Abstract
Animals often convey useful information, despite a conflict of interest between the signaller and receiver. There are two major explanations for such ‘honest’ signalling, particularly when the size or intensity of signals reliably indicates the underlying quality of the signaller. Costly signalling theory (including the handicap principle) predicts that dishonest signals are too costly to fake, whereas the index hypothesis predicts that dishonest signals cannot be faked. Recent evidence of a highly conserved causal link between individual quality and signal growth appears to bolster the index hypothesis. However, it is not clear that this also diminishes costly signalling theory, as is often suggested. Here, by incorporating a mechanism of signal growth into costly signalling theory, we show that index signals can actually be favoured owing to the cost of dishonesty. We conclude that costly signalling theory provides the ultimate, adaptive rationale for honest signalling, whereas the index hypothesis describes one proximate (and potentially very general) mechanism for achieving honesty.
Keywords: condition dependence, costly signalling, handicap principle, honest signalling, indices, sexual selection
1. Introduction
A remarkable feature of animal communication is the apparent honesty with which individuals advertise their quality. This observation is particularly puzzling when the interests of signallers and receivers conflict. Most famously, in competition over mates, males of the highest genetic quality or physical condition also tend to display the most exaggerated ornaments and weaponry [1–4]. This can give high-quality males greater access to mates, either because females prefer the showiest males [5] or because rival males yield to the most armoured opponents [6]. Given such benefits of signalling high quality, the problem is to explain why natural selection does not favour low-quality males who ‘fake’ a larger signal.
There are two major explanations for honest signalling despite a conflict of interest, typically presented as alternative hypotheses [7–14]. First, costly signalling theory (including the handicap principle [15]) predicts that honesty will be maintained when dishonest signals are too costly to fake [2,9,14,16]. Specifically, the maintenance of honesty does not require a realized cost paid by honest signallers, as in Zahavi's [15] view of the handicap principle, but rather a potential cost for dishonesty [17–19]. Second, the index hypothesis predicts that physiological or developmental mechanisms create a causal link between quality and signal size, such that dishonest signals cannot be faked [7,8,20].
Recently, the index hypothesis has been bolstered by studies of the growth of male sexual signals. These studies show that signal growth is regulated by a highly conserved mechanism—the insulin/insulin-like signalling (ILS) pathway—which closely tracks the quality (e.g. health or nutritional state) of individual animals [21,22]. By this mechanism, low-quality males have limited potential for growth, and so it can appear that their signal size is intrinsically ‘constrained’. This apparent constraint, coupled with the finding that signals can lack detectable costs [23–28], has been taken as evidence that honest signalling can be explained without costly signalling theory [21,22,29,30].
However, it is still not clear that the index hypothesis is a true alternative to costly signalling theory [10,31]. This is because, whereas costly signalling theory provides an adaptive explanation for honest signalling, indices have been treated as static constraints not subjected to natural selection [7,8,32]. Yet, recent evidence strongly suggests that the strength of a causal link between quality and the growth of a particular trait (e.g. coded by alleles that alter a trait's sensitivity to the ILS pathway) may indeed be adjusted by selection [21]. Consequently, a major gap in signalling theory is the lack of an adaptive explanation for the evolution and maintenance of index signals. This is particularly crucial in the light of recent suggestions that most sexual signals may in fact be examples of indices [21,29].
Here, we address the relationship between costly signalling theory and the index hypothesis by examining the adaptive evolution of index signals. Specifically, we incorporate into costly signalling theory a mechanism that causally links individual quality to signal size, and we determine the conditions that favour such a mechanism. We use this model as a formal argument that the honesty of index signals can actually be explained by the cost of dishonesty. Furthermore, in contrast to suggestions that index signals will represent only a special case of costly signalling theory [31], our model implies that indices may indeed be a very general mechanism for achieving honesty.
