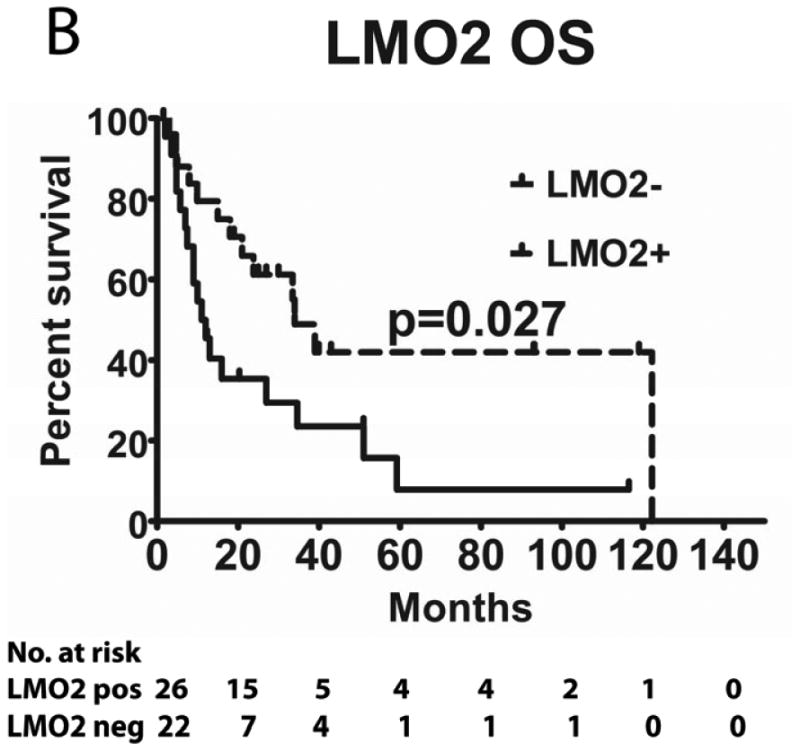
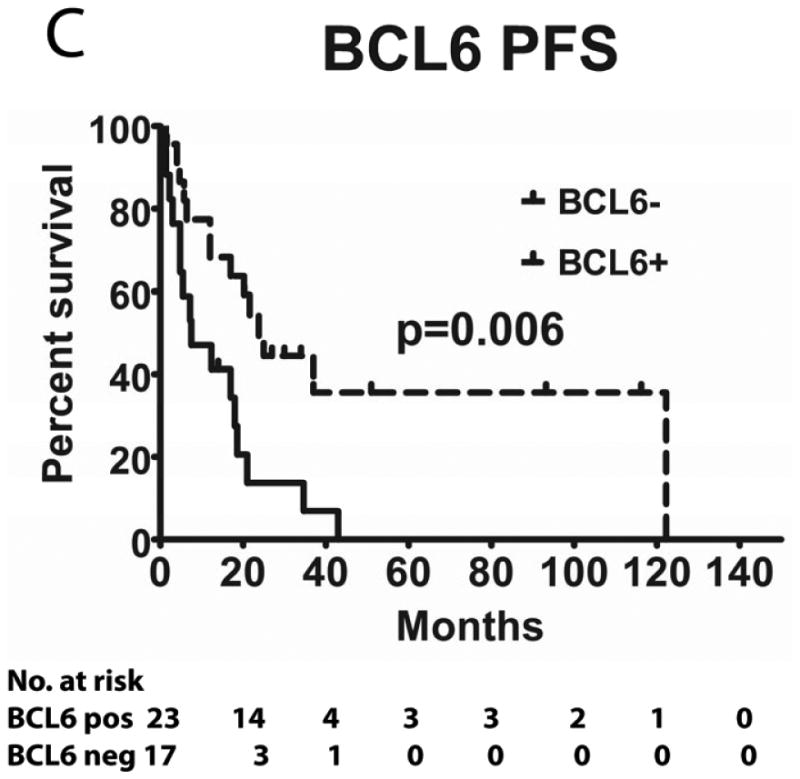
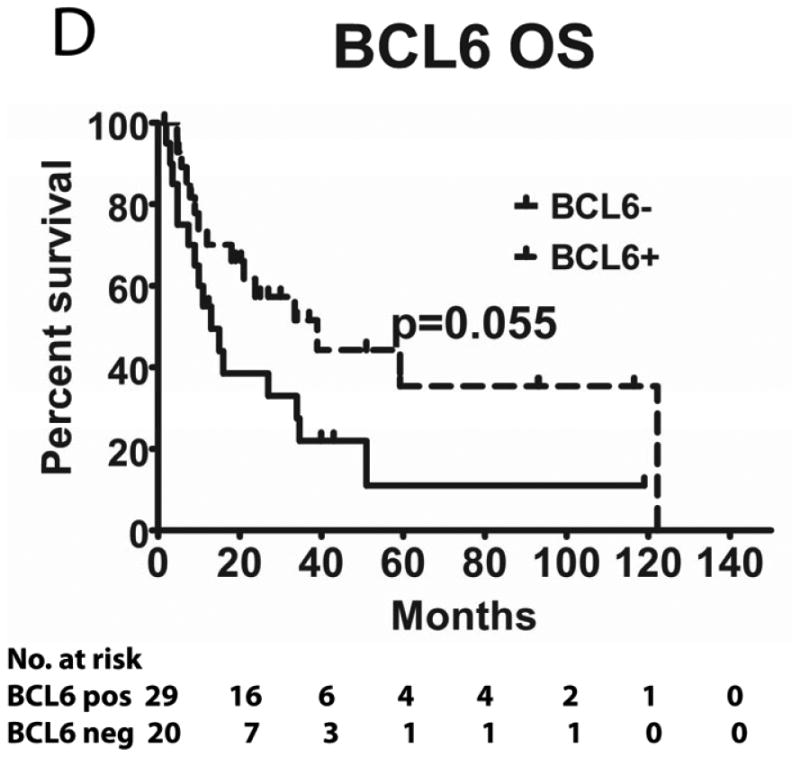
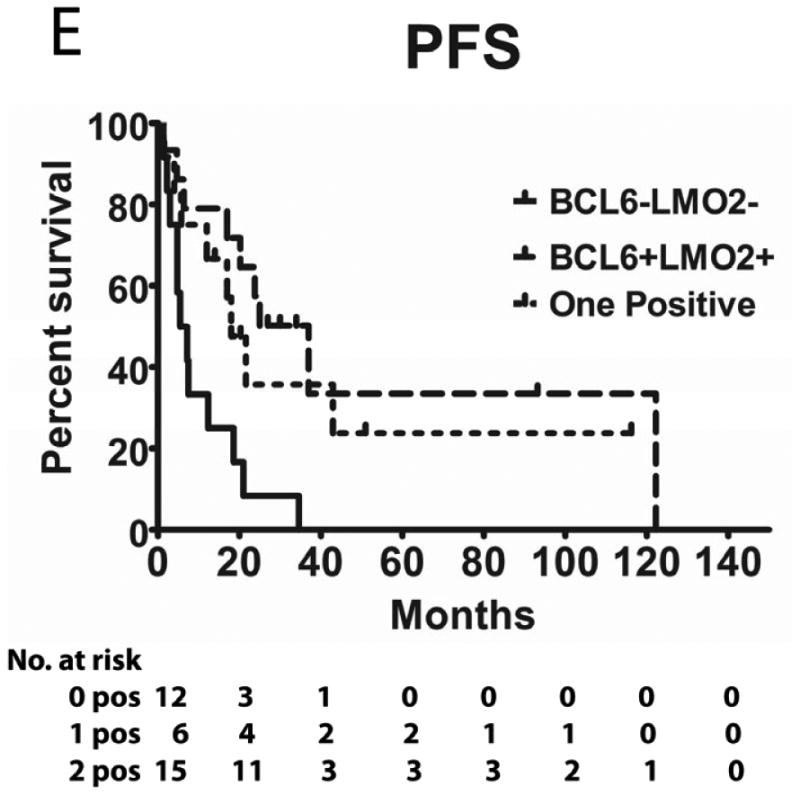
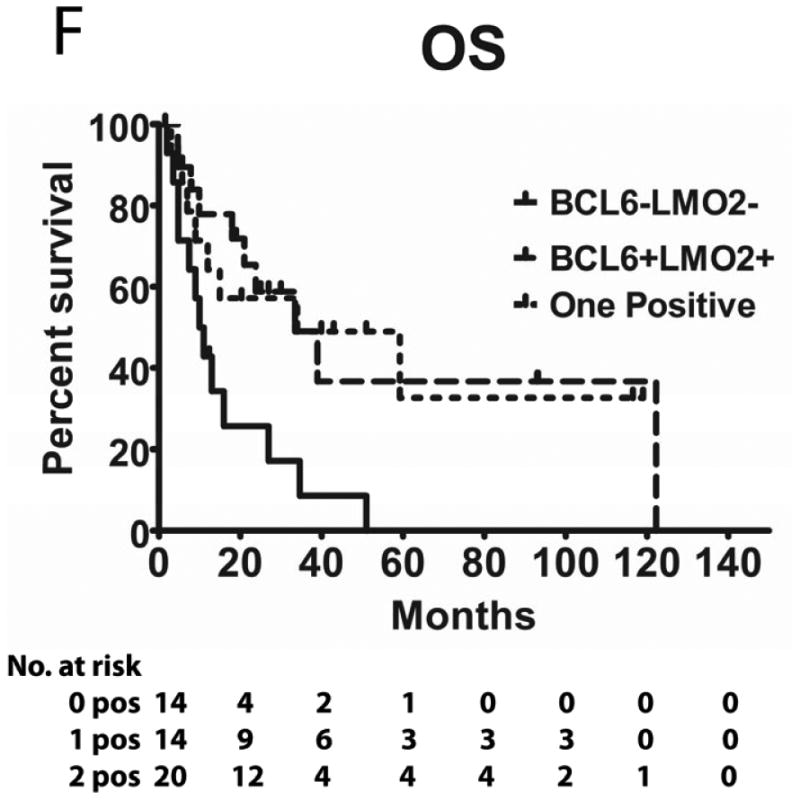
Tumours from 26 (54%) patients treated with MTX-based therapy exhibited positive staining for LMO2, 31 (66%) were positive for HGAL and 29 (59%) were positive for BCL6. A Pearson's correlation analysis demonstrated no correlation between the expression of LMO2, HGAL and BCL6 with patients' age, KPS and use of radiation or rituximab treatment (data not shown). The median PFS of patients with positive and negative HGAL staining was 17 (95% confidence interval [CI]: 2-32) and 19 months (95% CI: 9-28 p=0.99), respectively, and the median OS was 21 (95% CI: 1-41) and 27 months (95% CI: 3-51 p=0.76), respectively (Table II). The median PFS of patients with positive and negative LMO2 staining was 25 (95% CI: 8-42) and 8 months (95% CI: 0-16 p=0.071), respectively, while the median OS was 34 (95% CI: 25-43) and 11 months (95% CI: 7-15, p=0.027), respectively. The median PFS for BCL6-positive and -negative cases was 24 (95% CI: 17-31) and 8 (95% CI: 0-17, p=0.006), respectively and the median OS was 39 months (95% CI: 13-65), and 13 (95% CI: 5-21, p=0.055) months, respectively (Table II and Figure 2). Compared to patients with LMO2/BCL6 double negative tumours, simultaneous LMO2 and BCL6 expression was associated with significantly better OS (p=0.006) and PFS (p=0.004), but was not significantly different from tumours expressing only one of these biomarkers (Figure 2).
