Abstract
Objective
To examine the association of maternal and/or paternal smoking during pregnancy with offspring cardio-metabolic risk (CMR) factors at adolescence and early adulthood, taking into account socio-demographic, medical and lifestyle characteristics of parents and offspring, as well as offspring common genetic variation.
Methods
We used a population-based cohort of all 17 003 births in Jerusalem during 1974–76, with available archival data on parental and birth characteristics. Measurements at age 17 were assessed at military induction examinations for 11 530 offspring. 1440 offspring from the original 1974–1976 birth cohort were sampled using a stratified sampling approach, and were interviewed and examined at age 32. Parental smoking during pregnancy (i.e. maternal, paternal and any parent) was primarily defined dichotomously (any number of cigarettes smoked daily by mother or father during pregnancy vs. non-smokers). Additionally, smoking was assessed by quantity of cigarettes smoked daily. Linear regression models were used to evaluate the associations of parental smoking during pregnancy with various offspring CMR factors, after controlling for potential confounders and for genetic variation in candidate genes.
Results
Prevalence of exposure to parental smoking in-utero (i.e. smoking of any parent) was 53.2% and 48.4% among the 17 years old and 32 years old samples, respectively. At age 17, smoking of at least one parent during pregnancy was significantly associated with weight (B=1.39), height (B=0.59), BMI (B=0.32) and pulse rate (B=−0.78) (p-values<0.001). At age 32, parental smoking, adjusted for covariates, was associated with 2.22 kg higher mean offspring weight, 0.95 cm higher mean offspring height, 0.57 kg/m2 higher BMI, and 1.46 cm higher waist-circumference (p-values≤0.02). Similar results, reflecting a dose response, were observed when maternal and paternal smoking were assessed by number of cigarettes smoked daily.
Conclusions
This prospective study demonstrates a potential long-term adverse effect of parental smoking during pregnancy on offspring health and calls for increasing efforts to promote smoking cessation of both parents before pregnancy.
Keywords: cohort study, smoking, pregnancy, obesity, risk factors
Introduction
Active and passive cigarette smoking are major risk factors for multiple clinical conditions, such as cardiovascular disease, cancer, chronic lung disease, and mortality1,2. Hence, cigarette smoking is a major public health concern globally. Despite the known harmful effects of cigarette smoking on the health of mothers and their children, it is estimated that 22 to 34 percent of American women of reproductive age smoke cigarettes3. Smoking during pregnancy is one of the most important modifiable risk factors associated with adverse pregnancy outcomes. The negative impact of cigarette smoking on fetal health is well established and cigarette smoking has been associated with numerous adverse perinatal outcomes4,5. Low birth weight (LBW) (<2500 grams) is the best-studied complication of smoking during pregnancy. Women who smoke are 1.5 to 3.5 times more likely to have a LBW infant with increasing risk as cigarette consumption increases6. It has been estimated that at least 20% percent of LBW and small for gestational age infants are attributed to tobacco exposure during pregnancy7. Exposure to passive smoking during pregnancy also appears to have adverse effects on the fetus, child, and adult offspring8,9, including increased rate of stillbirth, and reduced mean birth weight9.
Additionally, parental smoking during pregnancy is associated with long-term offspring health risks. Associations of parental smoking with offspring cardiovascular risk factors have been demonstrated mostly in pre-pubertal children10,11. Only few studies have examined these associations in adolescents and adults and most of them were retrospective12–15. Prior studies have focused mostly on maternal smoking, rather than paternal smoking, and the associations of maternal smoking with offspring obesity16,17 and blood pressure18. Evidence regarding the associations of parental smoking with other cardio-metabolic risk (CMR) traits is sparse19–21.
It has been suggested that the long-term effects of both paternal and maternal smoking on offspring health are related to effects on the intrauterine environment as well as to postnatal characteristics that are associated with CMR22–24.
In our study, we aimed to assess the effect of maternal and/or paternal smoking during pregnancy on a range of cardio-metabolic risk factors at age 17 and 32, taking into account various socio-demographic, medical and lifestyle characteristics of the parents and the offspring, as well as common genetic variation in offspring.
Methods
The Jerusalem Perinatal Study (JPS), a population-based birth cohort, recorded data on all 17 003 births to residents of Jerusalem, between the years 1974 and 1976. Details regarding data collection methods were described previously25. Briefly, available data consisted of demographic, socioeconomic, medical conditions of the mother during current and previous pregnancies, and offspring birth weight, abstracted either from birth certificates or maternity ward logbooks. Parental smoking status as well as additional information on lifestyle and pregnancy-related and medical characteristics, such as gestational age, height and pre-pregnancy weight, end of pregnancy weight and gynecological history, was collected by interviews of mothers on the first or second day postpartum. Through data linkage with the Israeli military draft records, information from medical examinations at age 17, was obtained for approximately 70% of the JPS cohort26 (82% of male offspring, and 54% of female offspring, totaling 11 530 individuals). The adequacy of the linkage was ascertained by comparing offspring’s sex, date of birth and several other demographic characteristics. Military induction medical examinations included measurements of standing height (without shoes to the nearest centimeter), body weight (with indoor light clothes, to the nearest kilogram) and pulse rate. Blood pressure was measured in the right arm, in sitting position, with a mercury sphygmomanometer. The proportion of the birth cohort with military induction data was lower for females, as a result of exemption from military service of women due to religious practice.
The JPS Family Follow-Up study includes a sample of 1440 offspring from the original 1974–1976 birth cohort, who were interviewed and examined between 2007 and 200927. The sampling frame for the follow-up study included singletons and term (gestational age≥36 weeks) births without congenital malformations. Both low (≤2500 grams) and high (≥4000 grams) birth weight offspring as well as over-weight and obese mothers (BMI≥27) were over-sampled given the focus of the research on maternal and offspring body size. Standard procedures and training protocols were used to measure standing height, body weight, waist circumference (at the midpoint between the lower ribs and iliac crest in the midaxillary line) and blood pressure (measured three consecutive times in the right arm in sitting position following five minute rest). Blood samples at fasting (at least 8 hours of fasting) were taken using standard procedures. Samples were immediately spun and biochemical measurements were assayed in plasma, including insulin and glucose levels, total cholesterol, HDL-C and triglycerides27. Genomic DNA was extracted at Hebrew University using the salting-out method; and, high throughput genotyping was performed at the University of California, San Francisco using an Illumina, Inc., BeadArray™. Available genotypic data included 1384 SNPs from 180 candidate genes selected based on molecular pathways associated with CMR-related traits.
