Abstract
Receiver bias models of signal evolution are typically regarded as alternatives or complements to ornament evolution due to coevolving mate choice, whereas sexually or socially selected agonistic signals are rarely studied with respect to receiver psychology. Against the background of convergent evolution of red agonistic signals from yellow ancestors in the genus Euplectes (widowbirds and bishops), we experimentally test the function of a yellow signal in the montane marsh widowbird (E. psammocromius), as well as a hypothesized receiver bias for redder (longer wavelength) hues. In a field experiment in southern Tanzania, males that had their yellow wing patches blackened lost their territories or lost territorial contests more often than controls or reddened males, which together with a longer wavelength hue in territory holders, indicates an agonistic signal function. Males painted a novel red hue, matching that of red-signalling congeners, retained their territories and won contests more often than controls. To our knowledge, this is the first demonstration of a receiver bias driving agonistic signal evolution. Although the sensory or cognitive origin of this bias is yet unknown, it strengthens our view that genetically constrained signal production (i.e. carotenoid metabolism), rather than differential selection, explains the carotenoid colour diversification in Euplectes.
Keywords: pre-existing receiver bias, sexual selection, status signalling, signal evolution, Euplectes, carotenoid coloration
1. Introduction
Communication signals may evolve in response to pre-existing (as opposed to coevolving) receiver biases, originating from other adaptive contexts (e.g. foraging) or from inherent sensory or cognitive mechanisms [1–4]. Such ‘receiver–precursor models’ [5], however, are almost invariably discussed and tested with regard to female mating preferences. Given the preoccupation with evolution of mate choice in sexual selection research [6], this focus on epigamic (courtship) signals is understandable, but has also meant a neglect of other kinds of signalling to which receiver–precursor models equally apply. For example, the enormous diversity of agonistic (threat) signals [7,8], in social as well as sexual contexts, are virtually unstudied from a receiver bias perspective (but see [9]).
In this study, we address the evolution of carotenoid-based colour signals in birds, which apart from being classic quality indicators in mate choice [10], also have been frequently found to have an agonistic function [11,12–19]. Specifically, we study a striking carotenoid colour signal diversification with several convergent gains of red displays from ancestral yellow in the African widowbirds and bishops (Euplectes spp.) [20], which also has an intriguing parallel (although without the signal function established) in the North American Icteridae [21]. Moreover, unlike the situation in Icteridae, the convergent gains of red hues in Euplectes are based on distinctly different mechanisms, conversion of dietary yellow pigments to red ketocarotenoids in red ‘bishops’ versus concentration of yellow pigments in red ‘widowbirds’, respectively [22], which suggests different mechanistic solutions to a potentially pre-existing signal selection pressure.
Because receiver bias models of signal evolution entail the bias evolving prior to the signal, predictions and tests typically need to be based on resolved phylogenies and ancestral character state reconstructions [23], as well as on knowledge of the signal function (to design the appropriate experiments). These criteria are uniquely well met in Euplectes: (i) male breeding plumages are black with either carotenoid-based yellow or red (or in a few cases melanin brown) patches on wings (widowbirds) or body (bishops), and an agonistic signalling function of red has been shown in several species [14,18,24]; (ii) there is a robust phylogeny covering all species and 33 of the geographical subspecies [25]; and (iii) an ancestral character state reconstruction suggesting three convergent gains of red from ancestral yellow [20]. Two of these gains have occurred in the widowbird clade, in the fan-tailed widowbird (E. axillaris) and in the long-tailed widowbird (E. progne), whereas the other extant widowbirds (and their reconstructed ancestors) either retain the ancestral yellow hue or have lost carotenoid coloration entirely (E. jacksoni and a subspecies of E. albonotatus) (figure 1).
Figure 1.
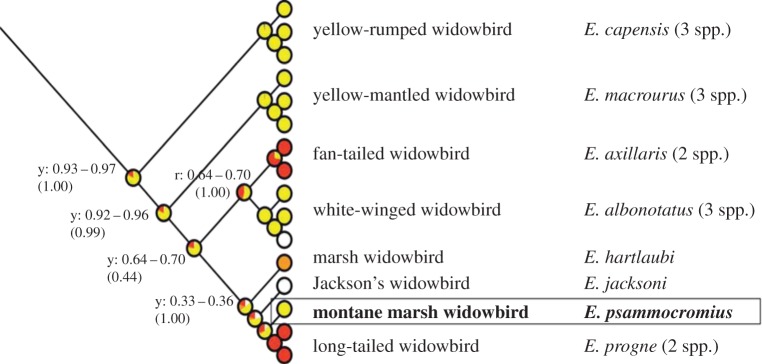
Part of ancestral character state reconstruction of discrete hue states (non-carotenoid (white), yellow (light grey in print), red (dark grey in print)) in Euplectes, showing the clade of eight species (with one to three subspecies) of ‘widowbirds’. Pie charts indicate the proportional likelihoods of the hue states, and number ranges by selected nodes give the posterior probabilities of the most likely hue state. Redrawn from fig. 2 in [16]. (Online version in colour.)