Table II. PCNSL survival analysis based on expression of HGAL, LMO2 and BCL6 proteins.
| Variable | PFS | PFS | OS | OS |
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| HGAL | ||||
| Negative (reference) | 1.0 | 0.99 | 1.0 | 0.76 |
| Positive | 1.0 (0.45 – 2.22) | 0.89 (0.42 – 1.88) | ||
| LMO2 | ||||
| Negative (reference) | 1.0 | 0.071 | 1.0 | 0.027 |
| Positive | 0.50 (0.23 – 1.06) | 0.44 (0.21 – 0.91 | ||
| BCL6 | ||||
| Negative (reference) | 1.0 | 0.006 | 1.0 | 0.055 |
| Positive | 0.35 (0.16 – 0.74) | 0.49 (0.24 – 1.02) | ||
| LMO2/BCL6 combined | ||||
| Double negative (reference) | 1.0 | 0.004 | 1.0 | 0.006 |
| One marker positive | 0.37 (0.15 – 0.90) | 0.31 (0.12 – 0.77) | ||
| Double positive | 0.24 (0.10 – 0.61) | 0.32 (0.13 – 0.75) | ||
PFS, progression-free survival; OS, overall survival
Figure 2. Kaplan Meier curves of PFS and OS in 49 PCNSL patients treated with methotrexate –based regimens.
(A) PFS stratified on expression of LMO2 protein; (B) OS stratified on expression of LMO2 protein; (C) PFS stratified on expression of BCL6 protein; (D) OS stratified on expression of BCL6 protein; (E) PFS as a function of combined LMO2 and BCL6 proteins expression; (F) OS as a function of combined LMO2 and BCL6 proteins expression. PFS, progression-free survival; OS, overall survival.
A multivariate regression analysis that included LMO2 and BCL6 with OS as the dependent variable demonstrated that LMO2 expression almost reached statistical significance (p=0.064) as an independent predictor of OS in PCNSL patients. In contrast, a multivariate regression analysis that included LMO2 and BCL6 with PFS as the dependent variable demonstrated that BCL6 expression is an independent predictor of PFS in PCNSL patients (P = 0.014). In multivariate regression analyses that included LMO2, BCL6, HGAL, age and KPS, with OS or PFS as the dependent variables, only BCL6 expression almost reached statistical significance (p=0.06) as an independent predictor of PFS in PCNSL patients (Table III). The relatively small number of analysed patients might limit the value of these multivariate analyses.
Table III.
Prognostic factors in multivariate analysis of overall survival and progression free survival in patients with PCNSL.
| Factor | P value for OS | P value for PFS |
|---|---|---|
| BCL6 | 0.40 | 0.06 |
| LMO2 | 0.19 | 0.32 |
| HGAL | 0.37 | 0.72 |
| Age (≤ 50 years versus > 50 years) | 0.23 | 0.85 |
| KPS (< 70 versus ≥70) | 0.22 | 0.57 |
OS, overall survival; PFS, progression-free survival; KPS, Karnofsky performance score.
Discussion
PCNSL is an aggressive variant of NHL with morphological and genetic similarities to DLBCL. The advent of MTX-based therapy has significantly improved patient prognosis. However, despite these therapeutic advances, response to treatment remains variable and the disease remains largely incurable, necessitating an improved biological understanding of the disease. While several clinical variables, notably age (<50 years) and KPS, have been shown to be associated with improved PFS and OS of PCNSL patients (Abrey, et al 2006), these factors do not provide indications about the molecular underpinnings of the disease. Therefore, finding molecular markers associated with improved patient survival may be important for understanding resistance to conventional therapy and the underlying etiology and pathogenesis of PCNSL.
