Abstract
Non-genetic transmission of information across generations, so-called parental effects, can have significant impacts on offspring morphology, physiology, behaviour and life-history traits. In previous experimental work using the two-spotted spider mite Tetranychus urticae Koch, we demonstrated that dispersal distances increase with local density and levels of genetic relatedness. We here show that manipulation of parental and grand-parental density has a significant effect on offspring dispersal distance, of the same order of magnitude as manipulation of offspring density. We demonstrate that offspring exposed to the same density disperse further if they were born to parents exposed to higher density compared with parents exposed to low density. Offspring dispersal distance also increases when grand-parents were exposed to higher density, except for offspring exposed to low densities, which disperse at shorter distances whatever the grand-parental density. We also show that offspring from mothers exposed to higher densities were overall larger, which suggests that parents in high densities invest more in individual offspring, enabling them to disperse further. We propose that our findings should be included in models investigating the spread rate of invasive species or when predicting the success of conservation measures of species attempting to track changing climates.
Keywords: density, maternal effects, plasticity, conditional dispersal, invasion
1. Introduction
Phenotypic plasticity occurs when different phenotypes are produced from a single genotype depending on the environment [1]. Behaviour is often regarded to be the most plastic aspect of animal phenotypes [1,2], and there is increasing evidence that behavioural plasticity, allowing natural populations to, e.g. shift distribution or temporal activity, helps to mitigate the impacts of global changes, such as climate warming and habitat fragmentation [2–4]. An important plastic behavioural trait is dispersal, the movement of individuals from one breeding site to another. It has evolved as a mechanism to avoid inbreeding, kin competition and in response to spatio-temporal heterogeneity [5,6]. As habitat fragmentation increases and as species are forced to shift their ranges in response to climate change, the distances organisms disperse and the mechanisms that affect dispersal distance decisions are becoming increasingly relevant in the fields of invasion ecology and range expansion [7]. It is likely that dispersal distance strategies, in addition to emigration strategies, can also be plastic [8–10]. In this regard, we recently showed that the distances individuals disperse can respond plastically to population density and the level of relatedness within a population [11,12].
One mechanism producing behavioural plasticity and driving epigenetic inheritance of behavioural traits is parental effects, i.e. effects that parents have on the phenotype of their offspring that are unrelated to the offspring's own genotype [13,14]. Parental effects have been found in a wide range of taxa and traits including dispersal [6,12], with most studies focusing specifically on maternal effects rather than paternal (but see [15,16]). This non-genetic (or epigenetic) transmission of information can also be transferred from grand-mothers to grand-offspring [17]. It has been predicted in particular that mothers exposed to higher densities [18], or reproducing in patches of deteriorating quality [19], should increase the proportion of dispersing offspring. Maternal effects, through changes in offspring sensitivity to density, could further affect population dynamics by introducing population cycling [20,21].
These theoretical developments on the evolution of emigration are supported by a wide variety of empirical literature available on the effects of maternal density on offspring emigration propensity. High density may indicate increased competition over various resources for the offspring, including space and food [10,22,23]. For example, pea aphid (Acyrthosiphon pisum) mothers increase the ratio of dispersal morphs in their progeny in anticipation of crowded offspring conditions [24]. Mothers have been shown to increase offspring body size in response to density or food availability [25,26], or produce more aggressive offspring in response to availability of nest sites [27]. In turn, these phenotypic traits may alter the offspring dispersal ability, as they are often associated in dispersal syndromes [28,29]. For example, in the marine bryozoan Bugula neritina, mothers from high-density environments produced fewer but larger offspring, enabling them to disperse from crowded conditions [22].
While the majority of theoretical and empirical studies examining the relationship between maternal environment and offspring dispersal focus on offspring emigration, other important aspects of dispersal were found to be affected by maternal effects, such as colonization success [30], range expansion [27] and dispersal distance [10]. In the sole theoretical study investigating the evolution of dispersal distances when the trait is under maternal versus offspring control, Kokko & Starrfelt [31] predicted that offspring dispersal distances should be longer when mothers were in control (see Motro [32] for similar findings on emigration rates). To the best of our knowledge, the effect of parental density on the dispersal distance of offspring in an actively dispersing species has not yet been empirically addressed (but see Donohue [23] and Wender [10] for a passively dispersed organism).
We use the two-spotted spider mite, Tetranychus urticae, to test whether parental and grand-parental density influences the dispersal distances of their offspring. In this species, maternal effects have been found to influence offspring fecundity, sex ratio, diapause incidence and aerial dispersal behaviour [33–35], demonstrating that mothers can alter the phenotype of their offspring based on their own environment. As a consequence of a short generation time, high population growth rates and the fact that mothers and offspring are often found on the same leaf [36], parents may use population densities as an indication of the future environmental conditions of their offspring. High-density conditions have been shown to negatively impact T. urticae growth rate and body size [37], and density was shown to be a cue used by mothers to control offspring diapause incidence [34], suggesting that maternal density is likely to be a reliable predictor of offspring environment. Moreover, we identified some trace of grand-maternal effects on offspring dispersal distance in a large-scale experiment of artificial selection aimed at increasing or decreasing dispersal distance [38]. Thus, we expect that mothers and grand-mothers use density as a cue to inform their offspring about how far they should disperse.
