Abstract
The 5 year survival rate of lung cancer is <20%, with most patients dying from distant metastasis. However, the molecular mechanisms underlying lung cancer invasion and metastasis have not been fully characterized. In this study, we found that fibulin-3, a fibulin family extracellular matrix protein, functions as a suppressor of lung cancer invasion and metastasis. Fibulin-3 was downregulated in large fractions of lung tumors and cell lines, and inhibited lung cancer cell invasion and the expression of matrix metalloproteinase-7 (MMP-7), a promoter of lung cancer invasion. The expression levels of fibulin-3 and MMP-7 were inversely correlated in lung tumors. Fibulin-3 inhibited extracellular signal-regulated kinase (ERK) to activate glycogen synthase kinase 3β and suppress Wnt/β-catenin signaling, which induces MMP-7 expression in lung cancer cells. Furthermore, fibulin-3 expression impeded the growth and metastasis of lung tumors in mice. Collectively, these results suggest that downregulation of fibulin-3 contributes to lung cancer invasion and metastasis by activating Wnt/β-catenin signaling and MMP-7 expression.
Introduction
The high modality rate and aggressive phenotypes of lung cancer are largely due to tumor invasion and metastasis (1). A number of genetic and epigenetic alterations in lung cancer have been shown to drive this process (2). One of the key steps in tumor metastasis is degradation of the basement membrane by proteolytic enzymes such as matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) (3). However, the mechanisms by which MMPs and TIMPs are activated to promote lung cancer invasion have remained unclear.
Accumulating evidence suggests that the fibulin family, a group of widely expressed extracellular matrix proteins, is involved in lung cancer invasion and metastasis. The fibulin family consists of seven members (fibulin-1–7) and are characterized by several epidermal growth factor-like domain repeats and a unique carboxyl terminal structure (4). These proteins serve as a structural component of extracellular matrix and are involved in regulating cell-to-cell and cell-to-matrix communication (4). Previous studies have shown that fibulin family members are aberrantly expressed in a variety of tumors and can either suppress or promote tumor cell growth depending on cell types and cellular contexts (5,6). Our previous work identified fibulin-3 and fibulin-5 as two frequently downregulated genes in lung cancer through epigenetic silencing by promoter methylation (7,8).
Activation of the Wnt/β-catenin pathway is a critical oncogenic event in tumor initiation, progression and metastasis (9). β-Catenin is an oncoprotein normally localized in the cytoplasm, where its expression is kept in check through ubiquitin/proteasome-mediated protein degradation mediated by the tumor suppressor adenomatous polyposis coli and glycogen synthase kinase 3β (GSK3β) (10). Oncogenic Wnt signaling promotes β-catenin accumulation and nuclear translocation, and allows it to form a complex with transcription factors such as T-cell factor 4 (TCF-4), leading to activation of downstream targets such as the oncogenes c-Myc and cyclin D1 (11,12). Wnt/β-catenin signaling is frequently activated in lung cancer (13,14), which promotes lung cancer cell proliferation, survival and metastasis (15,16). However, the mechanisms underlying the aberrant activation of Wnt/β-catenin signaling in lung cancer have not been fully characterized.
In this study, we found that fibulin-3 functions as a suppressor of lung cancer invasion and metastasis. It suppressed MMP-7 expression through its inhibitory effects on extracellular signal-regulated kinase (ERK) and Wnt/β-catenin signaling. Delineating the negative regulation of fibulin-3 on Wnt/β-catenin and MMP-7 in suppressing lung cancer invasion provides a potential clue for targeting metastatic lung cancer.
Materials and methods
Cell culture and transfection
Lung cancer cell lines were obtained from American Type Culture Collection
(Manassas, VA). Cells were maintained at 37°C and 5% CO2, and cultured in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% defined fetal bovine serum (HyClone, Logan, UT), 100U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Demethylation with 5-aza-2ʹ-deoxycytidine (5-aza-dC; Sigma, St Louis, MO) was performed as described (7).
Transfection was performed using Lipofectamine™ 2000 (Invitrogen) following the manufacturer’s instructions. To establish fibulin-3-expressing cell lines, A549, H1299 and H460 cells were transfected with fibulin-3 or control empty vector, and were selected by G418 (400ng/ml for A549 and H1299; 600ng/ml for H460). Stable clones expressing fibulin-3 were identified by western blotting. Transfection of small interfering RNA (siRNA) was performed as described previously (8). Fibulin-3 was knocked down using ON-TARGET plus siRNA J-011855-05 and -06 (ThermoFisher, Waltham, MA). GSK3β was knocked down by pre-made GSK3β siRNA (Santa Cruz Biotechnology, Santa Cruz, CA).
Tissue samples and immunostaining
Tissue microarray slides containing 101 non-small-cell lung cancer (NSCLC) and 46 normal samples, including 32 matched tumor/normal pairs, were purchased from US Biomax (Rockville, MD). The information of these samples was summarized in Supplementary Table 1, available at Carcinogenesis Online. An independent set of frozen specimens with known fibulin-3 promoter methylation status, including 32 randomly selected lung tumors and the matched histologically normal lung parenchyma samples, were described previously (7). Immunostaining was performed with the mouse antibodies against fibulin-3 (Abcam, Cambridge, MA) and MMP-7 (EMD BioSciences, Billerica, MA) as described previously (8). The staining distribution was scored based on the percentage of positive cells: 0, 0%; 1, 1–30%; 2, 31–60% and 3, 61–100%. The criteria used for scoring signal intensity were as follows: 0, no signal; 1, weak; 2, moderate and 3, marked. The staining was considered to be positive if the sum of distribution and intensity scores was >2 (7).
Reverse transcriptase–PCR
Total RNA was isolated from cells using the RNAgents Total RNA Isolation System (Promega, Madison, WI). First-strand complementary DNA was synthesized from 10 μg of total RNA using Superscript II reverse transcriptase (Invitrogen). Reverse transcriptase–PCR (RT–PCR) was done to amplify the candidate genes using the touchdown PCR conditions described previously (7), and primers listed in Supplementary Table 2, available at Carcinogenesis Online.
