To the Editor:
Routine blood cultures for all patients hospitalized with community-acquired pneumonia have limited utility, and false-positive results lead to inappropriate antimicrobial use and longer hospital stays.1 As a result, performance measures and practice guidelines that promoted obtaining blood cultures in all such patients were modified in 2005–2007 to recommend routine collection in only the sickest patients.1, 2 Using a national sample of emergency department (ED) visits, we examined patterns of obtaining cultures in adults hospitalized with community-acquired pneumonia.
METHODS
We analyzed data from the 2002–2010 National Hospital Ambulatory Medical Care Surveys (NHAMCS), a probability sample of ED visits in the US.3 Years 2005–2006 are omitted because NHAMCS did not collect blood culture use during this period.
We included all visits by patients 18 years or older with community-acquired pneumonia who were subsequently hospitalized. Community-acquired pneumonia was defined by having an ICD-9 code of 481–486. Blood culture collection during the visit was recorded as a checkbox on the NHAMCS data collection form. As a control group we examined the trend in collecting cultures in patients hospitalized for a urinary tract infection (UTI; ICD-9 codes 595.00, 599.00), a diagnosis with no change in recommendations during the study period.
Analyses accounted for the complex survey design to reflect national estimates. Trends in culture use were evaluated using linear regression. We used logistic regression to evaluate predictors of culture use after recommendation revisions, using combined data from years 2007–2010. This study was exempt from review by our institutional review boards.
RESULTS
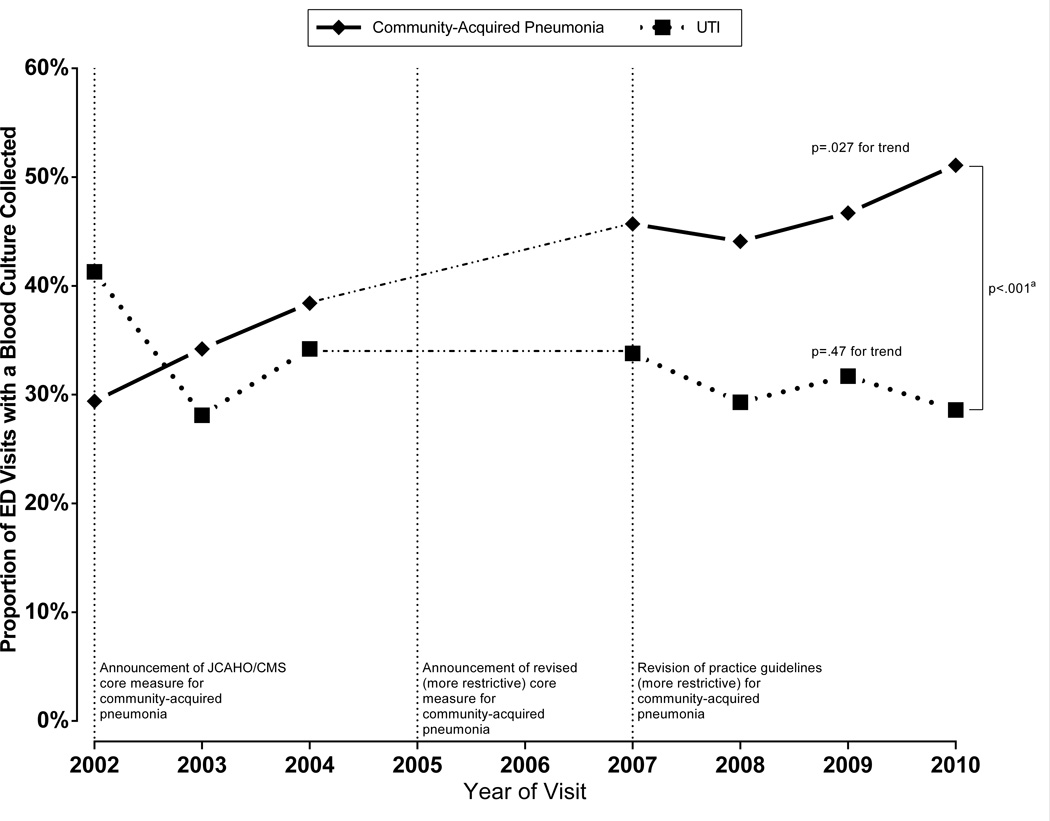
This study included 1,487 visits, representing 5.1 million visits by adult patients hospitalized with community-acquired pneumonia (more information in supplement). The proportion of cultures collected in patients hospitalized with community-acquired pneumonia increased from 29% (95% CI, 22%–38%) in 2002 to 51% (95% CI,42%–60%) in 2010 (p=.027 for trend), a 76% relative increase (Figure). In contrast, culture rates for UTI remained stable (p=.47), with a substantial difference in culture use between the two conditions over time (difference of 3.2% per year, 95% CI, 1.6%–4.8%).