2. Material and methods
(a). Organism
Tetranychus urticae is a haplo-diploid polyphagous mite that feeds on hundreds of plants and industrial crops. Females can lay up to 10 eggs per day for about 10 days, and the life cycle can complete in 10 days depending on host plant and temperature [39]. The base population (BP) was composed of the ‘LS-VL’ strain of T. urticae spider mites collected in October 2000 from roses in a garden near Ghent, Belgium and since then maintained on potted Phaseolus vulgaris plants, variety ‘Prelude’ (named ‘bean’ hereafter), in a climatically controlled room at 26.5 ± 1°C, 60% relative humidity, and 16 L : 8 D photoperiod with a population size of about 5000 mites [40].
In nature, population densities vary from less than 0.1 individual cm−2 to up to 50 individuals cm−2 depending on the host plant and the state of the infestation [39,41]. Under controlled conditions, when resources are scarce and population densities become too high, mites disperse individually either by ambulatory [42] or aerial means [43]. Newly emerged 1- to 2-day old mated adult females are considered to be the dispersers [43]. We tested parental effects using three densities: 2.5, 12.5 and 25 individuals cm−2 following Bitume et al. [11]. We considered the lowest experimental density as the most favourable environment, and the highest density as the most stressful environment.
(b). Manipulation of (grand)parental densities
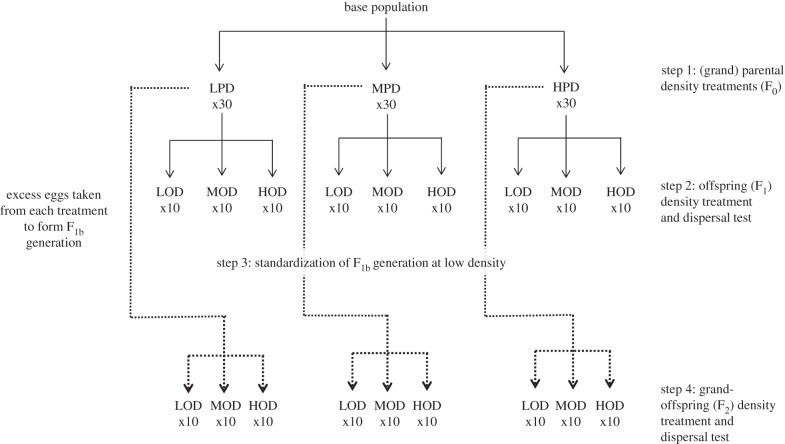
We aimed to measure the effect of density in the parental and grand-parental environments on the distance dispersed by their offspring. As offspring dispersal distance is known to vary with the density that they themselves experience [11], we further explored how information about (grand)parental density and offspring density are combined to influence dispersal distance. We carried out a full two-way factorial experiment in which we tested the distance dispersed by two successive generations of offspring whose parent (F1 offspring generation), or grand-parents (F2 offspring generation), had been exposed to one of three different densities. In short, all individuals developed and were kept at low density (less than 2.5 individuals cm−2) except when density was manipulated to produce the treatments (for detailed information on the protocol, please see the electronic supplementary material, I). Young adult females (F0 generation; figure 1) were placed at low (2.5 individuals cm−2; low parental density, LPD), medium (12.5 individuals cm−2; medium parental density, MPD) or high (25 individuals cm−2; high parental density, HPD) densities for 4 days, and males were added for 24 h to allow fecundation. On the fifth day, females were isolated and allowed to lay eggs for 24 h (F1 generation). To test for grand-parental effects on dispersal distance, the F0 females produced more offspring (F1b generation) that were allowed to lay eggs individually (F2 offspring generation; step 4). These F2 offspring thus had mothers whose density was low and common to all of them but whose grand-parents were exposed to the three different density treatments. Because we were unable to expose the F1b generation to all three densities owing to practical limitations, we chose to standardize the F1b generation at low density to represent the least stressful environment. Adult females from the F1 and the F2 generations were assessed for dispersal distance in the three densities (steps 2 and 4, respectively, in figure 1 and see §2c).
Figure 1.
Scheme of the experimental design. (Step 1) Families formed from the base population (parental generation, F0) were exposed to one of three densities (low, LPD: 2.5 individuals cm−2, medium, MPD: 12.5 individuals cm−2, and high, HPD: 25 individuals cm−2). (Step 2) Their offspring (generation F1) were then marked, divided and placed on a starting patch containing one of three densities (low, medium and high) and the dispersal distance of the offspring was measured. To determine whether there were grand-parental effects present on dispersal distance, excess offspring from the parental generation exposed to the different densities (generation F1b) were collected separately and allowed to develop at a low standardized density (step 3). (Step 4) These offspring then produced the F2 generation whose dispersal distance was measured at one of the three densities (see §2 and the electronic supplementary material 1 for a detailed explanation).
(c). Quantification of (grand)offspring dispersal distance
We measured the offspring (F1 or F2) dispersal distance in three densities. We placed the female mites on a 2 × 2 cm leaf with other female mites of the same age to create a total of either 10 (low offspring density, LOD), 50 (medium offspring density, MOD) or 100 (high offspring density, HOD) 1- to 2-day old females. The experimental set-ups used for testing dispersal distance, hereafter referred to as ‘trials’, were similar to that used in Bitume et al. [11]. Briefly, mites were left on the leaf for 30 min with a barrier preventing them from dispersing. The starting patch was connected with either 20 (for LOD and MOD) or 25 (for HOD) patches measuring 2 × 1 cm. Each patch was laid on wet cotton and connected via Parafilm bridges measuring 8 × 1 cm. After 30 min, the barrier was removed, and the mites were then allowed to disperse freely through the linear system. The number of patches used for each density were based on pilot experiments that showed no crowding at the end of the system with the respective number of patches. The total possible dispersal distance was 150 cm for the 20 patch system, and 200 cm for the 25 patch system.
