Abstract
Species with restricted distributions make up the vast majority of biodiversity. Recent evidence suggests that Drosophila species with restricted tropical distributions lack genetic variation in the key trait of desiccation resistance. It has therefore been predicted that tropically restricted species will be limited in their evolutionary response to future climatic changes and will face higher risks of extinction. However, these assessments have been made using extreme levels of desiccation stress (less than 10% relative humidity (RH)) that extend well beyond the changes projected for the wet tropics under climate change scenarios over the next 30 years. Here, we show that significant evolutionary responses to less extreme (35% RH) but more ecologically realistic levels of climatic change and desiccation stress are in fact possible in two species of rainforest restricted Drosophila. Evolution may indeed be an important means by which sensitive rainforest-restricted species are able to mitigate the effects of climate change.
Keywords: adaptation, desiccation, evolutionary potential, additive genetic variation, heritability, selection
1. Introduction
Global surface temperatures have risen by 0.2°C per decade over the past 30 years [1] and are predicted to rise a further 1.4–5.8°C before the turn of the next century [2]. Climate projections for one of the world's most biodiverse regions, the wet tropics of Australia, indicate average temperatures may increase by 0.5–1.4°C in 2030 and by 1–4.2°C in 2070 under low- and high-emission scenarios, respectively (Commonwealth Scientific and Industrial Research Organization (CSIRO), http://www.climatechangeinaustralia.gov.au). Rainfall is predicted to become more seasonal in the wet tropics, with a wetter wet season and a longer, drier dry season [3,4]. While there is more uncertainty surrounding projections for relative humidity (RH) compared to temperature in this region, it is predicted that RH may decrease by 0.5–1% in 2030 and by 2–4% in 2070 under low- and high-emissions scenarios, respectively [2] (CSIRO, http://www.climatechangeinaustralia.gov.au). Predicted rises in the average basal altitude of the orographic cloud layer [5,6], which will reduce cloud-moisture capture, are likely to exacerbate the effects of longer and drier dry seasons [6]. Species restricted to the tropics, particularly rainforests, have low levels of desiccation resistance, reflecting the continuous high humidity encountered in those habitats [7]. As conditions become drier under climate change and as fragmentation generates edge effects that alter the microclimate within fragments, desiccation stress experienced within rainforests is likely to increase. However, tropically restricted species are predicted to face a higher risk of extinction under climate change because they are thought to have limited capacity to evolve higher levels of desiccation resistance [7].
Specifically, Drosophila species restricted to the rainforests of the wet tropics of Australia have very low levels of genetic variation for resistance to extreme (less than 10% RH) desiccation stress [7,8] and are unable to respond to selection for increased desiccation tolerance at these humidity levels [8,9]. By contrast, widely distributed Drosophila species have much higher levels of genetic variation for this same trait [7]. These differences suggest that tropically restricted species lack genetic variation in the key trait of desiccation resistance and thus are fundamentally constrained in their ability to mount evolutionary responses to changing environmental conditions. These results are significant because the magnitude of ecological consequences to climate change will depend strongly on the rate of adaptation of species to their changing environment [10,11], which requires additive genetic variation. Kellermann et al. [7] provide the first evidence that some species might lack the genetic variation necessary to mount adaptive responses to climate change [12].
However, these assessments of fundamental evolutionary constraint in rainforest-restricted species considered resistance to extreme (less than 10% RH) levels of desiccation stress that extend well beyond current, and projected, humidity levels in the tropical rainforest habitats of Australia and are more reflective of RH experienced in temperate environments (electronic supplementary material, tables S1 and S2 and figure S2). Thus, it is possible that the results of Kellermann et al. [7] reflect historical constraints between tropical and temperate species that have arisen over long evolutionary timeframes, reflecting the evolutionary consequences of specialization to tropical environments, rather than the potential for tropical rainforest species to respond to levels of desiccation stress immediately beyond their current levels of resistance via contemporary evolution. It is therefore unclear whether tropical species are evolutionary constrained in their responses to less severe, but more ecologically realistic, levels of desiccation stress that are likely to occur under contemporary climatic change [2] (CSIRO, http://www.climatechangeinaustralia.gov.au).
Furthermore, there is an increasing recognition of the fact that additive genetic variance (and thus heritability and adaptive capacity) may change with environmental conditions [13–15]. The environmental dependency of the expression of additive genetic variance may complicate predictions of evolutionary constraint under climate change when based on data collected under laboratory conditions that do not reflect environmental conditions experienced in nature [13–16]. Desiccation resistance to less than 10% RH does not display any significant additive genetic variance at either 19°C [8] or 25°C [7], suggesting that the evolutionary constraint is not dependent on thermal environment. However, whether this holds true under thermal regimes that might be expected under climate change is not known.
