Abstract
Many prey species, from soil arthropods to fish, perceive the approach of predators, allowing them to escape just in time. Thus, prey capture is as important to predators as prey finding. We extend an existing framework for understanding the conjoint trajectories of predator and prey after encounters, by estimating the ratio of predator attack and prey danger perception distances, and apply it to wolf spiders attacking wood crickets. Disturbances to air flow upstream from running spiders, which are sensed by crickets, were assessed by computational fluid dynamics with the finite-elements method for a much simplified spider model: body size, speed and ground effect were all required to obtain a faithful representation of the aerodynamic signature of the spider, with the legs making only a minor contribution. The relationship between attack speed and the maximal distance at which the cricket can perceive the danger is parabolic; it splits the space defined by these two variables into regions differing in their values for this ratio. For this biological interaction, the ratio is no greater than one, implying immediate perception of the danger, from the onset of attack. Particular attention should be paid to the ecomechanical aspects of interactions with such small ratio, because of the high degree of bidirectional coupling of the behaviour of the two protagonists. This conclusion applies to several other predator–prey systems with sensory ecologies based on flow sensing, in air and water.
Keywords: sensory ecology, predator–prey interactions, flow sensing, danger perception, ground effect
1. Introduction
Predation involves a sequence of steps, starting with prey encounter, followed by prey detection, identification, attack and subjugation, and ending with consumption [1]. It is difficult to assess the chances of predation success for each of the steps in this sequence in natural conditions, as few studies have been carried out on entire predation sequences. One such study, a field study of over 2000 predator–prey interactions between crab spiders and their prey community, found that the probability of completing each step plummeted along the predation sequence, from over 50% for the first step after encounter (i.e. landing on an occupied flower), to much less than 10% for the final strike [2]. As these probabilities must be multiplied to give the overall probability of success, the probability of prey capture was estimated at 3.5% for prey alighting on an inflorescence harbouring a spider, consistent with the low success rates reported in other studies [3,4]. Studies on wild ranging carnivores and raptor birds have also yielded low estimates of capture success [5]. Thus, encountering a prey is only the first step; the predator must then subdue and capture the prey, and these steps are just as important as finding the prey in the first place.
For these and many other predator–prey interactions, the final steps of a close range interaction are often rapid and violent, stretching the physiological responses of both protagonists to their limits. For example, mantis shrimps exert extraordinary smashing forces on mollusc shells, to such a point that they may cause water cavitation [6]. The C-start of small fishes escaping predators is also very powerful [7,8]. It is during these final steps of behavioural interactions that ecomechanics—fluid dynamics and solid mechanics in an ecological context—connect sensing, moving and behavioural ecology, determining the final success or failure of capture [9]. A framework for understanding the conjoint trajectories of predator and prey and their ecomechanics during the final steps of the interaction is thus required, also to understand the longer term coevolutionary forces that shape the strategies used [10–12].
Such a framework can be developed from another recently developed framework focusing on predator sensory ecology [13]. This framework relates sensory distance, the distance at which a prey is sensed, to the motor distance of a predator, the distance that the predator requires to stop before swallowing a prey, for example. What really matters is the set of locations that can be reached in a given time. We therefore refer here to distances rather than volumes (as in the original study), to simplify presentation. The ratio of sensory distance to motor distance provides considerable insights into behavioural and biomechanical control strategies, such as the minimal turning radius and other kinematic aspects relating to predator inertia. This ratio can also be used to distinguish between different types of attack mode. In the ‘collision’ mode, the sensory distance is smaller than the motor distance required for the predator to come to a halt: predators literally crash into their prey. The ‘deliberative’ mode, typical of dolphin echolocation and of visual predators, for example, is essentially the opposite situation and occurs when the ratio is high. Other examples are provided by birds of prey high in the air swooping down on a rodent on the ground and cheetahs hunting impalas over hundreds of metres [14]. This mode is typical of predators using an active ‘find-and-destroy’ strategy in which the predator may change course during action (‘after deliberation’). The ‘reactive’ mode corresponds to a ratio of about one, as seen in knifefish, for example [13]. This mode of attack involves the rapid, direct coupling of sensation and action. Predators can also switch from a deliberative mode to a reactive mode at shorter distances from their target, as in echolocating porpoises [15].
This framework uses the predator as the focal point, but neglects the prey. It can be extended to pairs of predator and prey, by defining two different distances: the distance required for the predator to instigate an attack and the maximal distance at which the prey can perceive the danger. The first of these distances is observed during an interaction, whereas the second must be inferred from a combination of the conspicuousness of the predator and the sensory abilities of the prey. The reference frame for this new framework is thus shifted from the predator to the predator–prey pair. The ratio of these two distances (i.e. attack to perception) provides an appreciation of the combined ecomechanical factors influencing attack and escape strategies that no study focusing on a single participant can provide [12].