2. Models and results
We consider individuals' division of resources between a signalling trait and their own survival, or viability (after [32]). Signallers vary in quality q (0 ≤ q ≤ 1), which could be any trait that is of interest to receivers but that cannot be observed directly (e.g. fighting ability, good genes or health). We assume that receivers attempt to judge quality by measuring a focal individual's signal size s (0 ≤ s ≤ 1) relative to the mean signal size 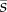 in the rest of the population (excluding the focal individual). We assume that by producing a large signal, an individual reduces its survival probability, which is given by t(s, q) = 1 − s1 +
kq and ranges from unity at s = 0 to zero at s = 1. Crucially, the shape of this cost function may depend on individual quality. A given signal size may be equally costly for individuals of any quality (k = 0), or it may be more costly for low-quality individuals (k > 0). This differential cost is a key mechanism that promotes honest signalling [16,32–35].
in the rest of the population (excluding the focal individual). We assume that by producing a large signal, an individual reduces its survival probability, which is given by t(s, q) = 1 − s1 +
kq and ranges from unity at s = 0 to zero at s = 1. Crucially, the shape of this cost function may depend on individual quality. A given signal size may be equally costly for individuals of any quality (k = 0), or it may be more costly for low-quality individuals (k > 0). This differential cost is a key mechanism that promotes honest signalling [16,32–35].
(a). The optimal quality-dependent strategy
To recover basic predictions of costly signalling theory, we first determine the optimal strategy for varying signal size with individual quality. This is the strategy that maximizes individual fitness w, which we measure as the product of an individual's relative signal size and its survival probability (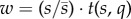 ). Using standard optimization techniques (see the electronic supplementary material), we find that the optimal signal size is
). Using standard optimization techniques (see the electronic supplementary material), we find that the optimal signal size is
| 2.1 |
This leads to the following results
Result 1. Consistent with previous models, quality dependence in the optimal signal size (ds*/dq > 0) requires that signalling is differentially costly for low-quality individuals (k > 0; figure 1a). By contrast, when there is no differential cost of signalling (k = 0), the model predicts that all signallers will express s* = 1/2, irrespective of quality. In reality, given that the signal no longer reflects quality at this point, we expect that receivers will ignore the signal trait, and so it may eventually be lost altogether (this is beyond the scope of our model).
Figure 1.
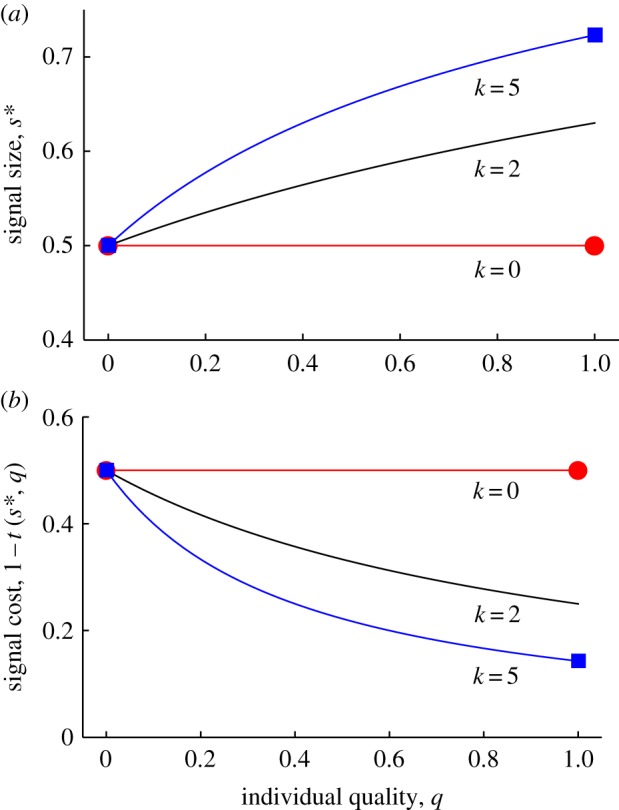
A simple model of costly signalling. Plots show the optimal quality-dependent signal size s* (a) and the associated cost paid by honest signallers (b). If a given signal size is equally costly for individuals of any quality (k = 0), then it is not adaptive for individuals to advertise their quality. However, if a given signal size is differentially costly for low-quality individuals (k > 0), then individuals will honestly signal their quality, with high-quality individuals producing the larger signals. As the magnitude of the differential cost increases in this model, s* tends to increase, whereas the cost of producing the optimal signal tends to decline. The outcome of the joint evolution of signal size and quality dependence, from figure 2, is shown by the red circles (for k = 0) and blue squares (for k = 5).