We extend on the idea of fundamental evolutionary limits in rainforest-restricted species [7] and suggest that significant adaptive capacity in rainforest-restricted species of Drosophila might be revealed by considering less extreme, more ecologically realistic changes in temperature and humidity (desiccation stress). To do this, we reared two species of rainforest-restricted Drosophila, Drosophila birchii and Drosophila bunnanda, under developmental temperatures predicted for Cairns, a site central to their distribution in the wet tropics of Australia under moderate carbon emissions [2] (CSIRO, http://www.climatechangeinaustralia.gov.au). This translates into a 1°C increase in average temperature by 2030 (electronic supplementary material, figure S1). We then assessed genetic variation for desiccation resistance at severe (less than 10% RH) and a more moderate, but ecologically realistic level of desiccation stress (35% RH) projected for the wet tropics by 2030 (CSIRO, http://www.climatechangeinaustralia.gov.au; electronic supplementary material, figure S2).
2. Material and methods
(a). Field collection and laboratory maintenance
We have previously shown [17] that populations from throughout the range of D. birchii and D. bunnanda show the same pattern of low, non-significant additive genetic variance for resistance to less than 10% RH. We thus focused this study on a single population for each species sampled from a site central to both species' distribution in the wet tropics of Australia. Populations of D. birchii and D. bunnanda were collected near Cairns in Queensland, Australia (latitude 16.522° S) in February 2010. Twenty field inseminated females were collected for each species and used to establish iso-female lines in the laboratory. Two generations after collection, a mass-bred population of each species was founded with 20 males and 20 females from each of the 20 iso-female lines. The mass-bred populations were maintained in discrete generations at 25°C under a 12 L : 12 D cycle in 3 × 250 ml bottles containing 60 ml of potato, yeast and sucrose media. Densities were approximately 300–400 flies per bottle to ensure a census population size of 1000+ individuals.
(b). Experimental conditions
Average temperature for Cairns, a site central to the distribution of both species in the wet tropics of Australia, is predicted to increase by 1°C by 2030 under all carbon emission scenarios ([2]; CSIRO, http://www.climatechangeinaustralia.gov.au). Emissions scenarios are from the Intergovernmental Panel on Climate Change Special Report on Emission Scenarios. Low emission is the B1 scenario (equivalent to RCP4.5), medium is A1B (equivalent to RCP6.0) and high is A1FI (equivalent to RCP8.5) [2,18,19]. These projections are given relative to the period 1980–1999, and give an estimate of the average climate around 2030, 2050 and 2070, taking into account consistency among climate models. Individual years will show variation from this average. The 50th percentile (the mid-point of the spread of model results) provides a best estimate result. We were interested in understanding the extent to which the additive genetic variance for desiccation resistance might change with thermal regimes predicted under climate change across seasons. To reflect this increase in temperature in a more ecologically realistic context, we took daily temperatures experienced in January (summer/dry season) in Cairns (hourly averages from 2001 to 2008; Bureau of Meteorology http://www.bom.gov.au/climate/), and added 1°C to these values to reflect a 1°C increase in temperature encompassing natural fluctuations throughout a 24 h cycle (electronic supplementary material, figure S1), to generate a summer/wet season experimental thermal regime. A winter/dry season thermal regime was generated by adding 1°C to daily temperatures experienced in July at Cairns (hourly averages from 2001–2008; Bureau of Meteorology, http://www.bom.gov.au/climate/) (electronic supplementary material, figure S1).
Average RH is predicted to decrease by 0.5% in 2030 under all emission scenarios (emission scenarios as above, CSIRO, http://www.climatechangeinaustralia.gov.au). These projections are given relative to the period 1980–1999. The projections give an estimate of the average climate around 2030, 2050 and 2070, taking into account consistency among climate models. Individual years will show variation from this average. The 50th percentile (the mid-point of the spread of model results) provides a best estimate result.
To see how these projections translate into changes in RH by 2030 in Cairns, we applied the projected 0.5% reduction in RH to the average RH for Cairns in January (summer/wet season) and July (winter/dry season) for 2012 (electronic supplementary material, table S2; Bureau of Meterology http://www.bom.gov.au/climate/). This projected decrease in humidity based on the 50th percentile is the same for the low-, medium- and high-emissions scenarios (CSIRO, http://www.climatechangeinaustralia.gov.au). To assess the extent to which both D. birchii and D. bunnanda might be able to adapt to a more realistic level of desiccation stress, we chose a level of 35% RH. This presents a level of RH that is well below the average summer and winter RH experienced in the wet tropics of Australia (electronic supplementary material, tables S1 and S2), and falls below the projected relative average and minimum RH for summer in Cairns 2030 (electronic supplementary material, figure S2).
(c). Desiccation resistance assays
To assess desiccation resistance to less than 10% RH, individual flies were placed into 5 ml glass vials covered with gauze which were then placed in a sealed glass tank with silica gel ([7,17] at 25°C. RH was confirmed with an i-button data logger (Maxim Integrated i-button DS1923). Flies were scored as dead when no movement was detected, and flies were scored every hour until 100% mortality.
