Abstract
Idiopathic osteonecrosis of femoral head (ION) is a painful disorder that progresses to collapse of the femoral head and destruction of the hip joint. Although its precise pathology remains unknown, the loss of blood supply causing the loss of living bone-forming cells is a hallmark of the pathophysiology of osteonecrosis. Transplantation of multipotent mesenchymal stromal cells (MSCs) is a promising tool for regenerating the musculoskeletal system. The aim of the present study was to assess the safety and efficacy of transplantation of cultured autologous bone marrow-derived MSCs mixed with β-tricalcium phosphate (β-TCP) in combination with vascularized bone grafts for the treatment of advanced stage ION in a clinical trial. Ten patients with stage 3 ION were enrolled in this study. Autologous bone marrow-derived MSCs were cultured with autologous serum, and cells (0.5–1.0×108) were transplanted after mixing with β-TCP granules in combination with vascularized iliac bone grafts. Patients were assessed 24 months after treatment. The primary and secondary endpoints were progression of the radiological stage and changes in bone volume at the femoral head, and clinical score, respectively. Nine of ten patients completed the protocol, seven of whom remained at stage 3, and the remaining two cases progressed to stage 4. The average bone volume increased from 56.5±8.5 cm3 to 57.7±10.6 cm3. The average clinical score according to the Japan Orthopaedic Association improved from 65.6±25.5 points to 87.9±19.0 points. One severe adverse event was observed, which was not related to the clinical trial. Although the efficacy of cell transplantation was still to be determined, all procedures were successfully performed and some young patients with extensive necrotic lesions with pain demonstrated good bone regeneration with amelioration of symptoms. Further improvements in our method using MSCs and the proper selection of patients will open a new approach for the treatment of this refractory disease.
Introduction
Idiopathic osteonecrosis of femoral head (ION) is a painful disorder that progresses to collapse of the femoral head and symptomatic osteoarthritis of the hip joint.1,2 This disease mainly affects individuals aged 30–40 years of age.3 ION includes steroid-induced, alcoholism-related, and true idiopathic conditions. The precise pathological mechanism of ION remains unknown, however, macro- or microscopic obstruction of blood supply to the femoral head is considered to be a hallmark of this condition, which causes the necrosis of bone-forming cells. Bone tissues without bone-forming cells gradually lose their mechanical properties and eventually collapse, causing articular surface deformities.1–3
Several staging and classification systems have been applied to diagnose ION, and the system proposed by the Specific Disease Investigation Committee (SDIC) has been used in Japan, which is a modified version of the system proposed by the Association Research Circulation Osseous (ARCO) Committee.4 This staging system includes stage 1 (specific findings of osteonecrosis are observed on magnetic resonance imaging [MRI], bone scintigram, or histology, not on X-ray images), stage 2 (demarcating sclerosis is seen without collapse of the femoral head), stage 3 (collapse of the femoral head, including the crescent sign, is seen without joint-space narrowing. Mild osteophyte formation of the femoral head or acetabulum may be seen), and stage 4 (osteoarthritic changes are seen). Stage 3 was subdivided into stage 3A (collapse of the femoral head being less than 3 mm) and stage 3B (collapse of the femoral head being 3 mm or greater).4 Although the natural history of ION depends on the etiological background of each case, this condition is considered to be a progressive disease from the early stage (stage 1), with minimum necrotic areas found only by MRI, to the advanced stage (stage 4), with painful osteoarthritis of the hip joint.4,5 Regarding the surgical treatment of patients at early stages (stage 1 or 2), core decompression has been widely used to decompress elevated pressure in the femoral head.5–7 Although this procedure may be effective for some patients, the results are unpredictable.7 Total hip arthroplasty (THA) is the only way to treat patients at the terminal stage (stage 4).1,3 The survival rate of THA has markedly improved owing to the advancement of materials and surgical technology.3 However, THA should be avoided as much as possible, because patients with ION are relatively young, therefore, joint-preserving treatment for patients at stage 3 is a critical issue. Sugioka's rotational osteotomy, which replaces the weight-bearing area with intact bone tissues, has been used for stage 3 patients and was shown to be effective in some patients.8 However, the application of this surgery is limited for patients with intact areas.9 Because the loss of blood supply and bone-forming cells are causative for this condition, grafting vascularized bones is a theoretically reasonable way to treat this condition.10 Clinical results appear to depend on the size of the necrotic area and the prolonged period required to generate new bone tissues,10 possibly due to the decreased number of bone-forming cells.11,12
The application of concentrated bone marrow cells (BMCs) to ION was initiated by Hernigou.13 He invented a method to transplant BMCs after core decompression and performed it on a large number of patients at early stages. The effect of transplantation was not clear because of the lack of controls in his studies.14 Gangji et al. conducted prospective randomized studies and reported that the application of concentrated BMCs significantly delayed progression of the disease,15,16 and its effect continued 5 years after transplantation.16 The clinical results of this method depended at least partly on the number of osteogenic cells in aspirated BMCs, which could not be controlled.11
Multipotent mesenchymal stromal cells (MSCs) are promising materials for regenerating the musculoskeletal system.17,18 MSCs are defined as plastic dish-adherent cells that can be differentiated to cells in osteogenic, chondrogenic, and adipogenic lineages in vitro.19,20 MSCs may also be able to differentiate to vascular endothelial cells.21 They are found in several different tissues,17 among which bone marrow stroma is a representative home of MSCs; therefore, the therapeutic effect of BMCs for ION may depend on the effect of MSCs. The application of MSCs expanded in vitro for ION was considered to overcome the issue of the unstable number of BMC treatment, and Zhao conducted a prospective randomized trial using in vitro-expanded MSCs and reported that cultured MSC transplantation in combination with core decompression surgery was effective in delaying and avoiding femoral head collapse.22 Although these findings suggest the promising role of MSCs on ION, this was also shown to be ineffective for patients at advanced stages such as stage 3.22
We previously designed a treatment with a combination of MSCs and vascularized bone grafts to apply MSCs for advanced stages, and performed a preclinical study using an animal model.23 Based on the promising results of that study, we proceeded to clinical trials and herein we report the result.