Bioinformatic analysis
Fibulin-3 (EFEMP1) and MMP-7 expression and DNA methylation in The Cancer Genome Atlas (TCGA) databases were analyzed by using The UCSC Cancer Genomics Browser (https://genome-cancer.soe.ucsc.edu/proj/site/hgHeatmap/) (17). The TCGA lung cancer (LUNG) RNAseq (IlluminaHiSeq; N = 965) and DNA methylation (HumanMethylation27; Illumina 27K platform; N = 312) data sets were used to compare fibulin-3 and MMP-7 expression and methylation because these data sets include data from control normal tissues. Heatmap mode was used to display the expression and methylation data, which are sorted using sample type (lung tumor or normal) and significance as the first and second criteria, respectively.
Antibodies and western blotting
Western blotting was performed as described previously (8). The antibodies included monoclonal antibodies against V5 (Invitrogen), fibulin-3 (Abcam), MMP-7, α-tubulin (EMD BioSciences), c-Myc (9E10), cyclin D1 (Santa Cruz Biotechnology), β-catenin (BD Biosciences, San Jose, CA), GSK3β, phospho-GSK3β (Ser9), and rabbit antibodies against ERK and phospho-ERK (Thr202/Tyr204; Cell Signaling Technology, Danvers, MA).
Matrigel invasion assay
Invasion assays were performed in triplicate in six-well trans-well units with 8 μm filters coated with Matrigel (BD Biosciences) at 1:6 dilution. Each well was loaded with ~2 × 106 cells. After incubation for 36 h, cells passing through the filters into bottom wells were fixed in formalin and stained with crystal violet (Sigma). Cell numbers in 10 randomly selected fields (×200) from each well were counted.
Luciferase assay
Wild-type (WT) MMP-7 luciferase reporter was constructed by cloning a 264 bp fragment in the promoter region of MMP-7 into pBV-Luc plasmid (18). Mutant MMP-7 reporter was generated by site-directed mutagenesis using QuickChange XL site-directed mutagenesis kit (Agilent Technologies, SantaClara, CA). For reporter assays, A549 and H1299 cells were co-transfected with fibulin-3 and the transfection control β-galactosidase reporter pCMVβ (Promega), along with the TCF-4 reporter plasmid pTOPFlash or the control inactive reporter pFOPFlash, and WT or mutant MMP-7 reporter. In some experiments, cells were transfected with mutant β-catenin with deletion of its first 45 amino acids (ΔN). After transfection, collection of cell lysates and measurement of luciferase activities were done as described (18). All reporter experiments were performed in triplicate and repeated three times.
Analysis of β-catenin nuclear localization
Nuclear β-catenin in H1299 and A549 cells was analyzed by western blotting of β-catenin in nuclear fractions, which were isolated from transfected cells using the NE-PER nuclear/cytoplasmic extraction kit (ThermoFisher) according to the manufacturer’s instructions. Nuclear β-catenin in SW480 colorectal cancer cells was analyzed using immunofluorescence as described previously (18). Mounted slides were subjected to microscopic analysis under a Nikon fluorescence microscope (TS800) equipped with a SPOT camera and imaging software.
Animal experiments
All animal experiments were approved by the institutional animal care and use committee at the University of Pittsburgh. Female 5- to 6-week-old BALB/c nude mice (Charles River, Wilmington, MA) were housed in a sterile environment with microisolator cages and allowed access to water and chow ad libitum. To analyze tumor growth, mice were injected subcutaneously in both flanks with parental and fibulin-3-expressing H1299 cells (5 × 106 cells/injection). Tumor volumes were calculated according to the formula 0.5 × length × width2. After killing of mice, tumors were dissected and fixed in 10% formalin and embedded in paraffin. Immunostaining was performed using V5 (Invitrogen) and MMP-7 (EMD BioSciences) antibodies as described (8). To analyze tumor metastasis, parental and fibulin-3-expressing H460 cells were injected intravenously by tail vein into nude mice. For each injection, 1 × 106 cells suspended in 200 μl phosphate-buffered saline were used. Following killing of mice, lung metastasis nodules were counted, and body weight was measured.
Statistical analysis
Statistical analyses were performed using GraphPad Prism V software. P-values <0.05 were considered to be statistically significant. The means ± one standard deviation were displayed in the figures.
Results
Downregulation of fibulin-3 in NSCLC
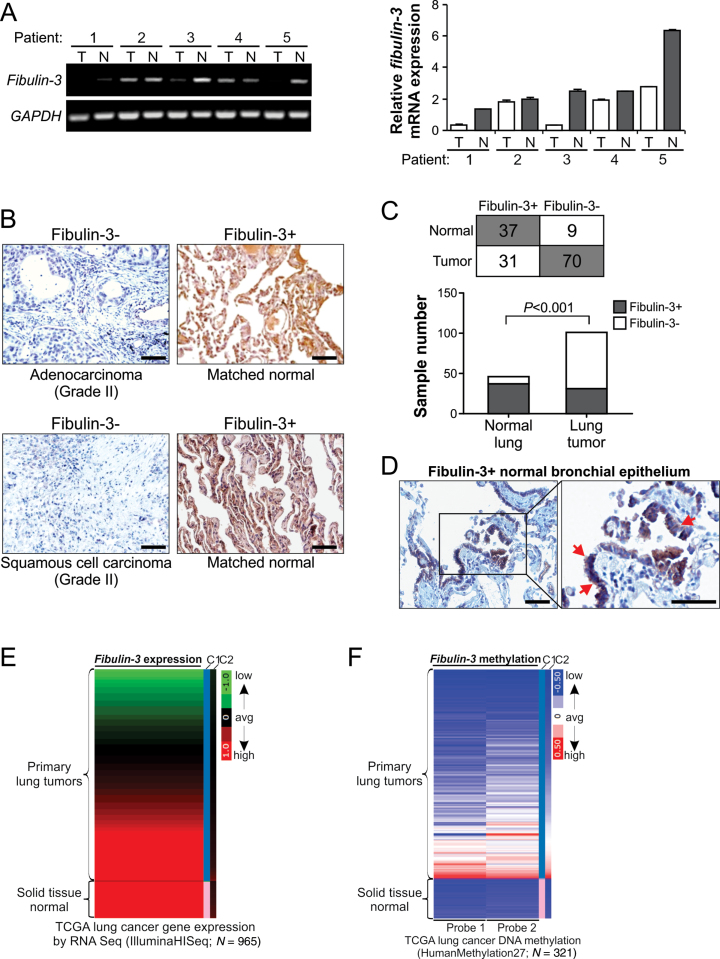
