Abstract
Objective
We set out to examine the short-term regulation of the intestinal sodium/glucose cotransporter SGLT1 by its substrate glucose and sweet taste analogs.
Summary Background Data
Intestinal SGLT1 is a putative target for antidiabetic therapy; however, its physiological regulation is incompletely understood, limiting its application as a pharmacological target. While it is clearly regulated by dietary composition over a period of days, its short-term regulation by nutrients is unknown.
Methods
Sprague-Dawley rats were anesthetized, and the duodenum cannulated. D-glucose, D-fructose, saccharin, D-mannitol, and water were infused for 3 hours, before harvest of proximal jejunum for SGLT1 analysis with Western blotting and quantitative polymerase chain reaction. In further experiments, the receptor region was identified by D-glucose infusion of isolated regions. Lastly, the vagus was de-afferented with capsaicin, and 5HT3-receptor activation of vagal afferents inhibited using ondansetron, before repeating experiments using water or D-glucose infusion.
Results
Infusion of D-glucose led to 2.9-fold up-regulation in SGLT1 compared with water or iso-osmotic D-mannitol; this effect was replicated by D-fructose or saccharin. This response was strongest following isolated infusions of duodenum and proximal jejunum, with a blunted effect distally; topography matched the expression profile of sweet taste receptor T1R2/T1R3. The reflex was abolished by capsaicin pretreatment, and blunted by ondansetron.
Conclusions
The agonist response implicates the luminal-based sweet-taste receptor T1R2/T1R3, with the reflex apparently involving vagal afferents. The proximal nature of the sensor coincides with the excluded biliopancreatic limb in Roux-en-Y gastric bypass, and this may provide a novel explanation for the antidiabetic effect of this procedure.
Type 2 diabetes mellitus (T2DM), and associated obesity, is a major economic and public health problem throughout the developed world.1,2 Multiple reports have now confirmed that patients with diabetes undergoing certain bariatric procedures, such as Roux-en-Y gastric bypass (RYGB), experience improvement or complete resolution of T2DM within a few days of the procedure, independent of any weight loss effects.3 Unfortunately, a large proportion of patients with diabetes who might benefit from bariatric surgery do not fulfill the criteria established by the National Institutes of Health and other professional bodies, and struggle with suboptimal medical treatments because the risks of surgery have been judged to outweigh the benefits. Leading limitations include the expense, life-changing ramifications, and invasive nature of surgical interventions, with associated morbidity and mortality.
As a way to develop a less invasive alternative to RYGB that procedures its metabolic benefits without exposing patients to significant risk and morbidity, we have focused our attention on the intestinal absorption of hexoses (the ultimate intestinal hydrolysis products of dietary carbohydrates) as a potential therapeutic target for the treatment of obesity, and associated T2DM. In particular, we have been interested in the sodium-glucose cotransporter SGLT1, which is dysregulated in obesity and T2DM.4 – 6 This transporter co-ordinates the secondary active transport of glucose across the apical membrane of enterocytes throughout the small bowel. SGLT1 is up-regulated approximately 3- to 4-fold in both patients and animal models with T2DM,7–10 while murine knock-outs of the SGLT1 regulatory subunit RS1, which over-express SGLT1, develop profound obesity.11 Lastly, pharmacological blockade of SGLT1 with phloridzin and related apple-derived phenolic compounds improves glucose tolerance and, surprisingly, peripheral insulin resistance.12–14 We hypothesize that suppression of SGLT1-mediated glucose absorption will slow intestinal glucose uptake, reducing postprandial hyperglycemia, thus influencing metabolic state.
Our current incomplete understanding of the physiological regulation of SGLT1 limits efforts to develop novel therapies modulating intestinal glucose uptake. It is clear however that SGLT1 expression is tightly matched to dietary composition, increasing in the face of diets rich in its own substrate, glucose.15 Thus consumption of diet rich in glucose leads to an increase in SGLT1 expression and glucose uptake capacity the subsequent day.16–18 Similarly, high-fructose diets also induce increased SGLT1 expression and function, although fructose is not itself a substrate of SGLT1.15
A well-studied model of SGLT1 regulation is sheep. These ruminants ferment almost all dietary carbohydrate to volatile fatty acids in the rumen and therefore little or no carbohydrate reaches the small intestine.19 As an adaptation to this metabolic circumstance, SGLT1 expression in the sheep small bowel is negligible. However, infusion of D-glucose into the sheep duodenum leads to a 50-fold increase in SGLT1 expression within 3 hours.20 Replication of this response by glucose bound to a nonabsorbable macromolecule, thus remaining intraluminal,20 implies the presence of a luminal glucose sensor on the apical surface of the mucosa regulating SGLT1 and thereby intestinal glucose transport.
While a rapid response mechanism for SGLT1 was initially thought restricted to ruminants, luminal sensing has been observed in nonruminant rodents. In particular, G-protein coupled sweet-taste receptors T1R2/T1R3 have recently been detected throughout the small intestine.21,22 Most pertinent, murine knock-outs of T1R3 or α-gustducin (the G-protein transducer) are unable to up-regulate SGLT1 in response to increases in dietary glucose.23 Furthermore, subdiaphragmatic vagal de-afferentation ablates both SGLT1 up-regulation in response to long-term increases in dietary glucose,24 and normal circadian increases in SGLT1 in response to anticipated feeding time.25 These studies suggest that vagal afferents may participate in a signaling loop detecting as yet unknown abdominal visceral cues and matching SGLT1 expression correspondingly.
We therefore set out to define the short-term regulation in response to glucose or glucose-analogs in a rodent model. We specifically focused on receptor characteristics, the topographical location of the sensor, and the role of vagal pathways in mediating glucose signaling.