PCNSL rarely express the GC marker CD10 and almost universally express MUM1, suggesting that they resemble activated B cell-like systemic DLBCL (Camilleri-Broet, et al 2006, Lin, et al 2006). However, CD10 expression is frequently downregulated in extranodal GC-derived lymphomas (Younes, et al 2011), making it difficult to precisely determine the cell of origin of PCNSL tumours based on classifications derived from systemic DLBCL. Molecular studies of PCNSL immunoglobulin genes showed the presence of somatic mutations, which were frequently ongoing, and biased use of immunoglobulin genes, suggesting a potential origin from antigen-experienced GC cells (Montesinos-Rongen, et al 1999, Thompsett, et al 1999). Gene expression profiling studies showed that PCNSL may exhibit gene expression signatures similar to GC-B cell-like and activated B cell-like DLBCL (Montesinos-Rongen, et al 2008). Herein we sought to determine whether HGAL, LMO2 and BCL6, GC-genes known to be prognostic markers and important in the pathogenesis of DLBCL (Lossos, et al 2003, Lossos, et al 2001, Natkunam, et al 2008), were expressed in and associated with prolonged PFS and OS in PCNSL.
We demonstrate that PCNSLs express HGAL, LMO2 and BCL6 proteins at frequencies not significantly different from systemic DLBCL. BCL6 expression was significantly associated with improved PFS and almost reached statistically significant association with prolonged OS. Previously, 7 studies examined the prognostic significance of BCL6 expression in PCNSL and showed contradicting results (Table IV). Two studies demonstrated a significantly shorter PFS in patients with PCNSL tumours expressing BCL6 protein (Momota, et al 2010, Rubenstein, et al 2013) while one study showed longer PFS (Levy, et al 2008), in accord with the current study. Prolonged OS was associated with BCL6 protein expression in 3 previous studies (Braaten, et al 2003, Levy, et al 2008, Lin, et al 2006), reaching statistical significance in one (Braaten, et al 2003), while one study showed statistically shorter OS (Rubenstein, et al 2013). The reasons for these discrepancies stem from the small number of tumours analysed in the majority of these studies. Further, different antibody clones and cut-off pointss for BCL6 expression were used. Only 3 studies used the 30% cut-off point established in systemic DLBCL and used herein. Most studies were retrospective, including our study. The limitations of retrospective studies include a heterogeneous patient population and non-uniform use of chemotherapeutic agents as well as radiation and rituximab, which may contribute to the conflicting results. However, the age and KPS distributions of our patients were quite characteristic for patients with PCNSL, and all patients received MTX-based therapy. A single prospective study of biomarkers in PCNSL has been reported, but analysed only 26 tumours- a number too small to draw any significant conclusions (Rubenstein, et al 2013). Moreover, preferentially prospective studies using the same antibody and cut-off value as in systemic DLBCL (as was done in the current study) are necessary to elucidate the role of BCL6 in the development of PCNSL and its association with improved patient PFS and OS.
Table IV. Summary of studies examining the prognostic role of BCL6 in PCNSL.
| Reference | Patients (n) | Treatment | Median OS of all patients (months) | Antibody | Cut-off for positivity (%) | % Positive | Association between BCL6 positivity and PFS (p=) | Association between BCL6 positivity and OS (p=) |
|---|---|---|---|---|---|---|---|---|
| Chang, et al (2003) | 14 | CT* | NR | Santa Cruz Biotechnology (Dallas, TX, USA) | 20 | 57 | NR | 0.16# |
| Camilleri-Broet, et al (2006) | 83 | HDMTX | 42 | Clone PG-B6p; Dako (Carpinteria, CA, USA) | 30 | 56 | NS | NS |
| Momota, et al (2010) | 27 | HDMTX | Not reached | Polyclonal; Dako (Carpinteria, CA, USA) | 30 | 48 | 0.038# | 0.124# |
| Rubenstein, et al (2013) | 26 | HDMTX | Not reached | Clone PG-B6p; Dako (Carpinteria, CA, USA) | 60 | 59 | 0.019#& | 0.009# |
| Lin, et al (2006) | 29 | HDMTX** | 19.8 | Clone PG-B6p; Dako (Carpinteria, CA, USA) | 20 | 61 | NR | 0.073## |
| Braaten, et al (2003) | 33 | HDMTX | 101 | Clone PG-B6p; Dako (Carpinteria, CA, USA) | 10 | 79 | NR | 0.002## |
| Levy, et al (2008) | 48 | HDMTX | 34.6 | Novocastra (Buffalo Grove, IL, USA) | 50 | 46 | 0.02## | 0.18## |
| Present study | 49 | HDMTX*** | 23.8 | Clone PG-B6p; Dako (Carpinteria, CA, USA) | 30 | 56 | 0.004## | 0.05## |
OS, overall survival; PFS, progression-free survival; CT, chemotherapy; NR, not reported; NS, not statistically significant; HDMTX, high dose methotrexate-based chemotherapy.