Study variables
We examined the associations of parental smoking during pregnancy with offspring cardio-metabolic outcomes at age 17 (n = 11 530) and 32 (range 30–35) years (n = 1440). Information on smoking was obtained from postpartum interviews of the mothers in response to the question: “Do you and/or your husband smoke cigarettes?” and was categorized into the following 4 groups: Never smoked; quit before this pregnancy; quit during this pregnancy; smoking now. Smoking was defined as any number of cigarettes smoked per day by the parent. To define smoking during pregnancy, we grouped those quitting before this pregnancy with parents who never smoked (i.e. non-smokers) and those quitting during this pregnancy with current smokers (i.e. smokers). In the models, exposure to parental smoking during pregnancy was primarily assessed by three separate explanatory variables: 1) maternal smoking (yes/no); 2) paternal smoking (yes/no); and 3) smoking of at least one parent (i.e. any smoker; yes/no). Additionally, we assessed maternal and paternal smoking by quantity of cigarettes smoked daily, grouped into intervals of 10 cigarettes (i.e. 0 (non-smoker), 1 (1–10 cigarettes smoked daily), 2 (11–20), 3 (21–30), 4 (31–40) and 5 (over 40 cigarettes).
The primary outcome variables measured at age 17 were weight [kg], height [cm], body-mass index (BMI, calculated by dividing weight by squared height [kg/m2]) (continuous), pulse rate (PR [bpm]), systolic blood pressure (SBP) and diastolic blood pressure (DBP [mm Hg]). The following cardio-metabolic outcomes were measured at age 32: weight, height, BMI, waist circumference (WC, mean of two consecutive measurements [cm]), PR, SBP and DBP (mean of three consecutive measurements), glucose [mg/dL], insulin (mean of two repeated measures [μU/mL], log-transformed (base 10) due to asymmetrical distribution), low-density lipoprotein cholesterol (LDL-C [mg/dL], high-density lipoprotein cholesterol (HDL-C [mg/dL]) and total cholesterol [mg/dL] and triglycerides (TG [mg/dL], log-transformed (base 10) due to asymmetrical distribution). All outcomes were treated as continuous variables.
All models were adjusted for offspring gender and ethnicity. Adjustment for ethnicity in the analyses examining cardio-metabolic outcomes measured at age 17 was based on mother’s country of birth (categorized as: Israel, other West Asia, North Africa and Europe/America and other industrialized countries). In the analyses examining outcomes at age 32, availability of more detailed information on ethnicity in this subcohort enabled us to follow an approach suggested by Thomas and Witte28, in which ethnicity was adjusted for by covariates reflecting nine major ethnicity strata based on country of origin of all four grandparents27.
In all analyses we addressed potential confounders at time of birth reflecting the early environment (i.e. pre- and peri-natal periods). Potential confounders were: (1) average parental age at child birth (years, continuous); (2) socioeconomic status (SES) based on father’s occupation at time of birth (grouped into three categories: low, medium, high); (3) average parental years of education at time of birth (years, continuous); (4) maternal medical condition (dichotomous, based on whether the mother had ever suffered from any of the following diseases: diabetes, hypertension, heart disease and toxemia); 5) birth weight (grams, continuous); and (6) maternal pre-pregnancy BMI (mppBMI; kg/m2, continuous).
To deal with a limited number of missing values for average parental age or average parental years of education (<2%) we either used information on only one parent when available or imputed the data based on the mean. Missing data for mppBMI (6% and 3% in the age 17 and age 32 analyses, respectively) were imputed as the mean. Results of analyses using imputed data for missing values and those obtained by excluding missing values were essentially identical and therefore the reported analyses included imputed data for missing values.
In the analyses examining the associations with cardio-metabolic outcomes measured at age 32, we could further address potential confounders at young adulthood (age 32). Potential confounders were: (1) smoking status (current smoker vs. never smoked or smoked in the past); (2) physical activity (grouped into 2 categories; 1 - moderate or vigorous physical activity that lasts at least 20 minutes, three or more times a week, 0 - less or no activity; and (3) years of education (continuous). The expected positive associations of physical activity with HDL-cholesterol and years of education, and the negative association with current smoking observed in our data provide support for the validity of these variables.
Genetic Scores
Genetic scores were created based on established methodology used to create composite scores to study the influence of the additive effect of genetic variations on given relationships 29–31. A similar approach was also applied in two previous studies conducted in JPS 32,33. Using 1384 SNPs from 180 CMR-related genes among offspring, we created genetic scores that were predictive of the cardio-metabolic outcomes, to be used as precision variables. Separate genetic scores were created for the different CMR traits by fitting linear regression models individually for each of the 1384 SNPs with each separate outcome. The final scores were constructed as the mean predicted value of these outcomes calculated across all relevant SNPs for each individual.
Statistical analyses
Analyses were carried out using the SPSS version 20.0 statistical package (SPSS, Inc., Chicago, Illinois) and Stata 10.0 (StataCorp, College Station TX).
Linear regression models were used to investigate the associations of maternal or paternal smoking, independent of each other, with cardio-metabolic outcomes measured at age 17 and at age 32, after controlling for potential confounders.
Maternal and paternal smoking were introduced into the models either separately (i.e. including both maternal and paternal smoking) or as smoking of at least one parent. At both ages (17 and 32) we constructed two models: 1) adjusted for gender and ethnicity, and 2) further adjusting for the aforementioned potential confounders at birth and at time of outcome measurement. In the analyses using outcomes measured at age 32 in addition to potential confounders, model 2 included also outcome-specific genetic score. To assess dose response, model 2 was repeated with maternal and paternal smoking defined by number of cigarettes smoked daily.
All analyses examining cardio-metabolic outcomes at age 32 used inverse probability weighting to account for the stratified sampling. Additionally, individuals who reported taking BP-lowering medication (n=11), lipid-lowering medication (n=13) or medication to treat diabetes (n=13) were excluded from the corresponding analyses.
This study was approved by the Institutional Review Board of the Hadassah-Hebrew University Medical Center and the University of Washington Human Subject Review Committee. All participants provided informed consent.
Results
Maternal, and offspring socio-demographic and offspring cardio-metabolic characteristics at age 17 and age 32 are listed in tables 1a and 1b, respectively.
Table 1a.