For each of the parental density treatments, we selected 10 F0 mothers and assessed the dispersal distance of three of their daughters (F1), each at a different density. For each of the grand-parental density treatments, we selected 10 F1 mothers, each born to a distinct F0 grandmother, and assessed the dispersal distance of three of their daughters (F2), each at a different density. Offspring from several mothers were tested in the same trial (see the electronic supplementary material, table S1 and S2). The F1 and F2 offspring were marked with water colour paint [38], so that they could be followed throughout the experiment, and are hereafter referred to as ‘focal’ offspring. The other females used to produce the different treatment environmental densities were obtained from the BP and are hereafter referred to as ‘surrounding’ population.
(d). Body size experiment
In a separate experiment, we tested whether parental environment can affect the body size of offspring. Parents were exposed to the exact same densities and experimental protocol as previously described (n = 80 females at low density; eight patches of 10 females, n = 100 females at medium density; two patches of 50 females, n = 200 females at high density; two patches of 100 females, all patches 2 × 2 cm). Groups of 20 mothers, previously exposed to the same density treatment, were allowed to lay eggs for 48 h on a leaf disc with an area of 50 cm2 to allow their offspring to develop at a low density of 1.5 cm2. When the female offspring were 1- to 2-days old, we measured three parameters of their body size: length, width and circumference of body (n = 70 females at low density, n = 60 females medium density and n = 60 females high density). The females were killed in alcohol and photographed under a microscope. The photos were analysed using ImageJ image processing and analysis software (v. 1.47).
(e). Statistical analyses
The variable of interest is the dispersal distance of the focal offspring, measured as its position on the seventh (last) day of the dispersal assay experiment. However, because spider mites are a subsocial species known to follow the movements of conspecifics [42], the movements of the population as a whole might affect the dispersal distance by the focal offspring. To test whether the focal offspring move according to the movement of the whole population, we included the mean distance moved by the surrounding population as a covariate in the analysis. The mean was calculated by taking the dispersal distances of surrounding mites and dividing by the total number of mites. As density and mean distance dispersed by the surrounding population were significantly correlated (distribution Spearman's rank correlation test, ρ14 = 0.72, p < 0.001), we used the residuals of the mean dispersal distance of the whole population after regression by the offspring density (called hereafter ‘population distance’ (PDist)) as a covariate. We also compared the Akaike information criterion (AIC) values of a model with and without PDist as a variable. We estimated the effects of parental density at emergence (PD), density of the offspring in the trial (OD), of PDist, and their interactions on the dispersal distance of the focal offspring. The response variable (dispersal distance of focal offspring) was loge transformed to meet the model assumptions of normalized residuals (Shapiro–Wilk tests on linear model residuals: all p > 0.05). Density (PD, OD) and population dispersal distance (PDist) were continuous fixed factors, and mother and trial were random factors. We expected dispersal distances to increase with densities as we found previously, and so we applied the same methodology as in previous work to analyse the data, using density as a non-transformed, continuous, variable [42]. Using the statistical software R [44], we used a linear mixed-effect model with the software package lme4 [45]. Model simplification was performed using the principle of backwards stepwise factor elimination, where variables were retained when p < 0.05 [46]. We report the estimate (±s.e.) of each fixed factor together with the chi-statistics of the comparison between the models with and without the tested factor. This analysis was repeated by replacing the F1 offspring with the F2 offspring to determine the presence of grand-parental effects (GD). We again used the residuals derived from a regression of mean dispersal distance of the population on density (PDist) since they were significantly correlated (Spearman's rank correlation test, ρ13 = 0.87, p < 0.001). All data are accessible at http://alturl.com/yv4gn.
To determine whether parental environment affects offspring body size, we first performed a repeatability test to determine which traits could be measured confidently to represent body size [47]. We tested the repeatability of body width, length and circumference by measuring each trait three times on different days on the same individual. Repeatability was calculated using the formula
where S2A is the between group variance and S2 is the within group variance. Groups are composed of the three measurements taken for each individual. Repeatability was over 90% for all traits (length = 94.5% ± 0.002, width = 92.1% ± 0.002 and circumference = 93.9% ± 0.002). We then performed a linear regression with each body size trait as the response variable and parental density as the continuous explanatory variable.
