Abstract
The importance of in situ lymphocyte proliferation for net accumulation of lung lymphocytes during pulmonary immune responses and in immunologic lung diseases remains uncertain. Accordingly, we studied the experimental pulmonary immune response of antigen-primed C57BL/6 mice to intratracheal challenge with the particulate antigen sheep red blood cells (SRBC). Uptake of nucleotide analogs (bromodeoxyuridine in vivo and tritiated thymidine in vitro), expression of the cell activation antigens CD25 and CD69 by flow cytometry, and response to the anti-mitotic agent hydroxyurea (in vivo) were measured. Although many lung lymphocytes and CD4+ T cells were CD25+ and CD69+, indicating recent activation, all techniques demonstrated that lung lymphocytes proliferated minimally in vivo. Blockade of cell division by hydroxyurea administration for 24 hours did not significantly decrease lung lymphocyte accumulation on day 3 after challenge. Lung lymphocytes also proliferated minimally in vitro (even on macrophage removal and despite addition of exogenous IL-2 or IL-4). However, lung lymphocytes responded vigorously to mitogens (immobilized anti-CD3, phytohemagglutinin or concanavalin A), excluding global unresponsiveness to restimulation. Thus, in this model of pulmonary immunity, accumulation of lung lymphocytes does not require local T cell proliferation and presumably depends instead on recruitment.
Index terms: Disease Models, Animal, Bromodeoxyuridine, Flow cytometry, Hydroxyurea, Mice, Inbred Strains, C57BL/6, Time Factors, Cell Division
Introduction
Generating appropriate pulmonary immune responses requires accumulation in the lungs of lymphocytes, the crucial immunoregulatory cells that recognize antigens (1). Net lymphocyte accumulation reflects the balance of factors that increase their numbers (recruitment and proliferation), and those that decrease their numbers (emigration and cell death). Each of these processes could profoundly influence lung lymphocyte numbers, hence the diversity of antigens recognized and thus, the ultimate outcome of pulmonary immune responses. The relative importance of these competing processes must be determined to devise effective immunomodulatory strategies to treat immunologic lung diseases or infections in immunocompromised patients.
The importance of local T cell proliferation in the lungs during pulmonary immune responses and in immunologic lung diseases remains uncertain. The lungs contain some elements necessary for T cell proliferation, such as dendritic cells which have been shown to be potent antigen-presenting cells in vitro (2). Overall, however, the lungs are believed to be a relatively anti-proliferative environment for lymphocytes, due to the effects of surfactant (3) and products of alveolar macrophages (4) and pulmonary epithelial cells (5). Moreover, the net function of alveolar macrophages, the predominant resident phagocyte, is to down-regulate pulmonary immune responses (6). Therefore, we hypothesized that lung lymphocytes are growth-arrested and that local proliferation is not essential for lymphocyte accumulation during pulmonary immune responses.
To proliferate, resting T cells must be activated by antigens, mitogens, or superantigens to re-enter the cell cycle and must receive progression factors to complete the cell cycle. T cell activation can be detected by rapid and transient induction of such immediate early gene products as CD69 and CD25. CD69 is a homodimeric type II membrane glycoprotein member of the C-type lectin family of unknown function; CD69 is the most rapidly induced of T cell activation receptors (7). CD25 is the inducible 55 kDa IL-2 receptor (IL-2R) subunit which, together with the constitutively expressed 75 kDa IL-2R subunit CD122, forms high-affinity IL-2 receptors (8). IL-2 is the major T cell progression factor (9). Once activated, most T cells can complete multiple rounds of cell division solely under the influence of IL-2.
To test directly the importance of in situ lung lymphocyte proliferation, we studied the well-characterized pulmonary immune response resulting from intratracheal challenge of primed inbred mice with the particulate T cell-dependent antigen, sheep red blood cells (SRBC) (10-12). Specifically, we measured uptake of nucleotide analogs (both in vivo and in vitro), surface expression of CD25 and CD69, and magnitude of lung lymphocyte accumulation when proliferation was blocked. We also examined the proliferative response of lung lymphocytes from these immunized mice or from normal mice in response to mitogens or exogenous cytokines. The results indicate that although a high percentage of lung lymphocytes has been activated recently, they proliferate minimally within the lungs. Nevertheless, lung lymphocytes are capable of proliferating vigorously in response to mitogenic stimulation, especially when removed from immunosuppressive macrophages.
Materials and Methods
Animals
Specific pathogen-free female C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME) and were used in experiments within 4-6 weeks. Mice were housed in the Animal Care Facility at Ann Arbor VA Medical Center, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. Mice were given water and food ad libitum.
Antibodies
Monoclonal antibodies (mAbs) B44 (mouse IgG1, kappa; FITC-conjugated for flow cytometry) and ZBU30 (mouse IgG1, kappa; biotin-conjugated for immunohistochemistry), which recognize bromodeoxyuridine (BrdU) incorporated in single-stranded DNA, were obtained from Becton Dickinson (Mountain View, CA) and Zymed (South San Francisco, CA), respectively. The following mAbs were obtained from PharMingen (San Diego, CA): RM4-4 (anti-murine CD4; rat IgG2b; FITC-conjugated); 7D4 (anti-murine CD25; rat IgM; biotinylated); 145-2C-11 (hamster anti-murine CD3; biotinylated); H1.2F3 (anti-murine CD69; hamster IgG; biotinylated); 107.3 (anti-TNP control mouse IgG1, kappa; biotinylated); biotinylated anti-TNP Hamster IgG; and biotinylated rat IgM, kappa. Optimal staining concentrations were determined empirically on appropriate lymphoid tissues.
Cell Lines
The cloned T cell line CTLL-2 (generously supplied by Dr. Stephen Chensue, University of Michigan) was used both as a positive control in some proliferation assays and for measurement of IL-2 by bioassay. EL-4 thymoma cells (ATCC, Rockville, MD) were used to produce a supernatant rich in IL-2 and other cytokines. For this purpose, EL-4 cells were cultured at a density of 1-10 × 106 viable cells/mL with a final concentration of 20 ng/mL phorbol myristate acetate (PMA) (Sigma; St. Louis, MO) for 40 hours. This supernatant had 400 units of IL-2/mL as determined by the CTLL-2 bioassay (13).
Experimental design
In all experiments, a secondary pulmonary immune response was induced by intratracheal challenge of primed mice using SRBC as antigen. At various times during the ensuing pulmonary response, cells were recovered from the lungs by bronchoalveolar lavage (BAL) and mincing of enzymatically-digested lung tissue, and in some experiments from the lymph nodes, bone marrow, and spleen. To obtain a sufficient number of cells for each assay, cells from groups of two to eight identically-treated mice were combined except as noted. For comparison, in some experiments cells recovered from the lungs of normal mice were analyzed. All procedures were performed according to a protocol approved by the Animal Care Committee of the VA Medical Center. This study complied with the NIH “Guide for the Care and Use of Laboratory Animals” {DHEW Publication No. (NIH) 80-23, Revised 1978, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205}.
Five types of experiments were performed. First, to detect in situ proliferation mice were injected with the nucleotide analog BrdU; flow cytometry and immunohistochemical staining were then used to determine BrdU incorporation into DNA of BAL and interstitial lymphocytes, respectively. Second, to verify the contribution of short-term lymphocyte proliferation to lung lymphocyte numbers, mice were injected with hydroxyurea, an S phase-specific inhibitor of cell division, and the magnitude of lymphocyte accumulation in the lungs was determined. Based on previous kinetic analyses (10, 11), in this experiment we chose to analyze the period over which lymphocyte accumulation increased most vigorously, days 2-3 after intratracheal challenge. Third, as a measure of recent lymphocyte activation, the percentage of total lung lymphocytes and CD4+ lung T cells expressing CD25 and CD69 was determined using two-color immunofluorescence staining and four-parameter flow cytometry. Fourth, to determine whether lymphocytes proliferate in vitro, cells were pulsed with tritiated thymidine (3H-Thd) either immediately upon removal from the mouse or after culture or exogenous cytokines; in each case, incorporated radioactivity was measured after overnight culture. Fifth, to determine the capacity of lung lymphocytes to proliferate in response to additional stimulation in vitro, cells from immunized mice or normal mice were activated with mitogens and analyzed for 3H-Thd uptake.
Induction of pulmonary immune response to SRBC
SRBC (initially sheep #602 and later #4151) (Colorado Serum Co.; Denver, CO) were washed three times in 10 mL normal saline before use; SRBC from a single sheep were used in each experiment. C57BL/6J mice were primed by intraperitoneal (IP) injection with 1 × 108 SRBC in 0.5 mL normal saline. Two weeks later, mice were intratracheally challenged with 5 × 108 SRBC in 50 μl normal saline as previously described (14). This technique causes the majority of antigen to be deposited and retained in alveoli (15) and results in a highly reproducible pulmonary immune response (10, 11, 14).
