Abstract
Current treatment modalities for soft tissue defects due to various pathologies and trauma include autologous grafting and the use of commercially available fillers. However, these treatment methods are associated with a number of limitations, such as donor site morbidity and volume loss over time. As such, improved therapeutic options are needed. Tissue engineering techniques offer novel solutions to these problems through development of bioactive tissue constructs that can regenerate adipose tissue with an appropriate structure and function. The recent advances in the derivation and characterization of hASCs have led to numerous studies of soft tissue reconstruction. In this chapter, we discuss methods in which our laboratory has used hASCs and silk scaffolds for adipose tissue engineering. The use of naturally occurring and clinically acceptable materials such as silk protein for tissue-engineering applications poses advantages with respect to biocompatibility and mechanical and biological properties.
Keywords: hASC, Adipose tissue, Silk, Scaffold, Lipogenesis, Lipolysis
1. Introduction
The utilization of 3D adipose tissue systems to study different physiological states provides several advantages over traditional 2D culture studies. The 3D environment provides a scaffold structure that functions as a physiologically like microenvironment, and thereby promotes cell attachment and migration, and cell–cell and cell–matrix interactions (1). Therefore, regenerative medicine strategies, and, more specifically, adipose tissue engineering, provide promising avenues for the study of adipose tissue in both the normal and diseased states.
Human adipose-derived stem cells (hASCs) are widely available, abundant, and easily accessible through procedures such as liposuction and biopsies (2). Remarkable progress has been made with respect to the use of hASCs with natural and synthetic scaffolds, in vitro and in vivo, and for various time frames. In addition to exploring hASC viability and differentiation capacity on various materials (3–10), the contributions of hASCs toward vascularization (11–14) and wound healing (15) have been investigated.
Silk is a versatile protein biomaterial for various tissue-engineering applications (16–19), with favorable characteristics such as low immunogenicity, slow degradation, absence of bioburdens, and strong mechanical properties (20–25). Additionally, we recently demonstrated the ability to engineer vascularized adipose tissue in vitro by cocultivating adipocytes derived from hASCs with endothelial cells in silk scaffolds (26). The ability to tailor silk with respect to biodegradation, mechanical strength, and ability to undergo surface modifications provides strong rationale for its use as a scaffold for soft tissue regeneration (27). Critically, this protein-based biomaterial offers sustained morphological and architectural support, from months to years in vivo during slow degradation, providing soft tissue reconstruction support without loss of size and shape with time until tissue regeneration occurs in vivo.
2. Materials
2.1. Cell Culture and Lysis
Dulbecco’s Modified Eagle’s Medium/F-12 (DMEM/F-12) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin–fungizone (PSF).
0.25% Trypsin–EDTA.
Biotin is dissolved in water at 33 mM (1,000×), stored in aliquots at −20°C, and added to medium as required.
Pantothenate is dissolved in water at 17 mM (1,000×), stored in aliquots at −20°C, and added to medium as required.
Dexamethasone is dissolved in water at 1 mM (1,000×), stored in aliquots at −20°C, and added to medium as required.
Human recombinant insulin is dissolved in water at 1 mM (1,000×), stored in aliquots at 4°C, and added to medium as required.
Thiazolidinedione (TZD) is dissolved in dimethyl sulfoxide (DMSO) at 5 mM (1,000×), stored in aliquots at −20°C, and added to medium as required.
3-Isobutyl-1-methylxanthine (IBMX) is dissolved in absolute ethanol at 500 mM (1,000×), stored in aliquots at −20°C, and added to medium as required.
DNA cell lysis buffer: 10 mM Tris, 1 mM EDTA, 1% Triton X-100, 1 mg proteinase K.
Intracellular triglyceride lysis buffer: sodium dodecyl sulfate (SDS) buffer (0.1% w/v) in water.
ASC expansion media: DMEM/F-12 supplemented with 10% FBS and 1% PSF.
Adipogenic induction medium: DMEM/F-12 supplemented with 3% FBS, 1% PSF, 33 μM biotin, 17 μM pantothenate, 1 μM dexamethasone, 1 μM insulin, 5 μM TZD, and 500 μM IBMX.
Adipogenic maintenance media: induction media minus TZD and IBMX.
Surgical microscissors.
2.2. Silk Scaffold Preparation
Bombyx mori silkworm cocoons are purchased from Tajima Shoji Co., LTD. (Naka-ku, Yokohama, Japan).