3. Results
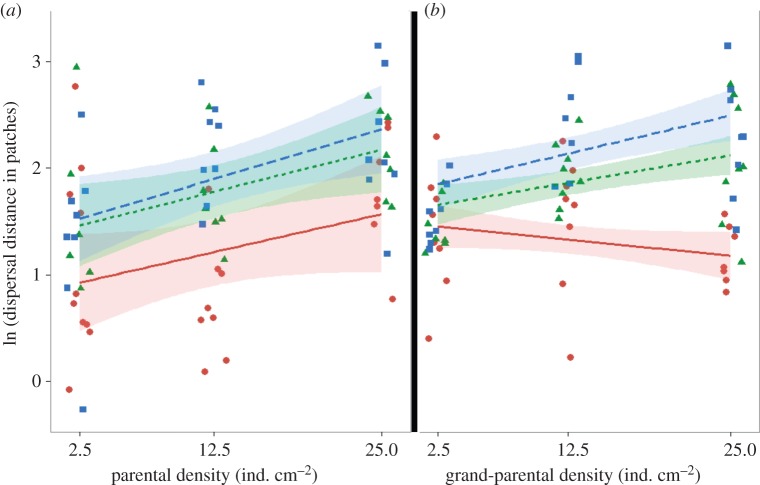
When testing the effect of parental density on loge-transformed dispersal distance of offspring, there were no significant interactions between any of the explanatory variables (see estimates and test statistics in table 1). Offspring dispersal distance was significantly increased by PD (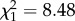 , p = 0.004, n = 70). As parental density increased from low to high, the distance dispersed by offspring was multiplied by 2.05 (figure 2). As expected [11], OD also had a significant effect on dispersal distance (
, p = 0.004, n = 70). As parental density increased from low to high, the distance dispersed by offspring was multiplied by 2.05 (figure 2). As expected [11], OD also had a significant effect on dispersal distance (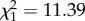 , p < 0.001, n = 70). As offspring density increased, the distance dispersed by offspring was multiplied by 1.92. The distance travelled by the population, after correction by offspring density, also had a significant positive effect on the distance dispersed by the offspring (
, p < 0.001, n = 70). As offspring density increased, the distance dispersed by offspring was multiplied by 1.92. The distance travelled by the population, after correction by offspring density, also had a significant positive effect on the distance dispersed by the offspring (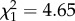 , p = 0.03, n = 70). However, there was no large difference in fit between models with and without PDist as an explanatory variable (with PDist, AIC = 155.2, without PDist, AIC = 153.4). This indicates that while there is a positive effect of the population movement, it is not strong.
, p = 0.03, n = 70). However, there was no large difference in fit between models with and without PDist as an explanatory variable (with PDist, AIC = 155.2, without PDist, AIC = 153.4). This indicates that while there is a positive effect of the population movement, it is not strong.
Table 1.
Results of the linear mixed-effects model testing the effects of offspring density, (grand)parental density and population distance on the log-transformed distance of focal offspring for the parental and grand-parental effects. (Terms were rejected when p > 0.05. All variables are fixed effects except for mother and trial, which are random effects.)
| estimate | s.e. | 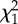 |
p | σ | |
|---|---|---|---|---|---|
| parental effects | |||||
| final model | |||||
| offspring density | 0.030 | 0.009 | 11.39 | <0.001 | |
| parental density | 0.032 | 0.008 | 8.48 | 0.004 | |
| population distance | 0.297 | 0.123 | 4.65 | 0.03 | |
| mother | 0.17 | 0.67 | 0.02 | ||
| trial | 0.67 | 0.41 | 0.03 | ||
| residual | 0.35 | ||||
| rejected terms | |||||
| parental density × population distance | 0.010 | 0.011 | 0.78 | 0.38 | |
| parental density × offspring density | 0.000 | 0.011 | 0.29 | 0.59 | |
| offspring density × population distance | 0.000 | 0.017 | 0.00 | 0.99 | |
| parental density × offspring density × population distance | −0.001 | 0.002 | 0.67 | 0.41 | |
| grand-parental effects | |||||
| final model | |||||
| grand-parental density | −0.010 | 0.008 | 1.67 | 0.20 | |
| offspring density | 0.013 | 0.007 | 3.22 | 0.07 | |
| population distance | 0.178 | 0.090 | 3.80 | 0.05 | |
| grand-parental density × offspring density | 0.002 | 0.000 | 12.78 | <0.001 | |
| mother | 0.00 | 1.00 | 0.00 | ||
| trial | 0.00 | 1.00 | 0.00 | ||
| residual | 0.10 | ||||
| rejected terms | |||||
| grand-parental density × population distance | −0.013 | 0.010 | 1.82 | 0.18 | |
| offspring density × population distance | 0.002 | 0.015 | 0.03 | 0.87 | |
| grand-parental density × offspring density × population distance | 0.001 | 0.002 | 0.68 | 0.41 | |
Figure 2.
Effect of (a) parental and (b) grand-parental density on offspring dispersal distance. Points are jittered for better visibility. (a) Slope values are derived from the estimates of the model: ln(dist) ∼ parental density + offspring density + population distance with mother and trial as random effects; (b) ln(dist) ∼ grand-parental density × offspring density + population distance with mother and trial as random effects. Red (solid) line with red circles, green (small dotted) line with green triangles and blue (thick dotted) line with blue squares indicates offspring exposed to low (2.5 individuals cm−2), medium (12.5 individuals cm−2) or high (25 individuals cm−2) densities, respectively. Shading represents 95% confidence intervals. (Online version in colour.)