Materials and Methods
Study design
This study was a prospective, open-labeled, proof-of-concept clinical trial conducted in the Kyoto University Hospital. Patient registration and data management were performed in the Department of Clinical Trial Design and Management, Translational Research Center, Kyoto University Hospital. This clinical study was registered to the UMIN Clinical Trials Registry (UMIN000001601). The study protocol was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine, Guidelines on Clinical Research using Human Stem Cells of Ministry of Health, Labour and Welfare, Japan (www.mhlw.go.jp/bunya/kenkou/iryousaisei06/pdf/111201_ 1.pdf) and was conducted according to the Declaration of Helsinki. Results were evaluated 24 months after the treatment, and the primary endpoint was the progression of the radiographic stage and the secondary endpoint was bone volume changes evaluated by computed tomography (CT), and clinical score.
Patients eligibility
Eligibility criteria were between 20 and 50 years of age and the presence of necrotic stage 3A or 3B according to the radiographic stage system by the SDIC.4 The radiographic stage was evaluated also by the Steinberg classification.24
Exclusion criteria were patients with a surgical history on the affected part, heavy smokers (Brinkman index>600) requiring the continued use of warfarin, diabetes mellitus (defined by HbA1c>9.0%), arteriosclerosis obliterans, pregnancy, malignant disease, myocardial infarction, brain infarction, rheumatoid arthritis, patients receiving dialysis, blood disease (leukemia, myeloproliferative disorder, and myelodysplastic disorder), patients with a limited life expectancy, hepatitis B, hepatitis C, human immunodeficiency virus and syphilis, hypotension (systemic volume<90 mmHg), low body weight (<40 kg), loss of marrow function (neutrophils<1500/mm3, hemoglobin<11.0 g/dL [men], 10.0 g/dL [women], platelets<100,000/mm3), patients whose drugs (osteoporosis and steroids) were changed within 3 months of the clinical study, and ineligible patients for the clinical study as decided by a doctor.
Assessment of necrotic lesions
The necrotic lesion and the size were assessed by the radiographic classification proposed by the Japanese Investigation Committee.4 Type A lesions occupied the medial one-third or less of the weight-bearing portion. Type B lesions occupied the medial two-thirds or less of the weight-bearing portion. Type C1 and type C2 lesions both occupied more than the medial two-thirds of the weight-bearing portion, wherein type C2 lesions extended laterally to the acetabular edge, whereas type C1 lesions did not.4
Preparation of autologous bone marrow-derived MSC
Peripheral blood (400 mL) was obtained from patients and incubated at room temperature for 3 h to proceed coagulation. Serum was then separated by centrifugation at 1500 g for 15 min, collected, and stored at −30°C. This procedure was repeated once after 7 days; therefore, autologous serum from 800 mL was prepared from each patient.
The isolation of bone marrow-derived MSCs from patients was performed as described previously.25 Briefly, bone marrow (100 mL) was aspirated under general anesthesia from two sites of each right and left posterior iliac crest using a bone marrow harvest needle (Angiotech, Gaineville, FL). Aspirated bone marrow (25 mL) was immediately mixed with 1000 IU heparin (Mochida Pharmaceutical Co., Tokyo, Japan) and transported to the cell processing center. Mononuclear cells containing MSCs were seeded at a density of 2.5×105 cells/cm2and cultured with the α-minimal essential medium with GlutaMAX (Invitrogen Co., Carlsbad, CA) supplemented with 10% autoserum, 100 U/mL penicillin, and 100 mg/mL streptomycin, under 20% pO2 and 5% pCO2 conditions at 37°C. When the total cell number reached 5.0×107, cells were dissociated by TripLE Select (Invitrogen Co.), frozen with cryoprotectant (CP-1; Kyokuto Pharmaceutical Ind. Co., Tokyo, Japan), and stored in liquid nitrogen. Frozen cells were thawed and recultured at a density of 1.35×104 cells/cm2 under the same condition for 4 days before transplantation surgery. The cells were dissociated by TripLE Select, washed by serum, and prepared in suspension 2 h before transplantation. The survival rate was calculated after thawing and at the timing of shipping.
Evaluation of multipotent MSCs
Differentiation potential
The potential for differentiation in osteogenic, adipogenic, and chondrogenic directions was examined as previously reported using the differentiation induction protocol provided by Cambrex (East Rutherford, NJ) in each MSC of the sampled cells from the preceding MSC. Osteogenic differentiation was evaluated by Alizarin Red S staining on day 14 of induction. Adipogenic differentiation was evaluated by Oil Red O staining on day 21 of differentiation, in which cells were stained with 40 mM pH 4.2 Alizarin Red S (Sigma-Aldrich, St. Louis, MO). After 21 days of adipogenic differentiation, cells were stained with 0.3% Oil Red O (NacalaiTesque, Kyoto, Japan). After 21 days of chondrogenic differentiation, pellets were stained with Alcian Blue (Muto Pure Chemicals Co., Ltd., Tokyo).
Cytogenetic analysis
G-band cytogenetic analysis was performed on isolated mononuclear cells and a sample of final transplanted MSCs. A minimum of 20 metaphases were analyzed in each case. The criteria used to describe a cytogenetic clone and description of the karyotype followed the recommendations of the International System for Human Cytogenetic Nomenclature.26
Measurement of endotoxin
The activity of endotoxin in the supernatant of final transplanted MSCs was assayed by the limulus test using the limulus ES-II single test kit (Wako, Osaka, Japan).