To assess resistance to 35% RH desiccation stress, flies were placed individually into empty 50 ml plastic vials stopped with a foam plug. The humidity in these vials was 35% RH (determined using a Maxim Integrated i-button DS1923). Room temperature was set to 25°C. As for the desiccation assay at less than 10% RH, flies were scored as dead when no movement was detected and were scored every hour until 100% mortality.
(d). Estimating additive genetic variances and covariances
We used a paternal half-sibling breeding design to estimate additive genetic variance for desiccation resistance. The species were assessed in different generations (see below). For each species, density was controlled in the parental generation by only allowing mated females to lay eggs over an 8 h period. Eggs were left to develop at 25°C. Within 6 h of adult emergence, virgin females and males were separated using light CO2 anaesthesia. Two-hundred and fifty males (sires) were each mated to three females (dams) and left together for 3 days. The females were then separated and placed individually into a 50 ml vial containing 15 ml of potato, yeast and sucrose media allowed to lay eggs for a 48 h period, after which time they were removed, and placed into a second vial to lay for another 48 h period, and the vials then left at 25°C for 24 h to allow eggs to hatch. These vials were then placed in control temperature cabinets set to the Cairns summer and winter thermal regimes (+1°C) to allow the flies to complete larval-to-adult development.
Females and males emerging from these vials were collected within 1 day of eclosion and held together in a fresh food vial for a further 48 h to ensure females were mated. After 48 h, females and males were separated using light CO2 anaesthesia and allowed to recover for a further 48 h before assessing desiccation resistance (flies were 6–7 days old).
Desiccation resistance in D. birchii was measured using two independent paternal half-sibling full-sibling experiments performed in February 2011 (F11 of laboratory culture) and June 2011 (F17 of laboratory). For the first paternal half-sibling full-sibling breeding design (February 2011), we examined desiccation resistance in adult flies exposed to less than 10% RH and 35% RH under the summer developmental thermal regime. Two females from each dam were assessed at 10% RH and 35% RH.
For the second paternal half-sibling full-sibling experiment (June 2011), we examined desiccation resistance in D. birchii to 35% RH in both male and female flies reared under the summer and winter thermal regimes. Desiccation resistance was assessed on two females and two males per dam as described above.
Desiccation resistance to 35% RH in D. bunnanda was assessed in July 2011 (F18 of laboratory culture) using a paternal half-sibling full-sibling breeding design in flies reared under the summer and winter thermal regimes using the same protocols as described above for D. birchii. Two females and two males from each dam were assessed.
The following mixed linear model was used to represent the half-sibling full-sibling breeding design following van Heerwaarden & Sgrò [20]:
where Yijkl is the trait value of an individual, μ is the population mean, Xi is effect of the ith run, Sj is the effect of the jth sire, Dk(j) is the effect of the kth dam nested within the jth sire and Wl(jk) is the effect of the lth progeny of the kth dam nested with the jth sire. Sex was modelled as a fixed effect, whereas all other terms were considered random. The total phenotypic variance for the breeding design for the purpose of estimating genetic parameters was represented by
Variance and covariance (COV) components were estimated using restricted maximum-likelihood implemented via the MIXED procedure of SAS (SAS Institute, Cary, NC, USA). Narrow sense heritability estimates for desiccation resistance for each developmental temperature were estimated as four times the paternal half-sibling intraclass correlation, tPHS, as outlined by Lynch & Walsh [21]. As we used a half-sibling full-sibling breeding design, the sire variance, 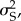 is one-fourth of the additive genetic variance (VA) [22,23]. Thus, to estimate VA, we multiplied the sire variance by four. To determine whether the sire variance and additive genetic variance were significantly different from zero, we used log-likelihood ratio tests, where the final model for each trait was compared to a model specifying
is one-fourth of the additive genetic variance (VA) [22,23]. Thus, to estimate VA, we multiplied the sire variance by four. To determine whether the sire variance and additive genetic variance were significantly different from zero, we used log-likelihood ratio tests, where the final model for each trait was compared to a model specifying 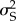 to be zero [23,24].
to be zero [23,24].
The additive genetic COVs between resistance to 10 and 35% RH (D. birchii experiment 1) and between desiccation resistance to 35% RH across the thermal regimes (D. birchii experiment 2, and D. bunnanda) were individually tested for significance from zero by performing log-likelihood ratio tests where the final models for each trait were compared to models specifying the sire-level COVS to be zero [23–25]. We estimated the additive genetic correlation between traits using the MIXED procedure of SAS (SAS Institute). Log-likelihood ratio tests were used to test whether any of the additive genetic correlations were significantly different from both zero and one [24,25].