Given a general agonistic function of carotenoid displays in Euplectes, or at least the wing patches in the widowbird clade studied here (figure 1), we may hypothesize that red signals have evolved and been retained as more effective agonistic signals than yellow. Apart from being suggested by the directional and convergent evolution of red in this and other bird taxa [20,21], there are several a priori reasons to expect a pre-existing receiver bias for red. First, demonstrations of generalization (supernormal stimuli) are frequently based on colour contrasts [26]. In particular, recent tests in chickens [27,28] employed stimulus variation in colour hue (although mistakenly termed ‘saturation’ or ‘intensity’ by the authors), in one case along the yellow–red hue axis explored in this study. Second, given generalized female responses to supernormal tail ornamentation in Euplectes [29–31], a generalized male response to supernormal agonistic signals seems quite likely. Third, in saturated carotenoid colours, yellow and red alike, hue (‘redness’) is the only or major colorimetric variable related to differential carotenoid pigmentation [32]. Disregarding whether there is an adaptive (e.g. honest or efficient) component to this hue variation (a discussion not elaborated on here), it sets the stage for red as a supernormal version of yellow. Finally, the colour red is quite commonly associated with threat or warning in birds [18,33–35], which may indicate a widespread perceptual bias that has affected colour signals in both intra- and interspecific contexts. An obvious first prediction from the hypothesized pre-existing bias for red is that experimental reddening of a yellow signal should increase competitive dominance. We test this prediction in the yellow-signalling montane marsh widowbird (Euplectes psammocromius), a close relative to the red-signalling long-tailed widowbird (figure 1). To our knowledge, this represents the first experimental test of a receiver bias behind the evolution of agonistic colour signals in birds.
2. Material and methods
(a). Study population and study species
The study was undertaken from December 2011 to February 2012 on a population of montane marsh widowbirds breeding in the Mtitu Valley, Southern Highlands, Tanzania (08°12′ S, 35°48′ E). The study site was situated at approximately 1900 m.a.s.l., with habitat consisting of a grassland—agriculture mosaic in the valley bottom, and a grassland–pine (planted) mosaic on the slopes. Montane marsh widowbirds are seasonally and sexually dimorphic, with adult males undertaking a prenuptial moult from a drab brown plumage to a breeding plumage consisting of entirely jet-black feathers (including a graduated elongated tail), with the exception of yellow carotenoid-pigmented lesser wing coverts flanked by light-brown (‘buff’) medium wing coverts. As with all Euplectes, montane marsh widowbirds are polygynous; males compete to occupy grassland territories (approx. 50 × 50 m; CE Ninnes 2012, unpublished data) from which they display to attract females to nest and breed (without male assistance except for, presumably, the initial nest ring or frame [36]).
(b). Experimental subjects and sampling
Potential experimental subjects (territory-holding males) were observed for 2 h prior to a catching attempt. The purpose of this was to establish that the focal individual was indeed the territory holder and to obtain an estimate of the territory boundaries to facilitate both the catching process, and subsequent observations of territorial boundary contests and territory retention. Target subjects were then captured on their territories using an ‘E-Z Catch’ bird trap (WCS) with a model intruder placed on it. Following successful capture, the subject was transferred to the nearby camp where it was immediately processed. Processing involved (i) ringing and collecting morphometric measurements (mass, tarsus length, wing length and tail length); (ii) measuring the spectral characteristics of the yellow colour patch (lesser wing coverts); and (iii) manipulation of the colour patch, using Copic art markers (Too Marker Products, Tokyo, Japan), to one of three treatments: red (R08), black (100) or a yellow control (Y13). Spectral reflectance measurements were then taken again after manipulation, whereupon the bird was returned to its territory and released. This process, from capture to release, was never longer than 60 min, and birds were offered water throughout.
Additionally, a ‘random’ sample of the male population, including both territorial and non-territorial (‘floater’) males in unknown proportions, was collected at dawn at the communal night roost (tall reeds in the valley bottom) using mist nets (ECOTONE). These random males were ringed and measured as above, but not colour manipulated.
(c). Reflectance and colorimetrics
Reflectance spectrometry methods and objective colorimetrics follow Andersson & Prager [32] and are identical to earlier studies (e.g. [5,15]). Briefly, we used a USB2000 spectroradiometer system (Ocean Optics Inc., Dunedin, FL, USA), including a fibre-optic reflectance probe, illumination from a HL2000 halogen light source and C-Spec software (Ancal Inc., Las Vegas, NV, USA). A WS-2 white reference was scanned prior to measuring each individual. We used a ‘coincident normal’ measuring configuration (coaxial illumination and reading beams, perpendicular to the plumage plane), with a homemade probe holder fitted on the probe, taking three scans and removing the probe between each. We computed brightness (RAV), carotenoid chroma (CChr) and hue (λR50, wavelength at which reflectance is halfway between its minimum and its maximum), but as in previous analyses of saturated carotenoid colours, hue (spectral location) was the most informative and relevant aspect of the signal variation, and the only colorimetric presented. For further details, see Andersson & Prager [32].