In vivo tumor formation assays
Nonobese diabetic (NOD)-severe combined immunodeficient (SCID) mice were purchased from Shimizu Laboratory Supplies (Kyoto, Japan). In vivo experiments were approved by the Institutional Animal Research Committee, and were performed according to the Guidelines for Animal Experiments of Kyoto University. A fraction of final transplanted MSCs (5.0×106) was sampled and inoculated subcutaneously into the right flank of each mouse. Tumor formation was monitored for 6 months, and mice were then euthanized and the existence of tumors was assessed.
Transplantation surgery
Patients were placed in a supine position on the table. A curved skin incision (modified Smith-Peterson approach) was prepared from the iliac crest to the anterior aspect of the proximal thigh. The anterior aspect of the femoral neck was explored and a cortical window (1.5×4 cm) was made, through which a bony trough connecting to the necrotic area was formed under X-ray fluoroscopy.27,28 Necrotic areas, including sclerotic bones, were extensively curetted under the fluoroscopy and endoscopy. MSCs (0.5–1.5×108) premixed with β-tricalcium phosphate (β-TCP) granules (Osferion; Olympus Terumo Biomaterials Co., Tokyo, Japan) were transplanted into the cavity. The tricortical iliac bone (1.5×2×5 cm) with a vascular pedicle (deep circumflex iliac vessels) was then harvested and grafted into the bone trough.27,28
Postoperative course
Patients were kept non-weight bearing for 6 weeks, followed by partial bearing for the next 6 weeks. The return to sports and work was allowed for the next 6 months confirming ossification. Clinical and radiological evaluations were performed according to the protocol until 24 months after surgery.
Evaluation of treatment
The primary endpoint in this study was radiographic progression of osteonecrosis by anteroposterior radiographs at the point of pretreatment and 2 years after treatment. Progression was evaluated according to the radiographic clinical stage established by the SDIC with modifications to the latest version of the ARCO staging system4 and Steinberg classification.24
Bone volume was calculated from CT images. CT images consisted of 400 slices with a voxel size of 68.224 μm in all three axes. Coronal and sagittal cross-sectional views of the femoral head were reconstructed using adjunctive software. Image reconstruction and quantification of the femoral head were performed with VG Studio MAX software (Nihon Visual Science, Tokyo, Japan). The femoral head was defined as an upper lesion on the line between the epiphysis edge of the inside and outside. The bone volume of the femoral head was calculated by VG Studio MAX software. These radiographic results were evaluated by three outside reviewers (two orthopedic surgeons and one radiologist) who were unrelated to this clinical trial.
Clinical symptoms were evaluated by the hip score defined by the Japanese Orthopaedic Association (JOA).29
Adverse events
In this clinical trial, adverse events were defined as any event occurred during the entire study period regardless of the relationship with the protocol, and monitored in the Department of Clinical Trial Design and Management, Translational Research Center. Serious adverse events were assessed by the External Data Monitoring Committee.
Statistical analysis
Continuous variables are described as the mean and standard deviation. Radiographic progression of osteonecrosis was assessed by the Wilcoxon's signed-rank test. Changes in bone volume and the clinical score according to the JOA score were examined by the paired t-test. All reported p-values were two sided, with a value below 0.05 being considered significant. All statistical analyses were conducted in the Department of Clinical Trial Design and Management, Translational Research Center using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Baseline data of patients
Between November 2007 and June 2009, 10 patients were recruited into the clinical trial. All patients were male and the average age was 31.7 (range 20–48) years, and previous history of steroid treatment was found in four patients (Table 1). The pretreatment radiographic stage was stage 3A on six hips and stage 3B on four hips according to the SDIC System, and stage 3 on six hips and stage 4 on four hips by the Steinberg classification (Table 2). Pretreatment bone volumes averaged 56.5 (range 45.3–66.4) cm3. Pretreatment clinical scores according to the JOA averaged 65.6 (range 33–95) points (Table 2).
Table 1.
Characteristics of Patients and Transplanted Cells
| Case | Age/gender | Past illness | History of steroid | Concentration of isolated MNCs from bone marrow (×107/mL) | Total number of seeded MNCs (×109) | Culture period (day) | Total number of stored MSCs (×107) | Fraction of living cells after thawing (%) | Population doubling after thawing | Population doubling time (hr) | Fraction of living cells at shipping (%) | Chromo somal aberration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27/M | Nephritis | Yes | 1.54 | 1.00 | 9 | 6.38 | 89.5 | 0.41 | 232.1 | 97.3 | No |
| 2 | 23/M | Cushing syndrome | Yes | 1.47 | 1.00 | 9 | 14.38 | 87.7 | 0.61 | 156.5 | 91.8 | No |
| 3 | 48/M | Meningioma | No | 2.25 | 1.37 | 9 | 9.88 | 93 | 0.92 | 104.5 | 98.0 | No |
| 4 | 20/M | Hepatitis | Yes | 2.28 | 1.14 | 8 | 11.94 | 92.6 | 1.15 | 83.5 | 92.0 | No |
| 5 | 35M | NP | No | 1.08 | 0.65 | 9 | 7.00 | 96.5 | 1.30 | 73.9 | 12.3 | No |
| 6 | 28/M | NP | No | 2.06 | 1.29 | 10 | 6.75 | 97.1 | 1.68 | 57.2 | 96.0 | No |
| 7 | 39/M | Leukemia | Yes | 2.31 | 1.31 | 9 | 14.00 | 95.5 | 0.82 | 117.7 | 98.0 | Yesa |
| 8 | 26/M | NP | No | 2.18 | 1.13 | 9 | 16.13 | 97.3 | 1.00 | 96.0 | 100.0 | No |
| 9 | 33/M | NP | No | 0.95 | 0.90 | 16 | 6.56 | 97.2 | 1.15 | 83.5 | 96.0 | No |
| 10 | 38/M | NP | No | 1.77 | 0.98 | 8 | 16.56 | 89.2 | 1.63 | 59.0 | 97.0 | No |
46,XY,add(1)(q11),−2,−4, add(7)(q11.1),−22, +3mar.