METHODS
Materials
D-Glucose, D-mannitol, D-fructose, saccharin, capsaicin, ondansetron, Tween 80 and protease inhibitors, were purchased from Sigma (St. Louis, MO); sodium pentobarbital from Ovation Pharmaceuticals (Deerfield, IL); buprenorphine from Bedford Labs (Bedford, OH). Antibodies were acquired from Chemicon (Temecula, CA; SGLT1 and GLUT2); Neomarkers (Fremont, CA; Actin) and Vector Laboratories (Burlingame, CA; horseradish peroxidase-conjugated α-rabbit and -mouse).
Whole Length Intestinal Infusion Studies
All animal studies were performed in accordance with protocols prospectively approved by the Harvard Medical Area Standing Committee on Animals. Male Sprague-Dawley rats (200 –210 g, Harlan, Indianapolis) were acclimatized for 7 days under a 12:12 light: dark cycle (lights-on 7 AM) with ad libitum access to standard rat chow. On the day of experimentation, animals were anesthetized at the same time of the day (10 AM, to avoid the confounding factor of the diurnal variation in intestinal function) with intraperitoneal sodium pentobarbital 50 mg/kg, with 5 to 10 mg/kg given as required for maintenance. To place a duodenal cannula, animals were inclined with 60° head-up tilt, as pilot studies showed high mortality rates from gastro-esophageal reflux and aspiration, without the head tilt. A 3 to 4 cm midline laparotomy performed and a fine silastic tube (0.04” internal diameter; Fisher Scientific, Pittsburg, PA) passed through the pylorus via a small incision made along the greater curve of the stomach, which was then closed with a 3– 0 silk purse-string suture. A moist swab was placed in the left upper quadrant to prevent reflux of infusate, and the whole intestine was then infused for 3 hours with: D-glucose (250 mM), D-fructose (250 mM), D-mannitol (250 mM), saccharin (0.3%), or water. Animals received a bolus of 1 mL infusate over 5 minutes, before infusing at a rate of 2.5 mL/h for the remaining 3 hours. Tissue was harvest as described below for all experiments.
Regional Intestinal Infusion Studies
For infusion of selective regions of intestine (duodenum, proximal jejunum, and midjejunum distally), clamps were positioned to isolate either the duodenum and stomach; 15 cm length of proximal jejunum immediately distal to the ligament of Treitz; or midjejunum distally, starting 15 cm distal to ligament of Treitz (n = 6 – 8 each group). A total of 250 mM D-glucose was again infused at similar rate to above for 3 hours. Jejunum segments corresponding to the segments analyzed above were harvested and analyzed as described below. Results were compared with whole intestinal infusions of water or glucose (Fig. 3).
FIGURE 3.
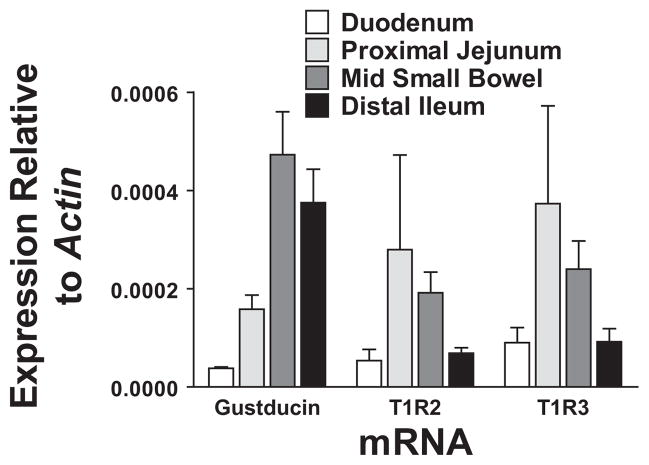
Expression of taste receptors (α-gustducin, T1R2, T1R3) were measured in proximal duodenum, proximal jejunum, mid small bowel, and terminal ileum, using qPCR. Both α-gustducin and T1R3 showed topographical variation, peaking in proximal to midsmall bowel. While T1R2 trended towards a regional variation, this did not meet significance.
Vagal and Inhibitor Studies
In subsequent studies, to determine the role of vagal afferents in SGLT1 control, animals were pretreated with capsaicin to selectively de-afferent the vagus, 10 days before experimentation. Animals were acclimatized for 7 days before undergoing laparotomy and vagal de-afferentation as described previously by our group.25 Briefly, 1 mg capsaicin in 1 mL vehicle solution (90% olive oil/10% Tween 80) was applied topically to the subdiaphragmatic vagal trunks for 30 minutes. The abdomen was then thoroughly lavaged before closure with vicryl 3– 0 suture, and recovery for 10 days with ad libitum access to food and water. Postoperative analgesia was provided for 48 hours (buprenorphine 0.05 mg/kg BD sc). Animals then underwent duodenal infusions with 250 mM D-glucose or water for 3 hours as described above, before harvest of proximal jejunum.
To identify whether vagal afferents were directly detecting glucose, or acting through enteroendocrine cells signaling via 5HT3 receptor-mediated pathways, we pretreated animals with ondansetron 1 mg/kg intraperitoneally 30 minutes before infusion with D-glucose or water as described.
Topography of Intestinal Sweet Taste Receptors
Four male Sprague-Dawley rats (200 –210 g, Harlan) were acclimatized before harvesting entire intestine from pylorus to ileocecal junction at 4 PM, the time at which mRNA levels peak for many intestinal brush border membrane proteins.26 Mucosa was harvested by scraping with glass microscope slides: aliquots were taken from the first 2 parts of the duodenum (as defined by the sphincter of Oddi); proximal jejunum; midsmall bowel; and distal ileum. These were subsequently assayed for sweet taste receptors T1R2 and T1R3, as well as α-gustducin mRNA.