To explore natural relationships between male characteristics and competitiveness, we compared pre-manipulation hue and morphometrics between territorial and random males.
(d). Behavioural observations
Subsequent to release, daily continuous focal sampling of each experimental subject (n = 27) was undertaken. Focal sampling involved observing (from more than 50 m) a territory for a maximum of 15 min, until the male was sighted, followed by a 15 min continuous sampling period. If the male was not sighted within 15 min, it was recorded as not present. The main categories recorded during observation periods were: outcomes of boundary contests (see below), presence of females on the territory and the number of courtship dances performed by focal males; a courtship dance is an elaborate perched display directed at a female in very close proximity, thus requiring the females receptiveness, and presumably reflecting interest in the male (CE Ninnes 2012, unpublished data).
(e). Territory retention
Territory retention was scored as ‘retained’ when the experimental subject was observed on his territory during the daily focal sampling, and successfully repelled any males that may have attempted to invade that territory. If the subject was not observed for two consecutive observations (days), the territory was classified as lost. There were no instances of an individual reappearing after being absent from its territory for two consecutive observations.
(f). Boundary contests
Boundary contests with neighbouring males were regularly observed during observation periods. These contests involved both males approaching the (estimated) boundary of their adjacent territories and performing rapid wing flicks (from the ground), and small advances and retreats towards each other, while in close proximity to each other. The loser of the contest was scored as the first male to abandon the contest and retreat (fly) back to the centre of his territory.
(g). Statistical analysis
Parametric tests were used to explore differences in morphometrics and colorimetrics between territorial and random males, as assumptions of normality and homogeneity of variances were met. A generalized linear model (binomial distribution) was used to test for differences in territory retention across treatments. To test for differences in the outcomes of boundary contests between treatments, we used a one-way ANOVA with ‘% wins’ (number of contests won divided by total number of contests) as the response variable. Because assumptions of normality were not fully satisfied, we also analysed these data using generalized linear models, and non-parametric tests (Kruskal–Wallis), as well as converting them to proportions, arcsine transforming them and repeating the analysis. Results were almost identical under these alternative analyses. Additionally, we performed the above analyses for boundary contests using ‘wins–losses’ as the response variable, which again, did not change the outcomes from the previous analyses.
Because we had strong a priori predictions regarding the effect directions (i.e. that blackening would be neutral or reduce dominance, and that reddening would be neutral or increase dominance), we calculated p-values from our test statistics that take these directional predictions into account [37], using ordered heterogeneity (OH) tests, as described by Rice & Gaines [38,39]. OH tests were not applied when the non-directional test statistic was highly significant.
3. Results
(a). Treatments
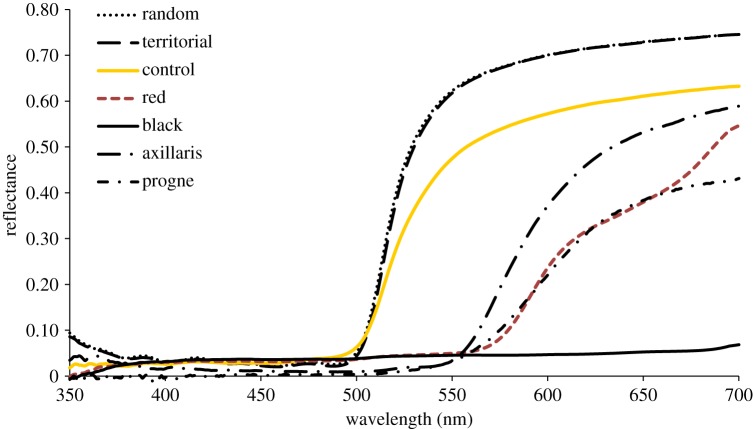
Figure 2 shows reflectance curves of the three manipulation treatments as compared to unmanipulated E. psammocromius, as well as to the two most closely related red-coloured species (E. progne and E. axillaris; see figure 1). The hue (spectral location) of the red treatment corresponded closely to that of E. progne. The rise in reflectance at the longwave end of the red manipulation spectra occurs beyond 650 nm, where spectral sensitivity steeply declines and hue discrimination is lost in both birds and man (e.g. [5]), and even if perceptible, the manipulation would simply be slightly ‘redder’ than our objective hue metric (λR50) implies.
Figure 2.
Mean reflectance curves for random, territorial (pre-treatment) and post-treatment (control, red, black) E. psammocromius, and for E. axillaris and E. progne (S Andersson 2004, unpublished data). (Online version in colour.)