Chemotherapy not specified
Bomes regimen (Cheng, et al 1998) in majority of patients
HDMTX with rituximab, temozolomide, etoposide and cytarabine
Shorter OS or PFS with BCL6 positivity;
Longer OS or PFS with BCL6 positivity;
- analysed as continuous variable
Our results have also demonstrated that LMO2 protein is expressed in PCNSL and that its expression is statistically associated with prolonged OS. A recent study from China detected LMO2 protein expression in only 16 of 66 (24%) PCNSL tumours (Chen, et al 2013). The study showed no statistical difference in the outcome of patients with LMO2-positive and -negative PCNSL, despite a tendency for longer survival of LMO2-positive patients. However, in this study no information on therapy was provided, and consequently it is unknown if all the patients received MTX-based therapy. LMO2 has been shown to be one of the best prognostic biomarkers of survival in systemic DLBCL (Alizadeh, et al 2011, Lossos, et al 2004). Although the function of LMO2 in DLBCL and PCNSL is unknown, its expression is associated with increased centrosome numbers and it is known to be part of a transcriptional regulatory complex (Cubedo, et al 2012). Further studies are needed to validate the association between LMO2 expression and OS in PCNSL as well as its function in these tumours.
Notably, while expression of BCL6 was significantly associated with improved PFS and most probably OS in MTX-treated patients, LMO2 expression was more significantly associated with OS. The latter observation suggests that LMO2 expression may be associated with better response to second line therapies resulting in patients' rescue and OS prolongation. LMO2 and BCL6 are both associated with improved survival in systemic DLBCL and PCNSL, suggesting that these genes may be important in the pathogenesis of both diseases (Lossos, et al 2001). This is strengthened by the observation that both genes are known to be functionally important in GC-derived B-cells, the presumed cell of origin for tumours expressing these proteins. Further studies are necessary to validate our observations and to determine the function of BCL6 and LMO2 in PCNSL pathogenesis and their contributions to prolonged PFS and OS.
Acknowledgments
I.S.L. is supported by National Institutes of Health (NIH) grants NIH CA109335, Lymphoma Research Foundation and the Dwoskin Family, Recio Family and Anthony Rizzo Family Foundations.
References
- Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, Smith JR, Korfel A, Soussain C, DeAngelis LM, Neuwelt EA, O'Neill BP, Thiel E, Shenkier T, Graus F, van den Bent M, Seymour JF, Poortmans P, Armitage JO, Cavalli F, International Primary CNS Lymphoma Collaborative Group Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M, DeAngelis LM. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- Alizadeh AA, Gentles AJ, Alencar AJ, Liu CL, Kohrt HE, Houot R, Goldstein MJ, Zhao S, Natkunam Y, Advani RH, Gascoyne RD, Briones J, Tibshirani RJ, Myklebust JH, Plevritis SK, Lossos IS, Levy R. Prediction of survival in diffuse large B-cell lymphoma based on the expression of 2 genes reflecting tumor and microenvironment. Blood. 2011;118:1350–1358. doi: 10.1182/blood-2011-03-345272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011;101:257–265. doi: 10.1007/s11060-010-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum T, Stadler EA, von Baumgarten L, Straube A. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. J Neurooncol. 2012;109:285–291. doi: 10.1007/s11060-012-0891-7. [DOI] [PubMed] [Google Scholar]
- Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, Harris NL, Batchelor TT. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9:1063–1069. [PubMed] [Google Scholar]