(e). Selection experiment
To determine whether D. bunnanda and D. birchii could evolve higher levels of desiccation resistance to 35% RH, we artificially selected for increased desiccation resistance in three selection lines per species in flies reared under the summer thermal regime. Selection was initiated at the F11 generation of laboratory culture for D. bunnanda and the F16 generation for D. birchii. Prior to each generation of selection, larval density was controlled by restricting laying to 24 h. After this time, flies were removed from the vials, and the vials placed at 25°C for 24 h to allow the eggs to hatch before being placed under the summer (wet season) rearing temperature regime (see above) to complete development. Flies to be selected were collected within 24 h of eclosion and the sexes were separated using light CO2 anaesthesia another 48 h later. After 48 h recovery, groups of 20 flies (300–500 per sex) were exposed to 35% RH (as described above) until at least 50–70% of flies had succumbed to desiccation stress (not showing any signs of movement). The surviving 30–50% of flies were removed from their desiccation vial and transferred into vials with fresh food, with a RH above 95%. Further mortality occurred, so the number of survivors was counted and recorded 24 h later and used to propagate the next generation. Flies were maintained at 25°C and transferred to fresh food every 2 days for 10 days. Selection was applied every other generation, and larvae only developed under summer conditions in the generation prior to selection. Three control lines were initiated for each species and were maintained as described above for the selected lines, but flies were randomly selected to propagate each generation.
To quantify the response to selection, desiccation resistance was assessed in the selected and control lines one generation after the final generation of selection (after 10 generations in D. bunnanda and after five generations in D. birchii). Larval density for this assessment was controlled by allowing approximately 500 male/female pairs per replicate line to lay eggs in a population cage with a lid containing the potato media stained with food dye and covered with a layer of live yeast to stimulate oviposition. Flies were allowed to lay eggs for approximately 12 h at 25°C, after which time flies were removed. Eggs were then placed into five replicate vials, at a density of 50 eggs per vial, per replicate selection and control line. Twenty-four hours later, vials were transferred to temperature cabinet to complete development under the summer thermal regime. Forty females and 40 males were assessed for desiccation resistance to 35% RH (as described above) in each selected and unselected control line. The sexes were separated 48 h after eclosion and allowed to recover for a further 72 h. Assessment of desiccation resistance was undertaken on 6-day-old flies.
A nested analysis of variance was used to test for significant responses to selection in each species, with treatment (selection or control) and replicate line nested within treatment as main effects. Sexes were combined in this analysis because males and females showed significant responses to selection (D. birchii females F1,234 = 46.45, p < 0.001 and males F1,234 = 69.36, p < 0.001; D. bunnanda females F1,233 = 21.49, p < 0.001 and males F1,234 = 14.63, p < 0.001) and there was no significant sex by treatment interaction (D. birchii F1,476 = 0.007, p = 0.932; D. bunnanda F1,475 = 1.06, p = 0.304).
3. Results
We first used a paternal half-sibling breeding design to estimate the additive genetic variance and narrow sense heritability for resistance to two levels of desiccation stress; extreme (less than 10% RH) and a more moderate, but ecologically realistic, (35% RH) level of desiccation stress projected for the wet tropics by 2030 in D. birchii reared under the summer temperature regime (electronic supplementary material, figures S1 and S2 and tables S1 and S2).
We confirm the fundamental evolutionary constraint identified by Kellermann et al. [7] and Hoffmann et al. [8]: the additive genetic variance and narrow sense heritability for desiccation resistance to less than 10% RH were not significantly different from zero (table 1). By contrast, we found significant additive genetic variance and narrow sense heritability for desiccation resistance to 35% RH (table 1). The additive genetic COV between desiccation resistance at less than 10% RH and 35% RH was not significantly different from zero (−0.094, p = 0.239), indicating that they reflect genetically independent traits. The genetic basis of desiccation resistance, and its capacity to evolve, therefore depends on the severity of the stress being experienced.
Table 1.
Mean desiccation resistance, narrow sense heritability (h2), additive genetic variance (VA), environmental variance (VE) (±1 s.e.) and number of individuals assayed (n) for desiccation resistance in D. birchii and D. bunnanda. (Standard errors estimated following [26]. *p < 0.05; **p < 0.001.)
| treatment | species | mean ± s.e. | h2 ± s.e. | VA ± s.e. | VE ± s.e. | n |
|---|---|---|---|---|---|---|
| < 10% RH summer | D. birchii | 7.051 ± 0.062 | 0.019 ± 0.181 | 0.057 ± 0.554 | 2.869 ± 0.208 | 812 |
| 35% RH summer | D. birchii | 11.232 ± 0.076 | 0.343 ± 0.163* | 2.082 ± 0.991* | 5.103 ± 0.327 | 1047 |
| 35% RH summer | D. birchii | 10.296 ± 0.056 | 0.216 ± 0.080** | 1.332 ± 0.490** | 5.415 ± 0.190 | 2034 |
| 35% RH winter | D. birchii | 9.278 ± 0.042 | 0.211 ± 0.079* | 0.700 ± 0.264* | 2.793 ± 0.103 | 2256 |
| 35% RH summer | D. bunnanda | 16.880 ± 0.089 | 0.159 ± 0.068** | 2.287 ± 0.967** | 12.734 ± 0.455 | 2085 |
| 35% RH winter | D. bunnanda | 21.970 ± 0.134 | 0.211 ± 0.085* | 5.892 ± 2.392* | 22.256 ± 0.827 | 1947 |
We then asked whether D. birchii and a second rainforest-restricted species, D. bunnanda displayed significant levels of additive genetic variance and narrow sense heritability for desiccation resistance to 35% RH when reared under the summer and winter developmental temperatures. Our earlier results in D. birchii were confirmed and extended. The additive genetic variance and heritability for desiccation resistance to 35% RH were significantly different from zero in both species under the summer and winter developmental temperature regimes (table 1).