Tissue Harvest and Analysis
At completion of all infusions, proximal jejunum, 15 cm proximal to the ligament of Treitz, was flushed on mesentery with ice-cold mammalian Ringer’s solution (128 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 20 mM NaHCO3; pH 7.38) before harvesting. Full thickness tissue sections were cut for functional assays as described below, and mucosa scraped from the remaining 10 cm for protein and mRNA assays. The scrapings were flash frozen in liquid nitrogen before storing at −80°C for later analysis.
Quantitative PCR
Total RNA was extracted from jejunal mucosa using a mirVana (Ambion, Austin, TX) commercial kit. A total of 5 mg RNA was reverse transcribed using Superscript III kit (Invitrogen, Carlsbad, CA). The cDNAs were quantified on an ABI7900HT thermal cycler using SYBR green master mix (Applied Biosciences, Foster City, CA) and respective primers (Supplemental Data Table 1, Supplemental Digital Content 1, available at: http://links.lww.com/SLA/A31). mRNA was measured semi-quantitatively compared with a standard sample, and then expressed relative to actin.
Western Immunoblotting
Our protocol for SGLT1 immunoblotting has been described previously.27 Proximal jejunal mucosa was thawed in 1 mL triton X lysis buffer containing protease inhibitors, homogenized, and debris removed by centrifugation at 11,000 g for 15 minutes. Proteins (60 μg extract) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and probed with α-SGLT1 or α-GLUT2 antibodies (1:4000). These were developed with horseradish peroxidase-conjugated α-rabbit secondary antibodies together with ECL chemiluminescence detection system (Amersham Biosciences, Buckinghamshire, UK) and exposed to Kodak Scientific Blue Imaging film (Eastman Kodak, Rochester, NY). After stripping (Alpha Diagnostics International, San Antonio, TX), membranes were blotted for actin (1:500) as a loading control. Images were scanned and semi-quantitative densitometry performed using Image J (NIH, Bethseda, MD).
Data Analysis and Statistics
Data were analyzed using GraphPad Prism V5 statistical software (GraphPad Software Inc, La Jolla, CA). Kolmogorov-Smirnov tests confirmed that all data were normally distributed except for taste receptor quantitative polymerase chain reaction (qPCR) results (for which numbers were too small for assessment). Therefore, data analysis used 2-tailed t tests for planned comparison of data pairs, or post hoc analysis of variance (ANOVA) when comparing multiple agonists to a single control. Nonparametric analysis of qPCR data was performed using Kruskal-Wallis ANOVA.
RESULTS
SGLT1 is Rapidly Up-Regulated in Response to Small Bowel Glucose Delivery
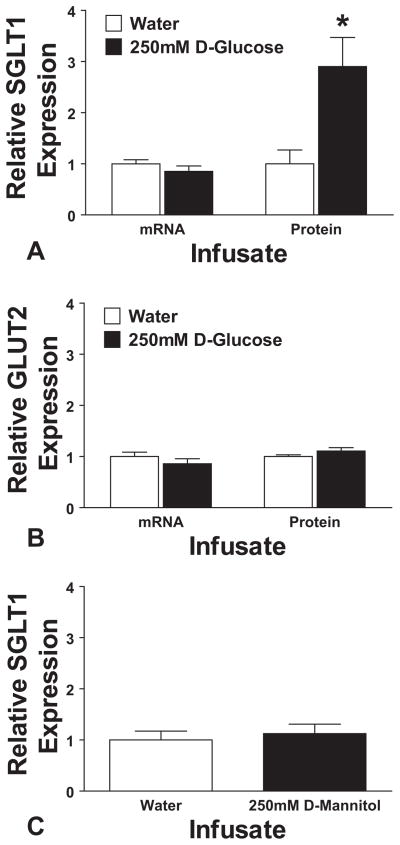
Infusion of the intestine with D-glucose for 3 hours during normal fasting periods led to a rapid up-regulation of total cellular SGLT1 (2.9 ± 0.6-fold increase, P = 0.013) compared with infusion of water alone (Fig. 1A). This was mediated by post-transcriptional mechanisms as there was no observed change in mRNA signal (0.85 ± 0.1-fold change, P = 0.29, Fig. 1A). This response was specific to SGLT1, with no measured change in the levels of facilitated glucose transporter GLUT2 mRNA or total cell protein (P ≥ 0.17, Fig. 1B). To confirm the specificity for glucose and to rule out an osmotic effect, we tested infusion of iso-osmotic D-mannitol (250 mM). Mannitol infusion yielded an SGLT1 protein signal indistinguishable from water infusion (P = 0.63, Fig. 1C).
FIGURE 1.
Infusion with 250 mM D-glucose leads to a 2.9-fold up-regulation in jejunal SGLT1 protein expression (A, *P < 0.05 compared with water infusion), but no change in SGLT1 mRNA. Expression is shown relative to water infusion. These effects are specific to SGLT1, with no effect when looking at GLUT2, either at transcriptional level or on Western blotting (B). Similarly, no effect is seen when infusing 250 mM D-mannitol compared with water: protein densitometry results are shown in (C).
Characteristics of Nutrient Sensor
The whole bowel infusion studies demonstrated the intestinal ability to detect luminal glucose. To characterize this nutrient sensor, we tested other hexoses and glucose analogs. Infusion of 250 mM D-fructose led to an up-regulation of SGLT1 protein similar to D-glucose, with a 2.3 ± 0.2-fold increase in SGLT1 expression after 3-hour (Fig. 2: P = 0.0035 vs. water; P = 0.42 vs. D-glucose). This result suggests that the sensor is not a member of the SGLT family, as these proteins have a very low affinity for D-fructose. To test the involvement of the T1R2/T1R3 sweet taste receptors,28 we infused saccharin. Saccharin binds to and activates T1R2/T1R3, but is neither absorbed nor metabolized. Infusion of 0.3% saccharin induced a 2.2 ± 0.2-fold increase in SGLT1 whole cell protein expression (Fig. 2: P = 0.003 vs. water; P = 0.29 vs. D-glucose). This result suggested that the nutrient sensor resides on the luminal face of the bowel, and does not require intracellular uptake of luminal nutrients nor metabolism. Importantly, this finding implicates the T1R2/T1R3 sweet taste receptors.