In vivo BrdU labeling
In some experiments, mice were injected IP with 4 mg BrdU (Sigma) in 0.5 mL Ca++, Mg++-free phosphate buffered saline (CMF-PBS), pH 7.4 (GIBCO; Grand Island, NY) {approximately 200 mg BrdU/kg of body weight) (16)}. BrdU injections were performed one to three times at 20 minute intervals before death.
Hydroxyurea treatment
In some experiments, mice were injected IP with hydroxyurea three times at 8 hour intervals, beginning 2 days after intratracheal antigen challenge; each injection consisted of 1 mg hydroxyurea/g body weight in 0.5 mL volume (17). Control mice were simultaneously injected with saline at the same time intervals. Seven hours and forty minutes after the final injection, mice were given an IP injection of BrdU as above and were killed humanely 20 minutes later. BAL samples were counted individually and differential cell analysis was performed. To confirm that cellular division was completely inhibited, BrdU incorporation in individual bone marrow samples of these mice was measured by flow cytometry.
Euthanasia of mice and collection of tissues
At various times from 2-9 days after intratracheal antigen challenge, mice were deeply anesthetized with pentobarbital (80 mg/kg IP) and killed by exsanguination. In most experiments, lungs were lavaged as previously described (10). Briefly, the trachea was cannulated with plastic tubing (PE-50, Clay-Adams, Parsippany, NJ) and the lungs washed with 10 mL of room temperature CMF-PBS in 1 mL aliquots. BAL cells were washed twice in CMF-PBS with centrifugation at 200 × g between washes. Samples from mice at identical time-points were pooled except for staining experiments (described below) or hydroxyurea-treated mice, from which samples were processed individually. BAL samples for cell culture experiments were resuspended in complete medium (RPMI 1640 containing 10% fetal bovine serum (GIBCO; catalog # 200-6140AJ), 50 μM 2-mercaptoethanol, and penicillin/streptomycin).
A cell preparation enriched for interstitial lymphocytes was prepared by first lavaging mice as above and by next perfusing the lungs with heparinized saline via right heart puncture until the great pulmonary vessels were grossly clear. Lungs were then excised at the pleural surface, minced finely with scissors, and incubated in RPMI 1640 containing 30 μg/mL DNAse-1 and 150 U/mL collagenase (CLS-1) (both from Worthington Biochemical Corp.; Freehold, NJ) for 35 minutes at 37° C with constant gentle swirling. Next, the lung fragments were forced through a SS80 mesh and washed twice in CMF-PBS. This population is referred to as lung mince. Portions of the resulting cell preparation were depleted of macrophages by passage over a Sephadex G-10 column (Sigma) (18). Treatment yielded a population which contained 88.1 ± 1.5% lymphocytes and 3.8 ± 0.3 % macrophages (n = 6 experiments).
Spleens, lymph nodes, thymi, femurs and tibiae were obtained by dissection. The capsule of the spleen was lacerated and the splenic parenchyma gently teased out using forceps. Lymph nodes and thymuses were mashed between the frosted ends of microscope slides. Femoral and tibular bone marrow was collected by removing the epiphyses and flushing with normal saline using a 27-gauge needle. Red blood cells were lysed from splenic and bone marrow preparations with ddH2O followed by 0.6 M KCl. All cell preparations were washed twice with CMF-PBS with centrifugation at 200 × g for 10 minutes at 4° C between washes. Aliquots of all samples were counted in a hemocytometer; cell viability was determined by exclusion of trypan blue (GIBCO), and cells were resuspended for culture in complete medium.
Detection of S phase lymphocytes
To detect lymphocytes that had incorporated BrdU into newly synthesized DNA, cells were fixed with 70% ethanol on ice for 30 minutes, treated with 2N HCl for 30 minutes at room temperature and neutralized using 0.1 M sodium tetraborate (Na2B4O7), pH 8.5. Cells were next aliquoted at a concentration of 5 × 105 cells/well into round bottom 96 well plates (Corning, Corning, NY) and stained with optimal concentrations of anti-BrdU for 30 minutes at room temperature. Cells were resuspended in 300 μL PBS containing calcium, magnesium, and 0.01% sodium azide (PBS++) for flow cytometric analysis.
Flow Cytometry
Staining and analysis for four-parameter flow cytometry were performed as described previously (10), except that data were collected by means of a FACSCAN flow cytometer, using Lysis II software {both from Becton Dickinson Immunocytometry Systems, (BDIS); Mountain View, CA} on a Hewlett-Packard 9133/9000-300 microcomputer operating under HP Pascal 3.12 System (Hewlett Packard; Fort Collins, CO). Data were analyzed using PC-Lysis II software on a Gateway 2000 4DX2-66 microcomputer (Gateway Corp; N. Sioux City, SD). A minimum of 10,000 cells were analyzed per sample, gating on lymphocytes by comparing light scatter to that of control lymphocytes from lymph nodes (10). The percentage of lymphocytes obtained from flow cytometry was used to calculate the total number of lymphocyte subsets in each mouse. Absolute numbers of CD4+ T cells per mouse of each phenotypic subset were calculated as the product of total cell count (obtained by hemocytometer count), the fraction of lymphocytes (obtained by differential cell count), and the fraction of each subset (obtained by flow cytometry).
Immunohistochemical analysis
Lungs were inflated without prior lavage using Millonig's modified phosphate buffered formalin (Surgipath Med. Industries; Richmond, IL) and were processed for paraffin embedding using standard histologic techniques. After removal of paraffin by incubation in xylene and graded alcohols, sections of 4-6 μm thickness were partially digested using dilute trypsin (Zymed) for 30 minutes at room temperature and rinsed in PBS. To denature DNA, sections were immersed in 2N HCl for 1 hour, rinsed in 0.1 M borate buffer, pH 8.5 for 5 minutes, and then rinsed in PBS. Sections were then incubated with CAS block (Zymed) to minimize background; after 10 minutes, excess CAS block was drained without rinsing and primary antibody was added. Sections were incubated with primary antibody (1:20 dilution PBS, pH 7.1 containing 0.05% Tween 20) for one hour at room temperature. After rinsing three times in PBS, sections were incubated with streptavidin conjugated to 5 nm gold particles and washed extensively in distilled water. Staining was enhanced with silver, rinsed in distilled water, air dried, and mounted in HISTOMOUNT (Zymed). The fraction of S phase mononuclear cells was determined by counting at least 200 cells in each of eight high power fields under oil immersion; this sample represents lungs from two mice.
In vitro lymphocyte proliferation assay
Lung mononuclear cell preparations were cultured in complete medium at a density of 2 × 105 cells/well in flat-bottomed 96-well tissue culture plates (#2586; Corning) in a 5% CO2 environment at 37° C. Two types of experiments were performed. To determine spontaneous proliferation, wells were immediately pulsed with 3H-TdR {10 μL per well of culture medium containing 1 μCi of 3H-TdR (specific activity = 5.0 Ci/mM; Amersham, Arlington Heights, IL)}, then cultured overnight (16-18 hours). As positive controls, lung cell preparation and splenocytes were stimulated with 2-10 μg/mL concanavalin A (Con-A; Sigma).
Alternatively, to determine whether previously activated lymphocytes could be stimulated to proliferate in vitro by additional cytokine stimulation, cells were pre-incubated with various cytokines for 24 hours, then pulsed with 3H-Thd and cultured for an additional 16-18 hours. The cytokines used were recombinant murine (rm) IL-2 (40 U/mL or 80 U/mL), rmIL-4 (40 U/mL or 80 U/mL) (both from Collaborative Research Inc.; Bedford, MA), and various concentrations of the supernatant from El-4 cells stimulated with PMA (described above). In both types of experiments, cells were harvested onto filters using a semi-automated harvester (PHD; Cambridge Technology, Inc., Watertown, MA). The amount of incorporated 3H-TdR was measured in counts per minute (cpm), determined on replicates of five identical wells using a liquid scintillation counter.
Viability assay
Some wells of the culture plates for proliferation assay were not pulsed with 3H-TdR and instead received trypan blue at the end of culture. Viability was assessed by exclusion of trypan blue, counting at least 100 cells per well.
Statistical Analysis
Data are expressed as mean ± SEM. Continuous ratio scale data were evaluated by Students t test (two samples) or analysis of variance (ANOVA) (three or more samples) with post hoc analysis by Fisher's protected least significant difference test (Fisher's PLSD) for comparisons between populations and by Dunnett's test for comparisons to a single reference population (19). Percentage data were arcsine transformed before analysis to convert them from a binomial to a normal distribution, using the formula .
Statistical calculations were performed on a Macintosh PowerPC 8100/80AV computer using the programs Statview 4.01 and SuperANOVA 1.11 (Abacus Concepts; Berkeley, CA). Statistically significant difference was accepted at p < 0.05.