Adaptive responses to climate change will also be influenced by genetic correlations across environments [10,11,27], so we asked whether desiccation resistance to 35% RH was genetically correlated across the summer and winter thermal regimes. A significant and positive additive genetic correlation was found for D. birchii (rg = 0.625, p < 0.05) but not D. bunnanda (not shown). Adaptation to a drier environment in D. birchii will occur via both direct and correlated responses to selection across thermal environments, while independent responses to selection will underpin the evolution of desiccation resistance across thermal regimes in D. bunnanda. Differences between closely related species in the genetic basis of response to changing environments may be common [28].
To demonstrate that the significant estimates of additive genetic variance and narrow sense heritability for resistance to 35% RH in D. birchii and D. bunnanda enable both species to evolve higher levels of desiccation resistance, we performed artificial selection for increased desiccation resistance in both species under the summer temperature regime. After just 10 generations of selection on D. bunnanda and five on D. birchii, desiccation resistance to 35% RH had significantly increased compared to the unselected controls by an average of 17 and 23%, respectively (figure 1; electronic supplementary material, table S3). These levels are comparable to the selection response seen for selection at less than 10% RH desiccation resistance in the closely related, but more widely distributed generalist species Drosophila serrata [8,29].
Figure 1.
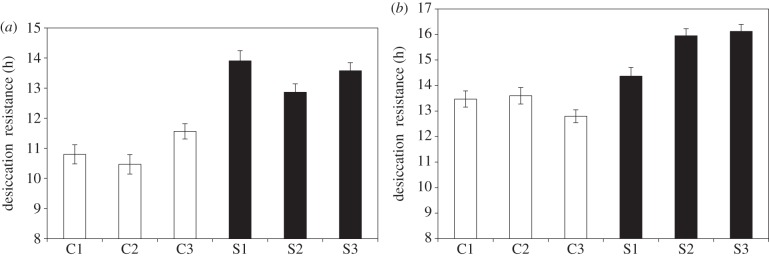
Response to selection for increased desiccation resistance to 35% RH in (a) D. birchii and (b) D. bunnanda for three replicate control (C1–3, open bars) and selected (S1–3, filled bars) lines. Error bars reflect standard errors. Response to selection was significant in D. birchii (F1,4 = 31.478, p < 0.001) and D. bunnanda (F1,4 = 12.775, p < 0.001) (electronic supplementary material, table S3).
4. Discussion
There is huge interest in the ecological fate of species under climate change [10,11,30]. Whether evolution will be rapid enough to rescue populations from extinction will depend on the presence of additive genetic variance for the traits under selection [10,11]. We show that the expression of additive genetic variance for ecologically important traits will depend on the severity of the stress experienced. Abrupt, severe reductions in humidity will probably lead to increased extinction because rainforest species may not harbour the genetic variation necessary to mount an evolutionary response to such abrupt and extreme change. However, we show that evolutionary responses to more moderate, ecologically realistic, reductions in humidity may in fact be possible.
The likelihood of evolutionary rescue has recently been shown to depend on the severity of the stress imposed by environmental change. Rapid adaptation and evolutionary rescue is possible following an abrupt shift to a historically lethal level of stress if populations have been exposed to prior selection to less severe levels of stress [28]. This evolutionary rescue under extreme environmental stress, however, requires a positive cross-environment genetic correlation of beneficial mutations selected at the lower stress level. Our results suggest that this will be unlikely to rescue rainforest-restricted species from severe (less than 10% RH) desiccation stress, because the additive genetic COV between this severe level of stress and the more moderately stressful 35% RH was not significantly different from zero. Different genes underlie the response to these different levels of desiccation stress. However in the context of rainforest habitats of the wet tropics of Australia and elsewhere, it seems unlikely that such severe and abrupt changes in humidity will occur over the next 30–60 years, even under high-emission climate change scenarios. The more likely scenario will be a gradual decrease in humidity over the next few decades. We show that rainforest species have the capacity to respond to these more moderate decreases in humidity via evolutionary adaptation.
Why might the expression of additive genetic variance depend on the severity of the stress experienced? It is possible that no mechanisms have evolved to detect and/or counter extreme levels of stress. Resistance to a severe desiccation stress (less than 10% RH) in Drosophila involves three different mechanisms, including increasing water storage, decreasing water loss and tolerating water loss [31]. In addition, the ability to detect desiccation stress may also be important. Using artificial selection for resistance to less than 10% RH in Drosophila melanogaster, Telonis-Scott et al. [32] identified two sensory organ genes that increased in frequency with increasing desiccation resistance. However, little is known about the genetic basis of desiccation resistance to less than 10% RH [32], and, genomic resources are not yet available for the two species examined here. Whether tropically restricted species lack the genes or sensory mechanisms to detect and respond to such extremely low levels of humidity remains to be examined.