All water used is ultrapure grade.
0.2 M Sodium carbonate in water.
9.3 M LiBr solution in water.
3.3.5-kDa Molecular weight cutoff dialysis cassette.
1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP).
Sieve salt particles to obtain the 500–600-μm range.
Teflon cylindrical molds.
Dermal biopsy punches.
2.3. Assay Kits
Quant-iTTM PicoGreen® dsDNA Reagent and kits.
Serum Triglyceride Determination kit.
Quantikine® Human Leptin Immunoassay.
2.4. Oil Red O Staining
Scaffold fixative: 2 M sucrose in water (see Note 1).
Staining fixative: 10% buffered neutral formalin.
Oil Red O solution: 0.700 g Oil Red O powder in 200 mL isopropranol. Make a 60% working solution with 0.2-μm filtered Oil Red O solution in phosphate-buffered saline (PBS).
Glycerol jelly mounting medium.
2.5. Cultureware
Non-tissue cultured-treated 6-well plates.
Glass-bottom dishes.
3. Methods
Mature adipocytes, the predominant cell type in the adipose tissue, contain microscopic intracellular lipid droplets, which are the first visible signs of adipose tissue formation. Oil Red O staining and enzymatic assays are conducted to visualize and quantify the intracellular lipid, respectively (7, 14, 28–31). As adipose tissue is recognized as a vital endocrine organ, the engineered tissue’s ability to secrete appropriate adipokines, such as leptin, has also been widely investigated (28, 32, 33). Another key measure of adipose tissue function is its sensitivity to hormone stimulation. Standard assays involve measurements on glucose uptake or glycerol and fatty acid release following stimulation with lipogenic (e.g., insulin) or lipolytic (e.g., catecholamine) hormones (5, 29, 33, 34).
In this section, we discuss detailed protocols for culture of adipogenic cells derived from hASCs on 3D silk scaffolds. In addition to hASC and 3D scaffold preparation, we include protocols for characterization of adipose tissue outcomes. See Fig. 1 for an overview of the methods discussed in this chapter.
Fig. 1.
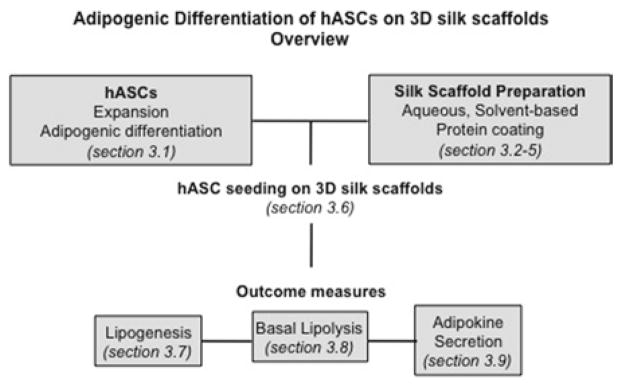
Overview of adipogenic differentiation of hASCs on 3D silk scaffolds, with the methods discussed in this chapter.
3.1. hASC Expansion and Adipogenic Differentiation
Prepare ASC expansion media.
Quickly thaw frozen hASCs in a 37°C water bath, add to the appropriate volume of expansion media, and allow cells to attach overnight.
The following day, aspirate all medium and replace with fresh expansion medium.
Exchange media after 3 days.
Prepare adipogenic induction medium.
Once cells are confluent, aspirate media, wash cells with PBS 2×, and add an appropriate volume of adipogenic induction medium.
Prepare adipogenic maintenance media.
Following 1-week induction, exchanging medium 2×, exchange induction media with adipocyte maintenance media.
3.2. Silk Biomaterial Preparation
Cut Bombyx mori silkworm cocoons into small pieces. Discard worm.
Boil 5 g of cocoons in 2 L of 0.2 M sodium carbonate for 20 min to degum and remove as much sericin as possible (see Note 2).
Rinse the silk fibroin in 1 L of ultrapure water three times for 20 min each time.
Dry overnight.
Solubilize the dry silk in 9.3 M lithium bromide at 60°C until dissolved (see Note 3), so that the final concentration is 20% w/v.
Load the silk solution into a 3.5-kDa molecular weight cutoff dialysis cassette in ultrapure water (see Note 4).
Exchange the water a total six times over 3 days.