The interaction between Pardosa sp., wolf spiders, and Nemobius sylvestris, wood crickets, is amenable to such analyses based on this ratio of predator attack and prey danger perception distances. Wolf spiders and wood crickets constitute a predator–prey system for which conjoint pursuit–escape trajectories and respective strategies are unusually well understood in both controlled and natural settings ([16] and references therein). Wolf spiders have a bimodal hunting strategy combining a true ‘sit-and-wait’ strategy, in which crickets must be within reach to trigger spider attack, with an active ‘sit-and-pursue’ strategy [17–19]. Crickets have developed a very sensitive early warning system [20]. This system, based on filiform hairs, senses the faintest of air flows produced by attacking spiders. It sets the danger perception threshold, which has been estimated at 30 μm s−1 [20], and is thus a key element determining the probability of the cricket escaping. Estimates of both spider attack distances and maximal cricket danger perception distances are required to estimate the ratio. Attack distances have already been measured in the laboratory and in the field [16]. Indeed, we previously showed that spiders attack their cricket prey at a distance of some 3 cm on leaf litter and that this distance is doubled if the predator and prey are located on a flat surface, such as a Petri dish [16]. In this study, we aimed to estimate the second, more difficult half of the ratio, the maximal danger perception distance of crickets as function of the speed of attacking spiders. This requires faithful predictions of the air disturbances upstream from an attacking spider from modelling of the relative contributions of the main body, legs and ground effect to flow amplitude as a function of the distance to a spider. Using the threshold for the perception of flow velocities by crickets cited above as a cutoff value, we can then calculate the second distance and, thus, the ratio.
We used a three-step approach. We first designed a three-sphere computational model of a running spider and estimated the deformations of air flow around it by computational fluid dynamics (CFD) with the finite-element method (FEM). This oversimplified, but classical approximation is discussed below. The CFD–FEM approach involves the efficient numerical solution of the Navier–Stokes equations describing fluid dynamics and is most suitable for complex problems for which analytical solutions do not exist [21]. These technical aspects are outlined in the electronic supplementary material, appendix. Once the computational algorithm was deemed appropriate, we then compared the computed flow disturbances of our running spider model with previously published experimental data for the flow disturbances created by spiders running at an intermediate speed of about 10 cm s−1. Once the contribution of the different body parts to air flow had been estimated and the complete model had been accepted, we explored the entire range of observed values for attack speed, running the model from 0 to 40 cm s−1. Each attack speed produced a different volumetric field of velocities, from which we extracted the cricket danger perception distance as a function of spider speed.
2. Material and methods
We will first describe the geometric elements used to model moving spiders. The model is highly simplified, but remains too complex for analytical calculation of the upstream flow. A CFD–FEM numerical scheme must therefore be used to solve the full Navier–Stokes equation. However, we first needed to check that our implementation of the FEM was correct. We did this by experimentally exploring the two limiting cases of very slow and fast translation of a single sphere and by testing our FEM model on these well-known cases. The observed flow disturbances and model predictions were then compared with analytical approximations of the Navier–Stokes equation available for these highly simplified situations. Once these comparisons had been carried out and we had confirmed that the FEM implementation was appropriate, we applied it to the full Navier–Stokes equations for the three-sphere model of a running spider.
(a). Spider kinematics and modelling of constitutive elements
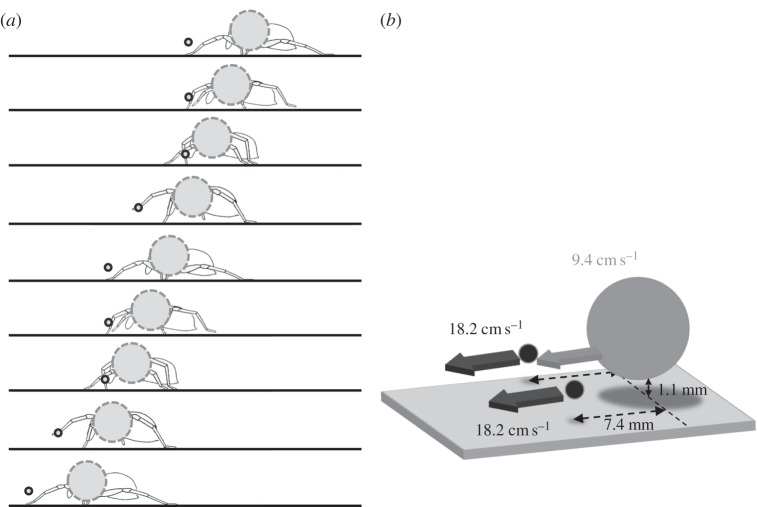
We describe here the rationale underlying our model of a running spider and the estimation of its parameters. A running spider is modelled as an ensemble of three spheres: one for the body trunk, and the other two for the front leg tips. Schematically, spider bodies move at a constant velocity, whereas the front legs alternate between high-speed movements and periods in a stationary position (figure 1a,b). A first approximation for the body is to consider it as a sphere with diameter identical to the spider body size diameter. The flow perturbations reaching the furthest distances from the spider are generated when the moving legs are in maximal extension. We modelled only the tips of the legs, using a diameter corresponding to the diameter of the tarsus. When the legs are motionless and close to the body, they act together as a single aerodynamic unit. When they move, the legs move at the same time, in a single plane, with constant speed (figure 1b).