In contrast to the parental effects experiment, in the grand-parental effects experiment there was a significant interaction between GD and OD (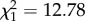 , p < 0.001, n = 69) on loge-transformed offspring dispersal distance (table 1). Offspring exposed to low density did not respond to grand-parental density (F1,21 = 3.294, p = 0.08). As grand-parental density increased from low to high, offspring dispersal distance was multiplied by 0.89 when offspring were at low density, and by 2.46 when offspring were at high density. As offspring density increased from low to high, offspring dispersal distance was multiplied by 1.50 when grand-parental density was low and by 4.13 when grand-paternal density was high. Similar to the parental effects experiment, population distance had a marginally significant positive effect on the distance dispersed by the offspring (
, p < 0.001, n = 69) on loge-transformed offspring dispersal distance (table 1). Offspring exposed to low density did not respond to grand-parental density (F1,21 = 3.294, p = 0.08). As grand-parental density increased from low to high, offspring dispersal distance was multiplied by 0.89 when offspring were at low density, and by 2.46 when offspring were at high density. As offspring density increased from low to high, offspring dispersal distance was multiplied by 1.50 when grand-parental density was low and by 4.13 when grand-paternal density was high. Similar to the parental effects experiment, population distance had a marginally significant positive effect on the distance dispersed by the offspring (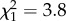 , p = 0.05, n = 69). There was no significant difference in fit between models with and without PDist as an explanatory variable (with PDist, AIC = 59.11, without PDist, AIC = 57.44), and thus we conclude that the effect is not strong.
, p = 0.05, n = 69). There was no significant difference in fit between models with and without PDist as an explanatory variable (with PDist, AIC = 59.11, without PDist, AIC = 57.44), and thus we conclude that the effect is not strong.
Parental density did not significantly affect offspring body length (β = 4 × 10−4 ± 3 × 10−4, F1,188 = 1.632, p = 0.20). Parental density did significantly increase offspring body width (β = 8 × 10−4 ± 2 × 10−4, F1,188 = 24.69, p < 0.001). Females from high density parents were on average 8% times wider than those from low density parents. The average body width of females from low density, medium density and high density mothers was 0.213 mm ± 0.004, 0.234 mm ± 0.003 and 0.232 mm ± 0.003, respectively. Parental density also significantly increased body circumference (β = 3 × 10−3 ± 7 × 10−4, F1,188 = 21.17, p < 0.001). As parental density increased from lowest to highest, the average body circumference increased by 9%. The average body circumference of low density, medium density and high density offspring was 0.91 mm ± 0.017, 0.98 mm ± 0.013 and 0.99 mm ± 0.014, respectively.
4. Discussion
Non-genetic transmission of information from parent to offspring has been recognized as having significant influence on offspring phenotype [14,48]. Here, we showed that parental and grand-parental density significantly affected offspring dispersal distance. Offspring exposed to higher density dispersed further, but these offspring dispersed even further when their parents were also exposed to high density. The same pattern was found for grand-offspring, except for grand-offspring exposed to low density that did not change their dispersal distance in response to increasing grand-parental density. We also found that offspring born to parents from a medium or high density were overall larger than offspring from low-density parents, indicating plasticity in body size in response to parental density.
Marshall & Uller [49] provide several criteria which, if fulfilled, explain the existence of adaptive maternally induced phenotypic plasticity in offspring. First, the environment must be spatio-temporally stochastic. In nature, the environment experienced by T. urticae is normally highly variable [50]. Tetranychus urticae can exploit and destroy their resources very quickly, and because many of their host plants are agricultural, the constant harvesting and regrowth imply a constantly changing habitat [51]. A second requirement is that the costs of transmitting the non-genetic information from mother to offspring must be low. Our experiment did not permit us to quantify the cost of information transmission from mother to offspring. However, because maternal effects are present in other life-history traits in this species, we can suppose that the cost is not prohibitive [34]. Lastly, and perhaps most relevant to our study, maternal environment or condition must be a good predictor of conditions that the offspring will experience. In T. urticae, if the mother is currently experiencing high densities, then there is virtually no chance that an F1 generation will consequently experience a low-density environment unless they disperse. This is owing to the fact that T. urticae populations can grow exponentially, even in the presence of predators [37]. Thus, a high-density environment is likely to be a reliable cue that should influence mothers to produce offspring with higher dispersal propensity.
There are several concurrent mechanisms that can explain how mothers modify the dispersal decisions of their offspring. One mechanism is that maternal density affects morphological attributes of the offspring that either constrain or encourage dispersal [52–54]. The adaptive parental hypothesis predicts that females exposed to high levels of competition or malnutrition should invest more in each individual offspring [17,55]. Indeed, our results show that offspring reared from parents exposed to medium and high density are larger. Larger individuals are often considered to be more capable and successful dispersers across a wide variety of taxa [56–58]. We did not test for paternal effects specifically, however, it is also possible that fathers are able to transmit information about density to offspring through their sperm [15,16]. In our experiments, paternal density was the same as maternal density during rearing and breeding, so we cannot exclude an effect of paternal density.
We observed that dispersal distance increased with grand-parental density. Across many insect taxa, larger mothers rear larger offspring [59]. Even though we standardized the F1b generation at a low density, the F2 offspring from medium- and high-density grand-parents were probably larger than those from grand-parents exposed to low density. An additive effect across generations of density on body size may explain how the information was transmitted from grand-parent to grand-offspring. We observed the same patterns in the grand-parental effects as for the parental effects, except in grand-offspring that were exposed to a low-density environment. These offspring dispersed based only on their own experienced densities. An explanation might be that offspring at a low density did not use or receive a signal when their grand-parents were in medium or high density because of conflict with information about their own density. If offspring find themselves in a high-density situation and their grandmothers were also in this situation, then all available information indicates that they should take the risk of dispersing further. However, because dispersal is costly [60], if the offspring are in a low-density situation, then they are better off to take into account their own density and their maternal density to more accurately assess how far they should disperse. In the case of our experiments, the mothers of the F2 generation were all exposed to low densities. Thus, because of this apparent conflict in information, lack of signal transmission or unpredictable environment, offspring that also find themselves in a low density situation did not disperse further.