No differences were detected across treatments in pre-manipulation hue (F = 2.15, p = 0.14), tail length (F = 0.29, p = 0.75), mass (F = 0.84, p = 0.44) or tarsus (F = 0.47, p = 0.63). In four cases, experimental males (two control, one red, one black) dropped their tails while in the bird-bag following capture. Although the sample size of these incidents was small, we examined the post-release outcomes for these individuals. All of these individuals retained their territories.
(b). Territory retention
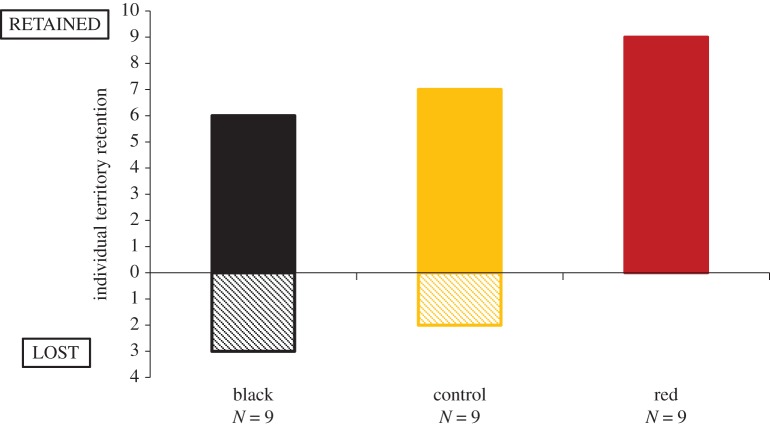
Significant differences in the hypothesized direction were found between treatments in territory retention (p < 0.02, OH test (of χ2 = 4.88, d.f. = 2, p = 0.087)), with 100% of red treatment birds, 78% of control birds and 67% of black treatment birds retaining their territories (figure 3).
Figure 3.
Territory retention across treatments. (Online version in colour.)
(c). Boundary contests
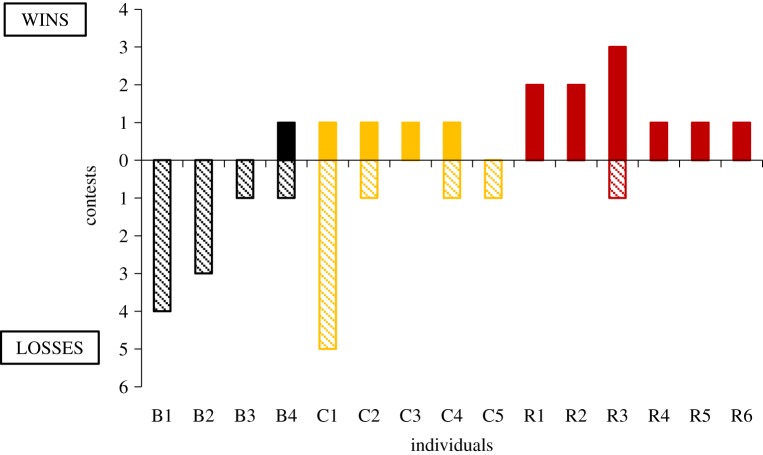
Highly significant differences in the hypothesized direction were found between treatments in the outcomes of boundary contests (F = 12.93, d.f. = 2, p = 0.001; figure 4). The mean percentage of wins across individuals within each treatment was: red 95.8%, control 43.4% and black 12.5%.
Figure 4.
Results of boundary contests for individuals from the three treatments (‘B’ = black, ‘C’ = control, ‘R’ = red). An LSD test revealed that males from the red treatment won significantly more contests than males from control (p = 0.006) or black (p < 0.001) treatments, and control males won contests more often than black treatments (p < 0.02, OH test (of LSD value of p = 0.1) [30]). (Online version in colour.)
(d). Differences between territorial and ‘random’ males
Territorial males had statistically significantly longer wavelength hue, longer tarsus, longer wing and larger mass, than did random males; however, no difference in tail length was found between territorial and random males (table 1).
Table 1.
Colour and morphometrics between territorial and random males.
| mean territorial | s.d. territorial | mean random | s.d. random | t | d.f. | p (two-tailed) | |
|---|---|---|---|---|---|---|---|
| hue (λR50) | 520.85 | 1.78 | 519.79 | 1.94 | 2.194 | 61 | 0.032 |
| tars (mm) | 32.27 | 0.72 | 31.71 | 1.12 | 2.292 | 64 | 0.025 |
| wing (mm) | 106.22 | 3.19 | 103.85 | 4.20 | 2.482 | 64 | 0.016 |
| mass (g) | 41.64 | 2.15 | 39.31 | 2.94 | 3.482 | 62 | 0.001 |
| tail (mm) | 277.22 | 31.19 | 275.16 | 27.49 | 0.267 | 58 | 0.790 |
(e). Female interest
Across treatments, there were no differences in the percentage of observations in which females were observed with the focal male (F = 1.49, p = 0.25), or in the number of courtship dances performed (F = 0.97, p = 0.4), indicating that female preferences were not affected by the colour manipulations.