Results
Determination of lung lymphocyte proliferation by BrdU labeling in vivo
Intratracheal antigen challenge of antigen-primed mice resulted in the prompt recruitment of large numbers of mononuclear cells and granulocytes into the lungs, in agreement with previous data in this model system (10, 12). Total BAL lymphocyte numbers increased 26-fold {from 0.14 ± 0.04 × 106 cells per mouse on day zero (mean ± SEM of groups of 8-12 mice pooled in each of three separate experiments) to 3.7 ± 0.7 × 106 cells per mouse on day 3 post IT challenge (mean ± SEM of 9 mice assayed individually in three separate experiments)}. Total BAL numbers of CD4+ T cells increased 20-fold (from 0.06 ± 0.02 × 106 cells per mouse to 1.2 ± 0.23 × 106 cells) within this same three day time period. Intratracheal challenge induced accumulation of inflammatory cells, which were very rare in the lung parenchyma of normal mice or antigen-primed mice without intratracheal challenge, in dense cuffs around pulmonary veins and venules and within bronchovascular bundles, chiefly centered adjacent to arteries (11).
Pulse labeling of cycling lymphocytes by in vivo BrdU administration was used to determine the fraction of lymphocytes proliferating within the lungs. Flow cytometric analysis easily detected BrdU+ cells in the positive control population bone marrow cells (figure 1a). Marked BrdU uptake was also seen on most days after intratracheal antigen challenge in cells from the paratracheal node (PTN) (figure 1b), the regional lymph node participating in this immune response. The low level of proliferation observed in PTN on day two after challenge was a reproducible finding seen in each of three separate experiments; it probably indicates initial entry of resting cells into the node shortly after antigen challenge. In contrast, lung lymphocytes showed much less BrdU uptake. Fewer than 5% of lymphocytes recovered from either BAL (figure 1c, d) or lung mince (figure 1d) of antigen challenged mice were BrdU+ by flow cytometry. To exclude the possibility that the dose or duration of BrdU might be inadequate, in some experiments mice were treated with BrdU for 60 minutes (three separate injections at twenty minutes intervals). Results were identical to those of mice injected once 20 minutes before death and data were pooled for analysis. In a separate control experiment, BAL lymphocytes of primed mice assayed four days after sham intratracheal challenge using saline showed 2.2 ± 0.1% BrdU positive cells (mean ± SEM of 11 mice assayed in three groups; bone marrows = 39.1 ± 4.1% BrdU positive).
Figure 1.
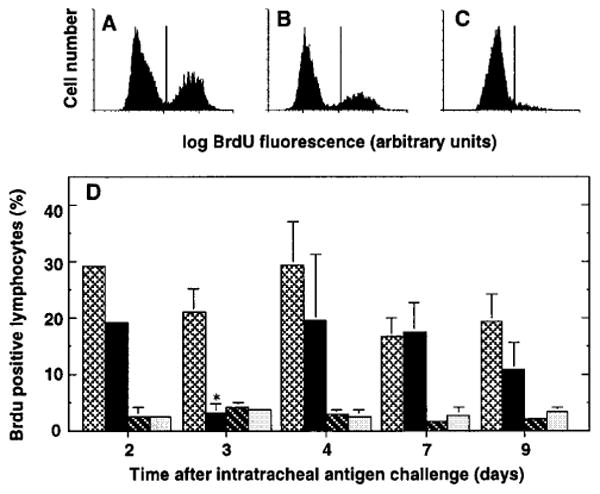
Flow cytometric analysis of in vivo lymphocyte proliferation. SRBC-primed C57BL/6 mice were challenged intratracheally with SRBC to induce a pulmonary immune response. On various days after challenge, BrdU (4 mgs/mouse IP) was administered in 1-3 doses at 20 minute intervals before the mouse was killed and cells harvested by BAL and dissection. Cells were stained for incorporated BrdU as described in Methods and were analyzed by flow cytometry. A-C. Representative histograms of cells within light scatter-defined gates from (A) bone marrow, (B) PTN, and (C) BAL. Data are from a single mouse four days after intratracheal antigen challenge. D. Kinetics of BrdU incorporation. Bars represent BrdU+ cells (defined by light scatter gating) in bone marrow (light cross hatching); paratracheal nodes (black); BAL (dark cross-hatching); and lung mince (light stippling). Data are mean ± SEM of 3 experiments, each consisting of pooled samples from 2-5 mice (except for bone marrows, which were assayed individually). *, significantly different from same tissue at other time-points, p <0.05, ANOVA with Fisher's PLSD post hoc testing.
Immunohistochemical analysis of separate groups of mice confirmed that BrdU positivity was minimal in mononuclear cells within perivascular inflammatory cell cuffs of BrdU-treated mice following intratracheal challenge (figure 2). Only 9.9 ± 1.1% (mean ± SEM of eight mice analyzed individually) of all perivascular inflammatory cells were BrdU+ on day 4 after intratracheal challenge. It should be noted that unlike the flow cytometric data which employed light scatter gating to identify lymphocytes (10), the immunohistochemical analysis did not distinguish between lymphocytes and mononuclear phagocytes. BrdU+ mononuclear cells were found scattered throughout the cuffs, demonstrating that BrdU had free access to cells within lung parenchyma (20). Thus, direct labeling demonstrated that both alveolar and interstitial lung lymphocytes proliferated minimally in vivo throughout this pulmonary immune response.
Figure 2.
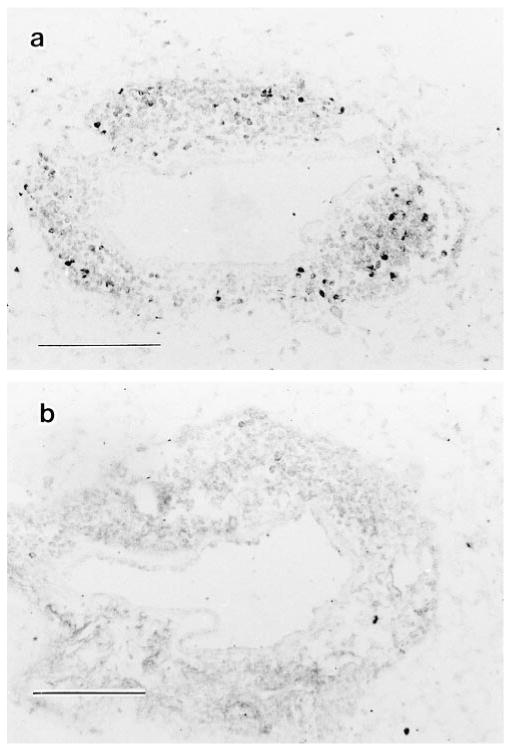
In situ demonstration of lung mononuclear cell proliferation. SRBC-primed C57BL/6 mice were challenged intratracheally with SRBC to induce a pulmonary immune response. BrdU (4 mgs/mouse) was administered IP in 1-3 doses at 20 minute intervals before the mouse was killed; lungs were processed histologically and sections were stained for BrdU incorporation by the immunogold technique as described in Methods. Representative serial photomicrographs of a single mouse four days after challenge. A. BrdU staining of individual cells within a perivenular inflammatory cell cuff. B. Control (irrelevant primary antibody). Similar results were obtained in three independent experiments. Scale indicates 100 μm.
For comparison, normal mice were also analyzed. By the flow cytometric method, very slight BrdU uptake was seen in lymphocytes recovered from the lungs of normal mice (BAL = 2.9 ± 0.9% BrdU positive versus 1.53 ± 0.43% isotype control positive; lung mince = 3.2 ± 1.0% BrdU positive versus 1.7 ± 0.7% isotype control positive; n = 10 mice assayed in groups of five). On immunohistochemical staining, virtually no BrdU+ inflammatory cells were seen in tissue sections of normal mice; the very low number of lymphocytes in the lungs of normal mice precluded accurate quantitation.
Ablation of dividing cells by hydroxyurea treatment in vivo
To determine directly the importance of short-term lymphocyte proliferation during the development of a pulmonary immune response, primed mice were treated with hydroxyurea to ablate all proliferating cells. The adequacy of hydroxyurea treatment was confirmed by demonstrating that in vivo BrdU uptake by bone marrow cells was abolished totally by the treatment regimen (figure 3a & 3b). Even though all proliferating cells were selectively killed, there was no significant difference in the number of total inflammatory cells nor of any of the major subpopulations accumulating in the lung three days after intratracheal challenge (figure 3c). This time-point was chosen as it is the day on which inflammatory cell numbers were consistently maximal in this model and because lung inflammatory cell numbers increased most markedly over the preceding 24 hours (10). Thus, maximal lung lymphocyte accumulation in response to intratracheal antigen challenge did not require cell division during the preceding 24 hours.
Figure 3.