FIGURE 2.
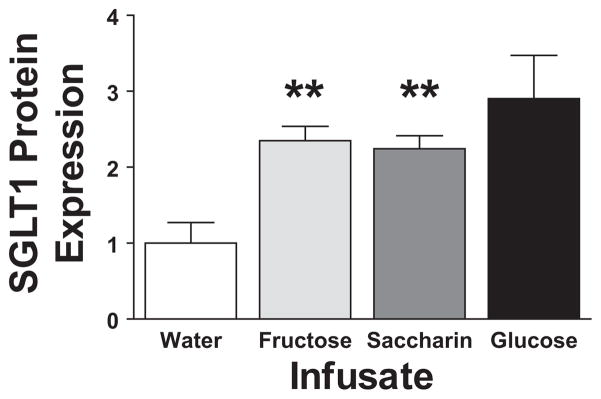
SGLT1 Western blot results are shown for whole bowel infusions of 250 mM D-fructose and 0.3% saccharin compared with infusions with water. Results for each show relative SGLT1 expression (as measured by densitometry) compared with water infusion (**P < 0.01 compared with water). Results from infusion of D-glucose are presented for comparison.
Taste Receptors Are Predominantly Located in Proximal Intestine
To demonstrate the presence of the proposed sweet taste receptors in our rodents, we assessed taste receptor expression (T1R2, T1R3, α-gustducin) in the small bowel by qPCR. Intestinal segments were harvested from duodenum through to terminal ileum to measure mRNA levels relative to actin. We detected mRNAs for both taste receptor subunits as well as α-gustducin in all intestinal segments examined, from duodenum to distal ileum (Fig. 3). T1R3 showed a clear regional variation, peaking in proximal jejunum (P = 0.0340, Kruskal-Wallis ANOVA), as did α-gustducin (P = 0.006, peaking in midsmall bowel). T1R2 was also predominantly expressed in the proximal bowel (Fig. 3), although this variation in expression topography did not reach significance (P = 0.081).
Nutrient Sensor Resides Predominantly in the Duodenum and Proximal Jejunum
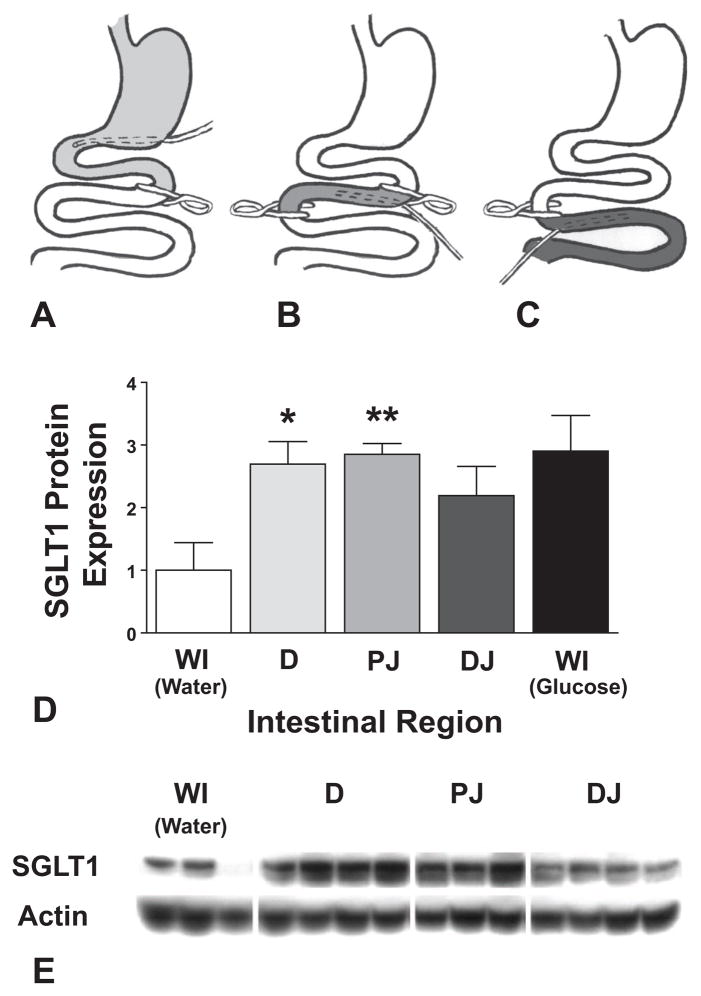
The substrate studies pointed to the involvement of taste receptors in the up-regulation of intestinal SGLT1 and the localization studies revealed that the T1R3 receptor, which is specifically involved in hexose detection, is preferentially expressed in the proximal jejunum. We therefore investigated the regional distribution of responsiveness to taste receptor stimulation to further emphasize involvement of taste receptors in SGLT1 up-regulation. Selective infusions of intestinal regions were performed as in Figures 4A to C. Infusion of the duodenum (first through to the fourth part) alone with 250 mM D-glucose almost entirely replicated infusion of the whole bowel, with a 2.7 ± 0.4-fold up-regulation in SGLT1 expression (P = 0.012 compared with whole intestinal infusion of water, P = 0.76 compared with whole intestinal infusion of glucose). Infusion of proximal jejunum gave similar results, with 2.9 ± 0.2-fold up-regulation of SGLT1 (P = 0.0029 vs. water, P = 0.94 vs. glucose). Infusion of the distal small jejunum onwards trended towards an increase in SGLT1 suggesting that glucose sensing is present in these segments too; however, the increase did not reach significance, indicating that the response is more modest in the distal segments (2.2 ± 0.5-fold up-regulation, P = 0.093 vs. water, P = 0.35 vs. glucose). These results are displayed graphically in Figures 4D, E.