4. Discussion
Based on the directional and convergent evolution of red agonistic signals in the genus Euplectes [20], including two likely independent gains of red (from yellow) in the subclade of eight widowbird species (figure 1), we hypothesized that a pre-existing receiver bias for red may be present in the yellow-signalling montane marsh widowbird. We experimentally tested and corroborated two predictions of this hypothesis, finding that reddened males were more successful at retaining their territories, and won boundary contests significantly more often.
Although many studies have found evidence of pre-existing receiver biases driving epigamic (courtship) signal evolution, e.g. swordtail fish [40–43], splitfins [44] and túngara frogs [45,46], very few studies have tested receiver–precursor models in the context of agonistic signalling, in which they are just as likely to apply. Ryan & Rand [47] found a receiver bias for enhanced responses to artificially elaborate calls in both sexes of túngara frogs, whereas no evidence for a pre-existing male bias for male dorsal coloration was found in Sceloporus minor [9]. This study is the first, to our knowledge, to experimentally indicate a receiver bias for a male–male contest signal. This reinforces that pre-existing receiver biases may be significant drivers of agonistic sexual signal design and exaggeration, perhaps even relatively more so than for epigamic (courtship) signals, in which genetic coevolutionary processes, such as Fisherian runaway selection, are likely to be strongly involved. Testing for this pre-existing bias in additional yellow-signalling Euplectes, as well as other weaverbirds (family Ploceidae), especially taxa representing older lineages, will help elucidate its evolutionary origins.
As regards the adaptive (in another context) versus inherent perceptual origin and maintenance of this receiver bias (see Introduction), we have little substantial evidence that, for example, redness is mechanistically linked to fighting ability, or that more longwave carotenoid hues increase male conspicuousness (efficacy). The generally assumed condition-dependence and honesty of carotenoid displays of course apply also to Euplectes and are best supported by (i) that wild-caught fan-tailed widowbird (E. axillaris) males with blackened wing patches, settled captive dominance interactions according to the original (‘pre-manipulation’) patch size and redness [14], and (ii) that there is an intraspecific trade-off between carotenoid signal redness and tail length, suggesting both signals to be costly and thus ‘honest’ signals [48].
Most pertinent to an adaptive origin of the receiver bias is whether yellow plumage patches in this and other Euplectes also are agonistic signals, and whether these also may be honest quality advertisements. Although not conclusively shown, an agonistic function is supported by the combination of descriptive and experimental results: first, the longer wavelength yellow hue of territorial males compared to random males, echoes the difference in red hue between territorial and floater males in the red-collared widowbird (E. ardens) [48], in which agonistic colour signalling is clearly shown. Although the hue difference was very small in this study, it represents a conservative estimate since the ‘random’ sample likely also included many territorial birds. Second, that experimentally blackened individuals more often failed to retain their territories, and more often lost contests than controls, also suggests a decrease in competitiveness (as in e.g. [15,24]) and a role of the yellow wing patch in agonistic signalling. However, even though consistent with the experimental studies of red ‘status signalling’ in E. axillaris [14] and E. ardens [24], the effect sizes in this study are notably smaller than in the studies of these red-coloured relatives, suggesting that yellow carotenoid signals, perhaps generally, may be less variable and less informative (of fighting ability) than are red signals; possibly because the latter are costlier to produce and thus have a larger ‘content’ or ‘strategic’ component [5,49]. There appears to be few studies of agonistic yellow plumage signals in birds (but see [50]), and further comparisons between yellow and red carotenoid signalling in Euplectes as well as other taxa would be interesting.
As regards epigamic versus agonistic sexual selection in this species, the fact that no differences were found in female interest across males in different treatments suggests that the carotenoid colour signal is of minor or no importance in mate choice, as seems to be the case in a more long-tailed congener [51]. Moreover, that no difference in tail length was found between territorial and random males (the only morphometric variable that was not significantly different), and that none of the experimental males that lost their tails lost their territories, suggests that tail length is not important in male–male competition (but see [52]) and corroborates the ‘multiple receiver hypothesis’ for the co-occurrence of multiple costly ornaments [48].
With this study suggesting an open-ended selection pressure for longer wavelength hues in montane marsh widowbirds, why does this species not display red coloration? Adaptive explanations, i.e. selection against red or redness in other contexts, such as species recognition (e.g. mate recognition; see [53]), which may explain the indication of stabilizing mate choice selection on tail elongation in a congener (Euplectes orix; [30]), seem unlikely here: first, because also males with blackened signals continued to attract and court females, and second, because all experimental individuals attracted attention only from conspecifics, and not from sympatrically occurring E. axillaris (even in cases where reddened experimental male montane marsh widowbirds had lost their tail and appeared, to a human observer, very similar to male E. axillaris).