Alternatively, the genetic basis of adaptation to severe, compared to moderate, levels of desiccation stress may differ, which will impact the evolution of desiccation resistance. Specifically, adaptation to severe or rapid environmental change, which requires large phenotypic changes in natural populations, may occur by the evolution of alleles of large effect. Compared to selection imposed by moderate stress levels, intense selection under severe stress may involve fewer genes of large effect [33–35]. Under such intense selection, only those individuals possessing resistance alleles conferring a high level of resistance (alleles of large effect) will survive. In the absence of the stress, these large effect alleles may be selected against, and reduced in frequency or absent from populations, because of large deleterious pleiotropic effects [33]. Building on these arguments in the context of the evolution of insecticide resistance, McKenzie [36] argued that where selection acts outside of the normal range of distribution of resistance phenotypes, selection will act on rare mutations of large effect. Conversely, when selection is operating within the normal distribution of phenotypic variation, pre-existing polygenic variation will be selected for. Thus, mutations of large effect for desiccation resistance at extreme levels of desiccation stress (less than 10% RH) may be rare or absent in rainforest species, where selection for this level of desiccation resistance does not occur, or because of strong pleiotropic effects. Conversely, resistance to moderate levels of stress may involve more alleles of smaller effect, with no pleiotropic effects on fitness, and so the observed frequency of these alleles is likely to be higher. The best evidence for this comes from studies of herbicide and pesticide resistance and studies of unicellular organisms. For example, Neve & Powles [37] found high levels of resistance evolved in the crop weed Lolium rigidum under selection at low herbicide doses, as multiple mechanisms were selected. Additional evidence comes from studies looking at the effect of rate of environmental change on adaptation, where faster rates of change equate to more abrupt and severe levels of stress. Adaptation in response to slower rates of environmental change in Chlamydomonas resulted in populations that grow faster and pay a lower cost of adaptation after 200 generations of selection than populations that adapt to a sudden change of the same magnitude [38]. Mutations of smaller effect that underpin adaptation to slower rates of environmental change may thus result in reduced levels of pleiotropy and historical constraints, and/or increased epistatic interactions, resulting in higher fitness and more successful adaptation [38].
Recent work in experimental populations of Escherichia coli confirms that evolutionary responses to environmental change can depend on the rate of environmental change experienced [39]. Some of the mutations beneficial at lower rates of change were not beneficial at higher rates of change, suggesting that independent mechanisms can play a role in adapting to rapid versus slower rates of environmental change, consistent with the non-significant additive genetic COV between resistance to less than 10% RH and 35% RH in this study. However, Lindsey et al. [39] focused on the contribution of new mutations, and not standing genetic variation, to the likelihood of adaptation to environmental change, although the latter is more likely to contribute to adaptive responses to rapid environmental change in higher organisms [40]. Whether their results extend to higher organisms is unknown.
Finally, it is also possible that genes underpinning resistance to less than 10% RH are no longer functional because of DNA decay [7,41–43], whereas genes underpinning resistance to 35% RH remain functional because of periodic selection imposed by intermittent exposure to moderately low levels of humidity. Comparative genomic studies of widespread generalist species like D. melanogaster and D. serrata, that display significant levels of additive genetic variance for resistance to less than 10% RH, and restricted, sensitive species like D. birchii and D. bunnanda that do not, are needed to answer this question.
Our results highlight the importance of understanding the underlying genetic basis of traits that will be key to underpinning adaptive responses to climate change. If many genes of small effect control the traits central to organismal responses to climate change [44], then evolutionary responses to climate change may be more likely than currently thought, provided that the distance the mean of the population needs to move to provide an adaptive shift is not too large ([34] but see [45]). In the presence of additive genetic variance for the trait under selection, then adaptation should occur, as illustrated by the significant selection response to selection for increased resistance to moderate (35% RH) desiccation resistance that we report here. If, on the other hand, the mean of the population needs to shift a large distance to provide an adaptive shift, then polygenic adaptation may be unable to achieve the necessary adaptation. Adaptation will only occur if genes exist at low frequencies that have a sufficiently large effect to achieve adaptation [34]. Current models of adaptation consider the evolution of populations faced with sudden and severe environmental change [33,34,45]. However, little is still known about how the genetic complexity underlying ecologically important traits will affect the capacity of populations to adapt and persist when abruptly faced with stressful environments [45], or when faced by moderately changing environments [46]. Genomic and quantitative genetic studies of ecologically important traits under a continuum of moderate to severe levels of stress are urgently needed to determine how the genetic basis of traits may change with the severity of stress, and how this in turn will affect the likelihood of subsequent adaptive shifts. The importance of demography in underpinning adaptation to environmental change must also be considered [47].