Figure. Trends in Collecting Blood Cultures During ED Visits by Patients Subsequently Hospitalized by Condition for Years, 2002–2010.
Abbreviations: ED, emergency department; UTI, urinary tract infection; JCAHO, Joint Commission on Accreditation of Healthcare Organizations; CMS, Centers for Medicaid and Medicare Services
Blood culture collection data was not recorded in the 2005 and 2006 surveys. In 2002, JCAHO/CMS announced a core measure for routine blood culture collection in the ED for all patients hospitalized with community-acquired pneumonia to benchmark the quality of care. This was subsequently revised in 2005 to focus only on ICU admissions. Practice guidelines for the management of pneumonia were revised at the beginning of 2007 to recommend routine blood cultures for only patients with severe community-acquired pneumonia.
aDifference in the trend lines was evaluated by testing the interaction term of year and condition in a regression model using the collection of a blood culture as the outcome variable. The difference in the rate of change of culture use between community-acquired pneumonia and UTI was 3.2% per year (95% CI, 1.6%–4.8%; p<.001).
In multivariable analysis (Table), disease severity did not predict culture collection and admission to the ICU was associated with a lower odds of obtaining cultures. Several non-clinical factors were strong predictors, including hospital ownership and region.
Table.
Predictors of Blood Culture Collection in the Emergency Department for Patients Hospitalized with Community-Acquired Pneumonia from 2007–2010
| Weighted % of visits with blood culture Unweighted N=792 |
Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| Demographics | |||
| Age, per 10 years | - | 0.95 (0.86–1.05) | 0.90 (0.77–1.05) |
| Sex | |||
| Male | 43 | 1 [Ref] | 1 [Ref] |
| Female | 51 | 1.41 (1.06–1.86) | 1.42 (1.01–2.00) |
| Race/ethnicity | |||
| White | 49 | 1 [Ref] | 1 [Ref] |
| Black | 39 | 0.66 (0.36–1.20) | 0.57 (0.28–1.14) |
| Other | 46 | 0.87 (0.52–1.48) | 1.09 (0.59–2.01) |
| Primary Payer | |||
| Commercial | 46 | 1 [Ref] | 1 [Ref] |
| Medicare | 47 | 1.04 (068–1.59) | 1.27 (0.77–2.11) |
| Medicaid | 36 | 0.68 (0.36–1.28) | 0.77 (0.37–1.59) |
| Other/Unknown | 60 | 1.81 (0.98–3.36) | 1.97 (1.05–3.69) |
| Clinical Characteristics | |||
| CRB-65b | |||
| 0 | 46 | 1 [Ref] | 1 [Ref] |
| 1 | 50 | 1.16 (0.76–1.78) | 1.22 (0.67–2.22) |
| 2–4 | 44 | 0.91 (0.50–1.67) | 0.98 (0.42–2.26) |
| Disposition Status | |||
| Non-ICU | 49 | 1 [Ref] | 1 [Ref] |
| ICU | 36 | 0.58 (0.35–0.96) | 0.53 (0.29–0.98) |
| Fever (≥ 100.4°F) | |||
| No | 46 | 1 [Ref] | 1 [Ref] |
| Yes | 50 | 1.15 (0.75–1.76) | 1.03 (0.64–1.65) |
| Hypoxia (< 90%) | |||
| No | 48 | 1 [Ref] | 1 [Ref] |
| Yes | 42 | 0.78 (0.50–1.24) | 0.83 (0.49–1.41) |
| Visit Characteristics | |||
| Triage status | |||
| Non-emergent | 45 | 1 [Ref] | 1 [Ref] |
| Emergent | 52 | 1.35 (0.92–1.99) | 1.41 (0.91–2.19) |
| Administered antibiotics in ED | |||
| No | 22 | 1 [Ref] | 1 [Ref] |
| Yes | 54 | 4.18 (2.57–6.80) | 3.30 (1.99–5.48) |
| Primary diagnosis is pneumonia | |||
| No | 35 | 1 [Ref] | 1 [Ref] |
| Yes | 53 | 2.09 (1.27–3.43) | 2.36 (1.46–3.80) |
| Number of tests/servicesc | |||
| 0–5 | 30 | 1 [Ref] | 1 [Ref] |
| 6–10 | 51 | 2.43 (1.51–3.93) | 2.04 (1.21–3.46) |
| > 10 | 62 | 3.83 (1.89–7.76) | 4.34 (2.00–9.43) |
| Year of visit | – | 1.09 (0.92–1.28) | 1.14 (0.94–1.38) |
| ED Characteristics | |||
| Region | |||
| West | 33 | 1 [Ref] | 1 [Ref] |
| Midwest | 53 | 2.11 (1.12–3.95) | 2.60 (1.35–5.00) |
| South | 49 | 2.26 (1.25–4.08) | 1.86 (0.98–3.51) |
| Northeast | 51 | 1.92 (1.15–3.49) | 2.90 (1.55–5.41) |
| Hospital Owner | |||
| Nonprofit | 46 | 1 [Ref] | 1 [Ref] |
| Government | 44 | 0.94 (0.48–1.81) | 1.14 (0.54–2.43) |
| Proprietary | 62 | 1.96 (1.05–3.65) | 2.92 (1.15–7.40) |
Abbreviations: ICU, intensive care unit; ED, emergency department
Weighted multivariate logistic regression model adjusted for all covariates listed above, accounting for complex survey design.