Age 17 analyses - Sample characteristics by gender
| Women (N=4387) | Men (N=7143) | Total (N=11 530) | ||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
|
| ||||||
| Characteristics obtained at birth* | ||||||
| Smoking any parent % | 59.5 | 49.3 | 53.2 | |||
| Smoking mothers % | 26.2 | 18.0 | 21.1 | |||
| Smoking fathers % | 51.9 | 44.5 | 47.4 | |||
| Maternal pre-pregnancy BMI, kg/m2 | 21.84 | (2.91) | 21.92 | (2.91) | 21.89 | (2.91) |
| Maternal ethnic origin % | ||||||
| Israel | 12.7 | 14.7 | 14.1 | |||
| Middle East | 31.0 | 29.4 | 28.8 | |||
| North Africa | 24.2 | 22.4 | 23.1 | |||
| Ashkenazi | 32.1 | 35.3 | 34.1 | |||
| Parents’ years of education, avg yrs | 11.85 | (3.37) | 12.09 | (3.41) | 12.0 | (3.39) |
| Parents’ age, avg yrs | 29.12 | (5.40) | 29.15 | (5.54) | 29.14 | (5.49) |
| Socioeconomic status % | ||||||
| Low | 24.9 | 42.4 | 23.1 | |||
| Medium | 44.8 | 35.6 | 39.1 | |||
| High | 30.3 | 22.0 | 37.8 | |||
| Birth weight, grams | 3188.54 | (472.99) | 3312.66 | (497.78) | 3265.43 | (492.1) |
| Mothers with any background disease† % | 4.9 | 4.8 | 4.8 | |||
| Cardio-metabolic outcomes at age 17* | ||||||
|---|---|---|---|---|---|---|
| Women | Men | Total | ||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
|
| ||||||
| Systolic BP, mmHg | 111.34 | (11.39) | 118.42 | (11.84) | 115.72 | (12.17) |
| Diastolic BP, mmHg | 70.32 | (8.01) | 72.78 | (8.10) | 71.84 | (8.15) |
| Weight, kg | 57.24 | (9.46) | 64.76 | (11.33) | 61.90 | (11.26) |
| Height, cm | 162.68 | (6.22) | 174.22 | (6.98) | 169.83 | (8.74) |
| BMI, kg/m2 | 21.62 | (3.30) | 21.30 | (3.33) | 21.43 | (3.32) |
| Pulse, BPM | 78.31 | (9.74) | 75.36 | (9.86) | 76.49 | (9.92) |
Values are expressed as mean (SD) or percent.
Diabetes, hypertension, heart disease or toxemia.
Table 1b.
Age 32 analyses - Sample characteristics, by gender
| Women (N=718) | Men (N=722) | Total (N=1440) | ||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
|
| ||||||
| Characteristics obtained at birth* | ||||||
| Smoking any parent % | 50.07 | 46.70 | 48.39 | |||
| Smoking mothers % | 17.41 | 17.87 | 17.64 | |||
| Smoking fathers % | 45.89 | 41.37 | 43.64 | |||
| Maternal pre-pregnancy BMI, kg/m2 | 24.40 | (3.89) | 23.76 | (3.60) | 24.08 | (3.76) |
| Maternal ethnic origin % | ||||||
| Israel | 13.09 | 13.85 | 13.47 | |||
| Middle East | 28.13 | 25.90 | 27.01 | |||
| North Africa | 23.26 | 23.13 | 23.19 | |||
| Ashkenazi | 35.52 | 37.12 | 36.32 | |||
| Parents’ years of education, avg yrs | 11.89 | (3.31) | 12.10 | (3.39) | 11.99 | (3.35) |
| Parents’ age, avg yrs | 30.31 | (6.04) | 30.10 | (5.77) | 30.20 | (5.90) |
| Socioeconomic status % | ||||||
| Low | 21.59 | 23.82 | 22.71 | |||
| Medium | 42.06 | 32.41 | 37.22 | |||
| High | 36.35 | 43.76 | 40.07 | |||
| Birth weight, grams | 3.27 | (0.61) | 3.53 | (0.62) | 3.40 | (0.63) |
| Mothers with any background disease† % | 10.45 | 10.11 | 10.28 | |||
| Characteristics obtained at age 32* | ||||||
| Education years | 14.91 | (2.57) | 15.30 | (3.64) | 15.11 | (3.16) |
| Smokers % | 18.53 | 35.28 | 26.88 | |||
| Physically active % | 48.36 | 53.57 | 50.96 | |||
| Cardio-metabolic outcomes at age 32‡ | ||||||
|---|---|---|---|---|---|---|
| Total | Women | Men | ||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
|
| ||||||
| Systolic BP, mmHg | 106.32 | (12.42) | 98.92 | (9.50) | 113.80 | (10.38) |
| Diastolic BP, mmHg | 71.61 | (8.52) | 68.52 | (7.92) | 74.73 | (7.95) |
| Weight, kg | 75.42 | (16.90) | 68.11 | (15.06) | 82.76 | (15.41) |
| Height, cm | 168.60 | (9.50) | 161.93 | (6.34) | 175.32 | (7.13) |
| BMI, kg/m2 | 26.44 | (5.16) | 25.98 | (5.57) | 26.91 | (4.67) |
| Waist circumference, cm | 86.64 | (13.35) | 81.75 | (12.60) | 91.54 | (12.25) |
| Pulse, BPM | 69.54 | (10.14) | 71.66 | (9.53) | 67.43 | (10.29) |
| Glucose§, mg/dL | 79.39 | (11.77) | 77.29 | (11.82) | 81.45 | (11.35) |
| Insulin§, μU/mL | 12.65 | (8.79) | 11.98 | (8.24) | 13.30 | (9.24) |
| LDL-C§, mg/dL | 112.74 | (28.84) | 107.95 | (28.49) | 117.51 | (28.41) |
| HDL-C§, mg/dL | 49.73 | (14.58) | 56.45 | (14.79) | 43.12 | (10.90) |
| Cholesterol§, mg/dL | 183.79 | (33.28) | 182.82 | (33.18) | 184.74 | (33.37) |
| Triglycerides§, mg/dL | 107.70 | (73.84) | 92.94 | (57.35) | 122.11 | (84.56) |
Values are expressed as mean (SD) or percent.
Diabetes, hypertension, heart disease or toxemia.
N=1438 for BP and adiposity (717 women, 721 men) and N=1371 for blood assays (677 women, 694 men).
Based on blood plasma assays at fasting (at least 8 hours).