The significance of our results is twofold. First, we show that rainforest species are fundamentally constrained by a very low level of additive genetic variance for very severe (less than 10% RH) levels of desiccation stress [7], independent of thermal regime. Second, and more importantly, we show that rainforest-restricted species can in fact mount significant and rapid evolutionary responses to the less severe levels of desiccation stress likely to occur with climate change over the next 30 years. Rather than focusing solely on organismal responses to severe and abrupt changes in environmental conditions, we should also be considering the ability of organisms to respond to more moderate, but perhaps more ecologically realistic, levels of environmental change projected over the short to medium term.
Supplementary Material
Acknowledgements
We thank Fiona Cockerell and Richard Foo Heng Lee for technical support.
Data accessibility
Data available on dryad doi:10.5061/dryad.8hv52.
Funding statement
This research was supported by grants from the Australian Research Council (DP110100665; DP1212045; FT1110951) and from the Science and Industry Endowment Fund. Monash University provided additional financial support.
References
- 1.Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M. 2006. Global temperature change. Proc. Natl Acad. Sci. USA 103, 14 288–14 293. ( 10.1073/pnas.0606291103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IPCC. 2013. Summary for policymakers. In Climate change 2013: the physical science basis contribution of Working Group I to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Stocker TF, Qin D, Plattner G-K, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM.), p. 32. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Walsh KJE, Ryan BF. 2000. Tropical cyclone intensity increase near Australia as a result of climate change. J. Clim. 13, 3029–3036. () [DOI] [Google Scholar]
- 4.Suppiah R, Hennessy K, Whetton PH, McInnes K, Macadam I, Bathols J, Ricketts J, Page CM. 2007. Australian climate change projections derived from simulations performed for the IPCC 4th assessment report. Aust. Meteorol. Mag. 56, 131–152. [Google Scholar]
- 5.Pounds JA, Fogden MPL, Campbell JH. 1999. Biological response to climate change on a tropical mountain. Nature 398, 611–615. ( 10.1038/19297) [DOI] [Google Scholar]
- 6.Still CJ, Foster PN, Schneider SH. 1999. Simulating the effects of climate change on tropical montane cloud forests. Nature 398, 608–610. ( 10.1038/19293) [DOI] [Google Scholar]
- 7.Kellermann V, van Heerwaarden B, Sgro CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246. ( 10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann AA, Hallas RJ, Dean JA, Schiffer M. 2003. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science 301, 100–102. ( 10.1126/science.1084296) [DOI] [PubMed] [Google Scholar]
- 9.van Heerwaarden B, Willi Y, Kristensen TN, Hoffmann AA. 2008. Population bottlenecks increase additive genetic variance but do not break a selection limit in rain forest Drosophila. Genetics 179, 2135–2146. ( 10.1534/genetics.107.082768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 12.Merila J. 2009. Genetic constraints on adaptation? Science 325, 1212–1213. ( 10.1126/science.1179326) [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann AA, Merila J. 1999. Heritable variation under favourable and unfavourable conditions. Trends Ecol. Evol. 14, 96–101. ( 10.1016/S0169-5347(99)01595-5) [DOI] [PubMed] [Google Scholar]
- 14.Husby A, Visser M, Kruuk L. 2011. Speeding up microevolution: the effects of increasing temperature on selection and genetic variance in a wild bird population. PLoS Biol. 9, e1000585 ( 10.1371/journal.pbio.1000585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann AA, Parsons PA. 1991. Evolutionary genetics and environmental stress, p. 284 New York, NY: Oxford University Press. [Google Scholar]
- 16.Charmantier A, Garant D. 2005. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425. ( 10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellermann VM, van Heerwaarden B, Hoffmann AA, Sgro CM. 2006. Very low additive genetic variance and evolutionary potential in multiple populations of two rainforest Drosophila species. Evolution 60, 1104–1108. ( 10.1554/05-710.1) [DOI] [PubMed] [Google Scholar]
- 18.van Vuuren DP, et al. 2011. The representative concentration pathways: an overview. Clim. Change 109, 5–31. ( 10.1007/s10584-011-0148-z) [DOI] [Google Scholar]
- 19.Nakicenovic N, et al. 2000. IPCC Special Report Emission Scenarios. Working Group III.