Our previous work identified fibulin-3, also known as EFEMP1, as an epigenetically silenced gene in lung cancer (7). To further investigate its role in suppressing lung cancer progression, we analyzed fibulin-3 expression in lung tumor and normal lung tissues. Analysis of fibulin-3 messenger RNA (mRNA) expression by RT–PCR confirmed fibulin-3 downregulation in several lung tumors compared with matched normal lung tissues (Figure 1A). Fibulin-3 expression was further analyzed by immunostaining using a tissue microarray containing 101 NSCLC and 46 matched normal samples (Supplementary Table 1, available at Carcinogenesis Online). Representative results were shown in Figure 1B and Supplementary Figure 1A, available at Carcinogenesis Online. A majority (37/46, 80.4%) of normal lung specimens were found to be positive for fibulin-3. In contrast, only 30.7% (31/101) NSCLC samples were positive for fibulin-3 (Figure 1C). The difference between tumor and normal samples was highly significant (P < 0.001, Fisher’s exact test). Fibulin-3 expression was detected in the cytoplasm of normal bronchial epithelial cells and fibulin-3-positive tumor cells (Figure 1B and D; Supplementary Figure 1A, available at Carcinogenesis Online). To further determine whether downregulation of fibulin-3 is due to epigenetic silencing, six lung cancer cell lines were treated with 5-aza-dC, a pharmacologic inhibitor of DNA methyltransferase. Fibulin-3 expression was significantly elevated in five lung cell lines following 5-aza-dC treatment, including H1299 and A549 cells, which contain fibulin-3 promoter methylation and lack endogenous fibulin-3 expression (Supplementary Figure 1B, available at Carcinogenesis Online) (7). The effect of 5-aza-dC on fibulin-3 expression was also observed in two prostate cancer cell lines, but not in two breast cancer cell lines analyzed (Supplementary Figure 1B, available at Carcinogenesis Online). The fibulin-3 promoter was shown previously to be methylated in 8 of 22 (36.4%) lung cancer cell lines (7). Furthermore, analysis of TCGA databases (19) confirmed fibulin-3 downregulation (Figure 1E) and increased DNA methylation (Figure 1F), in a substantial fraction of primary lung tumors compared with normal samples. Loss of fibulin-3 expression and fibulin-3 DNA methylation were also found in colon and prostate tumors, but not in head and neck and hepatocellular tumors in the TCGA databases (data not shown).
Fig. 1.
Downregulation of fibulin-3 in lung cancer. (A) RT–PCR analysis of fibulin-3 mRNA expression in five matched pairs of tumor (T)/normal (N) lung tissues. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a house-keeping gene, was used as an internal control. Left, analysis of PCR products by agarose gel electrophoresis; right, quantification of fibulin-3 expression by real-time RT–PCR. (B) Analysis of fibulin-3 expression in a tissue microarray (Supplementary Table 1, available at Carcinogenesis Online) by immunohistochemistry. Representative pictures of two matched tumor and normal pairs are shown (×200). Scale bar, 50 μm. (C) Summary of fibulin-3 staining results in 101 NSCLC and 46 normal lung tissues. The difference between NSCLC and normal lung tissues was statistically significant (P < 0.001, Fisher’s exact test). (D) Normal bronchial epithelium with positive fibulin-3 staining (left ×200; right, ×400). Arrows indicate example bronchial epithelial cells with positive staining. Scale bar, 50 μm. (E) Heatmap of fibulin-3 mRNA expression in the TCGA lung cancer (LUNG) RNAseq (IlluminaHiSeq; N = 965) data set. Red, high expression; black, average expression; green, low expression. C1 and C2, which represent sample type and significance, respectively, are the first and second criteria for sorting the data. (F) Heatmap of fibulin-3 gene methylation in the TCGA lung cancer (LUNG) HumanMethylation27 (Illumina 27K platform; N = 312) data set. Red, high methylation; white, average methylation; blue, low methylation. C1 and C2, which represent sample type and significance, respectively, are the first and second criteria for sorting the data.
Fibulin-3 suppressed lung cancer cell invasion and MMP-7 expression
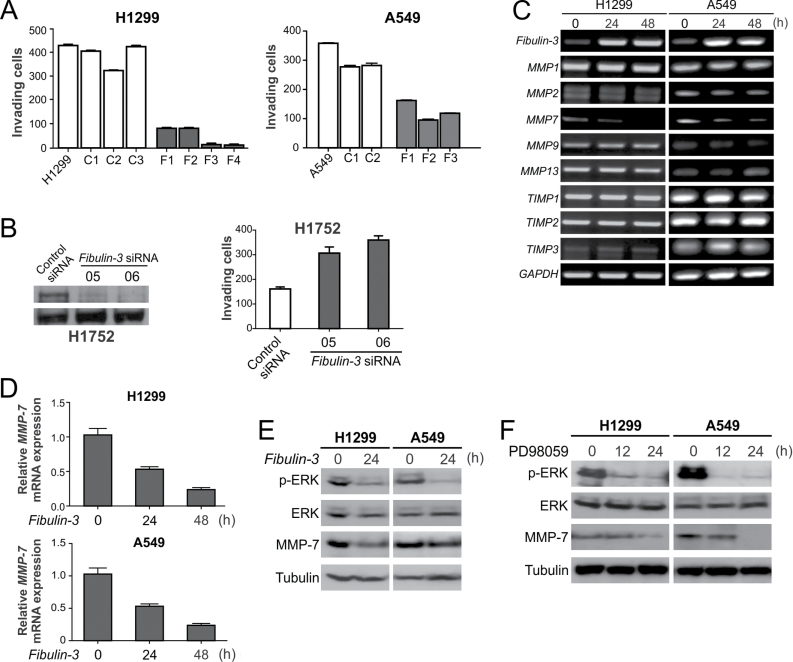
We then investigated the functional role of fibulin-3 in suppressing lung cancer progression. Transfecting H1299, A549 and H460 lung cancer cells with fibulin-3 significantly suppressed cell proliferation in colony formation assays (Supplementary Figure 2, available at Carcinogenesis Online). Stable transfection of fibulin-3 in A549 and H1299 cells significantly suppressed cell invasion analyzed by Matrigel assays (P < 0.01, Fisher’s exact test; Figure 2A and Supplementary Figure 3, available at Carcinogenesis Online). Conversely, knockdown of fibulin-3 by two independent siRNA in H1752 cells, which lack fibulin-3 promoter methylation and express endogenous fibulin-3 protein (7), promoted cell invasion (Figure 2B), suggesting that fibulin-3 downregulation alone is sufficient for stimulating lung cancer cell invasion.
Fig. 2.