Table 2 presents results of linear regression models examining the associations of maternal, paternal and any parent smoking with offspring cardio-metabolic outcomes at age 17. The coefficients in the table indicate the mean difference in cardio-metabolic outcome according to parental smoking status. After adjusting for potential confounders, maternal smoking and paternal smoking, independent of each other, were positively associated with offspring weight, height and BMI (p<0.01) and negatively associated with offspring PR (p<0.01) at age 17 (Table 2, Model 2). The coefficients of the associations between maternal smoking and anthropometric measures were at least 2-fold greater than those of paternal smoking. For example, compared to offspring of non-smoking parents, weight of offspring whose mothers had smoked during pregnancy was 1.71 kg higher at age 17 while weight of offspring of smoking fathers was 0.80 kg higher (Table 2, Model 2). When examining the effect of smoking of any parent (i.e. maternal, paternal or both) on cardio-metabolic outcomes at age 17, parental smoking was positively associated with weight, height and BMI (p<0.001) and negatively associated with PR (p<0.001). Our data show an increase of 1.39 kg in offspring weight, 0.59 cm in height, 0.32 kg/m2 in BMI and decrease of 0.78 heart bpm at age 17, in offspring of at least one smoking parent, as compared to offspring of non-smoking parents (Table 2, Model 2). To further examine dose response, associations of maternal and paternal smoking with offspring CMR outcomes measured at age 17, independent of each other and of potential confounders, were also assessed by quantity of cigarettes smoked daily (grouped into intervals of 10 cigarettes). Results showed a clear significant dose response for each additional 10 cigarettes smoked daily by both parents. The effect sizes of the associations of maternal smoking with offspring PR (B=−0.332±0.13, p=0.015), height (0.280±0.091, p=0.002), weight (1.019±0.143, p<0.001) and BMI (0.287±0.045, p<0.001) were larger than those observed for paternal smoking: PR (−0.224±0.072, p=0.002), height (0.200±0.048, p<0.001), weight (0.353±0.075, p<0.001) and BMI (0.067±0.024, p=0.005).
Table 2.
Associations* of maternal and paternal smoking with offspring cardio-metabolic outcomes at age 17
| Maternal smoking† | Paternal smoking† | Any parent smoking‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Model 1§ | Model 2§ | Model 1§ | Model 2§ | Model 1§ | Model 2§ | |||||||
| B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | |
| SYSTOLIC BP, mmHg | −0.37 (0.28) | 0.18 | −0.27 (0.28) | 0.33 | −0.47 (0.23) | 0.04 | −0.31 (0.23) | 0.18 | −0.50 (0.22) | 0.02 | −0.31 (0.22) | 0.17 |
| DIASTOLIC BP, mmHg | −0.15 (0.19) | 0.43 | −0.09 (0.19) | 0.63 | 0.03 (0.16) | 0.83 | −0.05 (0.16) | 0.77 | 0.01 (0.15) | 0.94 | 0.05 (0.16) | 0.75 |
| PULSE RATE, BPM | −0.80 (0.23) | 0.001 | −0.73 (0.23) | 0.002 | −0.49 (0.19) | 0.01 | −0.53 (0.20) | 0.007 | −0.78 (0.19) | <0.001 | −0.78 (0.19) | <0.001 |
| HEIGHT, cm | 0.48 (0.16) | 0.002 | 0.71 (0.15) | <0.001 | 0.23 (0.13) | 0.08 | 0.37 (0.13) | 0.004 | 0.39 (0.13) | 0.002 | 0.59 (0.13) | <0.001 |
| WEIGHT, kg | 1.36 (0.25) | <0.001 | 1.71 (0.24) | <0.001 | 0.73 (0.21) | <0.001 | 0.80 (0.20) | <0.001 | 1.23 (0.20) | <0.001 | 1.39 (0.20) | <0.001 |
| BMI, kg/m2 | 0.36 (0.08) | <0.001 | 0.43 (0.08) | <0.001 | 0.18 (0.07) | 0.005 | 0.17 (0.06) | 0.009 | 0.31 (0.06) | <0.001 | 0.32 (0.06) | <0.001 |
Linear regression models; coefficient indicates the mean difference in cardio-metabolic outcome between offspring of smoking and non-smoking parents.
Maternal and paternal smoking are included together in each model.
Al least one parent smokes.
Model 1: Adjusted for gender and ethnicity. Model 2: Model 1 plus age of parents, socioeconomic status, parent’s years of education, birth weight, maternal pre-pregnancy BMI and maternal health conditions
Table 3 presents results of associations between parental smoking and offspring cardio-metabolic outcomes at age 32. Offspring of at least one smoking parent had higher weight (B=2.22, p=0.004; i.e. mean weight for offspring of at least one smoking parent was higher by 2.22 kg as compared to that of offspring of nonsmoking parents), height (B=0.95, p=0.01), BMI (B=0.57, p=0.02) and WC (B=1.46, p=0.02). These associations were independent of characteristics at birth and at age 32 as well as of offspring common genetic variation (Table 3, Model 2). Association of at least one smoking parent with glucose was significant in the minimally adjusted model (B=1.89, p=0.04, model 1), yet was attenuated to the null in the fully adjusted model (model 2). No significant associations were demonstrated between parental smoking and offspring PR, insulin, lipids and lipoproteins at age 32. When assessed separately, there were significant associations between maternal smoking and glucose levels and between paternal smoking and offspring weight, height, BMI and WC. Analyses examining maternal and paternal smoking as number of cigarettes smoked daily yielded a similar picture. Each additional 10 cigarettes smoked daily by father were positively associated with offspring weight (0.089±0.031, p=0.005), height (0.033±0.016, p=0.035) and BMI (0.022±0.010, p=0.030), but not with WC. The associations between maternal smoking by number of cigarettes and offspring cardio-metabolic traits tended to be smaller.
Table 3.