MSCs, mesenchymal stromal cells; MNC, mono nuclear cell; NP, nothing particular.
Table 2.
Radiological and Clinical Results
| Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ARCOb | Steinberg | Bone volume (cm3)c | Clinical score (JOA) | ||||||
| Case | Classa | Before Tx | 2 year after Tx | Before Tx | 2 year after Tx | Before Tx | 2 year after Tx | Before Tx | 2 year after Tx |
| 1 | C2 | 3B | 3B | 3 | 4 | 45.3 | 46.8 | 33 | 93 |
| 2 | C2 | 3A | 3A | 3 | 3 | 65.4 | 68.8 | 96 | 100 |
| 3 | C2 | 3B | 4 | 4 | 5 | 43.6 | 38.3 | 36 | 39 |
| 4 | C1 | 3A | 3A | 4 | 4 | 66.4 | 69.7 | 95 | 100 |
| 5 | C2 | 3A | 3A | 3 | 3 | 58.3 | 60.2 | 79 | 95 |
| 6 | C2 | 3A | 3A | 3 | 4 | 60.4 | 60.6 | 52 | 95 |
| 7 | C2 | 3B | 4 | 4 | 6 | 57.1 | 51.0 | 84 | 84 |
| 8 | C2 | 3B | 3B | 4 | 4 | 56.0 | 58.0 | 56 | 90 |
| 9 | C2 | 3A | 3A | 3 | 3 | 64.8 | 65.9 | 88 | 95 |
| 10 | C2 | 3A | NA | 3 | NA | 47.4 | NA | 37 | NA |
Radiographic clinical classification proposed by Japanese Investigation Committee.
Latest modification of ARCO staging score by Japanese Investigation Committee.
Bone volume of femoral head calculated from computed tomography.
ARCO, Association Research Circulation Osseous; JOA, Japanese Orthopaedic Association; NA, not analyzed; Tx, treatment.
Cell characteristics of manufactured MSCs
The preparation of autoserum and harvesting, culturing, and storing of MSCs were safely conducted in all cases. The number of isolated mononuclear cells from bone marrow varied among cases (0.95–2.31×107/mL), with the expected number of cells (5.0×107) being obtained within 10 days in all cases, except for case 9, in which 16 days were required (Table 1). The mean survival rate of frozen MSCs after thawing was 93.6% (89.2–97.3%) (Table 1). The mean population doubling and doubling time after thawing was 1.07 (0.41–1.68) and 106.4 h (57.2–232.1 h), respectively, and the mean survival rate of shipping cells was 97.1% (92.0–100%) (Table 1). The differentiation properties to the osteogenic, adipogenic, and chondrogenic lineages were confirmed in MSCs (data not shown). No tumorous condition was found in any SCID mice during the 6 months following inoculation. A chromosomal aberration (46,XY, add(1)(q11), −2,−4, add(7)(q11.1), −22, +3mar was detected in one case (case 7, Table 1). An identical aberration was found in BMCs immediately after they were isolated, suggesting that this aberration may not have been related to the in vitro culture process. Endotoxin activity was not detected in the supernatant of MSCs.
Transplantation
During surgery, as much of the necrotic area was removed as possible. The average bone defect was 9.3 cm3 (2.0–20.0 cm3). The average number of transplanted MSCs was 1.2×108 (0.5–1.5×108) and the average volume of β-TCP granules was 3.3 g (1.5–5.0 g).
Radiographic progression of the stages
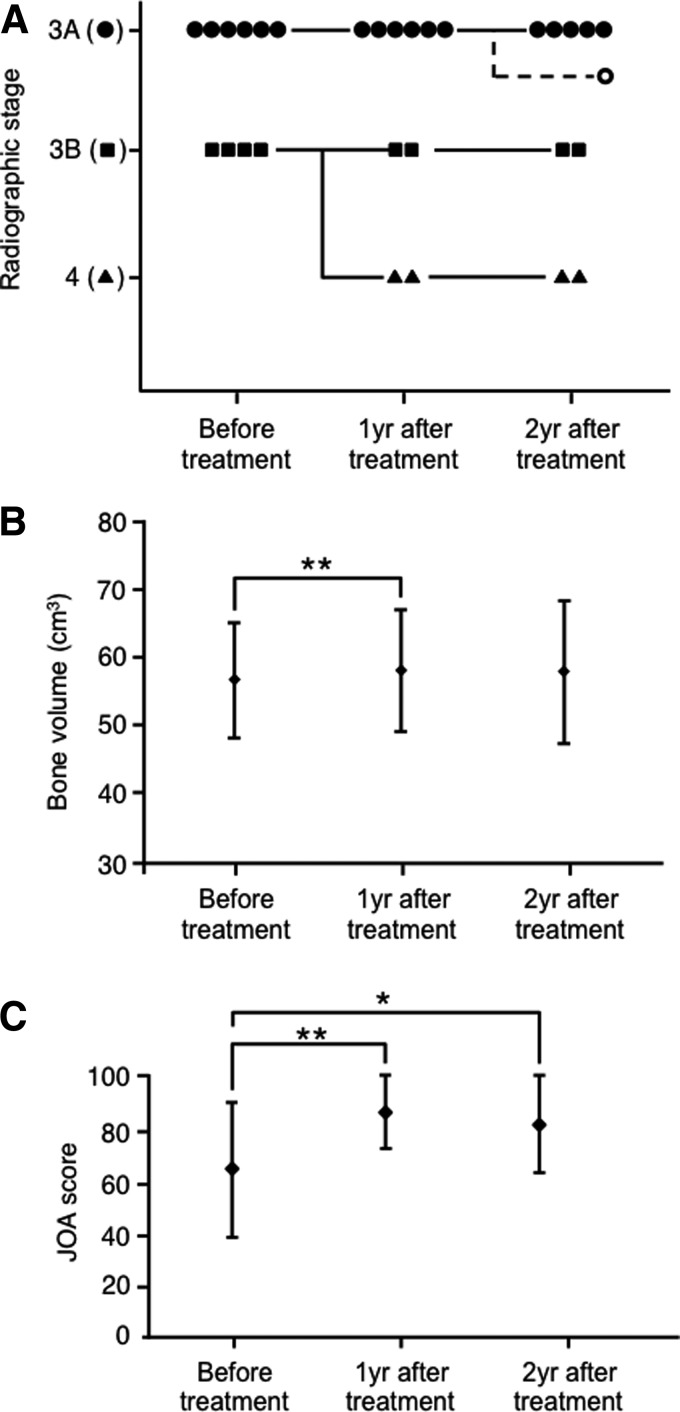
One patient who had bilateral lesions and decided to receive surgery for contralateral hip joints was excluded from this study, 12 months after the treatment. The other nine patients completed the planned follow-up course and were eligible for the evaluation. None of the stage 3A patients progressed to stage 3B or 4 (Figs. 1A and 2). Two hips of stage 3B (50%) did not progress (Fig. 1A), while the remaining two hips (50%) progressed to stage 4 twelve months after the treatment (Fig. 1A).