The emerging picture, and future challenge to unveil, is instead that physiological or genetic constraints are preventing the evolution of red in this and other yellow-signalling Euplectes. The presence of at least two, possibly more, ‘red-mechanisms’ (ketocarotenoid synthesis and pigment concentration, respectively; [22]), strongly suggests a qualitative or quantitative change in carotenoid metabolism that either has not appeared or is too costly to express in the yellow-coloured species.
In summary, by providing experimental evidence for a pre-existing receiver bias for a novel agonistic signal, this study has demonstrated the potential for receiver–precursor models to explain agonistic sexual selection and signal evolution. Further tests of both yellow and red signal mechanisms and signal selection pressures in other species, in particular lineages deeper in the Euplectes and weaverbird phylogenies, will extend on the findings reported here and better resolve this fascinating colour signal diversification.
Acknowledgements
We thank Leons Mlawila, Maneno, Jon Berge, Johan Berlin and Joel Almqvist for field assistance, and Neil and Liz Baker and the Phillips family for advice, logistic support and hospitality. Thanks to Mats Olsson for helpful comments on an early draft, and to the editor and two anonymous reviewers for thoughtful and constructive suggestions and improvements. This study was approved by the Tanzania Wildlife Research Institute (TAWIRI), kindly arranged and hosted by Professor Gabriel Mbassa, Sokoine University of Agriculture, Morogoro.
Data accessibility
Data will be made available 1 year after publication on Dryad: doi:10.5061/dryad.m69t2.
Funding statement
For financial support, we thank the Swedish Research Council (to S.A.), Swedish Academy of Sciences (K.V.A.), Helge Ax:son Johnsons Stiftelse, Wilhelm and Martina Lundgrens Vetenskapsfond, Lars Hiertas Minne Stiftelsen, Adlerbertska Stipendiestiftelsen (to C.E.N.).
References
- 1.Ryan MJ, Cummings ME. 2013. Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 44, 437–459. ( 10.1146/annurev-ecolsys-110512-135901) [DOI] [Google Scholar]
- 2.Endler JA, Basolo AL. 1998. Sensory ecology, receiver biases and sexual selection. TREE 13, 415–420. ( 10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- 3.Arak A, Enquist M. 1993. Hidden preferences and the evolution of signals. Phil. Trans. R. Soc. Lond. B 340, 207–213. ( 10.1098/rstb.1993.0059) [DOI] [Google Scholar]
- 4.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. ( 10.2307/2462431) [DOI] [Google Scholar]
- 5.Bradbury JW, Vehrencamp SL. 2011. Principles of animal communication, 2nd edn Sunderland, MA: Sinauer Associates. [Google Scholar]
- 6.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Andersson M. 1980. Why are there so many threat displays? J. Theor. Biol. 86, 773–781. ( 10.1016/0022-5193(80)90310-0) [DOI] [PubMed] [Google Scholar]
- 8.Butcher GS, Rohwer S. 1989. The evolution of conspicuous and distinctive coloration for communication in birds. In Current ornithology (ed. Power DM.), pp. 51–108. New York, NY: Springer. [Google Scholar]
- 9.Stephenson B, Ramírez-Bautista A. 2012. Did sexually dimorphic dorsal coloration evolve by a pre-existing bias in males in the lizard Sceloporus minor? Evol. Ecol. 26, 1277–1291. ( 10.1007/s10682-011-9551-1) [DOI] [Google Scholar]
- 10.Hill GE. 2006. Female mate choice for ornamental colouration. In Bird coloration (eds Hill GE, McGraw KJ.), pp. 137–200. Cambridge, UK: Harvard University Press. [Google Scholar]
- 11.Eckert CG, Weatherhead PJ. 1987. Ideal dominance distributions: a test using red-winged blackbirds (agelaius phoeniceus). Behav. Ecol. Sociobiol. 20, 43–52. ( 10.1007/bf00292165) [DOI] [Google Scholar]
- 12.Murphy TG, Hernandez-Mucino D, Osorio-Beristain M, Montgomerie R, Omland KE. 2009. Carotenoid-based status signaling by females in the tropical streak-backed oriole. Behav. Ecol. 20, 1000–1006. ( 10.1093/beheco/arp089) [DOI] [Google Scholar]
- 13.Peek FW. 1972. Experimental study of territorial function of vocal and visual display in male red-winged blackbird (Agelaius phoeniceus). Anim. Behav. 20, 112–118. ( 10.1016/s0003-3472(72)80180-5) [DOI] [PubMed] [Google Scholar]