Associations* of maternal and paternal smoking with offspring cardio-metabolic outcomes at age 32
| Maternal smoking† | Paternal smoking† | Any parent smoking‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Model 1§ | Model 2§ | Model 1§ | Model 2§ | Model 1§ | Model 2§ | |||||||
| B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | |
| SYSTOLIC BP, mmHg | −1.55 (1.05) | 0.14 | −0.94 (0.72) | 0.24 | 0.24 (0.87) | 0.78 | 0.56 (0.68) | 0.41 | −0.29 (0.79) | 0.72 | 0.04 (0.65) | 0.96 |
| DIASTOLIC BP, mmHg | −0.82 (0.85) | 0.33 | −0.56 (0.62) | 0.37 | −0.15 (0.72) | 0.83 | 0.46 (0.52) | 0.38 | −0.28 (0.65) | 0.67 | 0.27 (0.49) | 0.59 |
| WEIGHT, kg | −0.64 (1.39) | 0.65 | 0.84 (0.96) | 0.38 | 2.29 (1.12) | 0.04 | 2.12 (0.80) | 0.008 | 2.34 (1.06) | 0.03 | 2.22 (0.78) | 0.004 |
| HEIGHT, cm | −0.25 (0.67) | 0.70 | 0.27 (0.51) | 0.60 | 0.57 (0.51) | 0.27 | 0.81 (0.39) | 0.04 | 0.60 (0.50) | 0.23 | 0.95 (0.39) | 0.01 |
| BMI, kg/m2 | −0.16 (0.45) | 0.71 | 0.30 (0.29) | 0.30 | 0.76 (0.37) | 0.04 | 0.56 (0.25) | 0.03 | 0.75 (0.34) | 0.03 | 0.57 (0.24) | 0.02 |
| WAIST CIRCUMFERENCE, cm | 0.07 (1.12) | 0.95 | 0.78 (0.80) | 0.33 | 1.60 (0.91) | 0.08 | 1.34 (0.65) | 0.04 | 1.94 (0.86) | 0.02 | 1.46 (0.62) | 0.02 |
| PULSE RATE, BPM | 0.23 (0.99) | 0.82 | 0.17 (0.67) | 0.80 | −1.64 (0.76) | 0.03 | −0.98 (0.52) | 0.06 | −1.44 (0.77) | 0.06 | −0.74 (0.51) | 0.15 |
| GLUCOSE, mg/dL | 1.54 (1.16) | 0.18 | 1.70 (0.77) | 0.03 | 0.74 (1.00) | 0.46 | 0.16 (0.70) | 0.82 | 1.89 (0.92) | 0.04 | 0.71 (0.65) | 0.27 |
| INSULIN, μU/mL|| | 0.00 (0.03) | 0.91 | 0.01 (0.02) | 0.55 | 0.01 (0.02) | 0.63 | 0.01 (0.01) | 0.62 | 0.03 (0.02) | 0.10 | 0.02 (0.01) | 0.13 |
| LDL-C, mg/dL | −1.89 (2.84) | 0.50 | 0.29 (2.06) | 0.89 | 3.37 (2.37) | 0.16 | 2.02 (1.68) | 0.23 | 2.86 (2.26) | 0.21 | 1.99 (1.61) | 0.22 |
| CHOLESTEROL, mg/dL | 1.48 (3.17) | 0.64 | 1.80 (2.40) | 0.45 | 2.42 (2.68) | 0.37 | 1.12 (1.98) | 0.57 | 2.73 (2.60) | 0.29 | 1.46 (1.90) | 0.44 |
| HDL-C, mg/dL | 1.33 (1.44) | 0.36 | 0.27 (0.97) | 0.78 | −1.20 (1.03) | 0.24 | −1.09 (0.83) | 0.19 | −1.10 (1.04) | 0.29 | −1.12 (0.80) | 0.16 |
| TRIGLYCERIDES, mg/dL|| | 0.03 (0.02) | 0.29 | 0.01 (0.02) | 0.41 | 0.01 (0.02) | 0.57 | 0.01 (0.01) | 0.53 | 0.02 (0.02) | 0.20 | 0.01 (0.01) | 0.34 |
Linear regression models; coefficient indicates the mean difference in cardio-metabolic outcome between offspring of smoking and non-smoking parents.
Maternal and paternal smoking are included together in each model.
Al least one parent smokes.
Model 1: Adjusted for gender and ethnicity. Model 2: Model 1 plus socioeconomic status, age of parents, parent’s years of education, birth weight, maternal pre-pregnancy BMI, maternal health conditions, offspring smoking status, physical activity at age 32, education at age 32 and genetic propensity score
Log-transformed values (base 10) due to asymmetrical distribution
To further illustrate the findings, we used estimates from linear regressions adjusted for confounders presented in tables 2 and 3 to predict adjusted means for offspring weight and BMI by parents smoking (Figure 1). For example, adjusted mean weight at age 17 was 63.2 kg for offspring of non-smoking parents compared to 64.6 kg for offspring of at least one smoking parent. At age 32, adjusted weights were 68.3 kg and 70.5 kg for offspring of non-smoking and smoking parents, respectively.
Figure 1.
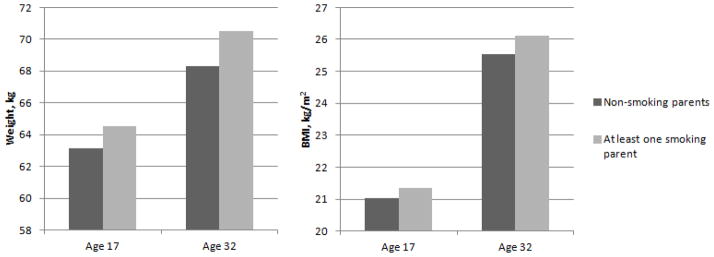
Adjusted means of offspring weight and BMI by parental smoking. Estimates from linear regressions adjusted for all confounders (presented in model 2, tables 2 and 3) were used to determine adjusted means for offspring weight and BMI at ages 17 and 32 by parents’ smoking status.
Discussion
Summary of findings
This study investigated the associations between parental smoking during pregnancy with a range of offspring cardio-metabolic risk factors in adolescence and early adulthood. We demonstrated that at age 17 maternal and paternal smoking were independently and positively associated with offspring weight, height and BMI and negatively associated with PR. At age 32, smoking of at least one parent was positively associated with offspring weight, BMI and WC, independent of potential confounders and genetic scores. These associations also showed a significant dose response at both time points when maternal and paternal smoking was assessed by number of cigarettes smoked daily, validating the findings based on the dichotomous smoking variables. We extend previous studies by assessing the associations with both maternal and paternal smoking during pregnancy, dichotomously and as number of cigarettes smoked daily, by examining a wide range of long term cardio-metabolic risk factors at two points of time: adolescence and early adulthood, and by taking into account various characteristics reflecting the pre-peri- and post-natal environment, including measures of lifestyle at young adulthood, as well as offspring common genetic variation.
Associations with offspring adiposity
Our findings that maternal smoking was positively associated with offspring adiposity are in accordance with other studies. Most studies have demonstrated this association in early childhood34, but the association has also been reported in adolescents35 and young adults16. These findings were also verified by two metaanalyses17,36. Evidence regarding the impact of paternal smoking on offspring adiposity is sparse and inconsistent. Several studies report a positive association of paternal smoking during pregnancy with childhood adiposity, albeit weaker than for maternal smoking37; however, a recent study failed to show an association of paternal smoking with growth characteristics at age 410
Associations with offspring BP
No associations were observed in our cohort for maternal, paternal or parental smoking and BP levels at ages 17 and 32. Few studies have shown a positive association between parental smoking and offspring BP, mainly in childhood38,39, while others have not18,24.