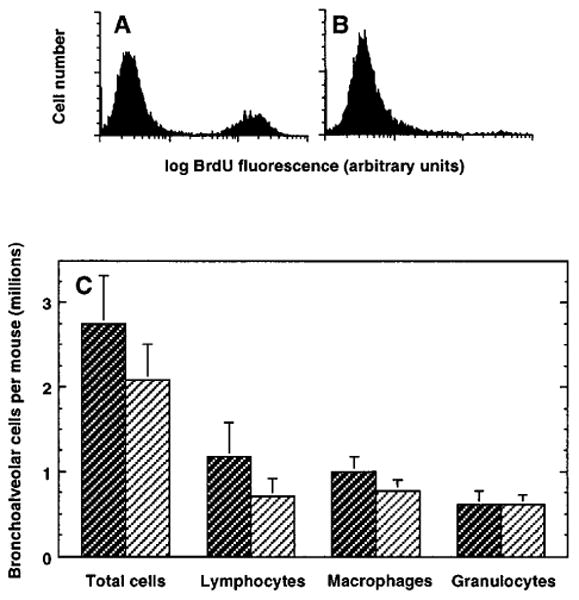
Effect of hydroxyurea treatment on lymphocyte accumulation in the lungs. SRBC-primed C57BL/6 mice were challenged intratracheally with SRBC to induce a pulmonary immune response. Two days later, hydroxyurea (1 mg/g body weight) or saline was administered IP for 24 hours (three separate injections at eight hour intervals). Mice were killed humanely 20 minutes after the last injection and cells were harvested from the lungs by BAL and from the bone marrow. A, B. Representative histograms of bone marrow from individual mice treated with saline (A) or hydroxyurea (B), showing effective systemic ablation of dividing cells in hydroxyurea-treated mice. C. Total and differential cell recovery in BAL. Control (saline injected) mice are represented by dark cross-hatching and hydroxyurea-treated mice by light cross-hatching. Data are mean ± SEM of ten mice per group assayed in two separate experiments. There are no significant differences between saline and hydroxyurea-treated groups, p >0.05, unpaired t test.
A similar protocol of hydroxyurea administration immediately before death had no effect on cell numbers or differential cell counts in the lungs of normal mice (2.8 ± 0.2 × 107 cells/mouse in lung minces of both hydroxyurea-treated and control mice; n = 3 mice/group).
Lung lymphocyte expression of activation markers
To determine whether the observed low fraction of proliferating lung lymphocytes reflected the fact that they had not been previously activated, surface expression of CD25 was measured. Two color immunofluorescence staining and flow cytometric analysis demonstrated increased expression of CD25 by lung lymphocytes compared to lymphocytes in peripheral blood (figure 4) or spleen (not shown). CD25 was expressed by a high percentage of the small numbers of cells recovered from the lungs by BAL at baseline (i.e., in normal mice or mice previously primed but not intratracheally challenged, graphed as day zero) (figure 4c). The percentage of cells expressing CD25 was greater among CD4+ T cells than among total lung lymphocytes. CD25 expression by the CD4+ T cells declined slightly on days 2-3 after challenge, then peaked at day 4 (figure 4d). There was no significant difference between alveolar cells and cells in a preparation enriched for lung interstitial leukocytes in the percentage of CD25+ cells, either among total lung lymphocytes or among CD4+ T cells. Hence, a substantial percentage of lung lymphocytes (30-60% of CD4+ T cells) expressed CD25 throughout the response. Moreover, most BAL lymphocytes also expressed the very early activation marker CD69 (51.6 ± 4.9% CD69 positive versus 3.1 ± 1.6% for isotype control; n = 5 mice in two experiments day 4 after intratracheal antigen challenge). These findings indicate that lung lymphocytes from antigen-challenged mice had been recently activated and had entered into the cell cycle.
Figure 4.
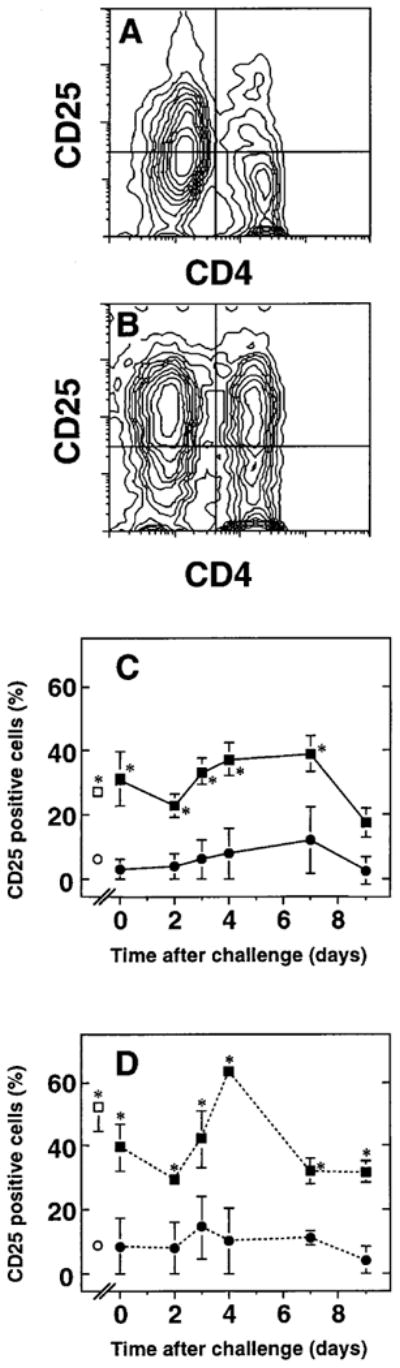
Intratracheal challenge results in CD25 expression by lung T cells. Peripheral blood lymphocytes (PBL) and BAL cells were obtained at various times after intratracheal challenge of SRBC-primed C57BL/6 mice. Cells were stained with pairs of conjugated monoclonal antibodies and analyzed by four parameter flow cytometry with light scatter gating to identify lymphocytes. A, B. Representative fluorescence data of (A) PBL and (B) BAL lymphocytes (defined by light-scatter gating). Data are depicted as 10% probability contour plots and represent cells from a single mouse. Horizontal axis is CD4 fluorescence and vertical axis is CD25 fluorescence; both axes are in arbitrary units on a four-decade logarithmic scale. The crossbars indicate the cut-offs for integration to determine double positive cells (upper right quadrant) as a percentage of all CD4+ T cells (upper right plus lower right quadrants). C, D. Time-course of CD25 expression by (C) total lymphocytes and (D) CD4+ T cells in lung and peripheral blood. Squares, BAL; circles, PBL. Open symbols denote results from normal mice. Data are mean ± SEM of 3-10 mice per time-point assayed individually in at least three separate experiments per time-point, except for normals and day zero, where lavage groups of 8-12 mice were pooled in each of four separate experiments. *, BAL sample significantly different from corresponding PBL sample, p < 0.05, unpaired t test.
Spontaneous in vitro proliferation of lung lymphocytes of antigen-challenged mice
Next, an in vitro system was employed to analyze determinants of lymphocyte proliferation. To mimic ongoing proliferation in vivo, in the first set of experiments lung mononuclear cell preparations were pulsed immediately with 3H-Thd, incubated for 16-18 hours at 37° C in a 5% CO2 environment and then assessed for incorporation of radioactivity. Three populations were examined at all time points: BAL cells, lung mince, and interstitial cells depleted of potentially immunosuppressive macrophages (post G-10 mince). The starting viability of all three lung cell populations was similar (>95%). As a control for the proliferation of a recently activated population of lymphocytes, we examined uptake of 3H-Thd by splenocytes stimulated with optimal concentrations of the lymphocyte mitogen Con-A. These activated splenocytes showed vigorous 3H-Thd uptake (day 3: 51,591 ± 4283 cpm; day 4: 48,850 ± 9,203 cpm; mean ± SEM of replicates of 5 wells containing 2 × 105 cells each).
In contrast, all lung cell populations showed minimal 3H-Thd uptake, never exceeding 10,000 cpm for 2 × 105 cells (figure 5). At all time points, the highest spontaneous 3H-Thd uptake in the lung populations was seen in the BAL cells. BAL cell 3H-Thd uptake varied somewhat with time after challenge and was significantly lower on days 3 and 7 in each of three experiments. Proliferation of the lung mince cells peaked on day 3. Removal of macrophages from the lung mince preparation did not increase 3H-Thd uptake. In fact, in all spontaneous proliferation experiments, 3H-Thd uptake of the macrophage-depleted cultures was negligible despite viability at the end of culture of at least 80%. Inspection did not disclose excessive acidity in the lung cell cultures, such as might develop after rapid proliferation, indicating that the low proliferative index was not due to medium exhaustion.
Figure 5.