- 14.Pryke SR, Andersson S. 2003. Carotenoid-based epaulettes reveal male competitive ability: experiments with resident and floater red-shouldered widowbirds. Anim. Behav. 66, 217–224. ( 10.1006/anbe.2003.2193) [DOI] [Google Scholar]
- 15.Pryke SR, Andersson S. 2003. Carotenoid-based status signalling in red-shouldered widowbirds (Euplectes axillaris): epaulet size and redness affect captive and territorial competition. Behav. Ecol. Sociobiol. 53, 393–401. ( 10.1007/s00265-003-0587-2) [DOI] [Google Scholar]
- 16.Pryke SR, Griffith SC. 2006. Red dominates black: agonistic signalling among head morphs in the colour polymorphic Gouldian finch. Proc. R. Soc. B 273, 949–957. ( 10.1098/rspb.2005.3362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryke SR, Lawes MJ, Andersson S. 2001. Agonistic carotenoid signalling in male red-collared widowbirds: aggression related to the colour signal of both the territory owner and model intruder. Anim. Behav. 62, 695–704. ( 10.1006/anbe.2001.1804) [DOI] [Google Scholar]
- 18.Santos ESA, Scheck D, Nakagawa S. 2011. Dominance and plumage traits: meta-analysis and metaregression analysis. Anim. Behav. 82, 3–19. ( 10.1016/j.anbehav.2011.03.022) [DOI] [Google Scholar]
- 19.Smith DG. 1972. Role of epaulets in red-winged blackbird, (Agelaius phoeniceus) social system. Behaviour 41, 251–268. ( 10.1163/156853972x00040) [DOI] [Google Scholar]
- 20.Prager M, Andersson S. 2010. Convergent evolution of red carotenoid coloration in widowbirds and bishops (Euplectes spp.). Evolution 64, 3609–3619. ( 10.1111/j.1558-5646.2010.01081.x) [DOI] [PubMed] [Google Scholar]
- 21.Friedman NR, Kiere LM, Omland KE. 2011. Convergent gains of red carotenoid-based coloration in the New World blackbirds. Auk 128, 678–687. ( 10.1525/auk.2011.11117) [DOI] [Google Scholar]
- 22.Andersson S, Prager M, Johansson EIA. 2007. Carotenoid content and reflectance of yellow and red nuptial plumages in widowbirds (Euplectes spp.). Funct. Ecol. 21, 272–281. ( 10.1111/j.1365-2435.2007.01233.x) [DOI] [Google Scholar]
- 23.Omland KE, Hofmann CM. 2006. Adding color to the past: ancestral-state reconstruction. In Bird coloration (eds Hill GE, McGraw KJ.), pp. 417–454. Cambridge, MA: Harvard University Press. [Google Scholar]
- 24.Pryke SR, Andersson S, Lawes MJ, Piper SE. 2002. Carotenoid status signaling in captive and wild red-collared widowbirds: independent effects of badge size and color. Behav. Ecol. 13, 622–631. ( 10.1093/beheco/13.5.622) [DOI] [Google Scholar]
- 25.Prager M, Johansson EIA, Andersson S. 2008. A molecular phylogeny of the African widowbirds and bishops, Euplectes spp. (Aves: Passeridae: Ploceinae). Mol. Phylogenet. Evol. 46, 290–302. ( 10.1016/j.ympev.2007.09.010) [DOI] [PubMed] [Google Scholar]
- 26.Ghirlanda S, Enquist M. 2003. A century of generalization. Anim. Behav. 66, 15–36. ( 10.1006/anbe.2003.2174) [DOI] [Google Scholar]
- 27.Jansson L, Enquist M. 2003. Receiver bias for colourful signals. Anim. Behav. 66, 965–971. ( 10.1006/anbe.2003.2249) [DOI] [Google Scholar]
- 28.Jansson L, Enquist M. 2005. Testing the receiver bias hypothesis empirically with ‘virtual evolution’. Anim. Behav. 70, 865–875. ( 10.1016/j.anbehav.2005.02.008) [DOI] [Google Scholar]
- 29.Pryke SR, Andersson S. 2002. A generalized female bias for long tails in a short-tailed widowbird. Proc. R. Soc. Lond. B 269, 2141–2146. ( 10.1098/rspb.2002.2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pryke SR, Andersson S. 2008. Female preferences for long tails constrained by species recognition in short-tailed red bishops. Behav. Ecol. 19, 1116–1121. ( 10.1093/beheco/arn100). [DOI] [Google Scholar]
- 31.Andersson M. 1982. Female choice selects for extreme tail length in a widowbird. Nature 299, 818–820. ( 10.1038/299818a0) [DOI] [Google Scholar]
- 32.Andersson S, Prager M. 2006. Quantifying colors. In Bird coloration (eds Hill GE, McGraw KJ.), pp. 41–89. Cambridge, MA: Harvard University Press. [Google Scholar]