Associations with offspring fasting glucose and insulin
Parental smoking, adjusted for all covariates, was not associated with offspring glucose levels. Yet, maternal smoking assessed separately was positively associated with fasting glucose. Data on the associations between parental smoking and offspring levels of glucose and insulin are scarce. Horta et al., using a Brazilian cohort, did not observe associations between maternal smoking and offspring glucose levels20. In a British cohort, maternal smoking was related to higher offspring glucose levels at age 45, yet, this association did not remain significant after further adjustment for adult adiposity40. In our study, adjusting for current offspring BMI had a minimal effect on the findings (data not shown).
Associations with offspring fasting lipids and lipoproteins
We did not find an association between parental smoking and offspring fasting lipids and lipoproteins levels. An association between maternal smoking and offspring total cholesterol was demonstrated in one study including 350 offspring14. However, this association was not observed in the large Brazilian cohort study20.
Associations with offspring pulse rate
We demonstrated a negative association between both maternal and paternal smoking and PR levels measured during adolescence. This association was not observed in adulthood. An increase in heart rate, as an indirect marker of adrenergic drive, has been shown in patients with metabolic syndrome41 and is receiving attention as a potential cardiovascular risk factor42. However, a recent study did not show an association, positive or negative, between maternal smoking and offspring PR in young adults, after controlling for confounders21. Therefore, our findings for PR may reflect chance and require replication in at least another Israeli study.
Mechanisms underlying the observed associations
While the pathophysiology of smoking related adverse outcomes of pregnancy is not completely understood, several possible mechanisms have been proposed. The best-studied cause of adverse outcome in pregnant women who smoke is impaired fetal oxygen delivery. Pathologic evaluations of placentas of smokers have shown structural changes, including a reduction in the fraction of capillary volume and increased thickness of the villous membrane when compared to nonsmokers43,44. Both of these factors may contribute to abnormal gas exchange within the placenta that in turn may have long-term cardiovascular consequences. Parental smoking may result in fetal growth retardation, which was shown to be associated with a more rapid postnatal weight gain45, with abnormal glucose tolerance and with insulin resistance in adults46. Other possible mechanisms responsible for adverse fetal outcomes in mothers who smoke include direct toxicity of numerous substances found in cigarettes47. Animal studies have suggested an association between maternal nicotine exposure and changes in adipose tissue and glucose metabolism48; nicotine may also affect neurotransmitter levels and in-utero hypothalamic development and function including longer term effects on appetite control49.
Whether these intrauterine mechanisms can explain solely long-term outcomes is a matter of debate. Some suggest that the observed long term effects are mainly related to familial and lifestyle factors23,24. Horta el al. failed to show any significant associations between maternal smoking and offspring cardiovascular risk factors after controlling for lifestyle factors and BMI and thus concluded that previously reported associations are likely due to lack of adjustment for postnatal exposure to lifestyle patterns20. Our findings, however, may suggest that the role of the intrauterine environment may change over time. First, our data show that the effect of maternal smoking on outcomes measured at age 17 is stronger than that of paternal smoking. A similar differential effect of parental smoking is seen for birth weight (data not shown), potentially pointing to an intrauterine mechanism. Second, the strengthening with age (from age 17 to 32) of the associations with anthropometric measurements, as was also demonstrated in the 1958 British cohort16, propose that the long term effect of early life tobacco exposure may be influenced by accumulating exposures to the post natal environment. The “mismatch concept” supports this line of reasoning by suggesting that it is the gap between the environment experienced by the offspring in utero and the one experienced later in life that exerts the observed effects on adult disease, regardless of size at birth50. It is thus reasonable to assume that the gaps between the early and later environments grow over time leading to a larger effect on outcomes measured at age 32, independent of birth weight. Alternatively, differences between the associations observed at the two time points may also be attributed to differences in sample sizes, sampling differences or the presence of different determinants of CMR in adolescence and adulthood.
Previous studies have shown that genetic variation in genes encoding enzymes that detoxify the products of cigarette smoking modify the associations between maternal cigarette smoking and infant birth weight and preterm delivery51,52. We, however, used a limited set of candidate genes related specifically to CMR outcomes. This set of genes was used to add to precision of our estimates rather than to explore a genetic mechanism underlying the examined associations. It is noteworthy that the contribution of epigenetic mechanisms, rather than genetic, may potentially underlie the long-term effects of maternal smoking53.
Strengths and limitations
The major strength of our study is the combination of high-quality detailed records of pre- and peri-natal maternal and offspring characteristics with comprehensive long-term follow-up data on offspring at ages 17 and 32 years. Availability of information collected in early life, including both pregnancy-related factors and socio-demographic characteristics, together with characteristics of offspring at early adulthood, improved the characterization of the environment during pregnancy, birth, and adulthood, permitting control for these important factors. We also added precision to our estimates by taking into account offspring common genetic variation.
There are several limitations to this study. Confounding by socioeconomic status remains a concern in studies of the relationship of parental smoking and adult offspring cardio-metabolic risk factors. Unlike many studies, we had information that allowed us to characterize and control for the socioeconomic environment at birth and young adulthood.
To assess the reliability of smoking information provided by the mother at time of delivery, we recently interviewed 784 fathers of offspring who completed the 32 year follow-up study, regarding their own and their wife’s (i.e. offspring’s mother) smoking status during the time of the pregnancy. For maternal smoking status, the agreement percentage between data reported by fathers in the interview and data reported by the wives at time of birth was 85% and the κ estimate was 0.66. The agreement percentage between reports of paternal smoking status from fathers and their wives was 93% and the κ estimates was 0.68. Smoking information ascertained by self-report or through a spouse has been shown by others to have a relatively strong concordance54.
In addition, in the original cohort of offspring born during 1974–76, we have examined the relation between maternal and paternal smoking during pregnancy and birth weight of their offspring. Offspring born to either mothers or fathers that smoked during pregnancy had a significantly lower mean birth weight, compared to offspring born to non-smoking parents55. These associations lend support to the predictive validity of the reported parental smoking during pregnancy.
Conclusions
Our study adds importantly to the limited evidence for long-term relationships of parental smoking during pregnancy with offspring cardio-metabolic health in adolescence and adulthood. Our results emphasize the potential adverse impact of exposure to active and passive smoking in-utero on various cardio-metabolic risk factors that are associated with sub-clinical and clinical disease, such as diabetes, myocardial infarction and stroke. The findings highlight the importance of health care interventions to reduce smoking of mothers and fathers before pregnancy, not only for the prevention of known immediate complications, but also of long term outcomes related to cardio-metabolic risk in their adolescent and adult children.