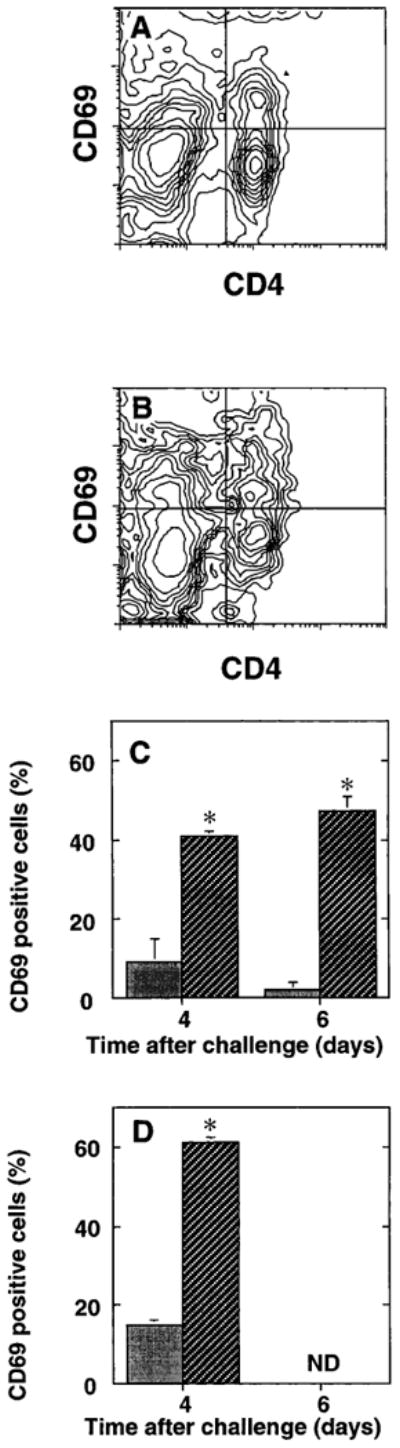
Expression of the early activation antigen CD69 by BAL T cells. Splenic lymphocytes (PBL) and BAL cells were obtained at various times after IT challenge of SRBC primed C57BL/6 mice. Cells were stained with pairs of conjugated monoclonal antibodies and analyzed by four-parameter flow cytometry with light-scatter gating to identify lymphocytes. Representative fluorescence data of (A) splenic lymphocytes and (B) BAL lymphocytes (defined by light-scatter gating). Data are depicted as 10% probability contour plots and represent cells from a single mouse. Horizontal axis is CD4 fluorescence and vertical axis is CD69 fluorescence; both axes are in arbitrary units on a four decade logarithmic scale. Crossbars indicate the cutoffs for integration to determine CD69+ cells (upper right quadrant) as a percentage of all CD4+ T cells (upper right plus lower right quadrants). CD69 expression by (C) total lymphocytes and (D) CD4+ T cells in spleen ( ) and BAL ( ). N.D., not determined. Data are means 6 SEM of three mice per time point assayed individually in two to three separate experiments per time point. * BAL sample significantly different from corresponding splenic sample, P , 0.05, unpaired t test.
To exclude the possibility that the observed low proliferation resulted from inadequate antigen priming, mice received three IP injections of 1 × 108 SRBC at weekly intervals followed by IT antigen challenge. Repeated IP priming did not increase either the numbers of lymphocytes recovered from the lungs or the magnitude of in vitro proliferation compared to a single IP priming (n = two experiments, data not shown). Thus, lymphocytes removed immediately from the lungs over the period of maximal increases in lung lymphocyte number showed only modest spontaneous proliferation in vitro, in agreement with results of BrdU labeling experiments.
For comparison, BAL of normal mice was also cultured with immediate addition of 3H-Thd but without additional stimulation (Table 1). Interestingly, the greatest degree of proliferation was seen within the first 24 hours. It should be noted that due to the very small numbers of both total cells and of lymphocytes in normal mice, it was not feasible to attempt removal of macrophages in these experiments.
Table 1. Spontaneous in vitro proliferation of BAL cells from normal C57BL/6 mice*.
| Day in Culture | Expt. #1 | Expt. #2 | ||||
|---|---|---|---|---|---|---|
| 1 | 5,356 | ± | 318† | 5604 | ± | 236 |
| 2 | 1,000 | ± | 222 | 830 | ± | 109 |
| 3 | 1,706 | ± | 194 | 2,380 | ± | 377 |
| 4 | 3,974 | ± | 563 | 2,170 | ± | 472 |
BAL cells from groups of 12–16 normal C57BL/6 mice were cultured at 2 × 105 cells per well in complete medium, and 3H-Thd was added for the final 16 h of culture.
Mean 6 SEM cpm.
Effect of cytokines on lung lymphocyte proliferation in vitro
Because a large percentage of lung lymphocytes from intratracheally challenged mice were CD25+, it was possible that the low spontaneous proliferation seen in vitro was attributable to inadequate production of IL-2 or other cytokines by lung lymphocytes. Accordingly, lung cells were cultured in various concentrations of rmIL-2, and proliferation was determined. Even with this additional cytokine stimulation, all lung lymphocyte populations displayed minimal 3H-Thd uptake, not exceeding 13,000 cpm on day 4, the maximal response (figure 6a). By comparison, an equal number of the IL-2 responsive T cell clone CTLL-2 showed over 10-fold greater proliferation (82,143 ± 1951 cpm; mean ± SEM of triplicate cultures). In these experiments, the highest response was seen in the minced lung and post G-10 minces; BAL cells responded the least regardless of the cytokine concentration. Similar results were obtained using lung lymphocytes obtained at 2 or 3 days after intratracheal challenge. Lung lymphocytes cultured with rmIL-4 likewise proliferated minimally when tested 2, 3, or 4 days after intratracheal challenge (figure 6b). Moreover, lung lymphocytes were not induced to proliferate by incubation with the cytokine-rich supernatant produced by EL-4 cells activated with PMA (not shown). By comparison, 50,000 CTLL-2 cells showed far greater proliferation in the same experiments (309,111 ± 13265 cpm; mean ± SEM for 40% EL-4 supernatant); the maximal difference was >100-fold on a per cell basis. As in the spontaneous proliferation experiments, there was no evidence of excessive acidity in the lung cell cultures in any of the cytokine coculture experiments, indicating that the low proliferative index was not due to medium exhaustion. Therefore, we concluded that the observed low spontaneous proliferation of lung lymphocytes was unlikely to result from inadequate cytokine stimulation.
Figure 6.
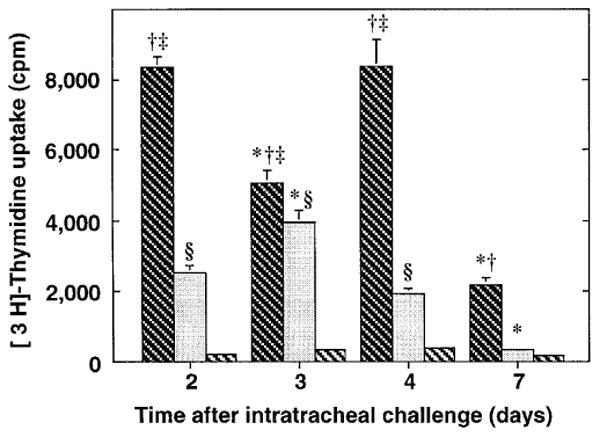
Spontaneous in vitro proliferation of lung lymphocytes from antigen-challenged mice. SRBC-primed C57BL/6 mice were challenged intratracheally with SRBC to induce a pulmonary immune response. On various days after challenge, cells obtained from BAL (dark cross-hatching), lung mince, (stippled), and lung mince post G-10 column (light cross-hatching) were placed in culture, pulsed immediately with 3H-Thd, and harvested 12-16 hours later. Values are expressed as mean ± SEM of at least three independent experiments at each time-point, each with replicates of 5-6 wells. *, significantly different from all other days within same cell-sample population; †, BAL significantly different from lung mince; ‡, BAL significantly different from post G-10 lung mince; § = lung mince significantly different from post G-10 lung mince; p < 0.05, ANOVA with Fisher's PLSD post hoc testing.
In these experiments, it was noted that the amount of proliferation in wells not pre-incubated with cytokines (figures 6) was less than that seen when cells were immediately pulsed with 3H-Thd (figure 5). This finding suggested that IL-2 may preserve lymphocyte viability even if it did not stimulate proliferation. To test this possibility, we determined the viability of lymphocytes in parallel cultures and found that wells cultured with IL-2 contained 10-20% more viable cells at the end of culture than those incubated without cytokines (data not shown). This result suggested that the large difference in proliferation over the additional 24 hours in culture did not result solely from cell death. Although lung lymphocytes showed a modest increased proliferation to cytokines (IL-2 and EL-4 supernatant to a larger degree than IL-4), only with the higher doses of exogenous IL-2 was proliferation even marginally greater than that seen in the spontaneous proliferation experiments (figure 5).
Response of lung lymphocytes to mitogen stimulation in vitro
To determine whether lung lymphocytes were refractory to stimulation in vitro, lung cell preparations from antigen challenged mice were next stimulated with the polyclonal T cell mitogens concanavalin A (Con-A) (figure 7), PHA (not shown), or immobilized anti-CD3 (figure 8). With Con-A and PHA, there was a modest response in the presence of macrophages which was greatly increased by their removal. With anti-CD3, a response was seen only after removal of macrophages.
Figure 7.