FIG. 1.
Radiological and clinical results of treatment. (A) Transition of the radiographic stage. The radiographic stage of each case at the indicated time points was evaluated by the system of Specific Disease Investigation Committee (SDIC), which is a modified version of the system proposed by the Association Research Circulation Osseous (ARCO) Committee.4 One case of stage 3A before treatment was off-protocol after 1 year. (B) Bone volume in the femoral head. Bone volume in each femoral head was calculated from data on computed tomography (CT) scans at each time point. **p<0.01. (C) Clinical results. Clinical results were evaluated by the Japanese Orthopaedic Association (JOA) hipscore29 at each time point. **p<0.01, * p<0.05.
FIG. 2.
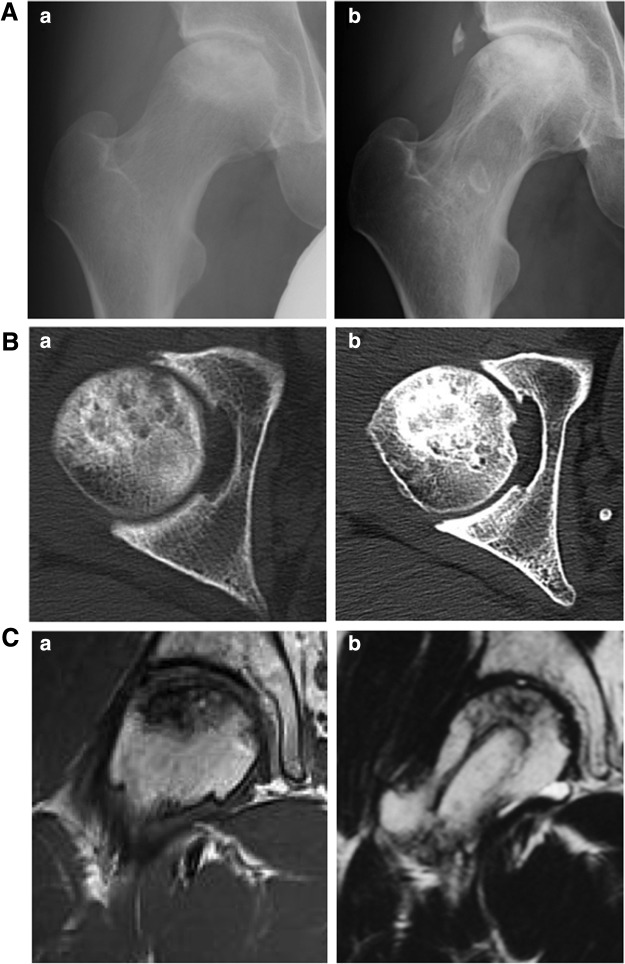
Case presentation. A 33-year-old man (case 9) with idiopathic osteonecrosis of the femoral head. (A) Anteroposterior radiographs of the femoral head before (a) and 2 years after surgery (b). The radiographic stage and classification before treatment were 3A and type C2, respectively (b). (B) Transverse CT image of the femoral head before (a) and 2 years after treatment (b). (C) Frontal view of the femoral head by T1-weighted magnetic resonance imaging before (a) and 2 years after treatment (b).
According to the Steinberg classification, two of six hips (33%) with stage 3 progressed to stage 4. One of four hips (25%) with stage 4 progressed to stage 5 and one hip (25%) progressed to stage 6. The remaining two hips (50%) maintained the shape of the femoral head. The etiology of the progressed hips was idiopathic in one hip and steroid induced in one hip, and past illnesses of these cases were meningioma in one hip and leukemia in one hip. Patient ages were 48 and 38 years. The radiographic clinical classification was type C2 in both cases.
Changes in the bone volume
Bone volume of the femoral head according to CT was evaluated before treatment, 1 year after treatment, and 2 years after treatment. Average bone volume before treatment was 56.5±8.4cm3 (Fig. 1B). Average bone volume significantly increased to 57.9±9.0 cm3 1 year after treatment (p=0.005, Fig. 1B). Average bone volume maintained at 57.7±10.6 cm3 2 years after treatment (Fig. 1B). However, bone volume decreased in progressed cases (43.6–38.3 cm3 and 57.1–50.9 cm3, Table 2).