Highlights.
We examine offspring cardio-metabolic risk in relation to parental smoking during pregnancy.
Parental smoking was positively associated with offspring adiposity at ages 17 and 32.
Associations with offspring insulin and lipid levels at age 32 were nonsignificant.
Results highlight potential long-term health impact of exposure to parental smoking.
Efforts to promote smoking cessation of parents before pregnancy may be warranted.
Acknowledgments
Funding sources
This work was supported by the National Institutes of Health research [grant numbers RO1CA80197, R01HL088884] and by the Israeli Science Foundation [grant number 1252/07].
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freund KM, Belanger AJ, D’Agostino RB, Kannel WB. The health risks of smoking. The Framingham Study: 34 years of follow-up. Annals of epidemiology. 1993 Jul;3(4):417–424. doi: 10.1016/1047-2797(93)90070-k. [DOI] [PubMed] [Google Scholar]
- 2.Pitsavos C, Panagiotakos DB, Chrysohoou C, et al. Association between passive cigarette smoking and the risk of developing acute coronary syndromes: the CARDIO2000 study. Heart and vessels. 2002 May;16(4):127–130. doi: 10.1007/s003800200008. [DOI] [PubMed] [Google Scholar]
- 3.Garrett BE, Dube SR, Trosclair A, et al. Cigarette smoking - United States, 1965–2008. Morbidity and mortality weekly report. Surveillance summaries. 2011 Jan 14;60( Suppl):109–113. [PubMed] [Google Scholar]
- 4.Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. American journal of epidemiology. 1996 Nov 1;144(9):881–889. doi: 10.1093/oxfordjournals.aje.a009022. [DOI] [PubMed] [Google Scholar]
- 5.Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy. Their effects on preterm birth. JAMA : the journal of the American Medical Association. 1986 Jan 3;255(1):82–84. [PubMed] [Google Scholar]
- 6.Jaddoe VW, Troe EJ, Hofman A, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatric and perinatal epidemiology. 2008 Mar;22(2):162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 7.(US) OoSaH. Women and Smoking: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2001. Mar, Available from: http://www.ncbi.nlm.nih.gov/books/NBK44303/ed. 2001. [PubMed] [Google Scholar]
- 8.Crane JM, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG : an international journal of obstetrics and gynaecology. 2011 Jun;118(7):865–871. doi: 10.1111/j.1471-0528.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- 9.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics. 2011 Apr;127(4):734–741. doi: 10.1542/peds.2010-3041. [DOI] [PubMed] [Google Scholar]
- 10.Durmus B, Kruithof CJ, Gillman MH, et al. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: the Generation R Study. The American journal of clinical nutrition. 2011 Jul;94(1):164–171. doi: 10.3945/ajcn.110.009225. [DOI] [PubMed] [Google Scholar]
- 11.Durmus B, Ay L, Hokken-Koelega AC, et al. Maternal smoking during pregnancy and subcutaneous fat mass in early childhood. The Generation R Study. European journal of epidemiology. 2011 Apr;26(4):295–304. doi: 10.1007/s10654-010-9544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RC, Mori TA, Beilin LJ. Early life programming of cardiometabolic disease in the Western Australian pregnancy cohort (Raine) study. Clinical and experimental pharmacology & physiology. 2012 Nov;39(11):973–978. doi: 10.1111/j.1440-1681.2012.05746.x. [DOI] [PubMed] [Google Scholar]
- 13.Hogberg L, Cnattingius S, Lundholm C, D’Onofrio BM, Langstrom N, Iliadou AN. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. Journal of hypertension. 2012 Apr;30(4):693–699. doi: 10.1097/HJH.0b013e32835168f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaddoe VW, de Ridder MA, van den Elzen AP, Hofman A, Uiterwaal CS, Witteman JC. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: A 27-years follow-up study. Atherosclerosis. 2008 Jan;196(1):42–48. doi: 10.1016/j.atherosclerosis.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Hunt KJ, Hansis-Diarte A, Shipman K, Korte JE, Fowler SP, Stern MP. Impact of parental smoking on diabetes, hypertension and the metabolic syndrome in adult men and women in the San Antonio Heart Study. Diabetologia. 2006 Oct;49(10):2291–2298. doi: 10.1007/s00125-006-0382-5. [DOI] [PubMed] [Google Scholar]
- 16.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. International journal of epidemiology. 2002 Apr;31(2):413–419. [PubMed] [Google Scholar]
- 17.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008 Feb;32(2):201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergel E, Haelterman E, Belizan J, Villar J, Carroli G. Perinatal factors associated with blood pressure during childhood. American journal of epidemiology. 2000 Mar 15;151(6):594–601. doi: 10.1093/oxfordjournals.aje.a010247. [DOI] [PubMed] [Google Scholar]
- 19.Haskins AE, Bertone-Johnson ER, Pekow P, Carbone E, Fortner RT, Chasan-Taber L. Smoking during pregnancy and risk of abnormal glucose tolerance: a prospective cohort study. BMC pregnancy and childbirth. 2010;10:55. doi: 10.1186/1471-2393-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horta BL, Gigante DP, Nazmi A, Silveira VM, Oliveira I, Victora CG. Maternal smoking during pregnancy and risk factors for cardiovascular disease in adulthood. Atherosclerosis. 2011 Dec;219(2):815–820. doi: 10.1016/j.atherosclerosis.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamun AA, O’Callaghan MJ, Williams GM, Najman JM. Maternal smoking during pregnancy predicts adult offspring cardiovascular risk factors - evidence from a community-based large birth cohort study. PloS one. 2012;7(7):e41106. doi: 10.1371/journal.pone.0041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howe LD, Matijasevich A, Tilling K, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. International journal of epidemiology. 2012 Jun;41(3):722–732. doi: 10.1093/ije/dys025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power C, Atherton K, Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis. 2010 Aug;211(2):643–648. doi: 10.1016/j.atherosclerosis.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Brion MJ, Leary SD, Smith GD, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007 Jun;49(6):1422–1428. doi: 10.1161/HYPERTENSIONAHA.106.085316. [DOI] [PubMed] [Google Scholar]
- 25.Harlap S, Davies AM, Deutsch L, et al. The Jerusalem Perinatal Study cohort, 1964–2005: methods and a review of the main results. Paediatric and perinatal epidemiology. 2007 May;21(3):256–273. doi: 10.1111/j.1365-3016.2007.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kark JD, Kedem R, Revach M. Medical examination of Israeli 17-year-olds before military service as a national resource for health information. Israel journal of medical sciences. 1986 Mar-Apr;22(3–4):318–325. [PubMed] [Google Scholar]