Result 2. The optimal signal size s* and the realized cost paid by honest signallers, measured by 1 − t(s*, q), depend on the marginal cost of faking a dishonest signal (figure 1a, b). If this marginal cost is low (small k), then s* tends to be relatively small, and the realized cost of honest signalling tends to be relatively large (i.e. the signal is a traditional ‘handicap’). If the marginal cost is high (large k), then s* tends to be relatively large, and the realized cost of honest signalling tends to be relatively small (see also [18,32]).
(b). Selection of a causal link between quality and signal size
Like most models in costly signalling theory, the above-mentioned model does not explicitly incorporate a mechanism for achieving signal honesty. Whereas the optimality model assumes full flexibility in adjusting signal size to individual quality, natural selection may instead favour a simple rule that approximates the optimal strategy. To address this, we consider a second model in which signal size is a function of two traits, s(x, c) = x(1 − (1 − q)e−1/c), where x represents the maximum signal size that an individual is able to produce (herein the ‘signalling strategy’), and c imposes a causal, linear relationship between quality and signal size, which limits the signal size of low-quality individuals (herein ‘quality dependence’). This captures the key mechanism of index signalling, where quality dependence can be understood as sensitivity to the ILS pathway, for example. In this scenario, we do not suppose that individuals can vary their expression of x and c in response to quality. Instead, we assume that natural selection acts on genetic variation for both x and c, and our aim is to find the joint evolutionarily stable strategy (ESS) x*, c* that best fits the distribution of signaller qualities in the population.
To analyse selection for signal size in this scenario, we specify a simple genetic basis for the signalling strategy and quality dependence. We assume that both traits are coded by alleles at a single locus and that there is no genetic correlation between the traits. Furthermore, we consider the fitness of a focal individual that carries mutant alleles for the traits xm, cm (producing a signal size sm) in an otherwise monomorphic population of individuals expressing x, c. The population mean signal size (excluding the focal individual) is therefore 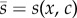 , and the fitness of the focal individual is
, and the fitness of the focal individual is
| 2.2 |
where survival probability t is the same quality-dependent function given above. Assuming that mutant alleles differ only slightly from the resident population, we find the local joint ESS x*, c* using an evolutionary invasion analysis (see the electronic supplementary material).
As an illustrative example, we consider a population in which signallers are either low or high quality (q = 0 or 1), each with probability 1/2. If the cost of dishonesty accounts for honest signalling in this scenario, then alleles for quality dependence will be favoured to avoid any differential cost to low-quality individuals for producing a large signal. Indeed, we find that the joint ESS is
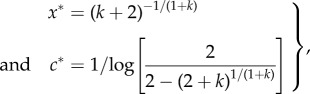 |
2.3 |
which leads to the following results
Result 3. If there is no differential cost of signalling (k = 0), then quality dependence is disfavoured (c* → 0), and no signalling of quality ultimately occurs (figure 2a). By contrast, if signalling is differentially costly for low-quality individuals (k > 0), then quality dependence is always favoured (c* > 0), and honest signalling is therefore enforced (figure 2b). We have checked a large number of numerical examples to confirm that c* gradually increases with increasing k, as expected.
Figure 2.