Clinical score
The clinical score according to the JOA was evaluated before treatment, 1 year after treatment, and 2 years after treatment. The average JOA score before treatment was 65.6±25.5 points (Fig. 1C). The JOA score significantly improved to 90.2±14.1 points with statistic difference at 1 year after treatment (Fig. 1C). The JOA score maintained at 87.9±19.0 points 2 years after treatment (p=0.028, Fig. 1C). The JOA score did not markedly improved in progressed cases (36–39 and 84–84, Table 2).
Adverse events
Sixty-one adverse events were observed in ten patients (Table 3). One serious adverse event was a case of medial meniscus tear in the contralateral knee joint. An external evaluation judged that this serious adverse event was not correlated to the current clinical study. Remaining sixty adverse events were nonserious adverse events. The frequently reported adverse events were an increase in creatine phosphokinase, increase in C-reactive protein, anemia, pain in the hip joint, decrease in albumin, complications at the wounded area, decrease in total protein, wound pain, and fever (Table 3). In one case, the vascular pedicle of the transplanted bone was injured during surgery, and the injured part of the vessels was microsurgically replaced by vein grafts and its patency was confirmed by a CT angiogram.
Table 3.
List of Adverse Events
| Type of event | No. of cases |
|---|---|
| Severe adverse event | |
| Meniscal teara | 1 |
| Nonsevere adverse event | |
| Increase of creatine phosphokinase, increase of C-reactive protein | 10 |
| Anemia | 9 |
| Pain of hip joint, decrease of albumin, complication of postoperative wound | 8 |
| Decrease of total protein, postoperative wound pain | 6 |
| Fever | 5 |
| Back pain, astriction, increase of glutamate oxaloacetate transaminase | 4 |
| Pain around injection, pain of extremity, hyposthesia of lower extremity | 3 |
| Decrease of body weight, increase of aspartate aminotransferase, inflammation around continuous epidural anesthesia catheter, cough, red patch around wound, insomnia, muscular pain, nausea, itching sensation, increase of white blood cell, stiffness of muscle, nausea after injection, secretion around the drainage, pharyngeal pain | 2 |
| Abdominal pain, itching around wound, rare around bandage, decrease of lactase dehydrogenase, increase of lactase dehydrogenase, increase of potassium, increase of blood pressure, dehydration, contact dermatitis, vertigo, difficulty of urination, headache, hypertension, increase of uric acid, keroid scar, dullness, stiffness around the clavicle, musculoskeletal pain, nasopharyngitis, orthostatic hypotension, fracture of the rib, dental pain, transient vocal cord paresis, increase of body weight, bleeding after injection, dyschezia, itching around eye, electrolyte abnormality, vomiting after surgery, headache after surgery | 1 |
Medial meniscus tear in the contralateral knee joint.
Discussion
The prognosis of ION is influenced by the size of the necrotic area, stage of the disease, time from the diagnosis, and etiological factors.4,9,30 According to the radiographic classification proposed by the SDIC, types A and B are less likely to progress to collapse than types C1 and C2.4,30 These results suggest that broad necrotic lesions on weight-bearing areas increase the risk of disease progression. The stage of disease is also an important factor that may influence the treatment.4,30 Core decompression surgery is commonly used in precollapse stages such as stage 2. Based on the clinical results reported by Hernigou, Gangji, and Zhao, the combination of core decompression with in vitro expanded MSCs was shown to be an appropriate approach for stage 2 with lesions defined as class A or B.12,14,22 Application of the biomaterial as a scaffold of MSCs reported by Nöth et al. may be an alternative choice for the treatment of patients with this condition.31
Core decompression surgery was performed less than vascularized bone graft surgery in the mild collapse stage corresponding to a subchondral fracture (stage 3).32,33 Vascularized bone graft surgery has the benefits of supplying living cells and mechanical strength. Kawate et al. published a case report on cell therapy combined with vascularized fibular grafts for ION.34 They cultured autologous MSCs with β-TCP granules in vitro, induced osteogenic differentiation and transplanted the necrotic area with a vascularized fibular graft.34 In the present study, we used undifferentiated MSCs instead of differentiated osteogenic cells, which was based on the findings of our previous preclinical study.23 A comparative study should be performed to investigate which type of cell therapy is suitable for the treatment of ION.
The safety and efficacy are critical points in any cell therapy, and the major purpose of our study is also to evaluate these points. As for the safety, we evaluated the characteristics of cultured cells and adverse effects on patients during the entire course of therapy. No serious events were observed in any case during establishing and expanding cells such as bacterial contamination. One serious risk of cell transplantation is the transformation during in vitro expansion.35 Previous studies have described the spontaneous transformation of MSCs during in vitro processing,36,37 which was shown to be caused by contaminated cancer cells.38–40 This may happen in laboratories in which cancer cells were cultured in the same area. Cells for clinical use should be prepared in an area defined by Good Manufacturing Practice, in which the culture of other cells, particularly established cancer cells, should be strictly forbidden.41 Cell transplantation in immunodeficient mice is one of standard methods used to evaluate transformation,35 and we did not observe tumorous conditions in NOD-SCID mice transplanted with MSCs in this series. Chromosome analysis is another method that can predict transformation. Physiological stress during in vitro culture has been suggested to induce chromosomal aberrations, which may be the case even if the cell processing system is of a clinical grade.42 In the present study, chromosomal aberrations were not found in transplanted MSCs, except for one case (case 7). This patient received intensive chemotherapy for leukemia 13 years ago and was free from the disease at the entry for the current study. The aberration was also detected in BMCs before expansion, excluding the effect of culture process. Cultured MSCs in this case showed no definite abnormalities during in vitro expansion or in their differentiation properties. A careful long-term follow-up should be undertaken in this case.