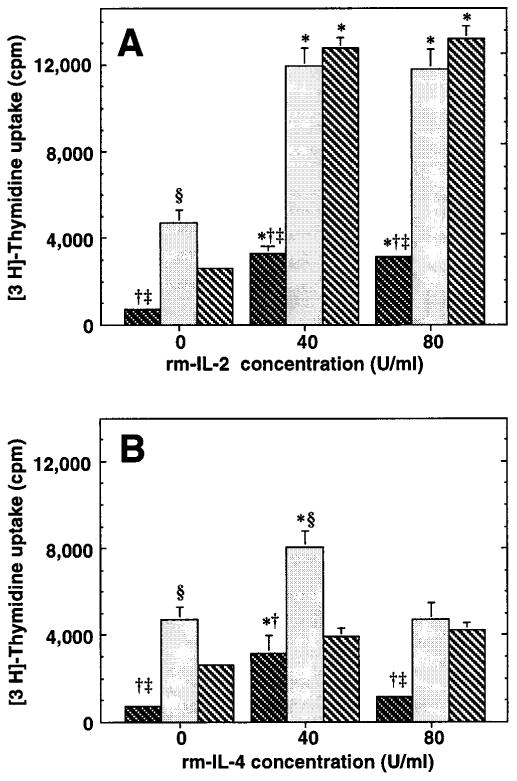
Effect of exogenous cytokines on lung lymphocyte proliferation in vitro. BAL (dark cross-hatching), lung mince (stippling), and lung mince post G-10 (light cross-hatching) were incubated for 24 hours with various concentrations of exogenous cytokines, then were pulsed with 3H-Thd and harvested after an additional 16-18 hour incubation. A. Incubation with rmIL-2. Data are mean ± standard error of 5-6 wells in a single experiment on cells obtained four days after intratracheal challenge, which is representative of three individual experiments. Similar results were obtained in each experiment using cells recovered at two and three days after intratracheal antigen challenge. B. Incubation with rmIL-4. Representative data of cells obtained at individual time-point post IT. Data are mean ± standard error of 5-6 wells in a single experiment on cells obtained four days after intratracheal challenge, which is representative of two individual experiments. *, significantly different from no cytokine incubation within same cell-sample population; p < 0.05, ANOVA with Dunnett's (two-tailed) post hoc testing; †, BAL significantly different from lung mince; ‡, BAL significantly different from post G-10 lung mince; § = lung mince significantly different from post G-10 lung mince; p < 0.05, ANOVA with Fisher's PLSD post hoc testing.
Figure 8.
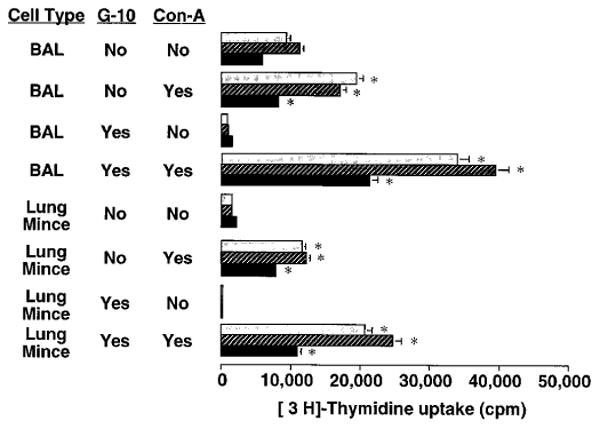
Proliferative response of lung lymphocytes to Con-A stimulation in vitro. BAL and lung mince mononuclear cells were harvested from SRBC-primed C57BL/6 mice four days after induction of a pulmonary immune response by IT challenge. Aliquots were depleted of phagocytic cells including macrophages by Sephadex G10 column. Cells were cultured for 2 days (light stippled bars), 3 days (cross-hatched bars), or 4 days (black bars) at 2 × 105 cells per well in flat-bottomed 96-well plates in complete medium in the absence or presence of Con-A 10 μg/mL (an optimal dose). Lymphocyte proliferation was assessed by uptake of 3H-Thd during the final 16 hours of culture. Splenocytes in the same experiment gave 18,000-98,000 cpm. *, significantly different compared to corresponding condition without Con-A stimulation, p < 0.05, unpaired t test. Similar results were obtained in two separate experiments.
To put the response of immunized mice into context, BAL cells from the lungs of normal mice were stimulated with mitogens. Because of the large number of mice needed to obtain sufficient cells, G-10 treatment was not performed in these experiments. Lung cells from normal mice responded vigorously to both PHA and Con-A. Thus, lung lymphocytes from normal mice and from immunized mice could be induced to proliferate in vitro when appropriately stimulated.
Discussion
There are five major results of this analysis of the murine pulmonary immune response to particulate intratracheal antigen. First, both alveolar and interstitial lung lymphocytes proliferated minimally in vivo as determined by pulse BrdU labeling. Second, blocking cell division in vivo for 24 hours using hydroxyurea had no significant effect on lung lymphocyte accumulation during the peak period of cellular influx. Third, many lung lymphocytes in both alveoli and lung interstitium displayed high levels of CD25 and CD69, indicating recent activation and entry into the cell cycle. Fourth, both lung lymphocyte populations proliferated minimally in vitro, either spontaneously or when further stimulated with a variety of exogenous cytokines. Proliferation was not increased in either experimental design by removal of potentially immunosuppressive macrophages. Fifth, nevertheless, lung lymphocytes were not refractory to polyclonal activation in vitro by mitogens. We conclude that in situ proliferation contributes minimally to net lung lymphocyte accumulation in this experimental model system.
This study utilized the classic T cell-dependent particulate antigen, SRBC, in an experimental model system that has the advantages of simplicity and reproducibility. The peribronchovascular cellular infiltrates induced by SRBC challenge are identical in location to those seen in two murine models of lung infection (21, 22), and in patients with hypersensitivity pneumonitis or pulmonary lymphomas, leading us to propose that the peribronchovascular regions are a stereotypic area of lymphocyte accumulation (23). Intratracheal SRBC challenge in primed A/J mice by an identical protocol has been studied as a model for asthma because it induces airway hyperreactivity and pulmonary eosinophilia (24, 25). Thus, our findings are relevant to understanding of a variety of infectious and immunologic lung diseases.
The finding that marked and rapid lung lymphocyte accumulation can occur in the absence of local proliferation is significant because it indicates that recruitment is chiefly responsible for the marked increases in lymphocyte numbers early in the development of this response. Hence, this model system can be used to study the molecular mechanisms of lymphocyte recruitment without the confounding influence of local lymphocyte proliferation. Because we have recently reported that a substantial fraction of lung lymphocytes in this model system undergo elimination by apoptosis (26), recruitment must be sustained to counterbalance ongoing losses. Based on analysis of adhesion receptor expression, we have previously reported that the overwhelming majority of lung CD4+ T cells in this model system have encountered antigen at some time in the past, ie., are primed or memory cells (27). We now conclude that most lung lymphocytes are in fact probably very recently reactivated cells, as CD69 expression peaks at 18-24 hours and decays with a half-life of 24 hours (28), and CD25 surface expression persists for only a few days after lymphocyte activation in vitro (29). Significant expression of CD25 by lung lymphocytes is a feature of several human immunologic lung diseases (30-32). Because the vast majority of lymphocytes at any site of inflammation are not specific for the inciting antigens, it is unlikely that the high percentage of CD25+ cells results from lymphocyte activation within lung parenchyma. Instead, these findings suggest ongoing recruitment to the lungs of activated lymphocytes of a wide range of antigen specificities. Our results are also consistent with in vitro observations that recently activated lymphocytes are the most invasive and motile cells (33).
Accurate direct measurement of in situ proliferation requires labeling only of cells proliferating within the lung, and not those proliferating elsewhere and recruited to the lung during the labeling period. Hence, the labeling procedure must be brief and efficient. For this purpose, the nucleotide analog BrdU, which is incorporated into DNA via the same pyrimidine salvage pathway as thymidine, is ideally suited. BrdU is rapidly degraded in vivo; >90% of an IP dose is degraded within 20 minutes and essentially no free BrdU is detectable in blood by one hour (34). A 20-60 minute labeling interval is sufficiently brief that cells in the process of recruitment should contribute little to the analysis, yet has been shown to efficiently label dividing cells within the lungs (20). In vivo labeling of dividing cells with BrdU yields results which are comparable to other methods (35, 36). Thus, it is likely that the BrdU treatment protocol used gave an accurate glimpse of instantaneous lymphocyte proliferation within the lungs. Although a slightly higher percentage of BrdU+ cells was detected in the lungs of antigen challenged mice by immunohistology than by flow cytometry, it is likely that the former technique yields an overestimate by failing to exclude BrdU+ macrophages. It is unlikely that the brief pulses of BrdU used in this study inhibited lymphocyte proliferation (37).