FIGURE 4.
A–C, are schematic representations of isolated regional infusions. In (A), a clip placed at the ligament of Trietz, and a drainage catheter in situ, allow infusion of just the stomach and duodenum (labeled D in [Fig. D, E]). In (B), a clip is placed at both ligament of Trietz and at 15 cm distal to this, infusing only proximal jejunum (PJ). Lastly, in (C), infusion is restricted to distal jejunum and ileum (DJ). D, shows densitometry results for Western blots of protein extracts taken from proximal jejunum in each case, compared with whole bowel water infusion. Whole bowel glucose infusion is also shown for comparison. E, shows a representative SGLT1 immunoblot from proximal jejunal protein extracts obtained after regional infusion. WI, Whole intestine; D, Duodenum; PJ, proximal jejunum; DJ, Distal jejunum. *P < 0.05 compared with water; **P < 0.01 compared with water.
Nutrient Sensing is Mediated by Vagal Afferents
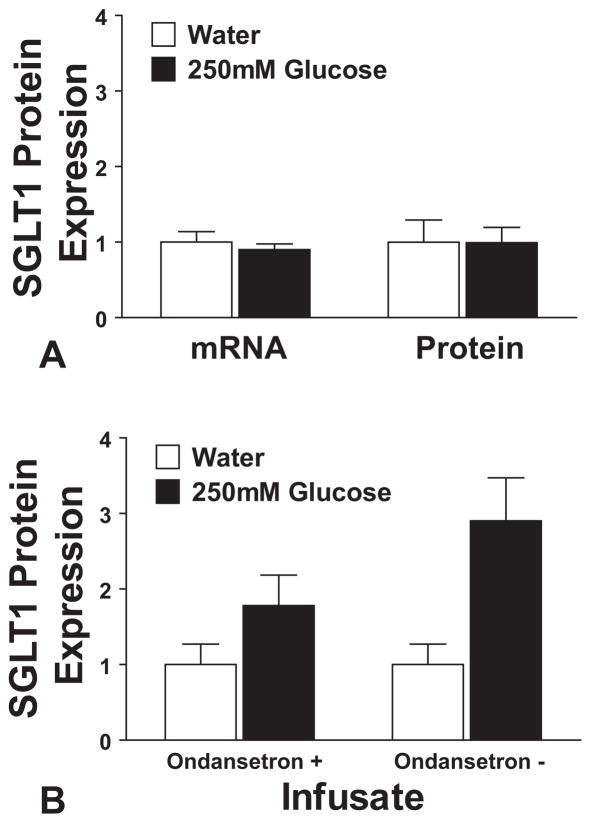
De-afferentation of the vagus entirely blocked the response to D-glucose infusion (1.0 ± 0.2-fold change, P = 0.98), as shown in Figure 5A. To see if the response was mediated through vagal afferents directly, or through 5-HT receptors identified on vagal afferents, standard infusion experiments were repeated in normal rats, after pretreatment with ondansetron to block 5HT3-mediated signaling. The expected effect was blunted by pretreatment with ondansetron, with D-glucose infusion in the presence of ondansetron inducing a nonsignificant 1.8± 0.4-fold increase in SGLT1 (P = 0.14) compared with water infusion under the same conditions. These results are shown in Figure 5B.
FIGURE 5.
A, shows the results of intestinal D-glucose infusion on jejunal SGLT1 expression, compared with water, in animals pretreated with capsaicin to de-afferent the vagus nerve. There is no significant difference in SGLT1 expression, either in terms of mRNA or lysate protein. B, examines the effects of pretreatment with ondansetron. The figure shows SGLT1 protein expression after infusion with D-glucose or water, in animals injected 30 minutes previously with ondansetron (1 mg/kg intraperitoneally; labeled “Ondansetron +”). This shows a blunted response compared with previous experiments without ondansetron pretreatment (labeled “Ondansetron −”; see Fig. 1A), reproduced alongside for comparison.
DISCUSSION
We have shown that intraluminal glucose, at physiological brush-border concentrations, is capable of inducing a rapid up-regulation (<3 hours) in the expression of its own intestinal transporter. This effect is replicated by fructose, which does not bind to SGLTs, suggesting SGLT1 is not acting as a transceptor. Furthermore, the effect is replicated by saccharin; given that saccharin binds to T1R2/T1R3,29 but is neither absorbed nor metabolized, this implicates these receptors in the activation pathway. The inability of iso-osmolar mannitol to reproduce this effect indicates that it is specific and not dependent on osmolality or luminal distension. Furthermore, vagal de-afferentation abolished, and ondansetron blunted, the reflex response, again suggesting that neither distension nor osmolality are inducing the effects. Finally, 0.3% saccharin is only 17 mM, markedly hypotonic compared with 250 mM D-glucose, and approaching the osmolality of the control water infusion, further ruling out osmolality as the impetus. The concentrations of glucose used may seem high, but the confinement of hydrolases to the brush-border generates very high local monosaccharide concentrations in the unstirred layer bathing SGLT1. Indirect measurements of the maltose hydrolysis rate suggest the brush border glucose concentration may approach 300 mM,30,31 a concentration used in previous experiments.32 Furthermore, it is worth noting that the infusate was free to mix with gastric and biliopancreatic secretions, which would reduce the effective concentration. Lastly, glucose diffusion into the unstirred layer would further dilute it yielding correspondingly lower glucose concentrations in the microenvironment at the brush-border.