3.3. Aqueous-Based Silk Scaffold Preparation
Dilute the aqueous solution to 6% w/v (see Note 5) using ultrapure water.
Pour the silk fibroin solution into a Teflon cylindrical mold and add 500–600-μm NaCl particles to the solution. Use 2 g of NaCl for every milliliter of solution.
Cover the mold and leave at room temperature for 2–3 days. Gently tap the mold to ensure even packing of the NaCl particles.
Remove the lid and place the scaffolds into ultrapure water to leach out the NaCl particles.
Exchange the water three times per day for 2 days.
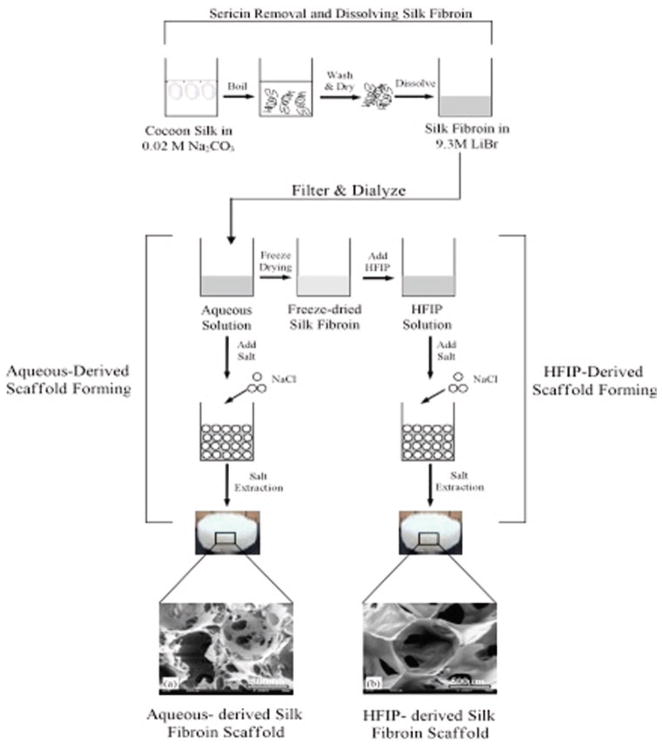
Cut the scaffolds to the desired size using a biopsy punch and autoclave to sterilize (see Note 6) (see Fig. 2).
Fig. 2.
Schematic of 3D silk fibroin scaffold formation. Processing methods for both aqueous-derived and HFIP-derived silk scaffolds are shown. Reproduced from Biomaterials with permission from Elsevier (27).
3.4. Solvent-Based Silk Scaffold Preparation
Lyophilize the aqueous silk solution for 2–3 days or until dry.
Dissolve the lyophilized silk in HFIP to yield a 17% w/v silk–HFIP solution.
Cover the solution immediately, seal with Parafilm, and keep at room temperature for 2–3 days until fully dissolved.
To cast the scaffolds, 3.4 g of NaCl particles is used for each milliliter of silk–HFIP solution; leave at room temperature to dry for 2 days.
Uncover the scaffolds to allow for HFIP evaporation for 1 day at room temperature and then place in methanol for 1 day.
Remove the scaffolds from the methanol and allow to dry.
Transfer to Ultrapure water to allow the salt to leach out over 2 days.
The scaffolds are cut and sterilized in the same way as the aqueous based scaffolds (see section 3.3) (see Fig. 2).
3.5. Protein Coating
Coating solutions were prepared by dissolving VEGF and/or laminin in sterile distilled water at a concentration of 10 μg/mL.
Incubate the sterile scaffolds for 15 min in the coating solution, allow to dry to adsorb proteins, and repeat two more times.
3.6. hASC Seeding on 3D Scaffolds
One day prior to cell seeding, presoak autoclaved silk scaffolds in adipogenic maintenance media in 37°C, 5% CO2, 95% relative humidity (see Note 7).
The following day, gently aspirate all media from culture wells and from within scaffolds using a 1,000-mL pipette tip (see Note 8). Place each scaffold in a well of a sterile, non-tissue culture-treated plate.
Detach differentiated adipocytes from culture flasks by incubating 4 mL of trypsin–EDTA per T185 culture flask for 5 min at 37°C, 5% CO2, 95% relative humidity. Add media (2× trypsin volume), collect and centrifuge for 10 min at 300 × g in 4°C.