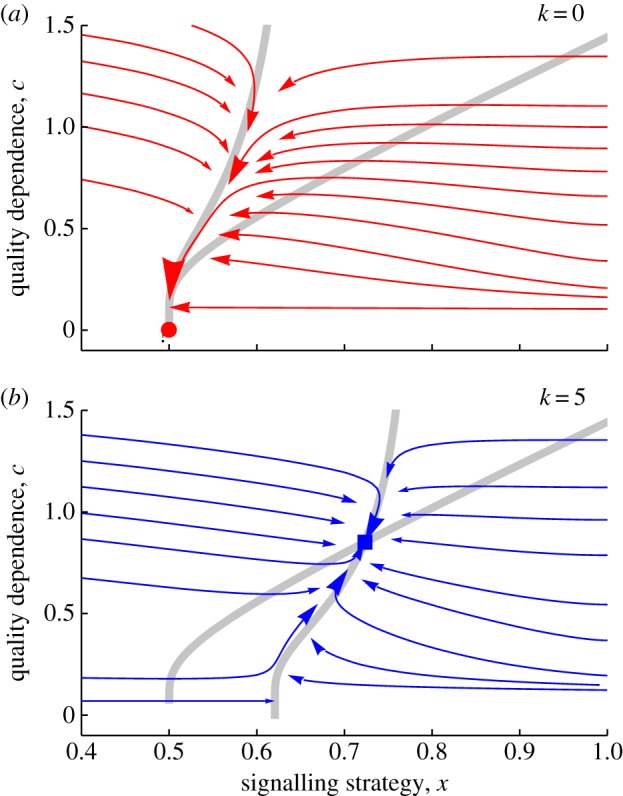
Index signals can evolve by a mechanism of costly signalling theory. Plots illustrate the joint evolutionary dynamics of a signalling strategy x, which sets the maximum signal size, and quality dependence c, which limits the signal size of low-quality individuals. In this example with individuals in either low or high quality (q = 0 or 1), each with probability 1/2, the arrows show the local direction of natural selection at a given trait combination (grey lines are null clines, and selection gradients are multiplied by rate constants vx = 1 and vc = 5; see electronic supplementary material). In (a), where a given signal size is equally costly for individuals of either quality (k = 0), quality dependence is disfavoured and honest signalling breaks down. In (b), where a given signal size is differentially costly for low-quality individuals (k = 5), quality dependence is favoured and honest signalling is therefore enforced. Realized signal sizes at the joint ESS x*, c* (filled symbols) are shown in figure 1.
Result 4. In this example, the signal size at the joint ESS (s* = s [x*, c*]), and hence the costs incurred by honest signallers, exactly matches the signal size and costs of the optimal conditional strategy (see filled symbols in figure 1). Hence, as found above, the equilibrium signal size s* and the realized costs associated with honest indices will depend on the marginal cost of faking a dishonest signal (mediated by k).
More generally, when the distribution of individual qualities is more complex, the joint ESS x*, c* will be an approximation of the optimal strategy. We illustrate this in the electronic supplementary material, using an example with three possible levels of quality in the population.
3. Discussion
Our results show that causal links between quality and signal size (i.e. index signals) can evolve by the same factors that favour an optimal, quality-dependent signalling strategy. We used a simple optimality model to show that (i) quality-dependent signals are favoured by a differential cost of signalling to low-quality individuals (k > 0; figure 1a), and (ii) the realized cost of honest signalling can depend on the marginal cost of faking a dishonest signal (figure 1b). We then included a mechanism that causally links individual quality and signal size and found that (iii) such index signals are favoured only when there is a differential cost of signalling (k > 0; figure 2), and (iv) the realized cost of index signalling will also depend on the marginal cost of dishonesty (figure 1b). Overall, these results suggest that the adaptive explanation for honest signalling, established by costly signalling theory (results 1 and 2), similarly applies to index signals (results 3 and 4).