Similar results have been described in ruminant species, using a wide range of sweet taste receptor analogues, including monosaccharides, D-glucose, D-galactose, a-methyl-D-glucose, D-fructose, and 2-deoxy-D-glucose.33–35 Although these are all sweet-taste analogues, not all are substrates for SGLTs nor are all transported or metabolized, suggesting they are acting through broad-spectrum sweet taste receptors rather than metabolic activity. Notably, these studies measured brush-border SGLT1, rather than whole-cell SGLT1 as measured here. As with the present study, the increase in SGLT1 protein was dissociated from changes in SGLT1 mRNA.36 A rapid increase in jejunal water and electrolyte transport in response to a meal has also been reported in nonruminant dogs.37–39 Using substrate-free and inhibitor solutions, this postprandial pro-absorptive state was identified as being due to increases in SGLT1 function.40 Correspondingly, whole cell SGLT1 protein was shown to increase significantly, with no changes in SGLT1 mRNA.
The identity of the glucose sensor or receptor remains less clear. Activation by saccharin suggests that the receptor is located on the luminal membrane and is supported by similar observations in sheep demonstrating activation by glucose covalently bound to nonabsorbable poly(ethylene glycol)600.20 Lingual sensing of the sweetness of saccharin is mediated by sweet-taste T1R2/T1R3 complexes.28,41 The identification of T1R2/T1R3 on discrete enteroendocrine-like cells throughout the small intestine23,42 makes this receptor a likely candidate. This conjecture is further supported by evidence that T1R3 knockout mice fail to up-regulate SGLT1 in response to a chronic high-carbohydrate diet.23 A further role for sweet-taste receptors in nutrient sensing and regulation of glucose transport has been identified in the coordination of apical trafficking of the facilitated glucose transporter GLUT2. In the presence of sweet-taste agonists, or high luminal concentrations of glucose, basolateral GLUT2 appears to be inserted in the apical membrane43 in a process dependent on sweet-taste receptors.44,45 However, these studies did not examine whole-cell SGLT1, and used a shorter time-scale, focusing rather on early brush-border measurements. It is further worth noting these authors have shown that the sweet taste receptor is expressed in Paneth cells and absorptive enterocytes, contrary to the majority of work that has indicated the location of the sweet taste receptor to be in the enteroendocrine cells. T1R2/T1R3 sweet-taste receptors may not be the only hexose sensors. Gastric emptying and intestinal fluid secretion are stimulated by SGLT substrates D-glucose and α-methyl-glucose, but not galactose or GLUT2 substrate 2-deoxy-D-glucose, characteristics that have been interpreted as implicating the intestinal glucose sensor SGLT3.46 It is not know whether these pathways are linked to the SGLT1 induction pathway. However, they do appear to be separate in sheep because neither 2-deoxy-D-glucose nor D-galactose inhibit gastric emptying, but do up-regulate SGLT1, in the ovine intestine.33–35,46 Furthermore, SGLT3 appears to be restricted to muscle and the myenteric plexus and thus unlikely to be responsible for luminal glucose sensing.
Although an increase in intestinal SGLT1 expression was noted even after infusions of distal intestine, our study showed that the sensitivity to glucose infusion was highest in the duodenum and proximal small intestine (first 15%). Nevertheless, SGLT1 increases were observed even when the region sampled for SGLT1 expression analyses was not actually infused. For example, when the duodenum was infused with glucose, SGLT1 expression was increased in the proximal jejunum even though this was not exposed. This suggests that sensors are located throughout the small bowel and may involve some form of humoral or neural reflex arc to signal the target tissue. Similarly, SGLT1 expression and function increases postprandially in Thiry-Vella loops in awake dogs, despite these being isolated from enteric continuity.40 The expression of sweet-taste receptor T1R3 and α-gustducin mRNA broadly matches the infusion data, where infusion of regions with high T1R3 expression resulted in a robust SGLT response. In contrast, infusion of regions with lower T1R3 expression produced a muted response. The duodenal data needs further explanation, as despite low taste receptor expression in the proximal half of the duodenum, infusion of the whole duodenum generated a robust up-regulation. However, as there is minimal or no taste receptor expression in the stomach,22 and high expression in the proximal jejunum, it may be that duodenal taste receptor expression is predominantly distally in the duodenum, in the third and fourth parts. Indeed, other studies broadly agree with this proposition, with high levels of duodenal taste-receptor expression identified when mucosa from whole of duodenal length is compared with that seen in the proximal jejunum.21,22
These results, demonstrating acute intestinal glucose sensing, shows that glucose sensing and regulation of intestinal glucose transport predominantly arises in the proximal small bowel. These observations are of particular relevance to the physiology underlying RYGB, and provide further support that isolation of the duodenum and proximal intestine is important in the efficacy of RYGB.47 As this region is responsible for the greatest nutrient sensing response, isolation of this region from enteric contents (and therefore sweet-taste sensing), may suppress SGLT1 expression and consequently improve oral glucose handling.
We have also demonstrated that vagal de-afferentation disrupts the normal signaling pathway. This again suggests that the underlying process is a coordinated reflex rather than a local response to a nonspecific stimulus such as osmolality or distension. In particular, experiments with Thiry-Vella loops in dogs demonstrated that topical local anesthesia delivered together with a test meal via a mid jejunal feeding jejunostomy could ablate postprandial pro-absorptive states in the isolated loop,48 suggesting the importance of afferent innervation. In contrast, if the animal was permitted to eat an oral meal together with midjejunal infusion of bupivicaine, the normal postprandial response was observed.49 Taken together, these support the presence of a proximal intestinal glucose sensing response mediated by the T1R2/T1R3 sweet taste receptor and dependent on afferent neural pathways. The identity of the efferent limb of the reflex, and specifically the role of the vagal motor fibers and the enteric nervous system, remains unclear. Again, in canine models, atropine and opioid-receptor antagonists prevent postprandial increases in absorption,39,50,51 implicating the efferent vagus and enteric nervous system respectively. Although concerns about the possibility that capsaicin had unanticipated effects on either efferent vagal fibers or on intrinsic neural pathways may be expressed, we have previously validated this technique and shown vagal efferent fibers persist after our topical capsaicin treatment.25 Although capsaicin was directly applied to the vagal trunks, it may have diffused into the peritoneal cavity and thus disrupted the intrinsic neural network. This was minimized by thorough lavage of the peritoneum after de-afferentation.