Following centrifugation, carefully aspirate media from cell pellet, resuspend in 5 mL media, and proceed to count cells using standard protocols via hemocytometer. Centrifuge again.
For each 8-mm × 4-mm (diameter × height) cylindrical silk scaffold, a cell volume of 60 μL is necessary, in which 1 × 106 cells are added per scaffold. Carefully pipette 60 μL cells drop-by-drop onto scaffold.
Place culture plates in incubator, and allow cells to attach for 2–3 h.
After 2–3 h, carefully add appropriate volume of adipocyte maintenance media to each well such that scaffolds are completely submerged.
The following day, carefully transfer seeded scaffolds to new non-tissue culture-treated plates with autoclaved tweezers (see Note 9). Add fresh media.
Exchange media every 3 days. See Fig. 3 for a confocal microscopy image of adipogenic-differentiated hASCs seeded on 3D silk scaffolds.
Fig. 3.
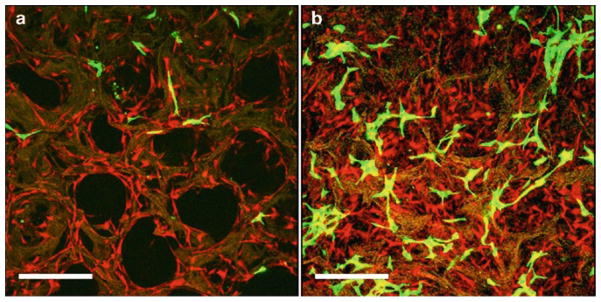
Confocal microscopy images of tomato red-transduced hASCs in coculture with green fluorescence protein-transduced endothelial cells in porous 3D aqueous silk fibroin scaffolds at day 4 (a) and 8 (b). Scale bar 375 μm. Reproduced from Tissue Engineering with permission from Mary Ann Liebert (26).
3.7. Measuring Lipogenesis of 3D Adipocyte-Seeded Scaffolds
Intracellular triglyceride can be quantified by harvesting scaffolds at various time points in 200 μL intracellular triglyceride lysis buffer, and storing in 1.5-mL Eppendorf tubes at −20°C until time of assay.
Thaw samples at room temperature, and chop scaffolds using autoclaved sterile surgical microscissors. Sonicate scaffolds briefly.
Centrifuge chopped scaffolds at 15,700 × g for 10 min at 4°C.
Collect supernatant and proceed to assay according to manufacturer’s protocols using TG quantification kit.
Accumulated lipid can also be visualized using Oil Red O staining. Fix scaffolds in 2 M sucrose overnight for histology. Embed scaffolds in OCT medium and freeze over dry ice, and store in −90°C. Frozen scaffolds can then be sectioned (10-μm sections) using a cryostat and stained.
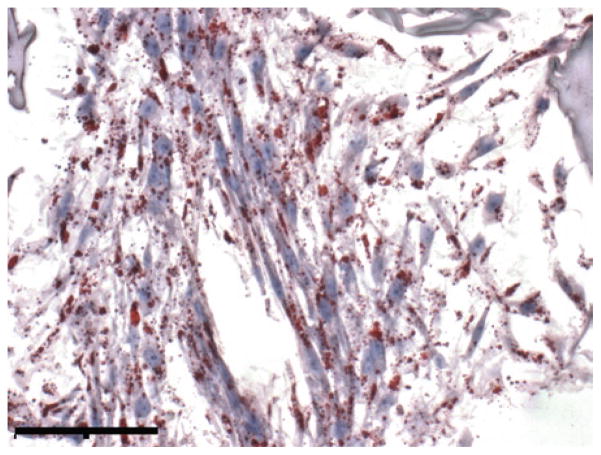
For Oil Red O staining, fix frozen sections in 10% buffered neutral formalin for 15 min, and rinse with distilled water. Pipette working solution onto frozen sections and incubate for 20 min. Following this, rinse slides with distilled water, and counterstain if desired. Cover using glass cover slips and glycerin jelly. Image with phase contrast microscope. An example image of a section from adipocyte-seeded silk scaffolds is shown in Fig. 4.
Fig. 4.
Oil Red O phase contrast images of differentiated hASCs on porous 3D aqueous silk fibroin scaffolds. Scale bar 500 μm; original magnification ×20.
3.8. Measuring Basal Lipolysis of 3D Adipocyte-Seeded Scaffolds
Levels of basal lipolysis can be measured by quantifying secreted glycerol from spent media samples.