(a). Index signals, costly signalling theory and the handicap principle
Our results undermine an influential classification of the explanations for honest signalling. Following Maynard Smith & Harper [7,8], three potential explanations are often cited: common interest between signaller and receiver; costs or handicaps (referring to a differential cost of signalling and/or a cost to honest signallers) and constraints (referring to the index hypothesis) [9]. In this classification, index signalling is seen as a distinct explanation, because the causal link between quality and signal size is treated as a constraint, or a modelling assumption [7,8]. By contrast, we show here that such links can emerge from a model of adaptive signalling, and only when signalling is differentially costly for low-quality individuals. This suggests that, contrary to recent claims [21,22,29,30], evidence for a strong causal link between individual quality and signal size does not diminish the role of costs in the maintenance of honesty.
Our results imply that index signals fit neatly into the framework of costly signalling theory (see also [31]). That is, whenever the interests of signallers and receivers conflict, costly signalling theory provides the ultimate, adaptive explanation for honest signalling, whereas the index hypothesis describes one proximate mechanism for achieving honesty. The fundamental aspect of costly signalling theory is the potential cost of faking a dishonest signal. This applies to honest signals that incur a realized cost at equilibrium (i.e. traditional ‘handicaps’) and to honest signals with minimal realized costs (e.g. when the cost of dishonesty is socially imposed) [13]. Similarly, index signals may or may not incur significant realized costs: as our model illustrates, this can depend on the marginal cost of faking a dishonest signal. It follows that, in contrast to the view of indices as ‘cheap’ signals [7], they should actually be defined by their mechanism of quality dependence rather than by the costs (or lack thereof) incurred by honest signallers.
Furthermore, in contrast to the suggestion that that index signals represent only a special case of costly signalling theory [31], our results imply that indices may be a very general mechanism for ensuring honesty. This supports recent claims that most sexual signals, including the most extravagant ornaments and weaponry, may be examples of indices [21,29]. Indeed, whenever signalling is differentially costly for low-quality individuals, simple rules that enforce honesty could often be the most efficient route to signal size plasticity. Sensitivity of signal growth to the ILS pathway, for example, may be more efficient than alternative forms of plasticity that would involve sensing and processing information about individual quality and then invoking the regulation of signal growth [36].
Our basic conclusions can also be extended to other examples of index signals (figure 3a). For example, a classic example is the roar of male red deer (Cervus elaphus), which rival stags use to assess each other's body size and hence fighting ability [37]. Roar frequency is negatively correlated with the length of the vocal tract, and therefore stags with longer necks—and correspondingly larger bodies—produce the lowest roars [38]. This causal relationship between neck length and roar frequency gives the appearance of a physical constraint that enforces honest signalling. However, one can imagine a mutation that increases neck length for a given body size, or a mutation that increases investment in overall body size. Presumably such a mutation would incur net fitness costs, because neck length and body size are probably subjected to selection in other contexts. Our model predicts that if low-quality males experience a disproportionate cost of exaggerating neck length or body size, then selection can favour a causal link between male quality and the ability to roar at low frequency.
Figure 3.