Although many studies have shown inhibition of lymphocyte proliferation when AMøs are added to mitogen-activated PBMC, there has been very little confirmatory analysis using lung lymphocytes. In the current study, spontaneous proliferation of lung lymphocytes in vitro was judged to be minimal in comparison with the marked proliferation of the same number of mitogen-activated cells in two reference populations: control splenocytes and lung lymphocytes themselves when stimulated with mitogens. Mitogen-activated splenocytes were chosen as one benchmark because their level of expression of CD25 and a variety of activation-dependent adhesion receptors is similar to that of lung lymphocytes over this time period (V.A. Maxwell & J.L. Curtis, unpublished observation). A low lymphocyte proliferative index despite high CD25 expression has been demonstrated in some other regional immune responses (38, 39), but to our knowledge not in experimental pulmonary immune responses. Interestingly, the degree of in vitro spontaneous proliferation we observed is similar to that found by Pinkston and associates for BAL lymphocytes in patients with high intensity alveolitis in sarcoidosis (approximately 1500 dpm/105 lymphocytes and 6-9% labeling by autoradiography) (40, 41). Because the fraction of proliferating lymphocytes was 10-fold higher in those patients than among BAL cells recovered from normal subjects or patients with low intensity alveolitis, those authors concluded that local proliferation is one possible explanation for lung lymphocyte accumulation in that disease. In contrast, by working in an experimental animal model system, we have been able in the current study to directly test both the actual fraction of cells proliferating in vivo, and the importance of cell division over the 24 hours preceding assay. These direct measurements lead us to conclude that local proliferation contributes minimally to net lung lymphocyte accumulation in this model system. Our results support those of Holt's laboratory which found that addition of IL-2 to mitogen-stimulated lung lymphocyte cultures did not increase proliferation (42). We extend those findings by examining the response of immunized mice and by examining IL-4 and EL-4 supernatants, which contain a variety of cytokines.
The observed brisk proliferative response of lung lymphocytes to mitogen stimulation in vitro is noteworthy for two reasons. First, this finding indicates that the culture conditions employed in this study were conducive to proliferation of lung lymphocytes when properly stimulated. Second, this finding indicates that following emigration from the lungs, these lymphocytes could potentially be stimulated to participate in immune responses elsewhere in the body or possibly to generate immunologic memory. Response to immobilized anti-CD3 would be anticipated based on the predominance of primed cells in the lung lymphocyte pool, and agrees with data on human BAL lymphocytes (43). The fact that we measured such a good response to ConA contrasts with findings from normal human BAL lymphocytes (43), probably reflecting the somewhat greater percentage of naive lymphocytes in this induced response (44). These data should not be construed to deny any role for in situ proliferation in the generation of the pulmonary immune response. Some of the observed 3-5% BrdU+ lung lymphocytes are probably SRBC-specific clones. Previous work in this model system has shown that the fraction of lymphocytes secreting specific anti-SRBC antibody (per million lung lymphocytes) peaks at day seven after intratracheal antigen challenge, at a time when total lymphocyte numbers in the lung are falling (10). Such enrichment could reflect either proliferation of antigen-specific B cell clones or their selective retention within lung parenchyma.
The most likely reason that, despite evidence of previous activation, lung lymphocytes failed to complete the cell cycle spontaneously or with cytokine stimulation is that they are in a refractory state following repeated cell division outside the lungs. It is known that after 6-10 cycles of cell division, T cell clones become refractory to the stimulating effect of IL-2 despite continued high level expression of IL-2R (45). Although it is currently unclear whether a similar IL-2 refractory state occurs in vivo, such an interpretation would be consistent with our results of in vitro stimulation with rmIL-2. Alternative explanations appear less likely. It is possible that lung lymphocyte proliferation depends on other exogenous factors not supplied by the cytokine mixtures we used. However, it should be noted that PMA-stimulated EL-4 cells have been used to clone the genes for a variety of lymphokines which support murine T cell proliferation; hence, although we did not assay for factors such as IL-7, IL-9, and IL-15, it is likely that the supernatants used in this study contained them. It is also possible that in vitro lung lymphocyte proliferation could be supported by the costimulatory action of cell-cell or cell-matrix interactions. Although we did not directly examine this issue, we believe that the low in vivo proliferative rate determined by BrdU labeling renders this question moot.
Our findings have important implications for the design of therapies to treat immunologic lung diseases including allograft rejection. Aside from the use of steroids, which have diverse and incompletely understood effects on the immune system, most current therapies for immunologic lung diseases depend on inhibition of lymphocyte proliferation. However, because transcription of a variety of lymphokine genes is coordinated with activation, accumulation of large numbers of activated memory cells in the lungs might lead to secretion of proinflammatory lymphokines by T cells not specific for the inciting antigens. Therefore, if accumulation of activated memory T cells within the lungs does not depend on local proliferation, as our data suggest, one reason for the limited success of these therapies may be that they do not prevent cytokine secretion. Modulating recruitment of memory T cells and their subsequent secretion of cytokines are alternative approaches to control of immunologic lungs diseases and lung allograft rejection.
Figure 9.
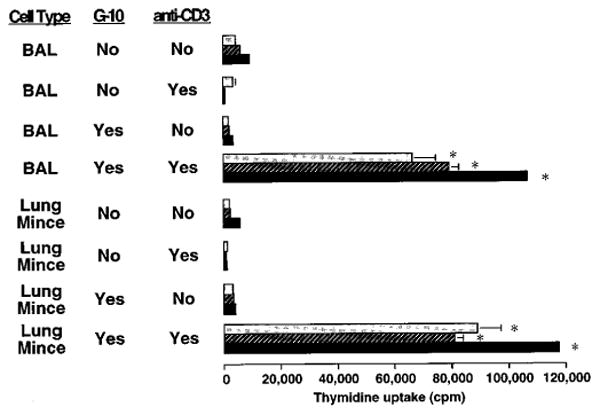
Proliferative response of lung lymphocytes to anti-CD3 stimulation in vitro. BAL and lung mince mononuclear cells were harvested from SRBC-primed C57BL/6 mice four days after induction of a pulmonary immune response by IT challenge. Aliquots were depleted of phagocytic cells including macrophages by Sephadex G10 column. Cells were cultured for 3 days (light stippled bars), 4 days (cross-hatched bars), or 5 days (black bars) at 2 × 105 cells per well in flat-bottomed 96-well plates in complete medium in the absence or presence of immobilized hamster anti-murine CD3 (clone 145-2C-11). Lymphocyte proliferation was assessed by uptake of tritiated thymidine during the final 16 hours of culture. *, significantly different compared to corresponding condition without anti-CD3 stimulation, p < 0.05, unpaired t test. Note difference in scale from Figure 7. Similar results were obtained in two separate experiments.
Acknowledgments
The authors thank Jodi Kauffman and Margaret A. Morris for technical assistance; Frances Wolber for instruction in immunogold staining; Robert McKnight for photography; Joyce O'Brien for secretarial support; Dr. Stephen Chensue for CTLL-2 cells; David Choiniere, Kelly Warmington, and Drs. Gwo-Hsiao Chen, Paul J. Christensen, Alicja M. Milik, Virginia M. Maxwell, Robert Paine III, and Lloyd M. Stoolman for helpful suggestions; and Dr. Paine for critiquing the manuscript.
This research was supported by Specialized Center of Research in Occupational and Immunologic Lung Diseases HL46487 and by RO-1 HL56309 from the USPHS; by Research Funds from the Department of Veterans Affairs; and by the University of Michigan Medical School Student Biomedical Research Fund (G.D.S.). Dr. Kim was the recipient of an Individual National Research Service Award # HL08763 from the USPHS.
Abbreviations Used
- 3H-Thd
tritiated thymidine
- APC
antigen-presenting cell
- BAL
bronchoalveolar lavage
- CMF-PBS
Ca++, Mg++-free phosphate buffered saline
- ddH2O
double distilled water
- mAb
monoclonal antibody
- PBL
peripheral blood lymphocyte
- PBS++
PBS containing calcium, magnesium, and 0.01% sodium azide
- Fisher's PLSD
Fisher's protected least significant difference test
- PTN
paratracheal node lymphocyte
- SRBC
sheep red blood cell
Footnotes
Portions of these data were presented to the Southern Society for Clinical Investigation (New Orleans, LA; February 5, 1994), at the Keystone Conference on Cellular & Molecular Immunology (Keystone, CO; April 14, 1994), and at the International Scientific Conference of the American Thoracic Society (Boston, MA; May 22, 1994), and have been published in abstract form (Clin Res 1993; 41:774A, 1993; and J Cell Biochem 1994; supplement 18D: 414).