Fibulin-3 suppressed lung cancer cell invasion and MMP-7 expression. (A) Matrigel invasion assay was used to analyze the invasion of H1299 and A549 cells with or without fibulin-3 expression. Numbers of invading cells were counted and the results are the average of three independent experiments (P < 0.01, Fisher’s exact test). (B) H1752 cells were transfected with control siRNA, or two independent fibulin-3 siRNA. Left, fibulin-3 was analyzed by western blotting 48h after siRNA transfection. Right, Matrigel assay was used to analyze cell invasion 36h after siRNA transfection. (C) H1299 and A549 cells were transfected with fibulin-3 or control pcDNA vector. Indicated MMPs and TIMPs were analyzed by RT–PCR at 24 and 48h after transfection. (D) MMP-7 expression in cells transfected as in (A) was confirmed by real-time RT–PCR with Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control. The results were normalized to those without fibulin-3 transfection (0h), which were defined as 1.0. (E) Western blot analysis of phospho-ERK (p-ERK; Thr202/Tyr204) and MMP-7 expression in A549 and H1299 cells at 24h after transfection with fibulin-3 or the control empty vector. (F) Western blot analysis of p-ERK and MMP-7 in H1299 and A549 cells treated with the ERK inhibitor PD98059 (50 μmol/l) for 12 or 24h.
To determine how fibulin-3 inhibits lung cancer cell invasion, we used RT–PCR to analyze the expression of several proteolytic enzymes involved in degrading the basement membrane, including three TIMPs and five MMPs, in H1299 and A549 cells following fibulin-3 transfection. Only the expression of MMP-7 was consistently downregulated by 50–80% following fibulin-3 transfection (Figure 2C and 2D). Our previous study showed that the ERK kinase is involved in regulating MMP-7 expression in lung cancer cells (8). We found that ERK activity is markedly downregulated in H1299 and A549 cells after fibulin-3 transfection (Figure 2E). Inhibiting ERK activity using the ERK inhibitor PD98059 was sufficient to downregulate MMP-7 expression in H1299 and A549 cells (Figure 2F), suggesting that fibulin-3 downregulates MMP-7 through ERK inhibition.
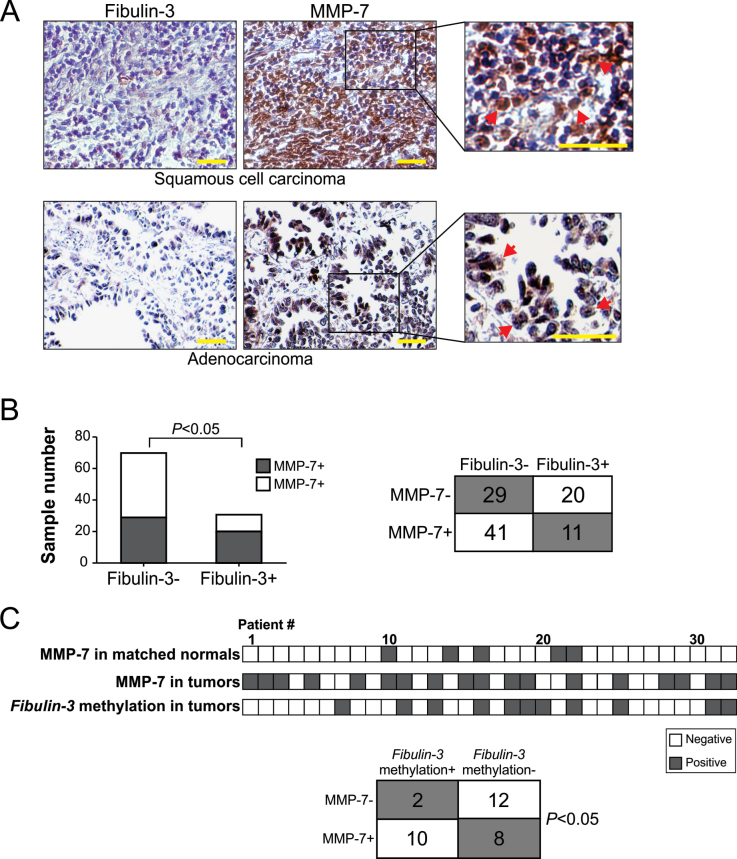
Fibulin-3 downregulation and promoter methylation were correlated with MMP-7 expression
To further determine the functional relationship between fibulin-3 and MMP-7 in NSCLC, MMP-7 expression was analyzed by immunohistochemistry using the aforementioned tissue microarray (Supplementary Table 1, available at Carcinogenesis Online), in comparison with fibulin-3 expression. More than 50% (52/101) of NSCLC samples were positive for MMP-7 (Figure 3A; Supplementary Table 1, available at Carcinogenesis Online). A statistically significant (P < 0.05, two-tailed χ2 test) inverse correlation was found between fibulin-3 and MMP-7 expression, with 60.4% (61/101) of tumors expressing either MMP-7 or fibulin-3, but only 10.9% (11/101) expressing both proteins (Figure 3B). We also analyzed MMP-7 expression in an independent set of 32 matched tumor and normal pairs with known fibulin-3 promoter methylation status (7). MMP-7 expression was positive in 83.3% (10/12) of fibulin-3-negative tumors containing fibulin-3 promoter methylation, but in only 40% (8/20) tumors without fibulin-3 promoter methylation (Figure 3C; Supplementary Figure 4, available at Carcinogenesis Online). The correlation between MMP-7 expression and fibulin-3 promoter methylation was also statistically significant (P < 0.05, Fisher’s exact test). Furthermore, analysis of the TCGA databases confirmed increased MMP-7 expression in lung tumors (Supplementary Figure 5A, available at Carcinogenesis Online). These results, along with the previous finding that induction of MMP-7 promotes lung cancer cell invasion (8), suggest that fibulin-3 suppresses lung cancer cell invasion by inhibiting MMP-7 expression.
Fig. 3.
Fibulin-3 downregulation and promoter methylation were correlated with MMP-7 expression in lung tumors. (A) Comparison of immunostaining of fibulin-3 and MMP-7 in NSCLC. Representative pictures of two fibulin-3-/MMP-7+ tumors are shown (×400). Arrows indicate examples of MMP-7-positive cells. Scale bar, 25 μm. (B) Summary of fibulin-3 and MMP-7 expression in 101 NSCLC samples (Supplementary Table 1, available at Carcinogenesis Online). The inverse correlation between fibulin-3 and MMP-7 expression was statistically significant (P < 0.05, two-tailed χ2 test). (C) Top: Summary of fibulin-3 and MMP-7 expression and MMP-7 promoter methylation in an independent set of 32 pairs of lung tumors and matched pathologically normal lung tissues. Bottom: Correlation of MMP-7 expression and fibulin-3 promoter methylation in lung tumors (P < 0.05, two-tailed χ2 test).