During the entire course, one serious and sixty adverse events were observed (Table 3). There were two adverse events correlated with cell preparation. One was vertigo (1/10) after blood sampling for preparation of autoserum, and the anemia (9/10) for preparation of autoserum and bone marrow aspiration. One serious adverse event (knee meniscus tear) was evaluated not to be related to the clinical study. Although it is difficult to completely deny the effect of transplanted MSCs, almost all other adverse events were those found in surgical procedures without cell transplantation. For example, the increase of creatine phosphokinase was almost always observed after the surgery involving muscles, and in the current surgery, muscles attaching iliac bone were severed for harvesting bone grafts. The increase of C-reactive protein, anemia, and decrease in albumin and total protein were also observed in major orthopedic surgeries. Without saying, even if the effect of adverse events is minimum, it is rational to consider these adverse events based on the risk–benefit balance. For example, we used general anesthesia for harvesting bone marrow, which potentially has a considerable risk. When the efficacy of the current method is precisely evaluated, the risk associated with each procedure should be reconsidered.
Because of the small sample number and lack of control, only limited information was obtained for efficacy in this study. Two of nine cases in this study showed the progression of stages evaluated by the SDIC system. In both cases, we observed the formation of cystic lesions in the femoral head 12 months after surgery. β-TCP degradation began from two to 3 weeks in vivo.43,44 An imbalance between bone resorption and formation may cause such a condition, and further study is required to define the suitable amount of β-TCP to be used in cell transplantation therapy. We compared the current results with those of simple vascularized iliac bone grafting performed during 1988 to 1997 in the affiliated hospital. The proportion of nonprogressed case in that series was 26/38 (68%), while the proportion of nonprogressed case in the current series was 7/9 (78%, 95% CI: 40–97%). Although it is clear that the prospective randomized trial using enough number of patients is necessary to draw any conclusion, the result in this study suggested the feasibility of our method. Based on the results in this study, we re-evaluate the risk–benefit value of each step and will modify to improve the value in the next trials.
Whether transplanted cells exert therapeutic effects through direct differentiation to osteogenic cells remain to be investigated. Most initial cell transplantation studies were designed and performed with the aim of engrafting transplanted cells to regenerate the tissue. However, recent studies showed that this was not the case. Only a small proportion of MSCs, locally or systemically administrated, will actually be incorporated into injured tissues, which indicates that the beneficial effects of tissue repair and regeneration are more likely indirect and depend on the paracrine activity of MSCs.45,46 This should be also considered in our case, although it is difficult to trace the fate of transplanted cells in humans.
As a conclusion, we showed that our procedure was performed safely, but the efficacy was still to be determined. In addition to the increase in the sample number, future modification should be considered to increase the efficacy, such as the combination with suitable degradable artificial components and growth factors. Information from the current clinical trial may lead to the successful result for the advanced stage of ION.
Acknowledgments
The authors are grateful to Drs. Kei-ichiro Kawanabe and Haruhiko Akiyama for their technical advice on ION, Drs. Kazumi Miura, Tatsuya Ito, Akira Shimizu, and Ryota Asada for promoting the clinical study, and Drs. Moritoshi Furu, Akira Nasu, Kenichi Fukiage, Seiji Otsuka, Takashi Kasahara, Tatsuya Sueyoshi, Kinya Ito, Yonghui Jin, and Hiroto Mitsui for their assistance.
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) with a project entitled Development of Evaluation Technology for Early Introduction of Regenerative Medicine, and also by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, from the Ministry of Education, Culture, Sports, Science, and Technology, and from the Ministry of Health, Labor, and Welfare.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mankin H.J.Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med 326,1473, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Malizos K.N., Karantanas A.H., Varitimidis S.E., Dailiana Z.H., Bargiotas K., and Maris T.Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol 63,16, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kaushik A.P., Das A., and Cui Q.Osteonecrosis of the femoral head: an update in year 2012. World J Orthop 3,49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugano N., Atsumi T., Ohzono K., Kubo T., Hotokebuchi T., and Takaoka K.The 2001 revised criteria for diagnosis, classification, and staging of idiopathicosteonecrosis of the femoral head. J Orthop Sci 7,601, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ficat R.P.Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 67-B,3, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Hopson C.N., and Siverhus S.W.Ischemic necrosis of the femoral head. Treatment by core decompression. J Bone Joint Surg Am 70-A,1048, 1988 [PubMed] [Google Scholar]
- 7.Koo K.H., Kim R., Ko G.H., Song H.R., Jeong S.T., and Cho S.H.Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br 77-B,870, 1995 [PubMed] [Google Scholar]
- 8.Sugioka Y.Transtrochanteric anterior rotational osteotomy of the femoral head in the treatment of osteonecrosis affecting the hip: a new osteotomy operation. Clin Orthop Relat Res 130,191, 1978 [PubMed] [Google Scholar]
- 9.Miyanishi K., Noguchi Y., Yamamoto T., Irisa T., Suenaga E., Jingushi S., Sugioka Y., and Iwamoto Y.Prediction of the outcome of transtrochanteric rotational osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Br 82,512, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Marciniak D., Furey C., and Shaffer J.W.Osteonecrosis of the femoral head. A study of 101 hips treated with vascularized fibular grafting. J Bone Joint Surg Am 87,742, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hernigou P., Beaujean F., and Lambotte J.C.Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br 81,349, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Gangji V., Hauzeur J.P., Schoutens A., Hinsenkamp M., Appelboom T., and Egrise D.Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol 30,348, 2003 [PubMed] [Google Scholar]