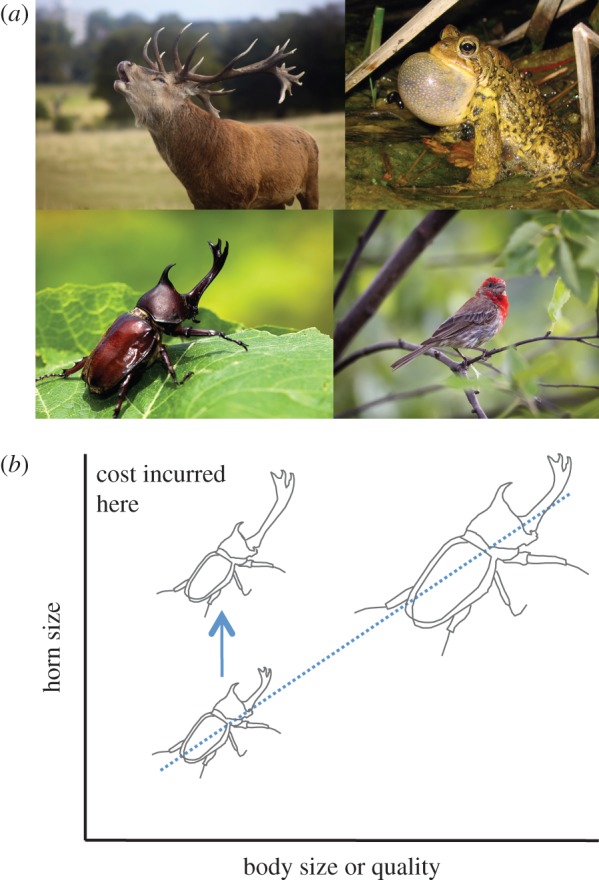
Classic and contemporary examples of index signals can be explained by costly signalling theory. Index signals, clockwise from top left (a): the roar of male red deer (C. elaphus) signals body size and hence fighting ability; the calls of male toads (Bufo bufo) signal their body size to potential mates; the carotenoid-pigmented plumage of male house finches (Carpodacus mexicanus) signals their health to females; and male rhinoceros beetles (Allomyrina dithotomus) use enlarged horns to fight for access to mates. In all cases, it is possible to imagine a mutation that increases the signal size (or intensity) of low-quality individuals; however, the expected cost incurred by these dishonest signallers may explain the maintenance and stability of honest signalling. This cost of dishonest signalling could be tested experimentally (b). For example, small (low-quality) male beetles could build a larger horn through a change in the sensitivity of horn growth to the ILS pathway, and this could be mimicked by upregulating horn growth in the smallest males. Whereas some have predicted that such dishonest signals will be favoured [29], our model predicts that dishonest males will suffer a fitness cost that exceeds the cost incurred by larger (high-quality) males with a comparable horn size. Images obtained from www.shutterstock.com and used with permission.
On the other hand, our model will not apply to examples of so-called revealing handicaps [39–41], where signallers are thought to undergo some task that reveals a quality of interest to receivers. If revealing handicaps exist, they are traits that are truly unfakable, because the content of the message is directly observed. Hence, they do not actually operate as signals, and—as opposed to index signals—costs play no role in their honesty [16].
(b). Implications for empirical tests
It has long been a challenge to test for the relevant costs of animal signals [2,17,18,25,42]. This is because, as stressed above, the modern view of costly signalling theory does not necessarily imply that observed signals will be costly; instead, the key prediction is that a given signal size will be differentially costly for low-quality individuals [2,13,17,31]. This has been supported by a few experiments that forced individuals to bear a signal size that exceeded their true quality [43–45]. However, no study has also tested for the potentially high production costs of growing the most extravagant signals, such as the peacock's tail or the horns and antlers of insects and mammals.
Fortunately, recent advances in the study of index signals could actually lead to long-awaited tests of costly signalling theory. In particular, a detailed knowledge of the pathways that control signal growth [21,22] should lead to genetic tools for upregulating signals in individuals of different quality. Theory predicts that the targeted upregulation of signalling traits in low-quality individuals will reveal the disproportionate production cost of faking a dishonest signal (figure 3b). Such tests may finally shed light on the ultimate explanation for the link between quality and extravagance in the animal world.
Supplementary Material
Acknowledgements
We thank Pau Carazo, Locke Rowe, Stuart West, Gregory Wyatt and two anonymous referees for comments and advice.
Data accessibility
Details of the model are available in the electronic supplementary material. The manuscript does not discuss other data.
Funding statement
J.C.P. was supported by the Natural Sciences and Engineering Research Council and Jesus College (University of Oxford). J.M.B. was supported by the European Research Council (via Stuart West).