- Camilleri-Broet S, Criniere E, Broet P, Delwail V, Mokhtari K, Moreau A, Kujas M, Raphael M, Iraqi W, Sautes-Fridman C, Colombat P, Hoang-Xuan K, Martin A. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q, Mo T, Murty VV, Dalla-Favera R. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7:445–455. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Chang CC, Kampalath B, Schultz C, Bunyi-Teopengco E, Logan B, Eshoa C, Dincer AP, Perkins SL. Expression of p53, c-Myc, or Bcl-6 suggests a poor prognosis in primary central nervous system diffuse large B-cell lymphoma among immunocompetent individuals. Arch Pathol Lab Med. 2003;127:208–212. doi: 10.5858/2003-127-208-EOPMOB. [DOI] [PubMed] [Google Scholar]
- Chen BB, Xu XP, Shen L, Han TJ, Lin ZG, Chen Z, Kang H, Huang B, Lin GW. Prognostic value of clinical characteristics and immunophenotypic biomarkers in 115 patients with primary central nervous system lymphoma. Chin Med J (Engl) 2013;126:482–487. [PubMed] [Google Scholar]
- Cheng AL, Yeh KH, Uen WC, Hung RL, Liu MY, Wang CH. Systemic chemotherapy alone for patients with non-acquired immunodeficiency syndrome-related central nervous system lymphoma: a pilot study of the BOMES protocol. Cancer. 1998;82:1946–1951. doi: 10.1002/(sici)1097-0142(19980515)82:10<1946::aid-cncr19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Cox D. Regression models and life tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. with discussion. [Google Scholar]
- Cubedo E, Gentles AJ, Huang C, Natkunam Y, Bhatt S, Lu X, Jiang X, Romero-Camarero I, Freud A, Zhao S, Bacchi CE, Martinez-Climent JA, Sanchez-Garcia I, Melnick A, Lossos IS. Identification of LMO2 transcriptome and interactome in diffuse large B-cell lymphoma. Blood. 2012;119:5478–5491. doi: 10.1182/blood-2012-01-403154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118:510–522. doi: 10.1182/blood-2011-03-321349. [DOI] [PubMed] [Google Scholar]
- Gregory G, Arumugaswamy A, Leung T, Chan KL, Abikhair M, Tam C, Bajel A, Cher L, Grigg A, Ritchie D, Opat S. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol. 2013;15:1068–1073. doi: 10.1093/neuonc/not032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Levy O, Deangelis LM, Filippa DA, Panageas KS, Abrey LE. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer. 2008;112:151–56. doi: 10.1002/cncr.23149. [DOI] [PubMed] [Google Scholar]
- Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, Hsu HC, Tien HF, Cheng AL. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12:1152–1156. doi: 10.1158/1078-0432.CCR-05-1699. [DOI] [PubMed] [Google Scholar]
- Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Zehnder JL, Tibshirani R, Levy R. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98:945–951. doi: 10.1182/blood.v98.4.945. [DOI] [PubMed] [Google Scholar]
- Lossos IS, Alizadeh AA, Rajapaksa R, Tibshirani R, Levy R. HGAL is a novel interleukin-4-inducible gene that strongly predicts survival in diffuse large B-cell lymphoma. Blood. 2003;101:433–440. doi: 10.1182/blood-2002-06-1931. [DOI] [PubMed] [Google Scholar]
- Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, Levy R. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- Lu X, Kazmierczak K, Jiang X, Jones M, Watt J, Helfman DM, Moore JR, Szczesna-Cordary D, Lossos IS. Germinal center-specific protein human germinal center associated lymphoma directly interacts with both myosin and actin and increases the binding of myosin to actin. FEBS J. 2011;278:1922–1931. doi: 10.1111/j.1742-4658.2011.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momota H, Narita Y, Maeshima AM, Miyakita Y, Shinomiya A, Maruyama T, Muragaki Y, Shibui S. Prognostic value of immunohistochemical profile and response to high-dose methotrexate therapy in primary CNS lymphoma. J Neurooncol. 