Collect spent media samples at desired time points, and store at −80°C.
At time of assay, thaw samples, centrifuge at 15,700 × g for 10 min at 4°C. Collect supernatants.
Detected glycerol can be measured (540 nm) using the TG kit described earlier, however excluding the hydrolysis step. Glycerol levels detected in media samples from blank scaffolds should also be subtracted from all media samples.
3.9. Measuring Leptin Secretion from 3D Adipocyte-Seeded Scaffolds
Levels of leptin secretion can be measured as an indicator of mature adipocyte differentiation.
Collect spent media samples at the desired time point, and store at −80°C.
At time of assay, thaw samples and centrifuge at 15,700 × g for 10 min at 4°C. Collect supernatants.
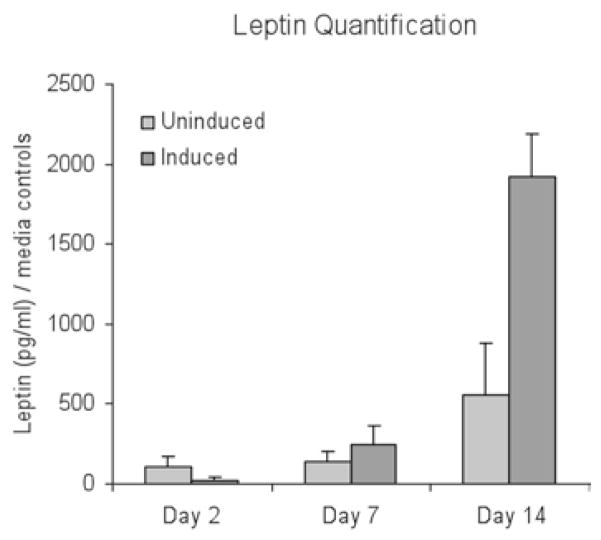
Leptin can then be detected using a quantitative sandwich enzyme immunoassay technique (ELISA) for human leptin. See Fig. 5 for example leptin measurements taken from adipogenic cells derived from hASCs in 3D aqueous silk scaffolds.
Fig. 5.
Leptin secretion measured in undifferentiated hASC/endothelial cocultures (uninduced) and differentiated hASC/endothelial cocultures (induced). Induced cocultures secreted significantly more leptin than uninduced cocultures at day 14 (*p < 0.05) and increased secretion at days 7 and 14 (+p < 0.05), indicative of the presence of more mature adipocytes. Uninduced cocultures increased leptin secretion at day 14 as well (++p < 0.05). Reproduced from Tissue Engineering with permission from Mary Ann Liebert (26).
Acknowledgments
We would like to thank the NIH P41 Tissue Engineering Resource Center (P41 EB002520) and AFIRM for support of soft tissue engineering research.
Footnotes
Prepare 2 M sucrose solution while stirring, slowly adding water to obtain final volume. It may take up to an hour for the sucrose to fully dissolve.
Publications from our lab have used varying boiling times. However, for our purposes, we chose to boil for 20 min. This boiling time yielded consistent batches of scaffolds, whereas longer boiling times do not always produce robust scaffolds.
Dissolving the silk in LiBr usually takes between 1 and 4 h. It is generally better to pack the dry silk into a beaker that just fits all the silk, and then slowly pour the LiBr solution over the top.
The silk solution will be viscous, and shearing of the solution may prematurely induce β-sheet formation. Use the largest needle diameter possible when loading and unloading the dialysis cassette.
The concentration was calculated by drying 1 mL of silk solution in a preweighed weigh boat and placing into a 60°C oven till fully dry and then measuring.
The scaffolds can be sterilized by autoclaving or by ethanol sterilization, however, degradation properties may be altered by the sterilization technique.
When presoaking scaffolds, due to the hydrophobicity exhibited by the protein structure, be sure to gently submerge the scaffolds into the media to ensure that all sides of the scaffold are wetted.
Aspirate media from scaffolds right before cell seeding to prevent completely drying scaffolds out.
Transferring scaffolds to new plates ensures that any nutrient-competing cells that may have attached onto the culture plate during seeding are eliminated.