Lastly, we have shown that ondansetron blunts the normal increase in SGLT1 after glucose infusion, suggesting that 5-HT3-receptor mediated signaling is involved in the glucose-sensing pathway. Previous work has demonstrated that 5-HT3-receptor signaling mediates glucose-induced inhibition of gastric emptying, and is inhibitable by ondansetron.52,53 Recent work has also identified a large subpopulation of α-gustducin-expressing enteroendocrine cells throughout the small intestine that colocalize with 5-HT.54 Given vagal afferents also express 5-HT3-receptors,52 this supports the concept that taste-receptors may indirectly stimulate vagal afferent firing.
Supplementary Material
Acknowledgments
Supported by National Institute of Health 5 R01 DK047326 (to A.T.), Harvard Clinical Nutrition Center Grant P30-DK040561; American Diabetes Association 7–05-RA-121 (to D.B.R.), Berkeley Fellowship, George Herbert Hunt Travelling Fellowship (to A.T.S.), and Nutricia Foundation Fellowship (to A.B.).
The authors thank Jan Rounds, BS, for invaluable managerial support.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
Dr. Stanley W. Ashley, MD, Professor of Surgery at Brigham and Women’s Hospital, Boston, provided supervisory support and training for A.T.S. and A.B., as well as critical review of the manuscript.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596– 615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Runge CF. Economic consequences of the obese. Diabetes. 2007;56:2668–2672. doi: 10.2337/db07-0633. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Morton AP, Hanson PJ. Monosaccharide transport by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1984;69:117–126. doi: 10.1113/expphysiol.1984.sp002772. [DOI] [PubMed] [Google Scholar]
- 5.Bihler I, Freund N. Sugar transport in the small intestine of obese hyperglycemic, fed and fasted mice. Diabetologia. 1975;11:387–393. doi: 10.1007/BF00429905. [DOI] [PubMed] [Google Scholar]
- 6.Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr. 1995;62:540–546. doi: 10.1093/ajcn/62.3.540. [DOI] [PubMed] [Google Scholar]
- 7.Dyer J, Garner A, Wood IS, et al. Changes in the levels of intestinal Na+/glucose co-transporter (SGLT1) in experimental diabetes. Biochem Soc Trans. 1997;25:479S. doi: 10.1042/bst025479s. [DOI] [PubMed] [Google Scholar]
- 8.Burant CF, Flink S, DePaoli AM, et al. Small intestine hexose transport in experimental diabetes. Increased transporter mRNA and protein expression in enterocytes. J Clin Invest. 1994;93:578–585. doi: 10.1172/JCI117010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedorak RN, Cheeseman CI, Thomson AB, et al. Altered glucose carrier expression: mechanism of intestinal adaptation during streptozocin-induced diabetes in rats. Am J Physiol. 1991;261(4 pt 1):G585–G591. doi: 10.1152/ajpgi.1991.261.4.G585. [DOI] [PubMed] [Google Scholar]
- 10.Dyer J, Wood IS, Palejwala A, et al. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 11.Osswald C, Baumgarten K, Stumpel F, et al. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-glucose cotransport in small intestine and develop obesity. Mol Cell Biol. 2005;25:78– 87. doi: 10.1128/MCB.25.1.78-87.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston KL, Clifford MN, Morgan LM. Possible role for apple juice phenolic, compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J Sci Food Agric. 2002;82:1800–1805. [Google Scholar]
- 13.Rossetti L, Smith D, Shulman GI, et al. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn BB, Shulman GI, Defronzo RA, et al. Normalization of blood-glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose-transport in adipose-cells without restoring glucose transporter gene-expression. J Clin Invest. 1991;87:561–570. doi: 10.1172/JCI115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solberg DH, Diamond JM. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 1987;252(4 pt 1):G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- 16.Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev. 1997;77:257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- 17.Ferraris RP, Diamond J. Crypt-villus site of glucose transporter induction by dietary carbohydrate in mouse intestine. Am J Physiol. 1992;262(6 pt 1):G1069–G1073. doi: 10.1152/ajpgi.1992.262.6.G1069. [DOI] [PubMed] [Google Scholar]
- 18.Karasov WH, Pond RS, III, Solberg DH, et al. Regulation of proline and glucose transport in mouse intestine by dietary substrate levels. Proc Natl Acad Sci U S A. 1983;80:7674–7677. doi: 10.1073/pnas.80.24.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton JD. Carbohydrate digestion and glucose supply in the gut of the ruminant. Proc Nutr Soc. 1971;30:243–248. doi: 10.1079/pns19710047. [DOI] [PubMed] [Google Scholar]
- 20.Dyer J, Vayro S, King TP, et al. Glucose sensing in the intestinal epithelium. Eur J Biochem. 2003;270:3377–3388. doi: 10.1046/j.1432-1033.2003.03721.x. [DOI] [PubMed] [Google Scholar]
- 21.Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346. doi: 10.1136/gut.2008.148932. [DOI] [PubMed] [Google Scholar]
- 22.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41– 49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 23.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates SL, Sharkey KA, Meddings JB. Vagal involvement in dietary regulation of nutrient transport. Am J Physiol. 1998;274(3 pt 1):G552–G560. doi: 10.1152/ajpgi.1998.274.3.G552. [DOI] [PubMed] [Google Scholar]