In both the parental and grand-parental experiments, the mean dispersal distance of the population had an effect on the movement of the focal offspring. Yet, we found no significant difference in AIC values when including the residuals of population distance on density (PDist) compared with a model without this variable. This suggests that the movement of the population may slightly influence the dispersal distances of the mites. Tetranychus urticae is a subsocial organism that prefers to live in small groups rather than individually or in large groups. Group living enhances fecundity, provides protection from predation and other abiotic factors, and also easy access to mates [39,61]. Additionally, in natural populations, the spatial distribution of mites tends to be slightly aggregated [37,41]. Thus, it is likely that T. urticae change their dispersal behaviour based on their present group size, but this effect is weak.
Interestingly, the parental effects have as much influence on dispersal distance as the offspring's own environment. We can envision how maternal effects might play a central role in the population dynamics of dispersing individuals in the context of an invading wave. We have previously shown that populations experiencing high densities and high levels of genetic relatedness will have individuals that disperse further away from the source patch [11]. Population densities at the invading front are usually low [62], and thus genetic relatedness will ultimately increase. Maternal effects can thus counteract or accelerate the speed of an invasion depending on the environmental conditions experienced in the previous generation. For example, if density at the front is low, but relatedness is high, and the mothers of these individuals come from a high-density environment, then the maternal effect could amplify the speed of invasion by acting simultaneously with genetic relatedness to increase the dispersal distances of offspring. It is thus necessary to take into account the effect of maternal environment to predict with any accuracy the speed at which a species will expand its range.
In conclusion, while theoretical models investigating the rate of, for example, range expansion, rarely acknowledge parental influence on offspring dispersal distance, our results indicate that parental effects may similarly affect two key components of dispersal strategies: emigration and, for the first time, dispersal distance. Furthermore, we show that parental influence on offspring dispersal distance is as important as the offspring's environment on dispersal decisions [22,53]. Parents can use a cue that normally promotes dispersal, such as density, and transmit that information to their offspring so that the offspring have a ‘head-start’ in their response to their own environmental conditions. Parents can augment offspring body size in response to various density conditions, affording their offspring the capability to make appropriate dispersal decisions.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Hannele Penson for efficient work and mite counting, and Gilles San Martin, Guillaume Chome and Marie-Jeanne Holveck for statistical consulting. E.B. and C.N. designed the experiments, E.B. performed the experiments, and E.B., I.O. and D.B. analysed the data. E.B. wrote the first draft of the manuscript, and C.N., O.R., I.O. and D.B. contributed substantially to revisions.
Funding statement
This research was supported by the Belgian ‘Fonds national pour la recherche scientifique’ (F.R.I.A. grant no. 1.EO25.09 to E.B.), by the Université catholique de Louvain-la-Neuve (grant no. ARC 10/15-031 and FSR grant no. 605031 to C.N.), by the Wallonian exchange programme ‘Tournesol’, by the CNRS ‘Programme International de Coopération Scientifique’ PICS 2010–2012 IO. D.B. was supported by the Research Network EVENET and FWO projects G.0057.09 and G.0610.11. O.R. was supported by the Evorange project (ANR-09-PEXT-011). This paper is publication BRC316 of the Biodiversity Research Centre (Académie Louvain) and publication ISEM 2014-070 of the Institut des Sciences de l'Evolution, Montpellier.
References
- 1.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Ledon-Rettig CC, Richards CL, Martin LB. 2013. Epigenetics for behavioral ecologists. Behav. Ecol. 24, 311–324. ( 10.1093/beheco/ars145) [DOI] [Google Scholar]
- 3.Richards EJ. 2006. Opinion: inherited epigenetic variation. Revisiting soft inheritance. Nat. Rev. Genet. 7, 395–401. ( 10.1038/nrg1834) [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669. [Google Scholar]
- 5.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 6.Clobert J, Baguette M, Benton TG, Bullock JM. 2012. Dispersal ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Kokko H, Lopez-Sepulcre A. 2006. From individual dispersal to species ranges: perspectives for a changing world. Science 313, 789–791. ( 10.1126/science.1128566) [DOI] [PubMed] [Google Scholar]