- 33.Pryke SR. 2009. Is red an innate or learned signal of aggression and intimidation? Anim. Behav. 78, 393–398. ( 10.1016/j.anbehav.2009.05.013) [DOI] [Google Scholar]
- 34.Senar JC. 2006. Color displays as intrasexual signals of aggression and dominance. In Bird coloration (eds Hill GE, McGraw KJ.), pp. 87–136. Cambridge, UK: Harvard University Press. [Google Scholar]
- 35.Svadova K, Exnerova A, Stys P, Landova E, Valenta J, Fucikova A, Socha R. 2009. Role of different colours of aposematic insects in learning, memory and generalization of naive bird predators. Anim. Behav. 77, 327–336. ( 10.1016/j.anbehav.2008.09.034) [DOI] [Google Scholar]
- 36.Craig AJFK. 1980. Behaviour and evolution in the genus Euplectes. J. Ornithol. 121, 144–161. [Google Scholar]
- 37.Rice WR, Gaines SD. 1994. ‘Heads I win, tails you lose’: testing directional alternative hypotheses in ecological and evolutionary research. TREE 9, 235–237. ( 10.1016/0169-5347(94)90258-5) [DOI] [PubMed] [Google Scholar]
- 38.Rice WR, Gaines SD. 1994. Extending nondirectional heterogeneity tests to evaluate simply ordered alternative hypotheses. Proc. Natl Acad. Sci. USA 91, 225–226. ( 10.1073/pnas.91.1.225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice WR, Gaines SD. 1994. The ordered-heterogeneity family of tests. Biometrics 50, 746–752. ( 10.2307/2532788) [DOI] [Google Scholar]
- 40.Basolo AL. 1990. Female preference predates the evolution of the sword in swordtail fish. Science 250, 808–810. ( 10.1126/science.250.4982.808) [DOI] [PubMed] [Google Scholar]
- 41.Basolo AL. 1995. A further examination of a pre-existing bias favouring a sword in the genus Xiphophorus. Anim. Behav. 50, 365–375. ( 10.1006/anbe.1995.0252) [DOI] [Google Scholar]
- 42.Basolo AL. 1995. Phylogenetic evidence for the role of a pre-existing bias in sexual selection. Proc. R. Soc. Lond. B 259, 307–311. ( 10.1098/rspb.1995.0045) [DOI] [PubMed] [Google Scholar]
- 43.Basolo AL. 2002. Congruence between the sexes in preexisting receiver responses. Behav. Ecol. 13, 832–837. ( 10.1093/beheco/13.6.832) [DOI] [Google Scholar]
- 44.Garcia CM, Ramirez E. 2005. Evidence that sensory traps can evolve into honest signals. Nature 434, 501–505. ( 10.1038/nature03363) [DOI] [PubMed] [Google Scholar]
- 45.Ryan MJ, Fox JH, Wilczynski W, Rand AS. 1990. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 343, 66–67. ( 10.1038/343066a0) [DOI] [PubMed] [Google Scholar]
- 46.Ryan MJ, Rand AS. 1993. Sexual selection and signal evolution: the ghost of biases past. Phil. Trans. R. Soc. Lond. B 340, 187–195. ( 10.1098/rstb.1993.0057) [DOI] [Google Scholar]
- 47.Ryan MJ, Rand AS. 1998. Evoked vocal response in male túngara frogs: pre-existing biases in male responses? Anim. Behav. 56, 1509–1516. ( 10.1006/anbe.1998.0928) [DOI] [PubMed] [Google Scholar]
- 48.Andersson S, Pryke SR, Ornborg J, Lawes MJ, Andersson M. 2002. Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 160, 683–691. ( 10.1086/342817) [DOI] [PubMed] [Google Scholar]
- 49.Andersson S. 2000. Efficacy and content in avian colour signals. In Animal signals: signalling and signal design in animal communication (eds Epsmark Y, Amundsen T, Rosenqvist G.), pp. 47–60. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 50.Beck ML. 2013. Nest-box acquisition is related to plumage coloration in male and female Prothonotary warblers (Protonotaria citrea). Auk 130, 364–371. ( 10.1525/auk.2013.12157) [DOI] [Google Scholar]
- 51.Pryke SR, Andersson S, Lawes MJ. 2001. Sexual selection of multiple handicaps in the red-collared widowbird: female choice of tail length but not carotenoid display. Evolution 55, 1452–1463. ( 10.1554/0014-3820(2001)055[1452:ssomhi]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 52.Savalli UM. 1994. Tail length affects territory ownership in the yellow-shouldered widowbird. Anim. Behav. 48, 105–111. ( 10.1006/anbe.1994.1216) [DOI] [Google Scholar]
- 53.Mendelson TC, Shaw KL. 2012. The (mis)concept of species recognition. TREE 27, 421–427. ( 10.1016/j.tree.2012.04.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available 1 year after publication on Dryad: doi:10.5061/dryad.m69t2.