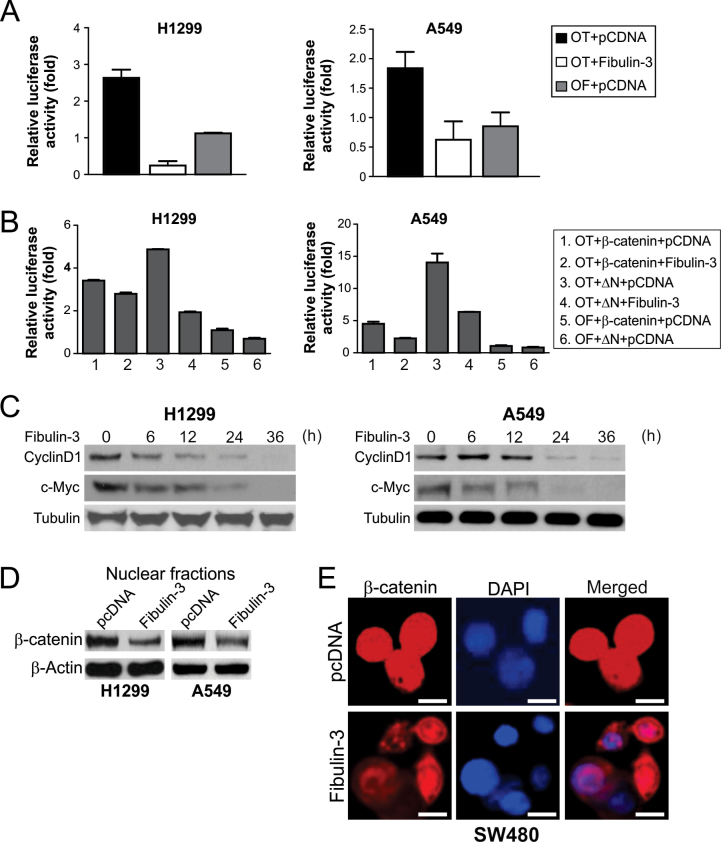
Fibulin-3 inhibited Wnt/β-catenin signaling in lung cancer cells
It has been shown that MMP-7 can be regulated by the Wnt/β-catenin pathway in colorectal cancer cells (20). We, therefore, investigated whether the effects of fibulin-3 on MMP-7 are mediated by Wnt/β-catenin signaling. Transfection of fibulin-3, but not the control vector, significantly inhibited the activity of TCF-4 reporter in A549 and H1299 cells (Figure 4A). Fibulin-3 also inhibited transactivation of TCF-4 reporter by WT β-catenin, as well as that by the mutant β-catenin lacking the N-terminal phosphorylation sites required for its degradation (ΔN; Figure 4B). The protein and mRNA expression of c-Myc and cyclin D1, two well-known TCF-4 downstream targets (11,12), was significantly suppressed after fibulin-3 transfection (Figure 4C; Supplementary Figure 6, available at Carcinogenesis Online). Furthermore, fibulin-3 expression decreased the level of nuclear β-catenin in H1299 and A549 cells (Figure 4D), and in SW480 colorectal cancer cells with abundant expression of nuclear β-catenin (Figure 4E). These results suggest that fibulin-3 inhibits Wnt/β-catenin signaling by preventing the nuclear translocation of β-catenin.
Fig. 4.
Fibulin-3 inhibited β-catenin nuclear translocation and expression of TCF-4 targets c-Myc and cyclin D1. (A) Fibulin-3 inhibited TCF-4 reporter activity. A549 and H1299 cells were transfected with fibulin-3 along with the TCF-4 reporter pTOPFlash (OT) or the control inactive reporter pFOPFlash (OF). Normalized luciferase activities were determined 24h after transfection. The activity of the OF was defined as 1.0. (B) The effects of fibulin-3 on TCF-4 reporter activities induced by WT or mutant β-catenin (ΔN). ΔN: The mutant β-catenin with N-terminal 45 amino acids deleted. Reporter assays were performed as in (A). (C) c-Myc and cyclin D1 expression at the indicated time points after fibulin-3 transfection in A549 and H1299 cells were analyzed by western blotting. (D) Western blot analysis of β-catenin in nuclear fractions isolated from H1299 and A549 cells at 24h after transfection with fibulin-3 or the control empty vector. (E) Representative pictures of β-catenin immunostaining in SW480 colorectal cancer cells at 48h after transfection with fibulin-3 or the control empty vector (×400). 4ʹ6-Diamidino-2-phenylindole (DAPI; blue) was used to counterstain the nuclei. Scale bar, 5 μm.
Fibulin-3 suppressed MMP-7 expression through Wnt/β-catenin signaling
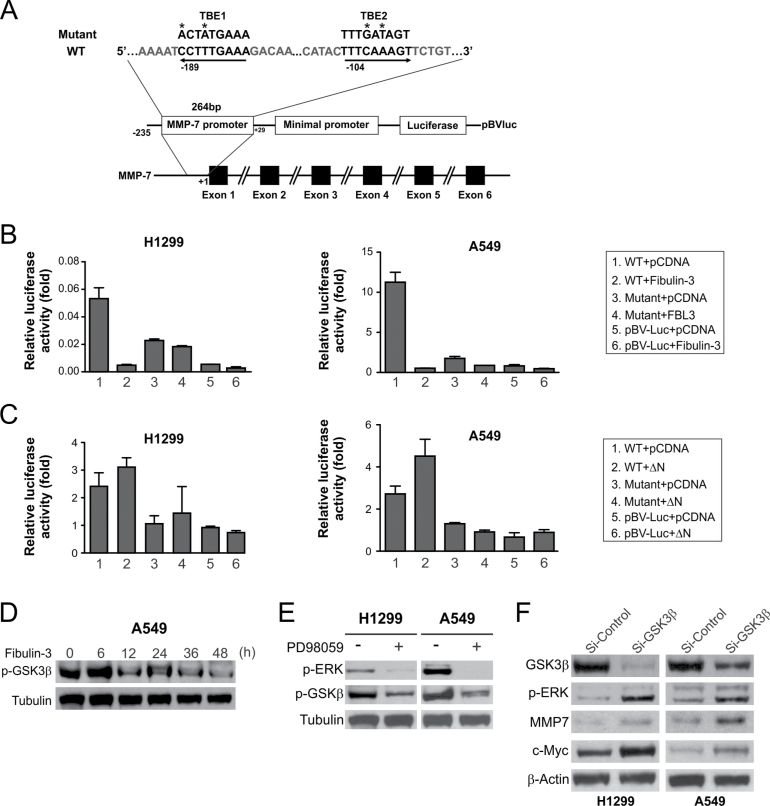
We then determined whether fibulin-3 suppresses MMP-7 expression through its inhibitory effects on Wnt/β-catenin signaling. There are at least two TCF-4 binding elements (TBEs) in the promoter region of MMP-7 (Figure 5A). The activities of an MMP-7 reporter containing these TBEs were markedly suppressed after fibulin-3 transfection (Figure 5B). The MMP-7 reporter activity was dependent on Wnt/β-catenin signaling, as mutations of both TBEs abolished the MMP-7 reporter activities in A549 and H1299 cells (Figure 5B). The activities of WT MMP-7 reporter, but not the mutant, were also enhanced upon transfection of the β-catenin ΔN mutant (Figure 5C). These results indicate that fibulin-3 suppresses MMP-7 expression by inhibiting the activities of β-catenin/TCF-4, which directly bind to TBEs in the promoter of MMP-7 to activate its transcription.