CRB-65 is a validated clinical prediction index that grades the severity of community-acquired pneumonia by 30-day mortality using four criteria: “C”onfusion, “R”espiratory rate ≥ 30/minute, systolic “B”lood pressure < 90 mmHg or diastolic “B”lood pressure < 60 mmHg, and age ≥ “65” years of age.6 Higher scores equate to greater risk of mortality. Confusion was defined by having one the following criteria: Glasgow Coma Scale < 15 (for years 2009–2010), not oriented to person, place, or time (for years 2007–2008), patient’s reason for visit coded as confusion, cognitive decline, change in mental status, disoriented, or altered level of consciousness, or a visit diagnosis was for alteration of consciousness (ICD-9 code 780.0) or altered mental status (ICD-9 code 780.97).
Tests or services included blood tests (i.e. electrolytes, liver function tests), imaging studies (i.e. radiography, ultrasound, computed tomography), and miscellaneous tests (i.e. urine studies, electrocardiogram). Panel blood tests, such as electrolytes, were counted as a single test. To avoid endogeneity of our predictor-outcome relationship, blood cultures were not included in the number of tests or services.
COMMENT
In this national study, we found that the collection of blood cultures in patients hospitalized with community-acquired pneumonia continued to increase despite recommendations for a more narrow set of indications. Furthermore, non-clinical factors were powerful predictors of blood culture use rather than disease severity and ICU admission status.
One potential explanation for increasing culture rates is that the JCAHO/CMS core measure (PN-3b) announced in 2002 mandated that if a culture is collected in the ED, it should be collected prior to antibiotic administration. This measure may encourage providers to reflexively order cultures in all patients admitted with community-acquired pneumonia in whom antibiotic administration is anticipated, even though cultures are strongly indicated in only the sickest patients. Given rising trends in obtaining cultures in low-risk patients, we advocate for JCAHO and CMS to reexamine this measure with consideration of eliminating it entirely to discourage overuse.
One limitation of our study was the omission of 2005–2006 data, prohibiting an evaluation of whether culture rates slowed down after revisions in recommendations. Also, there may be misclassification of culture use, but this would likely be non-differential and bias our findings for ICU status towards the null.
The appropriate use of cultures could reduce potential harms from inappropriate antibiotic use and longer hospital stays,4 and reduce the summative cost of the test itself.5 Further attention is warranted to the judicious use of blood cultures in the management of pneumonia.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge DB Grinsfelder for his assistance in creating the figure. Dr. Makam’s work on this project was completed while he was a Primary Care Research Fellow at the University of California San Francisco, funded by an NRSA training grant (T32HP19025-07-00).
Footnotes
We have no conflicts of interest to disclose.
Dr. Makam had full access to the data in the study and takes responsibility for the integrity of the date and accuracy of the data analysis. Study concept and design: Makam, Auerbach, Steinman. Acquisition of Data: Makam. Analysis and Interpretation of Data: Makam, Auerbach, Steinman. Drafting of the manuscript: Makam.Critical revision of the manuscript: Makam, Auerbach, Steinman. Statistical Analysis: Makam.
REFERENCES
- 1.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walls RM, Resnick J. The Joint Commission on Accreditation of Healthcare Organizations and Center for Medicare and Medicaid Services community-acquired pneumonia initiative: what went wrong? Ann Emerg Med. 2005;46(5):409–411. doi: 10.1016/j.annemergmed.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. NHAMCS scope and sample design. [Accessed May 27, 2013]; http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#nhamcs_scope.
- 4.Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365–369. [PubMed] [Google Scholar]
- 6.McNally M, Curtain J, O'Brien KK, Dimitrov BD, Fahey T. Validity of British Thoracic Society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: systematic review and meta-analysis. Br J Gen Pract. 2010;60(579):e423–e433. doi: 10.3399/bjgp10X532422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.