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Johnstone RA. 1995. Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol. Rev. Camb. Philos. Soc. 70, 1–65. ( 10.1111/j.1469-185X.1995.tb01439.x) [DOI] [PubMed] [Google Scholar]
- 3.Cotton S, Fowler K, Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783. ( 10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421. ( 10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 5.Andersson M. 1986. Evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution 40, 804–816. ( 10.2307/2408465) [DOI] [PubMed] [Google Scholar]
- 6.Steger R, Caldwell RL. 1983. Intraspecific deception by bluffing: a defense strategy of newly molted stomatopods (Arthropoda: Crustacea). Science 221, 558–560. ( 10.1126/science.221.4610.558) [DOI] [PubMed] [Google Scholar]
- 7.Maynard Smith J, Harper D. 2004. Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Maynard Smith J, Harper DGC. 1995. Animal signals: models and terminology. J. Theor. Biol. 177, 305–311. ( 10.1006/jtbi.1995.0248) [DOI] [Google Scholar]
- 9.Davies NB, Krebs JR, West SA. 2012. An introduction to behavioural ecology, 4th edn West Sussex, UK: Wiley-Blackwell. [Google Scholar]
- 10.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Vehrencamp SL. 2000. Handicap, index, and conventional signal elements of bird song. In Animal signals: signalling and signal design in animal communication (eds Espmark Y, Amundsen T, Rosenqvist G.), pp. 277–300. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 12.Bradbury J, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer. [Google Scholar]
- 13.Fraser B. 2012. Costly signalling theories: beyond the handicap principle. Biol. Philos. 27, 263–278. ( 10.1007/s10539-011-9297-8) [DOI] [Google Scholar]
- 14.Ruxton GD, Schaefer HM. 2011. Resolving current disagreements and ambiguities in the terminology of animal communication. J. Evol. Biol. 24, 2574–2585. ( 10.1111/j.1420-9101.2011.02386.x) [DOI] [PubMed] [Google Scholar]
- 15.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 16.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546. ( 10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 17.Számadó S. 2011. The cost of honesty and the fallacy of the handicap principle. Anim. Behav. 81, 3–10. ( 10.1016/j.anbehav.2010.08.022) [DOI] [Google Scholar]
- 18.Lachmann M, Számadó S, Bergstrom C. 2001. Cost and conflict in animal signals and human language. Proc. Natl Acad. Sci. USA 98, 13 189–13 194. ( 10.1073/pnas.231216498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurd PK. 1995. Communication in discrete action–response games. J. Theor. Biol. 174, 217–222. ( 10.1006/jtbi.1995.0093) [DOI] [Google Scholar]
- 20.Hill GE. 2011. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 14, 625–634. ( 10.1111/j.1461-0248.2011.01622.x) [DOI] [PubMed] [Google Scholar]
- 21.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. 2012. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337, 860–864. ( 10.1126/science.1224286) [DOI] [PubMed] [Google Scholar]
- 22.Kuo T-H, Fedina TY, Hansen I, Dreisewerd K, Dierick HA, Yew JY, Pletcher SD. 2012. Insulin signaling mediates sexual attractiveness in Drosophila. PLoS Genet. 8, e1002684 ( 10.1371/journal.pgen.1002684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough EL, Weingarden PR, Emlen DJ. 2012. Costs of elaborate weapons in a rhinoceros beetle: how difficult is it to fly with a big horn? Behav. Ecol. 23, 1042–1048. ( 10.1093/beheco/ars069) [DOI] [Google Scholar]
- 24.Husak JF, Swallow JG. 2011. Compensatory traits and the evolution of male ornaments. Behaviour 148, 1–29. ( 10.1163/000579510X541265) [DOI] [Google Scholar]
- 25.Kotiaho JS. 2001. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. Camb. Philos. Soc. 76, 365–376. ( 10.1017/S1464793101005711) [DOI] [PubMed] [Google Scholar]