- 27.Hochner H, Friedlander Y, Calderon-Margalit R, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012 Mar 20;125(11):1381–1389. doi: 10.1161/CIRCULATIONAHA.111.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas DC, Witte JS. Point: population stratification: a problem for case-control studies of candidate-gene associations? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002 Jun;11(6):505–512. [PubMed] [Google Scholar]
- 29.Lawlor DA, Fraser A, Macdonald-Wallis C, et al. Maternal and offspring adiposity-related genetic variants and gestational weight gain. The American journal of clinical nutrition. 2011 Jul;94(1):149–155. doi: 10.3945/ajcn.110.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 31.Zhao H, Rebbeck TR, Mitra N. Analyzing genetic association studies with an extended propensity score approach. Statistical applications in genetics and molecular biology. 2012;11(5) doi: 10.1515/1544-6115.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence GM, Shulman S, Friedlander Y, et al. Associations of maternal pre-pregnancy and gestational body size with offspring longitudinal change in BMI. Obesity. 2014 Apr;22(4):1165–1171. doi: 10.1002/oby.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wander PL, Hochner H, Sitlani CM, et al. Maternal Genetic Variation Accounts in Part for the Associations of Maternal Size during Pregnancy with Offspring Cardiometabolic Risk in Adulthood. PloS one. 2014;9(3):e91835. doi: 10.1371/journal.pone.0091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. International journal of epidemiology. 2006 Feb;35(1):121–130. doi: 10.1093/ije/dyi218. [DOI] [PubMed] [Google Scholar]
- 35.Al Mamun A, Lawlor DA, Alati R, O’Callaghan MJ, Williams GM, Najman JM. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. American journal of epidemiology. 2006 Aug 15;164(4):317–325. doi: 10.1093/aje/kwj209. [DOI] [PubMed] [Google Scholar]
- 36.Ino T. Maternal smoking during pregnancy and offspring obesity: metaanalysis. Pediatrics international : official journal of the Japan Pediatric Society. 2010 Feb;52(1):94–99. doi: 10.1111/j.1442-200X.2009.02883.x. [DOI] [PubMed] [Google Scholar]
- 37.Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity. 2006 Dec;14(12):2284–2293. doi: 10.1038/oby.2006.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geerts CC, Grobbee DE, van der Ent CK, et al. Tobacco smoke exposure of pregnant mothers and blood pressure in their newborns: results from the wheezing illnesses study Leidsche Rijn birth cohort. Hypertension. 2007 Sep;50(3):572–578. doi: 10.1161/HYPERTENSIONAHA.107.091462. [DOI] [PubMed] [Google Scholar]
- 39.Lawlor DA, Najman JM, Sterne J, Williams GM, Ebrahim S, Davey Smith G. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University study of pregnancy and its outcomes. Circulation. 2004 Oct 19;110(16):2417–2423. doi: 10.1161/01.CIR.0000145165.80130.B5. [DOI] [PubMed] [Google Scholar]
- 40.Thomas C, Hypponen E, Power C. Prenatal exposures and glucose metabolism in adulthood: are effects mediated through birth weight and adiposity? Diabetes care. 2007 Apr;30(4):918–924. doi: 10.2337/dc06-1881. [DOI] [PubMed] [Google Scholar]
- 41.Mancia G, Bombelli M, Corrao G, et al. Metabolic syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study: daily life blood pressure, cardiac damage, and prognosis. Hypertension. 2007 Jan;49(1):40–47. doi: 10.1161/01.HYP.0000251933.22091.24. [DOI] [PubMed] [Google Scholar]
- 42.Grassi G, Arenare F, Quarti-Trevano F, Seravalle G, Mancia G. Heart rate, sympathetic cardiovascular influences, and the metabolic syndrome. Progress in cardiovascular diseases. 2009 Jul-Aug;52(1):31–37. doi: 10.1016/j.pcad.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Burton GJ, Palmer ME, Dalton KJ. Morphometric differences between the placental vasculature of non-smokers, smokers and ex-smokers. British journal of obstetrics and gynaecology. 1989 Aug;96(8):907–915. doi: 10.1111/j.1471-0528.1989.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 44.Bush PG, Mayhew TM, Abramovich DR, Aggett PJ, Burke MD, Page KR. A quantitative study on the effects of maternal smoking on placental morphology and cadmium concentration. Placenta. 2000 Mar-Apr;21(2–3):247–256. doi: 10.1053/plac.1999.0470. [DOI] [PubMed] [Google Scholar]
- 45.Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatric research. 1995 Aug;38(2):267–271. doi: 10.1203/00006450-199508000-00022. [DOI] [PubMed] [Google Scholar]
- 46.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993 Jan;36(1):62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 47.Tuthill DP, Stewart JH, Coles EC, Andrews J, Cartlidge PH. Maternal cigarette smoking and pregnancy outcome. Paediatric and perinatal epidemiology. 1999 Jul;13(3):245–253. doi: 10.1046/j.1365-3016.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- 48.Somm E, Schwitzgebel VM, Vauthay DM, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008 Dec;149(12):6289–6299. doi: 10.1210/en.2008-0361. [DOI] [PubMed] [Google Scholar]
- 49.Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000 Oct;141(10):3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- 50.Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatric research. 2007 May;61(5 Pt 2):5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA : the journal of the American Medical Association. 2002 Jan 9;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 52.Grazuleviciene R, Danileviciute A, Nadisauskiene R, Vencloviene J. Maternal smoking, GSTM1 and GSTT1 polymorphism and susceptibility to adverse pregnancy outcomes. International journal of environmental research and public health. 2009 Mar;6(3):1282–1297. doi: 10.3390/ijerph6031282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suter M, Ma J, Harris A, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics : official journal of the DNA Methylation Society. 2011 Nov;6(11):1284–1294. doi: 10.4161/epi.6.11.17819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedlander Y, Siscovick DS, Weinmann S, et al. Family history as a risk factor for primary cardiac arrest. Circulation. 1998 Jan 20;97(2):155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 55.Rush D, Cassano P, Harlap S. Perinatal outcome, maternal weight gain, cigarette smoking and social status in Jerusalem. Revue d’epidemiologie et de sante publique. 1988;36(3):186–195. [PubMed] [Google Scholar]