- 13.Henigou P., Bernaudin F., Reinert P., Kuentz M., and Vernant J.P.Bone marrow transplantation in sickle cell disease: effect on osteonecrosis. J Bone Joint Surg Am 79,1726, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P., and Beaujean F.Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 405,14, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gangji V., Hauzeu J.P., Matos C., De Maertelaer V., Toungouz M., and Lambermont M.Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am 86-A,1153, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gangji V., De Maertelaer V., and Hauzeur J.P.Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 49,1005, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Caplan A.I.Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 11,1198, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Steinert A.F., Rackwitz L., Gilbert F., Nöth U., and Tuan R.S.Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 1,237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D.J., and Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Casteilla L., Planat-Bénard V., Cousin B., Laharrague P., and Bourin P.Vascular and endothelial regeneration. Curr Stem Cell Res Ther 5,141, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Zhao D., Cui D., Wang B., Tian F., Guo L., Yang L., Liu B., and Yu X.Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 50,325, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ikeguchi R., Kakinoki R., Aoyama T., Shibata K.R., Otsuka S., Fukiage K., Nishijo K., Ishibe T., Shima Y., Otsuki B., Azuma T., Tsutsumi S., Nakayama T., Otsuka T., Nakamura T., and Toguchida J.Regeneration of osteonecrosis of canine scapho-lunate using bone marrow stromal cells: possible therapeutic approach for Kienböck disease. Cell Transplant 15,411, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Steinberg M.E.Classification of avascular necrosis: a comparative study. Acta Orthop Belg 65Suppl 1,45, 1999 [PubMed] [Google Scholar]
- 25.Shibata K.R., Aoyama T., Shima Y., Fukiage K., Otsuka S., Furu M., Kohno Y., Ito K., Fujibayashi S., Neo M., Nakayama T., Nakamura T., and Toguchida J.Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 25,2371, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Brothman A.R., Persons D.L., and Shaffer L.G.Nomenclature evolution: changes in the ISCN from the 2005 to the 2009 edition. Cytogenet Genome Res 127,1, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Ishizaka M., Sofue M., Dohmae Y., Endo N., and Takahashi H.E.Vascularized iliac bone graft for avascular necrosis of the femoral head. Clin Orthop Relat Res 337,140, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Leung P.C., and Chow Y.Y.Reconstruction of proximal femoral defects with a vascular-pedicled graft. J Bone Joint Surg Br 66-B,32, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Kuribayashi M., Takahashi K.A., Fujioka M., Ueshima K., Inoue S., and Kubo T.Reliability and validity of the Japanese Orthopaedic Association hip score. J Orthop Sci 15,452, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Mont M.A., Zywiel M.G., Marker D.R., McGrath M.S., and Delanois R.E.The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am 92-A,2165, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Nöth U., Rackwitz L., Steinert A.F., and Tuan R.S.Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev 62,765, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Korompilias A.V., Lykissas M.G., Beris A.E., Urbaniak J.R., and Soucacos P.N.Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J Bone Joint Surg Br 91,287, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Aldridge J.M., 3rd, Berend K.R., Gunneson E.E., and Urbaniak J.R.Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. Surgical technique.J Bone Joint Surg 86-ASuppl 1,87, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kawate K., Yajima H., Ohgushi H., Kotobuki N., Sugimoto K., Ohmura T., Kobata Y., Shigematsu K., Kawamura K., Tamai K., and Takakura Y.Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs 30,960, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Barkholt L., Flory E., Jekerle V., Lucas-Samuel S., Ahnert P., Bisset L., Büscher D., Fibbe W., Foussat A., Kwa M., Lantz O., Mačiulaitis R., Palomäki T., Schneider C.K., Sensebé L., Tachdjian G., Tarte K., Tosca L., and Salmikangas P.Risk of tumorigenicity in mesenchymal stromal cell-based therapies-Bridging scientific observations and regulatory viewpoints. Cytotherapy 15,753, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Rubio D., Garcia-Castro J., Martín M.C., de la Fuente R., Cigudosa J.C., Lloyd A.C., and Bernad A.Spontaneous human adult stem cell transformation. Cancer Res 65,3035, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Røsland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H., Mysliwietz J., Tonn J.C., Goldbrunner R., Lønning P.E., Bjerkvig R., and Schichor C.Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69,5331, 2009 [DOI] [PubMed] [Google Scholar]
- 38.de la Fuente R., Bernad A., Garcia-Castro J., Martin M.C., and Cigudosa J.C.Retraction: spontaneous human adult stem cell transformation. Cancer Res 70,6682, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Garcia S., Bernad A., Martín M.C., Cigudosa J.C., Garcia-Castro J., and de la Fuente R.Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res 316,1648, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Torsvik A., Røsland G.V., Svendsen A., Molven A., Immervoll H., McCormack E., Lønning P.E., Primon M., Sobala E., Tonn J.C., Goldbrunner R., Schichor C., Mysliwietz J., Lah T.T., Motaln H., Knappskog S., and Bjerkvig R.Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track - letter. Cancer Res 70,6393, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Sensebé L., Bourin P., and Tarte K.Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther 22,19, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Tarte K., Gaillard J., Lataillade J.J., Fouillard L., Becker M., Mossafa H., Tchirkov A., Rouard H., Henry C., Splingard M., Dulong J., Monnier D., Gourmelon P., Gorin N.C., Sensebé L.; Société Française de Greffe de Moelle et Thérapie Cellulaire. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115,1549, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Kondo N., Ogose A., Tokunaga K., Ito T., Arai K., Kudo N., Inoue H., Irie H., and Endo N.Bone formation and resorption of highly purified beta-tricalcium phosphate in the rat femoral condyle. Biomaterials 26,5600, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Nasu T., Takemoto M., Akiyama N., Fujibayashi S., Neo M., and Nakamura T.EP4 agonist accelerates osteoinduction and degradation of beta-tricalcium phosphate by stimulating osteoclastogenesis. J Biomed Mater Res A89,601, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Doorn J., Moll G., Le Blanc K., van Blitterswijk C., and de Boer J.Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev 18,101, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Wang J., Liao L., and Tan J.Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther 11,893, 2011 [DOI] [PubMed] [Google Scholar]