References
- 1.Berman JS, Beer DJ, Theodore AC, Kornfeld H, Bernardo J, Center DM. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990;142:238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- 2.Sertl K, Takemura T, Tshachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma and visceral pleura. J Exp Med. 1986;163:436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sitrin RG, Ansfield MJ, Kaltreider HB. The effect of pulmonary surface-active material on the generation and expression of murine B- and T- lymphocyte effector functions in vitro. Exp Lung Res. 1985;9:85–97. doi: 10.3109/01902148509061530. [DOI] [PubMed] [Google Scholar]
- 4.Roth MD, Golub SH. Human pulmonary macrophages utilize prostaglandins and transforming growth factor β1 to suppress lymphocyte activation. J Leukocyte Biol. 1993;53:366–371. doi: 10.1002/jlb.53.4.366. [DOI] [PubMed] [Google Scholar]
- 5.Paine R, III, Chavis A, Gaposchkin D, Christensen P, Mody CH, Turka LA, Toews GB. A factor secreted by a human pulmonary alveolar epithelial-like cell line blocks T-cell proliferation between G1 and S phase. Am J Respir Cell Mol Biol. 1992;6:658–666. doi: 10.1165/ajrcmb/6.6.658. [DOI] [PubMed] [Google Scholar]
- 6.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Testi R, D'Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 8.Dukovich M, Wano Y, Thuy LB, Katz P, Cullen BR, Kehr JH, Greene WC. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987;327:518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- 9.Morgan D, Ruscetti F, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 10.Curtis JL, Kaltreider HB. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am Rev Respir Dis. 1989;139:393–400. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- 11.Curtis JL, Warnock ML, Arraj SM, Kaltreider HB. Histologic analysis of an immune response in the lung parenchyma of mice: angiopathy accompanies inflammatory cell influx. Am J Pathol. 1990;137:689–699. [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis JL, Byrd PK, Warnock ML, Kaltreider HB. Requirement of CD4+ T cells for cellular recruitment to the lungs of mice in response to intratracheal antigen. J Clin Invest. 1991;88:1244–1254. doi: 10.1172/JCI115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillis S, Ferm MM, Ou W, Smith KA. T-cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 14.Kaltreider HB, Curtis JL, Arraj SM. The mechanism of appearance of specific antibody-forming cells in lungs of inbred mice after intratracheal immunization with sheep erythrocytes. II. Dose-dependence & kinetics. Am Rev Respir Dis. 1987;135:87–92. doi: 10.1164/arrd.1987.135.1.87. [DOI] [PubMed] [Google Scholar]
- 15.McLeod E, Caldwell J, Kaltreider HB. Pulmonary immune responses of inbred mice: appearance of antibody-forming cells in C57BL/6 mice after intrapulmonary or systemic immunization with sheep erythrocytes. Am Rev Respir Dis. 1978;118:561–571. doi: 10.1164/arrd.1978.118.3.561. [DOI] [PubMed] [Google Scholar]
- 16.DeFazio A, Leary J, Headly D, Tattersall M. Immunohistochemical detection of proliferating cells in vivo. J Histochem Cytochem. 1987;32:571–577. doi: 10.1177/35.5.3549891. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson GS, Bradley TR, Martin RF, Sumner M, Fry P. Recovery of proliferating haematopoietic progenitor cells after killing by hydroxyurea. Cell Tissue Kinet. 1975;8:51–60. doi: 10.1111/j.1365-2184.1975.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 18.Ly IA, Mishell RI. Separation of mouse spleen cells by passage through columns of Sephadex G-10. J Immunol Methods. 1974;5:239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- 19.Zar JH. Biostatistical analysis. Prentice-Hall, Englewood Cliffs; 1974. p. 718. [Google Scholar]
- 20.Kawamoto M, Fukuda Y. Cell proliferation during the process of bleomycin-induced pulmonary fibrosis in rats. Acta Pathol Jpn. 1990;40:227–238. doi: 10.1111/j.1440-1827.1990.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 21.Beck JM, Warnock ML, Curtis JL, Sniezek MJ, Arraj-Peffer SM, Kaltreider HB, Shellito JE. Inflammatory responses to Pneumocystis carinii pneumonia in mice selectively depleted of helper T lymphocytes. Am J Respir Cell Mol Biol. 1991;5:186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JL, Huffnagle GB, Chen GW, Warnock ML, Gyetko MR, McDonald RA, Scott PJ, Toews GB. Experimental murine pulmonary cryptococcosis: differences in pulmonary inflammation and lymphocyte recruitment induced by two encapsulated strains of Cryptococcus neoformans. Lab Invest. 1994;170:113–126. [PubMed] [Google Scholar]
- 23.Curtis JL, Warnock ML. New concepts in the pathogenesis of immune lung injury. Semin Respir Med. 1990;12:158–176. [Google Scholar]
- 24.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 25.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milik AM, Beuchner-Maxwell VA, Kim S, Sonstein J, Seitzman GD, Beals TF, Curtis JL. Lung lymphocyte elimination by apoptosis in the murine response to intratracheal particulate antigen. J Clin Invest. 1997;99:1082–1091. doi: 10.1172/JCI119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Scott PJ, Curtis JL. CD4+ T cell subsets in pulmonary lymphocyte recruitment. Chest. 1993;103:94S. doi: 10.1378/chest.103.2_supplement.94s. [DOI] [PubMed] [Google Scholar]
- 28.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 29.Cantrell DA, Smith KA. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983;158:1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller-Quernheim J, Kronke M, Strausz J, Schykowski M, Ferlinz R. Interleukin-2 receptor gene expression by bronchoalveolar lavage lymphocytes in pulmonary sarcoidosis. Am Rev Respir Dis. 1989;140:82–88. doi: 10.1164/ajrccm/140.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JW, Djukanovic R, Howarth PH, Holgate ST. Lymphocyte activation in bronchoalveolar lavage and peripheral blood in atopic asthma. Am Rev Respir Dis. 1992;145:958–960. doi: 10.1164/ajrccm/145.4_Pt_1.958. [DOI] [PubMed] [Google Scholar]
- 32.Hol BE, Hintzen RQ, Van Lier RA, Alberts C, Out TA, Jansen HM. Soluble and cellular markers of T cell activation in patients with pulmonary sarcoidosis. Am Rev Respir Dis. 1993;148:643–649. doi: 10.1164/ajrccm/148.3.643. [DOI] [PubMed] [Google Scholar]
- 33.Masuyama J, Berman JS, Cruikshank WW, Morimoto C, Center DM. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992;148:1367–1374. [PubMed] [Google Scholar]
- 34.Kriss JP, Revesz L. The distribution and fate of bromodeoxyuridine and bromodeoxycytidine in the mouse and rat. Cancer Res. 1962;22:254–265. [PubMed] [Google Scholar]
- 35.Wynford-Thomas D, Williams ED. Use of bromodeoxyuridine for cell kinetic studies in intact animals. Cell Tissue Kinet. 1986;19:179–182. doi: 10.1111/j.1365-2184.1986.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Furth R, Van Zwet TL. immunocytochemical detection of 5-bromo-2 deoxyuridine incorporation in individual cells. J Immunol Methods. 1988;108:45–51. doi: 10.1016/0022-1759(88)90401-2. [DOI] [PubMed] [Google Scholar]
- 37.Von Boehmer H, Hafen K. The life span of naive alpha/beta T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergroth V, Konttinen YT, Nykänen PN, Von Essen R, Koota K. Proliferating cells in the synovial fluid in rheumatic disease: an analysis with autoradiography -immunoperoxidase double staining. Scand J Immunol. 1985;22:383–388. doi: 10.1111/j.1365-3083.1985.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 39.Ohmori K, Hong Y, Fujiwara M, Matsumoto Y. In situ demonstration of proliferating cells in the rat central nervous system during experimental autoimmune encephalomyelitis. Evidence suggesting that most infiltrating T cells do not proliferate in the target organ. Lab Invest. 1992;66:54–62. [PubMed] [Google Scholar]
- 40.Pinkston P, Bitterman P, Crystal R. Spontaneous release of IL-2 by lung lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983;308:793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- 41.Pinkston P, Saltini C, Muller-Quernheim J, Crystal RG. Corticosteroid therapy suppresses spontaneous interleukin 2 release and spontaneous proliferation of lung T lymphocytes of patients with active pulmonary sarcoidosis. J Immunol. 1987;139:755–760. [PubMed] [Google Scholar]
- 42.Upham JW, Strickland DH, Bilyk N, Robinson BWS, Holt PG. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 1995;84:142–147. [PMC free article] [PubMed] [Google Scholar]
- 43.Becker SD, Harris T, Koren HS. Characterization of normal human lung lymphocytes and interleukin-2-induced lung T-cell lines. Am J Respir Cell Mol Biol. 1990;3:441–448. doi: 10.1165/ajrcmb/3.5.441. [DOI] [PubMed] [Google Scholar]
- 44.Curtis JL, Kim S, Scott PJ, Buechner-Maxwell VA. Adhesion receptor phenotypes of murine lung CD4+ T cells during a pulmonary immune response to sheep erythrocytes. Am J Respir Cell Mol Biol. 1995;12:520–530. doi: 10.1165/ajrcmb.12.5.7537969. [DOI] [PubMed] [Google Scholar]
- 45.Churilla A, Braciale V. Lack of IL-2-dependent proliferation despite significant expression of high-affinity IL-2 receptors on murine cytolytic T lymphocyte clones late after antigenic stimulation. J Immunol. 1987;138:1338–1345. [PubMed] [Google Scholar]