- 8.Poethke HJ, Gros A, Hovestadt T. 2011. The ability of individuals to assess population density influences the evolution of emigration propensity and dispersal distance. J. Theor. Biol. 282, 93–99. ( 10.1016/j.jtbi.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 9.Rousset F, Gandon S. 2002. Evolution of the distribution of dispersal distance under distance-dependent cost of dispersal. J. Evol. Biol. 15, 515–523. ( 10.1046/j.1420-9101.2002.00430.x) [DOI] [Google Scholar]
- 10.Wender NJ, Polisetty CR, Donohue K. 2005. Density-dependent processes influencing the evolutionary dynamics of dispersal: a functional analysis of seed dispersal in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 92, 960–971. ( 10.3732/ajb.92.6.960) [DOI] [PubMed] [Google Scholar]
- 11.Bitume EV, Bonte D, Ronce O, Bach F, Flaven E, Olivieri I, Nieberding CM. 2013. Density and genetic relatedness increase dispersal distance in a subsocial organism. Ecol. Lett. 16, 430–437. ( 10.1111/ele.12057) [DOI] [PubMed] [Google Scholar]
- 12.Qvarnstrom A, Price TD. 2001. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 16, 95–100. ( 10.1016/s0169-5347(00)02063-2) [DOI] [PubMed] [Google Scholar]
- 13.Danchin E, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486. ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 14.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Crean AJ, Dwyer JM, Marshall DJ. 2013. Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance. Ecology 94, 2575–2582. ( 10.1890/13-0184.1) [DOI] [PubMed] [Google Scholar]
- 16.Curley JP, Mashoodh R, Champagne FA. 2011. Epigenetics and the origins of paternal effects. Hormones Behav. 59, 306–314. ( 10.1016/j.yhbeh.2010.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 18.Fowler MS. 2005. Interactions between maternal effects and dispersal. Oikos 110, 81–90. ( 10.1111/j.0030-1299.2005.13704.x) [DOI] [Google Scholar]
- 19.Ronce O, Brachet S, Olivieri I, Gouyon PH, Clobert J. 2005. Plastic changes in seed dispersal along ecological succession: theoretical predictions from an evolutionary model. J. Ecol. 93, 431–440. ( 10.1111/j.1365-2745.2005.00972.x) [DOI] [Google Scholar]
- 20.Ginzburg LR, Taneyhill DE. 1994. Population cycles of forest Lepidoptera: a maternal effect hypothesis. J. Anim. Ecol. 63, 79–92. ( 10.2307/5585) [DOI] [Google Scholar]
- 21.Kendall BE, Ellner SP, McCauley E, Wood SN, Briggs CJ, Murdoch WW, Turchin P. 2005. Population cycles in the pine looper moth: dynamical tests of mechanistic hypotheses. Ecol. Monogr. 75, 259–276. ( 10.1890/03-4056) [DOI] [Google Scholar]
- 22.Allen RM, Buckley YM, Marshall DJ. 2008. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am. Nat. 171, 225–237. ( 10.1086/524952) [DOI] [PubMed] [Google Scholar]
- 23.Donohue K. 1999. Seed dispersal as a maternally influenced character: mechanistic basis of maternal effects and selection on maternal characters in an annual plant. Am. Nat 154, 674–689. ( 10.1086/303273) [DOI] [PubMed] [Google Scholar]
- 24.Sutherland ORW. 1969. The role of crowding in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 15, 1385–1410. ( 10.1016/0022-1910(69)90199-1) [DOI] [Google Scholar]
- 25.Sinervo B, Svensson E, Comendant T. 2000. Density cycles and an offspring quantity and quality game driven by natural selection. Nature 406, 985–988. ( 10.1038/35023149) [DOI] [PubMed] [Google Scholar]
- 26.Plaistow SJ, St. Clair JJH, Grant J, Benton TG. 2007. How to put all your eggs in one basket: empirical patterns of offspring provisioning throughout a mother's lifetime. Am. Nat. 170, 520–529. ( 10.1086/521238) [DOI] [PubMed] [Google Scholar]
- 27.Duckworth RA. 2009. Maternal effects and range expansion: a key factor in a dynamic process? Phil. Trans. R. Soc. B 364, 1075–1086. ( 10.1098/rstb.2008.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209. ( 10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- 29.Buoro M, Carlson SM. 2014. Life-history syndromes: integrating dispersal through space and time. Ecol. Lett. 17, 756–767. ( 10.1111/ele.12275) [DOI] [PubMed] [Google Scholar]
- 30.Cote J, Clobert J, Fitze PS. 2007. Mother–offspring competition promotes colonization success. Proc. Natl Acad. Sci. USA 104, 9703–9708. ( 10.1073/pnas.0703601104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starrfelt J, Kokko H. 2010. Parent–offspring conflict and the evolution of dispersal distance. Am. Nat. 175, 38–49. ( 10.1086/648605) [DOI] [PubMed] [Google Scholar]
- 32.Motro U. 1983. Optimal rates of dispersal. III. Parent-offspring conflict. Theor. Popul. Biol. 23, 159–168. ( 10.1016/0040-5809(83)90011-4) [DOI] [Google Scholar]
- 33.Li J, Margolies DC. 1993. Quantitative genetics of aerial dispersal behaviour and life-history traits in Tetranychus urticae. Heredity 70, 544–552. ( 10.1038/hdy.1993.78) [DOI] [Google Scholar]
- 34.Oku K, Yano S, Takafuji A. 2003. Different maternal effects on diapause induction of tetranychid mites, Tetranychus urticae and T. kanzawai (Acari: Tetranychidae). Appl. Entomol. Zool. 38, 267–270. ( 10.1303/aez.2003.267) [DOI] [Google Scholar]
- 35.Macke E, Magalhães S, Bach F, Olivieri I. 2011. Experimental evolution of reduced sex ratio adjustment under local mate competition. Science 334, 1127–1129. ( 10.1126/science.1212177) [DOI] [PubMed] [Google Scholar]
- 36.Hinomoto N, Takafuji A. 1994. Studies on the population structure of the two-spotted spider mite, Tetranychus urticae Koch, by allozyme variability analysis. Appl. Entomol. Zool. 29, 259–266. [Google Scholar]