- 26.McCullough EL, Tobalske BW. 2013. Elaborate horns in a giant rhinoceros beetle incur negligible aerodynamic costs. Proc. R. Soc. B 280, 20130197 ( 10.1098/rspb.2013.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trappett A, Condon CH, White C, Matthews P, Wilson RS. 2013. Extravagant ornaments of male threadfin rainbowfish (Iriatherina werneri) are not costly for swimming. Funct. Ecol. 27, 1034–1041. ( 10.1111/1365-2435.12097) [DOI] [Google Scholar]
- 28.McCullough EL, Emlen DJ. 2013. Evaluating the costs of a sexually selected weapon: big horns at a small price. Anim. Behav. 86, 977–985. ( 10.1016/j.anbehav.2013.08.017) [DOI] [Google Scholar]
- 29.Warren IA, Gotoh H, Dworkin I, Emlen DJ, Lavine LC. 2013. A general mechanism for conditional expression of exaggerated sexually-selected traits. Bioessays 35, 889–899. ( 10.1002/bies.201300031) [DOI] [PubMed] [Google Scholar]
- 30.Shingleton AW, Frankino WA. 2012. New perspectives on the evolution of exaggerated traits. Bioessays 35, 100–107. ( 10.1002/bies.201200139) [DOI] [PubMed] [Google Scholar]
- 31.Higham JP. 2014. How does honest costly signaling work? Behav. Ecol. 25, 8–11. ( 10.1093/beheco/art097) [DOI] [Google Scholar]
- 32.Holman L. 2012. Costs and constraints conspire to produce honest signaling: insights from an ant queen pheromone. Evolution 66, 2094–2105. ( 10.1111/j.1558-5646.2012.01603.x) [DOI] [PubMed] [Google Scholar]
- 33.Spence M. 1973. Job market signaling. Q. J. Econ. 87, 355–374. ( 10.2307/1882010) [DOI] [Google Scholar]
- 34.Enquist M. 1985. Communication during aggressive interactions with particular reference to variation in choice of behaviour. Anim. Behav. 33, 1152–1611. ( 10.1016/S0003-3472(85)80175-5) [DOI] [Google Scholar]
- 35.Iwasa Y, Pomiankowski A, Nee S. 1991. The evolution of costly mate preferences II. The ‘handicap’ principle. Evolution 45, 1431–1442. ( 10.2307/2409890) [DOI] [PubMed] [Google Scholar]
- 36.DeWitt TJ, Sih A, Sloan Wilson D. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 37.Clutton-Brock TH, Albon SD. 1979. The roaring of red deer and the evolution of honest advertisement. Behaviour 69, 145–170. ( 10.1163/156853979X00449) [DOI] [Google Scholar]
- 38.Reby D, McComb K. 2003. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 65, 519–530. ( 10.1006/anbe.2003.2078) [DOI] [Google Scholar]
- 39.Maynard Smith J. 1985. Sexual selection, handicaps and true fitness. J. Theor. Biol. 115, 1–8. ( 10.1016/S0022-5193(85)80003-5) [DOI] [PubMed] [Google Scholar]
- 40.van Doorn GS, Weissing FJ. 2006. Sexual conflict and the evolution of female preferences for indicators of male quality. Am. Nat. 168, 742–757. ( 10.1086/508634) [DOI] [PubMed] [Google Scholar]
- 41.Pomiankowski A. 1987. The costs of choice in sexual selection. J. Theor. Biol. 128, 195–218. ( 10.1016/S0022-5193(87)80169-8) [DOI] [PubMed] [Google Scholar]
- 42.Zollman KJS, Bergstrom C, Huttegger SM. 2013. Between cheap and costly signals: the evolution of partially honest communication. Proc. R. Soc. B 280, 20121878 ( 10.1098/rspb.2012.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in paper wasp. Nature 432, 218–222. ( 10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 44.Kotiaho JS. 2000. Testing the assumptions of conditional handicap theory: costs and condition dependence of a sexually selected trait. Behav. Ecol. Sociobiol. 48, 188–194. ( 10.1007/s002650000221) [DOI] [Google Scholar]
- 45.Møller AP, de Lope F. 1994. Differential costs of a secondary sexual character; an experimental test of the handicap principle. Evolution 48, 1676–1683. ( 10.2307/2410256) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Details of the model are available in the electronic supplementary material. The manuscript does not discuss other data.