Fig. 5.
MMP-7 was downregulated by fibulin-3 through its inhibitory effects on the β-catenin pathway. (A) Schematic representation of MMP-7 promoter region containing two TBEs. Asterisks indicate the mutated nucleotides. (B) H1299 and A549 cells were transfected with fibulin-3 along with WT or mutant MMP-7 reporter construct. Luciferase activities were determined 24h after transfection and normalized to that of empty reporter pBV-Luc. (C) H1299 and A549 cells were transfected with WT or ΔN β -catenin along with WT or mutant MMP-7 reporter. Luciferase activities were analyzed as in (B). (D) Western blot analysis of p-GSK3β (Ser9) at the indicated time points in A549 cells after fibulin-3 transfection. (E) Western blot analysis of p-GSK3β and p-ERK in H1299 and A549 cells treated with 50 μM of the ERK inhibitor PD98059 for 24h. (F) Western blot analysis of GSK3β, p-ERK, MMP-7 and c-Myc in H1299 and A549 cells at 24h after transfection with GSK3β or control siRNA.
We further investigated how fibulin-3 regulates MMP-7 expression through the Wnt/β-catenin pathway. It is known that ERK can inhibit the activity of GSK3β, a key negative regulator of β-catenin, through inhibitory Ser9 phosphorylation of GSK3β (21). We found that Ser9 phosphorylation of GSK3β was decreased in lung cancer cells after fibulin-3 transfection (Figure 5D). Treating cells with the ERK inhibitor PD98059, which suppressed MMP-7 (Figure 2F), also decreased the level of Ser9 phosphorylation GSK3β (Figure 5E). Furthermore, knockdown of GSK3β by siRNA led to induction of MMP-7 and c-Myc expression, and also increased ERK phosphorylation in both H1299 and A549 cells (Figure 5F). Together, these results suggest that fibulin-3 inhibits ERK activity to activate GSK3β and suppress Wnt/β-catenin signaling, which in turn inhibits MMP-7 expression.
Fibulin-3 suppressed lung cancer growth and metastasis in mice
We then used xenograft models to investigate the in vivo tumor suppressive effects of fibulin-3. Fibulin-3 expression significantly (P < 0.001) suppressed the growth of H1299 xenograft tumors in nude mice (Figure 6A; Supplementary Figure 7A, available at Carcinogenesis Online) and also downregulated MMP-7 expression analyzed by immunostaining (Figure 6B). The NCI-H460 metastasis model described previously was then applied to determine whether fibulin-3 can suppress tumor metastasis (22). Parental and fibulin-3-expressing H460 cells were injected intravenously into nude mice. After 7 weeks, we detected a number of lung metastasis nodules in the mice receiving the parental H460 cells, but much fewer and smaller nodules in those receiving fibulin-3-expressing H460 cells (Figure 6C and 6D). The difference between the two groups was statistically significant (Figure 6D). Furthermore, the mice receiving the parental H460 cells lost significantly more weight than those receiving fibulin-3-expressing cells (Supplementary Figure 7B, available at Carcinogenesis Online). These results indicate that fibulin-3 functions as a suppressor of tumor growth and metastasis in vivo.
Fig. 6.
Fibulin-3 inhibited lung tumor progression and metastasis in mice. (A) Parental and fibulin-3-expressing H1299 cells were injected subcutaneously into BALB/c nude mice. Tumor volumes at indicated time points after inoculation were calculated and plotted (n = 5 in each group). The difference between parental and fibulin-3-expressing tumors was statistically significant (P < 0.001, Fisher’s exact test). (B) Representative pictures of fibulin-3 and MMP-7 immunostaining in tumors from (A) using anti-V5 and anti-MMP-7 antibodies, respectively (×200). Scale bar, 20 μm. (C) Parental and fibulin-3-expressing H460 cells were injected intravenously by tail vein into BALB/c nude mice. Representative pictures of fixed lungs at 7 weeks after injection were shown. Arrows indicate metastasis nodules. Scale bar, 5mm. (D) Quantification of metastasis nodules in the parental and fibulin-3-expressing H460 tumors from (C). The difference between the groups with and without fibulin-3 expression was statistically significant (P < 0.01, Fisher’s exact test). (E) A model of fibulin-3-mediated suppression of MMP-7 and invasion in lung cancer.
Discussion
Our previous and current studies demonstrate frequent downregulation of fibulin-3 in lung cancer (Figure 1) (7). Fibulin-3 is expressed in most (80%) normal lungs, but loses its expression in ~40% lung cancer cell lines and ~70% lung tumors, at least in part due to promoter methylation (Figure 1) (7). Silencing of fibulin-3 has been reported in colorectal (23), liver (24), prostate (25) and nasopharyngeal tumors (26). Fibulin-3 promoter methylation can be used as a biomarker for lung cancer detection (27). Serum levels of fibulin-3 were recently identified as an indicator of malignant mesothelioma (28). However, the role of fibulin-3 in tumor progression is cell type- and context-dependent. Tumor-promoting activities of fibulin-3 have been reported in cervical (29), brain (30) and pancreatic tumors (31).