- 37.Mitchell R. 1973. Growth and population dynamics of a spider-mite (Tetranychus. urticae K Acarina - Tetranychidae). Ecology 54, 1349–1355. ( 10.2307/1934198) [DOI] [Google Scholar]
- 38.Bitume EV, Bonte D, Magalhães S, San Martin G, Van Dongen S, Bach F, Anderson JM, Olivieri I, Nieberding CM. 2011. Heritability and artificial selection on ambulatory dispersal distance in Tetranychus urticae: effects of density and maternal effects. PLoS ONE 6, e26927 ( 10.1371/journal.pone.0026927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helle W, Sabelis MW. 1985. Spider mites: their biology, natural enemies, and control. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 40.Van Leeuwen T, Vanholme B, Van Pottelberge S, Van Nieuwenhuyse P, Nauen R, Tirry L, Denholm I. 2008. Mitochondrial heteroplasmy and the evolution of insecticide resistance: non-Mendelian inheritance in action. Proc. Natl Acad. Sci. USA 105, 5980–5985. ( 10.1073/pnas.0802224105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.So PM. 1991. Distribution patterns of and sampling plans for Tetranychus urticae Koch (Acarina: Tetranychidae) on roses. Res. Popul. Ecol. 33, 229–243. ( 10.1007/BF02513551) [DOI] [Google Scholar]
- 42.Yano S. 2008. Collective and solitary behaviors of twospotted spider mite (Acari : tetranychidae) are induced by trail following. Ann. Entomol. Soc. Am. 101, 247–252. ( 10.1603/0013-8746(2008)101[247:CASBOT]2.0.CO;2) [DOI] [Google Scholar]
- 43.Li JB, Margolies DC. 1993. Effects of mite age, mite density, and host quality on aerial dispersal behavior in the 2-spotted spider-mite. Entomol. Exp. Appl. 68, 79–86. ( 10.1111/j.1570-7458.1993.tb01691.x) [DOI] [Google Scholar]
- 44.R Development Core Team 2011. R: a language and environment for statistical computing, 2.15.1 ed. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 45.Bates D, Maechler M, Bolker B, Walker S. 2014. lme 4: Linear mixed-effects models using Eigen and S4. R package v. 1.1-5. See http://cran.r-project.org/package=lme4.
- 46.Crawley M. 2007. The R book. Chichester, UK: Wiley. [Google Scholar]
- 47.Lessells CM, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. The Auk 104, 116–121. ( 10.2307/4087240) [DOI] [Google Scholar]
- 48.Bernardo J. 1996. The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am. Zool. 36, 216–236. [Google Scholar]
- 49.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 50.Ellner SP, et al. 2001. Habitat structure and population persistence in an experimental community. Nature 412, 538–543. ( 10.1038/35087580) [DOI] [PubMed] [Google Scholar]
- 51.Hussey NW, Parr WJ. 1963. Dispersal of the glasshouse red spider mite Tetranychus urticae Koch (Acarina, Tetranychidae). Entomol. Exp. Appl. 6, 207–214. ( 10.1111/j.1570-7458.1963.tb00619.x) [DOI] [Google Scholar]
- 52.Diss AL, Kunkel JG, Montgomery ME, Leonard DE. 1996. Effects of maternal nutrition and egg provisioning on parameters of larval hatch, survival and dispersal in the gypsy moth, Lymantria dispar L. Oecologia 106, 470–477. ( 10.1007/BF00329704) [DOI] [PubMed] [Google Scholar]
- 53.Massot M, Clobert J. 1995. Influence of maternal food availability on offspring dispersal. Behav. Ecol. Sociobiol. 37, 413–418. ( 10.1007/BF00170589) [DOI] [Google Scholar]
- 54.Yanagi S, Saeki Y, Tuda M. 2013. Adaptive egg size plasticity for larval competition and its limits in the seed beetle Callosobruchus chinensis. Entomol. Exp. Appl. 148, 182–187. ( 10.1111/eea.12088) [DOI] [Google Scholar]
- 55.Badyaev AV, Uller T. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Phil. Trans. R. Soc. B 364, 1169–1177. ( 10.1098/rstb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Riain MJ, Jarvis JUM, Faulkes CG. 1996. A dispersive morph in the naked mole rat. Nature 380, 619–621. ( 10.1038/380619a0) [DOI] [PubMed] [Google Scholar]
- 57.Sinervo B, Calsbeek R, Comendant T, Both C, Adamopoulou C, Clobert J. 2006. Genetic and maternal determinants of effective dispersal: the effect of sire genotype and size at birth in side-blotched lizards. Am. Nat. 168, 88–99. ( 10.1086/505765) [DOI] [PubMed] [Google Scholar]
- 58.Fjerdingstad EJ, Schtickzelle N, Manhes P, Gutierrez A, Clobert J. 2007. Evolution of dispersal and life history strategies: Tetrahymena ciliates. BMC Evol. Biol. 7, 133 ( 10.1186/1471-2148-7-133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox CW, Czesak ME. 2000. Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 45, 341–369. ( 10.1146/annurev.ento.45.1.341) [DOI] [PubMed] [Google Scholar]
- 60.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. ( 10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 61.Le Goff GJ, Mailleux AC, Detrain C, Deneubourg JL, Clotuche G, Hance T. 2010. Group effect on fertility, survival and silk production in the web spinner Tetranychus urticae (Acari: Tetranychidae) during colony foundation. Behaviour 147, 1169–1184. ( 10.1163/000579510X510980) [DOI] [Google Scholar]
- 62.Holt RD. 2003. On the evolutionary ecology of species’ ranges. Evol. Ecol. Res. 5, 159–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.