2010;98:341–348. doi: 10.1007/s11060-009-0078-z. [DOI] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Kuppers R, Schluter D, Spieker T, Van Roost D, Schaller C, Reifenberger G, Wiestler OD, Deckert-Schluter M. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol. 1999;155:2077–2086. doi: 10.1016/S0002-9440(10)65526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos-Rongen M, Brunn A, Bentink S, Basso K, Lim WK, Klapper W, Schaller C, Reifenberger G, Rubenstein J, Wiestler OD, Spang R, Dalla-Favera R, Siebert R, Deckert M. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia. 2008;22:400–405. doi: 10.1038/sj.leu.2405019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y, Lossos IS, Taidi B, Zhao S, Lu X, Ding F, Hammer AS, Marafioti T, Byrne GE, Jr, Levy S, Warnke RA, Levy R. Expression of the human germinal center-associated lymphoma (HGAL) protein, a new marker of germinal center B-cell derivation. Blood. 2005;105:3979–3986. doi: 10.1182/blood-2004-08-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y, Hsi ED, Aoun P, Zhao S, Elson P, Pohlman B, Naushad H, Bast M, Levy R, Lossos IS. Expression of the human germinal center-associated lymphoma (HGAL) protein identifies a subset of classic Hodgkin lymphoma of germinal center derivation and improved survival. Blood. 2007a;109:298–305. doi: 10.1182/blood-2006-04-014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y, Zhao S, Mason DY, Chen J, Taidi B, Jones M, Hammer AS, Hamilton Dutoit S, Lossos IS, Levy R. The oncoprotein LMO2 is expressed in normal germinal-center B cells and in human B-cell lymphomas. Blood. 2007b;109:1636–1642. doi: 10.1182/blood-2006-08-039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkunam Y, Farinha P, Hsi ED, Hans CP, Tibshirani R, Sehn LH, Connors JM, Gratzinger D, Rosado M, Zhao S, Pohlman B, Wongchaowart N, Bast M, Avigdor A, Schiby G, Nagler A, Byrne GE, Levy R, Gascoyne RD, Lossos IS. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J Clin Oncol. 2008;26:447–454. doi: 10.1200/JCO.2007.13.0690. [DOI] [PubMed] [Google Scholar]
- Olson JE, Janney CA, Rao RD, Cerhan JR, Kurtin PJ, Schiff D, Kaplan RS, O'Neill BP. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, Van't Veer M, Hansen M, Soubeyran P, Taphoorn M, Thomas J, Van den Bent M, Fickers M, Van Imhoff G, Rozewicz C, Teodorovic I, van Glabbeke M, European Organization for, R. Treatment of Cancer Lymphoma, G High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- Romero-Camarero I, Jiang X, Natkunam Y, Lu X, Vicente-Duenas C, Gonzalez-Herrero I, Flores T, Garcia JL, McNamara G, Kunder C, Zhao S, Segura V, Fontan L, Martinez-Climent JA, Garcia-Criado FJ, Theis JD, Dogan A, Campos-Sanchez E, Green MR, Alizadeh AA, Cobaleda C, Sanchez-Garcia I, Lossos IS. Germinal centre protein HGAL promotes lymphoid hyperplasia and amyloidosis via BCR-mediated Syk activation. Nat Commun. 2013;4:1338. doi: 10.1038/ncomms2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Fridlyand J, Shen A, Aldape K, Ginzinger D, Batchelor T, Treseler P, Berger M, McDermott M, Prados M, Karch J, Okada C, Hyun W, Parikh S, Haqq C, Shuman M. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, Cheson BD, Kaplan LD. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- Thompsett AR, Ellison DW, Stevenson FK, Zhu D. V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood. 1999;94:1738–1746. [PubMed] [Google Scholar]
- Younes SF, Beck AH, Ohgami RS, Lossos IS, Levy R, Warnke RA, Natkunam Y. The efficacy of HGAL and LMO2 in the separation of lymphomas derived from small B cells in nodal and extranodal sites, including the bone marrow. Am J Clin Pathol. 2011;135:697–708. doi: 10.1309/AJCP7Z2BIBUNQPLZ. [DOI] [PMC free article] [PubMed] [Google Scholar]