Downregulation of fibulin-3 has significant functional effects, as fibulin-3 suppresses the growth and invasion of NSCLC cells lacking fibulin-3 expression, and depleting fibulin-3 in NSCLC cells expressing fibulin-3 was sufficient to promote cell invasion (Figure 2). The effects of fibulin-3 on lung cancer invasion are mediated by the inhibition of MMP-7, which plays an important role in cancer invasion (32,33). It has been shown that MMP-7 is overexpressed in lung cancer (34,35), and its overexpression is associated with poor prognosis of NSCLC (36). Our previous work demonstrated that upregulation of MMP-7 is sufficient to promote lung cancer invasion (8). The frequent downregulation of fibulin-3 (Figure 1), the inverse correlation between fibulin-3 and MMP-7 expression (Figure 3B), and the significant association between fibulin-3 promoter methylation and MMP-7 expression (Figure 3C) suggest that epigenetic silencing of fibulin-3 is an underlying mechanism of MMP-7 overexpression in NSCLC.
Fibulin-3 appears to have similar functions as another fibulin family member, fibulin-5, which is also epigenetically silenced in lung cancer and can suppress lung cancer invasion by inhibiting ERK signaling and MMP-7 expression (8). Both fibulin-3 and fibulin-5 have antiangiogenic activities (37), which may contribute to their inhibitory effects on lung cancer invasion. Unlike fibulin-5, fibulin-3 lacks an integrin-binding RGD domain, which mediates the effects of fibulin-5 on tumor invasion and angiogenesis (8,37). In addition to MMP-7, fibulin-3 and fibulin-5 functionally interact with other MMP/TIMP family proteins. For example, fibulin-3 is a binding pattern of TIMP-3 (38), and fibulin-5 modulates the expression of MMP-2, MMP-3, TIMP-1 and TIMP-3 in some tumor cells (37). These findings suggest concerted deregulation of multiple fibulins in regulating the activities of the MMP/TIMP family proteins.
Our results indicate that the downregulation of fibulin-3 promotes MMP-7 expression and lung cancer invasion through aberrant activation of Wnt/β-catenin signaling, which has recently emerged to play an important role in lung cancer (39). Lung cancer cells consistently express high levels of nuclear β-catenin, which cause activation of TCF-4 and overexpression of cyclin D1 to enhance lung cancer cell proliferation (13,14). Wnt/β-catenin and MMP-7 are also functionally linked in normal pulmonary physiology and pathological conditions such as lung injury response (40). Our results revealed for the first time an inhibitory effect of fibulin-3 on the Wnt/β-catenin pathway. Fibulin-3 did not appear to be primarily involved in β-catenin degradation, as it inhibited the activities of both WT and mutant β-catenin without the N-terminal phosphorylation sites required for its degradation. However, it is possible that keeping β-catenin in the cytoplasm facilitates its interactions with other proteins and subsequent turnover. Mechanistically, our data and results from other groups suggest a pathway in which fibulin-3, likely through its binding to a cell surface receptor via extracellular matrix (4), inhibits ERK activity and indirectly activates GSK3β to restrain β-catenin in the cytoplasm, which prevents MMP-7 expression and invasion (Figure 6E). A feedback loop may be involved as well, as alterations in GSK3β can modulate ERK activity (Figure 2F). However, we cannot rule out the possibility that fibulin-3 may directly modulate GSK3β activity in the cytoplasm. Future studies are necessary to further delineate this novel pathway and its role in suppressing lung tumor progression in vivo.
Tumor metastasis is stimulated upon inactivation of a group of metastasis suppressors, such as NM23, KAI1 and MKK4 (41). These genes are characterized by downregulation in metastatic tumors and activities of reducing the metastatic propensity of cancer cells in animal tumor models. Our in vitro and in vivo data strongly suggest that fibulin-3 functions as a metastasis suppressor of lung cancer. It has been shown that fibulin-5 is involved in regulating ‘epithelial–mesenchymal transition’ (42), a critical process in cancer metastasis during which epithelial cells acquire phenotypes of motile fibroblasts. ERK signaling and tumor-associated MMPs can stimulate epithelial–mesenchymal transition (43,44). Therefore, epigenetic silencing of fibulin-3 and fibulin-5 and subsequent activation of ERK and MMP-7 may be epithelial–mesenchymal transition-related events.
Collectively, our results demonstrate an important functional role of fibulin-3 in suppressing lung cancer invasion, which is mediated through the inhibition of Wnt/β-catenin signaling and MMP-7 expression. Further investigation of this pathway may provide potentially useful information for developing biological or pharmacological agents to target metastatic lung cancer.
Supplementary material
Supplementary Tables 1 and 2 and Figures 1–7 can be found at http://carcin.oxfordjournals.org/
Funding
Ministry of Science and Technology of China (2009CB918903 to Z.Y.); National Institutes of Health (CA172136 and CA106348 to L.Z.; CA129829 to J.Y.); Tianjin Municipal Education Commission Foundation (2012ZD01 to Z.Y.); American Cancer Society (RSG-10-124-01-CCE to J.Y.).
Supplementary Material
Acknowledgements
The authors would like to thank our lab members for critical reading.
Conflict of Interest Statement: L.Z. is a scholar of the V Foundation for Cancer Research.
Glossary
Abbreviations:
- 5-aza-dC
5-aza-2ʹ-deoxycytidine
- ERK
extracellular signal-regulated kinase
- GSK3β
glycogen synthase kinase 3β
- MMP
matrix metalloproteinase
- mRNA
messenger RNA
- NSCLC
non-small-cell lung cancer
- RT–PCR
reverse transcriptase–PCR
- siRNA
small interfering RNA
- TBE
T-cell factor 4 binding element
- TCF-4
T-cell factor 4
- TCGA
The Cancer Genome Atlas
- TIMP
tissue inhibitor of metalloproteinase
- WT
wild-type.