- 25.Stearns AT, Balakrishnan A, Rounds J, et al. Capsaicin-sensitive vagal afferents modulate posttranscriptional regulation of the rat Na+/glucose cotransporter SGLT1. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1078–G1083. doi: 10.1152/ajpgi.00591.2007. [DOI] [PubMed] [Google Scholar]
- 26.Stearns AT, Balakrishnan A, Rhoads DB, et al. Diurnal rhythmicity in the transcription of jejunal drug transporters. J Pharmacol Sci. 2008;108:144–148. doi: 10.1254/jphs.08100sc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stearns AT, Balakrishnan A, Rhoads DB, et al. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery. 2009;145:294–302. doi: 10.1016/j.surg.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson G, Hoon MA, Chandrashekar J, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 29.Chen XM, O’Hara SP, Huang BQ, et al. Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc Natl Acad Sci U S A. 2005;102:6338–6343. doi: 10.1073/pnas.0408563102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531(pt 3):585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pappenheimer JR. Scaling of dimensions of small intestines in non-ruminant eutherian mammals and its significance for absorptive mechanisms. Comp Biochem Physiol A Mol Integr Physiol. 1998;121:45–58. doi: 10.1016/s1095-6433(98)10100-9. [DOI] [PubMed] [Google Scholar]
- 32.Lane JS, Whang EE, Rigberg DA, et al. Paracellular glucose transport plays a minor role in the unanesthetized dog. Am J Physiol. 1999;276(3 pt 1):G789–G794. doi: 10.1152/ajpgi.1999.276.3.G789. [DOI] [PubMed] [Google Scholar]
- 33.Shirazi-Beechey SP. Intestinal sodium-dependent D-glucose co-transporter: dietary regulation. Proc Nutr Soc. 1996;55(1B):167–178. doi: 10.1079/pns19960018. [DOI] [PubMed] [Google Scholar]
- 34.Shirazi-Beechey SP, Hirayama BA, Wang Y, et al. Ontogenic development of lamb intestinal sodium-glucose co-transporter is regulated by diet. J Physiol. 1991;437:699–708. doi: 10.1113/jphysiol.1991.sp018620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyer J, Barker PJ, Shirazi-Beechey SP. Nutrient regulation of the intestinal Na+/glucose co-transporter (SGLT1) gene expression. Biochem Biophys Res Commun. 1997;230:624– 629. doi: 10.1006/bbrc.1996.6018. [DOI] [PubMed] [Google Scholar]
- 36.Lescale-Matys L, Dyer J, Scott D, et al. Regulation of the ovine intestinal Na+/glucose co-transporter (SGLT1) is dissociated from mRNA abundance. Biochem J. 1993;291(pt 2):435–440. doi: 10.1042/bj2910435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastidas JA, Orandle MS, Zinner MJ, et al. Small-bowel origin of the signal for meal-induced jejunal absorption. Surgery. 1990;108:376–383. [PubMed] [Google Scholar]
- 38.Sarr MG, Kelly KA, Phillips SF. Canine jejunal absorption and transit during interdigestive and digestive motor states. Am J Physiol. 1980;239:G167–G172. doi: 10.1152/ajpgi.1980.239.3.G167. [DOI] [PubMed] [Google Scholar]
- 39.Yeo CJ, Bastidas JA, Schmieg RE, Jr, et al. Meal-stimulated absorption of water and electrolytes in canine jejunum. Am J Physiol. 1990;259(3 pt 1):G402–G409. doi: 10.1152/ajpgi.1990.259.3.G402. [DOI] [PubMed] [Google Scholar]
- 40.Hines OJ, Whang EE, Bilchik AJ, et al. Role of Na+-glucose cotransport in jejunal meal-induced absorption. Dig Dis Sci. 2000;45:1–6. doi: 10.1023/a:1005477204960. [DOI] [PubMed] [Google Scholar]
- 41.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350(pt 1):155–162. [PMC free article] [PubMed] [Google Scholar]
- 44.Mace OJ, Affleck J, Patel N, et al. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mace OJ, Lister N, Morgan E, et al. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. 2009;587(pt 1):195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman SL, Bohan D, Darcel N, et al. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291:G439–G445. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- 47.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anthone GJ, Wang BH, Zinner MJ, et al. Meal-induced jejunal absorption requires intact neural pathways. Am J Surg. 1992;163:150–156. doi: 10.1016/0002-9610(92)90268-v. [DOI] [PubMed] [Google Scholar]
- 49.Anthone GJ, Bastidas JA, Zinner MJ, et al. Meal-stimulated canine jejunal ionic absorption. Influence of mucosal neural blockade. Dig Dis Sci. 1994;39:75–82. doi: 10.1007/BF02090064. [DOI] [PubMed] [Google Scholar]
- 50.Bastidas JA, Yeo CJ, Schmieg RE, Jr, et al. Endogenous opiates in the mediation of early meal-induced jejunal absorption of water and electrolytes. Am J Surg. 1989;157:27–32. doi: 10.1016/0002-9610(89)90415-7. [DOI] [PubMed] [Google Scholar]
- 51.McFadden DW, Jaffe BM, Ferrara A, et al. Jejunal absorptive response to a test meal and its modification by cholinergic and calcium-channel blockade in the awake dog. Surg Forum. 1984;35:174–176. [Google Scholar]
- 52.Raybould HE, Glatzle J, Robin C, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 53.Sharp JW, Dulake M, Vincent KM, et al. Intestinal glucose-induced phosphorylation of calcium-calmodulin kinase if (CaMKII) in 5-HT-secreting enterochromaffin cells, myenteric neurons and vagal afferents in the rat. Gastroenterology. 2008;134:A395. [Google Scholar]
- 54.Sutherland K, Young RL, Cooper NJ, et al. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–G1428. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.