- 20.van Heerwaarden B, Sgro CM. 2013. Multivariate analysis of adaptive capacity for upper thermal limits in Drosophila simulans. J. Evol. Biol. 26, 800–809. ( 10.1111/jeb.12090) [DOI] [PubMed] [Google Scholar]
- 21.Lynch M, Walsh B. 1998. Genetics and the analysis of quantitative traits. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 22.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. Harlow, UK: Pearson Education Ltd. [Google Scholar]
- 23.McGuigan K, Nishimura N, Currey M, Hurwit D, Cresko WA. 2011. Cryptic genetic variation and body size evolution in threespine stickleback. Evolution 65, 1203–1211. ( 10.1111/j.1558-5646.2010.01195.x) [DOI] [PubMed] [Google Scholar]
- 24.Littell R, Milliken A, Stroup W, Wolfinger R. 1996. SAS system for mixed models. Cary, NC: SAS Institute Inc. [Google Scholar]
- 25.Simonsen AK, Stinchcombe JR. 2010. Quantifying evolutionary genetic constraints in the ivyleaf morning glory, Ipomoea hederacea. Int. J. Plant Sci. 171, 972–986. ( 10.1086/656512) [DOI] [Google Scholar]
- 26.Becker WA. 1984. Maual of quantitiatve genetics. Washington, DC: Pulman. [Google Scholar]
- 27.Etterson JR, Shaw RG. 2001. Constraint to adaptive evolution in response to global warming. Science 294, 151–154. ( 10.1126/science.1063656) [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez A, Bell G. 2013. Evolutionary rescue and adaptation to abrupt environmental change depends upon the history of stress. Phil. Trans. R. Soc. B 368, 20120079 ( 10.1098/rstb.2012.0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blows M, Hoffman AA. 1993. The genetics of marginal populations Drosophila serrata. I. Genetic variation for stress resistance and species borders. Evolution 47, 1255–1270. ( 10.2307/2409990) [DOI] [PubMed] [Google Scholar]
- 30.Merila J. 2012. Evolution in response to climate change: in pursuit of the missing evidence. Bioessays 34, 811–818. ( 10.1002/bies.201200054) [DOI] [PubMed] [Google Scholar]
- 31.Gibbs AG, Matzkin LM. 2001. Evolution of water balance in the genus Drosophila. J. Exp. Biol. 204, 2331–2338. [DOI] [PubMed] [Google Scholar]
- 32.Telonis-Scott M, Gane M, DeGaris S, Sgro CM, Hoffmann AA. 2012. High resolution mapping of candidate alleles for desiccation resistance in Drosophila melanogaster under selection. Mol. Biol. Evol. 29, 1335–1351. ( 10.1093/molbev/msr294) [DOI] [PubMed] [Google Scholar]
- 33.Lande R. 1983. The response to selection on major and minor mutations affecting a metrical trait. Heredity 50, 47–65. ( 10.1038/hdy.1983.6) [DOI] [Google Scholar]
- 34.Macnair MR. 1991. Why the evolution of resistance to anthropogenic toxins normally involves major gene changes: the limits to natural selection. Genetica 84, 213–219. ( 10.1007/bf00127250) [DOI] [Google Scholar]
- 35.Lenski RE, Travisano M. 1994. Dynamics of adaptation and diversification: a 10,000 generation experiment with bacterial populations. Proc. Natl Acad. Sci. USA 91, 6808–6814. ( 10.1073/pnas.91.15.6808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKenzie JA. 2000. The character or the variation: the genetic analysis of the insecticide-resistance phenotype. Bull. Entomol. Res. 90, 3–7. ( 10.1017/s000748530000002x) [DOI] [PubMed] [Google Scholar]
- 37.Neve P, Powles S. 2005. Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum. Theor. Appl. Genet. 110, 1154–1166. ( 10.1007/s00122-005-1947-2) [DOI] [PubMed] [Google Scholar]
- 38.Collins S, de Meaux J. 2009. Adaptation to different rates of environmental change in Chlamydomonas. Evolution 63, 2952–2965. ( 10.1111/j.1558-5646.2009.00770.x) [DOI] [PubMed] [Google Scholar]
- 39.Lindsey HA, Gallie J, Taylor S, Kerr B. 2013. Evolutionary rescue from extinction is contingent on a lower rate of environmental change. Nature 494, 463–467. ( 10.1038/nature11879) [DOI] [PubMed] [Google Scholar]
- 40.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann AA, Willi Y. 2008. Detecting genetic responses to environmental change. Nat. Rev. Genet. 9, 421–432. ( 10.1038/nrg2339) [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann AA. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880. ( 10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann AA. 2010. A genetic perspective on insect climate specialists. Aust. J. Entomol. 49, 93–103. ( 10.1111/j.1440-6055.2010.00744.x) [DOI] [Google Scholar]
- 44.Rockman MV. 2012. The QTN program and the alleles that matter for evolution: all that's gold does not glitter. Evolution 66, 1–17. ( 10.1111/j.1558-5646.2011.01486.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomulkiewicz R, Holt RD, Barfield M, Nuismer SL. 2010. Genetics, adaptation, and invasion in harsh environments. Evol. Appl. 3, 97–108. ( 10.1111/j.1752-4571.2009.00117.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw RG, Etterson JR. 2012. Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol. 195, 752–765. ( 10.1111/j.1469-8137.2012.04230.x) [DOI] [PubMed] [Google Scholar]
- 47.Gomulkiewicz R, Houle D. 2009. Demographic and genetic constraints on evolution. Am. Nat. 174, E218–E229. ( 10.1086/645086) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on dryad doi:10.5061/dryad.8hv52.