References
- 1. Minna J.D., et al. (2002). Focus on lung cancer. Cancer Cell, 1, 49–52 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen D.X., et al. (2007). Genetic determinants of cancer metastasis. Nat. Rev. Genet., 8, 341–352 [DOI] [PubMed] [Google Scholar]
- 3. Brinckerhoff C.E., et al. (2002). Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol., 3, 207–214 [DOI] [PubMed] [Google Scholar]
- 4. Timpl R., et al. (2003). Fibulins: a versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol., 4, 479–489 [DOI] [PubMed] [Google Scholar]
- 5. Obaya A.J., et al. (2012). The dual role of fibulins in tumorigenesis. Cancer Lett., 325, 132–138 [DOI] [PubMed] [Google Scholar]
- 6. Gallagher W.M., et al. (2005). Fibulins and cancer: friend or foe? Trends Mol. Med., 11, 336–340 [DOI] [PubMed] [Google Scholar]
- 7. Yue W., et al. (2007). Frequent inactivation of RAMP2, EFEMP1 and Dutt1 in lung cancer by promoter hypermethylation. Clin. Cancer Res., 13(15 Pt 1), 4336–4344 [DOI] [PubMed] [Google Scholar]
- 8. Yue W., et al. (2009). Fibulin-5 suppresses lung cancer invasion by inhibiting matrix metalloproteinase-7 expression. Cancer Res., 69, 6339–6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vogelstein B., et al. (2004). Cancer genes and the pathways they control. Nat. Med., 10, 789–799 [DOI] [PubMed] [Google Scholar]
- 10. Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 11. He T.C., et al. (1998). Identification of c-MYC as a target of the APC pathway. Science, 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 12. Tetsu O., et al. (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature, 398, 422–426 [DOI] [PubMed] [Google Scholar]
- 13. Winn R.A., et al. (2002). gamma-Catenin expression is reduced or absent in a subset of human lung cancers and re-expression inhibits transformed cell growth. Oncogene, 21, 7497–7506 [DOI] [PubMed] [Google Scholar]
- 14. Lim J.H., et al. (2006). Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res., 66, 10677–10682 [DOI] [PubMed] [Google Scholar]
- 15. You L., et al. (2004). Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene, 23, 6170–6174 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen D.X., et al. (2009). WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell, 138, 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cline M.S., et al. (2013). Exploring TCGA Pan-Cancer data at the UCSC Cancer Genomics Browser. Sci. Rep., 3, 2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yue W., et al. (2008). Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis, 29, 84–92 [DOI] [PubMed] [Google Scholar]
- 19. Cancer Genome Atlas Research. (2012). Comprehensive genomic characterization of squamous cell lung cancers. Nature, 489, 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crawford H.C., et al. (1999). The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene, 18, 2883–2891 [DOI] [PubMed] [Google Scholar]
- 21. Ding Q., et al. (2005). Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol. Cell, 19, 159–170 [DOI] [PubMed] [Google Scholar]
- 22. Soon L.L., et al. (2003). Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J. Biol. Chem., 278, 11465–11470 [DOI] [PubMed] [Google Scholar]
- 23. Tong J.D., et al. (2011). Downregulation of fibulin-3 gene by promoter methylation in colorectal cancer predicts adverse prognosis. Neoplasma, 58, 441–448 [DOI] [PubMed] [Google Scholar]
- 24. Nomoto S., et al. (2010). Epidermal growth factor-containing fibulin-like extracellular matrix protein 1, EFEMP1, a novel tumor-suppressor gene detected in hepatocellular carcinoma using double combination array analysis. Ann. Surg. Oncol., 17, 923–932 [DOI] [PubMed] [Google Scholar]
- 25. Kuo P.L., et al. (2012). CXCL1/GROa increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-?B/HDAC1 epigenetic regulation. Carcinogenesis, 33, 2477–2487 [DOI] [PubMed] [Google Scholar]
- 26. Hwang C.F., et al. (2010). Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J. Pathol., 222, 367–379 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y., et al. (2011). Methylation of multiple genes as a candidate biomarker in non-small cell lung cancer. Cancer Lett., 303, 21–28 [DOI] [PubMed] [Google Scholar]
- 28. Pass H.I., et al. (2012). Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N. Engl. J. Med., 367, 1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song E.L., et al. (2011). EFEMP1 expression promotes angiogenesis and accelerates the growth of cervical cancer in vivo . Gynecol. Oncol., 121, 174–180 [DOI] [PubMed] [Google Scholar]
- 30. Hu B., et al. (2012). Fibulin-3 promotes glioma growth and resistance through a novel paracrine regulation of Notch signaling. Cancer Res., 72, 3873–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seeliger H., et al. (2009). EFEMP1 expression promotes in vivo tumor growth in human pancreatic adenocarcinoma. Mol. Cancer Res., 7, 189–198 [DOI] [PubMed] [Google Scholar]
- 32. Egeblad M., et al. (2002). New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer, 2, 161–174 [DOI] [PubMed] [Google Scholar]
- 33. López-Otín C., et al. (2007). Emerging roles of proteases in tumour suppression. Nat. Rev. Cancer, 7, 800–808 [DOI] [PubMed] [Google Scholar]
- 34. Safranek J., et al. (2007). Expression of mRNA MMP-7 and mRNA TIMP-1 in non-small cell lung cancer. Anticancer Res., 27, 2953–2956 [PubMed] [Google Scholar]
- 35. Lin T.S., et al. (2004). Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol. Rep., 12, 717–723 [PubMed] [Google Scholar]
- 36. Liu D., et al. (2007). Overexpression of matrix metalloproteinase-7 (MMP-7) correlates with tumor proliferation, and a poor prognosis in non-small cell lung cancer. Lung Cancer, 58, 384–391 [DOI] [PubMed] [Google Scholar]
- 37. Albig A.R., et al. (2006). Fibulins 3 and 5 antagonize tumor angiogenesis in vivo . Cancer Res., 66, 2621–2629 [DOI] [PubMed] [Google Scholar]
- 38. Klenotic P.A., et al. (2004). Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J. Biol. Chem., 279, 30469–30473 [DOI] [PubMed] [Google Scholar]
- 39. Van Scoyk M., et al. (2008). Wnt signaling pathway and lung disease. Transl. Res., 151, 175–180 [DOI] [PubMed] [Google Scholar]
- 40. Villar J., et al. (2011). Activation of the Wnt/ß-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS ONE, 6, e23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steeg P.S. (2003). Metastasis suppressors alter the signal transduction of cancer cells. Nat. Rev. Cancer, 3, 55–63 [DOI] [PubMed] [Google Scholar]
- 42. Lee Y.H., et al. (2008). Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis, 29, 2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giehl K., et al. (2008). Microenvironmental regulation of E-cadherin-mediated adherens junctions. Front. Biosci., 13, 3975–3985 [DOI] [PubMed] [Google Scholar]
- 44. Radisky D.C., et al. (2008). Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell, 2, 511–512 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.