References
- 1.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27(36):6052–63. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Dubois SG, et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008;449:69–79. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- 3.Choi YS, et al. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem Biophys Res Commun. 2006;345(2):631–7. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
- 4.Clavijo-Alvarez JA, et al. A novel perfluoroelastomer seeded with adipose-derived stem cells for soft-tissue repair. Plast Reconstr Surg. 2006;118(5):1132–42. doi: 10.1097/01.prs.0000221037.34883.0a. discussion 1143–4. [DOI] [PubMed] [Google Scholar]
- 5.Flynn LE, Prestwich GD, Semple JL, Woodhouse KA. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials. 2008;29(12):1862–71. doi: 10.1016/j.biomaterials.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006;183(3):133–40. doi: 10.1159/000095987. [DOI] [PubMed] [Google Scholar]
- 7.Uriel S, et al. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29(27):3712–9. doi: 10.1016/j.biomaterials.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Hong L, et al. 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007;13(6):1197–203. doi: 10.1089/ten.2006.0317. [DOI] [PubMed] [Google Scholar]
- 9.Mischen BT, et al. Metabolic and functional characterization of human adipose-derived stem cells in tissue engineering. Plast Reconstr Surg. 2008;122(3):725–38. doi: 10.1097/PRS.0b013e318180ec9f. [DOI] [PubMed] [Google Scholar]
- 10.Wall ME, Bernacki SH, Loboa EG. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007;13(6):1291–8. doi: 10.1089/ten.2006.0275. [DOI] [PubMed] [Google Scholar]
- 11.Amos PJ, et al. IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26(10):2682–90. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A. 2008;89(4):929–41. doi: 10.1002/jbm.a.32044. [DOI] [PubMed] [Google Scholar]
- 13.Hemmrich K, et al. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials. 2005;26(34):7025–37. doi: 10.1016/j.biomaterials.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 14.Lin SD, Wang KH, Kao AP. Engineered adipose tissue of predefined shape and dimensions from human adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14(5):571–81. doi: 10.1089/tea.2007.0192. [DOI] [PubMed] [Google Scholar]
- 15.Altman AM, et al. IFATS Series: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2008;27(1):250–8. doi: 10.1634/stemcells.2008-0178. [DOI] [PubMed] [Google Scholar]
- 16.Lovett M, et al. Silk fibroin microtubes for blood vessel engineering. Biomaterials. 2007;28(35):5271–9. doi: 10.1016/j.biomaterials.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinel L, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71(1):25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 18.Vunjak-Novakovic G, Meinel L, Altman G, Kaplan D. Bioreactor cultivation of osteochondral grafts. Orthod Craniofac Res. 2005;8(3):209–18. doi: 10.1111/j.1601-6343.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27(25):4434–42. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 20.Altman GH, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 21.Horan RL, et al. In vitro degradation of silk fibroin. Biomaterials. 2005;26(17):3385–93. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3(6):1233–9. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 23.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775–85. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 24.Meinel L, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26(2):147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 25.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–26. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 26.Kang JH, Gimble JM, Kaplan DL. In vitro 3D model for human vascularized adipose tissue. Tissue Eng Part A. 2009;15(8):2227–36. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27(36):6064–82. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Kang X, Xie Y, Kniss DA. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Eng. 2005;11(3–4):458–68. doi: 10.1089/ten.2005.11.458. [DOI] [PubMed] [Google Scholar]
- 29.Stosich MS, et al. Vascularized adipose tissue grafts from human mesenchymal stem cells with bioactive cues and microchannel conduits. Tissue Eng. 2007;13(12):2881–90. doi: 10.1089/ten.2007.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vashi AV, et al. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29(5):573–9. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Weiser B, et al. In vivo development and long-term survival of engineered adipose tissue depend on in vitro precultivation strategy. Tissue Eng Part A. 2008;14(2):275–84. doi: 10.1089/tea.2007.0130. [DOI] [PubMed] [Google Scholar]
- 32.Fischbach C, et al. Three-dimensional in vitro model of adipogenesis: Comparison of culture conditions. Tissue Eng. 2004;10(1–2):215–29. doi: 10.1089/107632704322791862. [DOI] [PubMed] [Google Scholar]
- 33.Vermette M, et al. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28(18):2850–60. doi: 10.1016/j.biomaterials.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Saidel GM, Kalhan SC. A computational model of adipose tissue metabolism: Evidence for intracellular compartmentation and differential activation of lipases. J Theor Biol. 2008;251(3):523–40. doi: 10.1016/j.jtbi.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]