Figure 1.
Establishment of the CFD model of a running spider. (a) A schematic of the kinematics of a running spider showing that the body trunk moves at constant speed, whereas the legs move intermittently, at a speed at most twice that of the body trunk (b). The body trunk is modelled by a sphere of the spider size and the leg tips are modelled as spheres with diameters identical to that of the metatarsus. Parameter values are set such that the computed flow disturbances correspond to the extreme cases defining a volume of flow velocities corresponding to the least favourable situation for spiders, i.e. the maximal danger perception distance for crickets.
The estimation of body and leg velocities and of the geometric relationships between the different elements of the model was based on 14 recorded runs made by six spiders in a previous study by Casas et al. [18]. Mean body size (3.6 mm, s.d. = 0.2 mm, n = 6) was estimated by adding coxa and trochanter lengths to the width of the prothorax at its widest part, as these three body parts act aerodynamically as a single unit. In the spiders studied, this unit was wider than the abdomen. The diameter of the metatarsus (0.37 mm, s.d. = 0.027 mm, n = 6) was estimated at the point at which the laser sheet crossed a leg. The distances between the two front legs and between the front legs and the body trunk were also measured at these points. Spider velocity was determined by measuring the mean velocity of the spider body on a run long enough for the extraction of a meaningful value. Speed measurements were made only if spider velocity remained constant for several centimetres and spiders were running in a straight line. Another set of six spiders was used to estimate height above the ground in the same set-up and conditions, but with a different, side of view. The distance from the ground to the lowest point of the opisthosoma was 1.1 mm (s.d. = 0.2 mm, n = 6).
Mean body speed was 9.44 cm s−1 (s.d. = 5.5 cm s−1, n = 14 measured on six spiders), with minimal and maximal observed values of 3 and 20 cm s−1, respectively. The legs moved at twice the speed of the body trunk (mean leg speed = 18.24 cm s−1, s.d. = 9.05 cm s−1, n = 18 on six spiders). The distance between the body and the legs, measuring from their respective centres, was 7.4 mm (s.d. = 0.2 mm, n = 11 on six spiders) at their most distant point in the air.
(b). Finite-element computations of the Navier–Stokes equations for the three spheres spider model
Once the CFD–FEM implementation of a single translating sphere had been validated (see the electronic supplementary material, appendix), we used this implementation to solve the full three-dimensional incompressible Navier–Stokes equation for a three-sphere spider model immersed in an immobile fluid (air: ηair = 1.56 × 10−5 m2 s−1 and ρair = 1.1774 kg m−3). The distance to the walls in the spider direction was set to 20 cm (corresponding to over 50 body lengths), as no change in velocity was observed with further expansions of the simulation volume. The distance between the centres of the three spheres and the ground was 2.9 mm. The simulations were run in a volume of 20 × 7 × 2 cm, subdivided into 6000 triangular elements by automatic recursive and adaptive meshing with the Delaunay algorithm. The Navier–Stokes equation was solved with a stationary solver (GMRES linear system solver) in the COMSOL Multiphysics package (COMSOL Multiphysics 3.3, COMSOL AB., 2006).
(c). Comparing model output between models and with observations
The output of the model is a volumetric field of velocities filling the entire simulation domain. Using an air velocity perception threshold for crickets of 30 μm s−1, we were able to define another volumetric field of velocities within the original simulation volume, the surface of which corresponded to this threshold value. The flow velocities contained within this new volume, itself smaller than the simulation volume and irregular in shape, are, by definition, all above the cricket's perception threshold. The maximal extent of this volume in the direction of the cricket is the maximal danger perception distance. It can be extracted by the definition of two additional planes crossing the volume. One of these planes is vertical, located between the legs of the spider and spanning the distance between the spider and the cricket. The other plane is horizontal and crosses the centre of the leg tips at the instant at which they are extended furthest from the body and moving (figure 1b). The maximal danger perception distance thus provides a snapshot within a cycle of continuous leg movements. From the spider's point of view, larger danger perception distances are the least advantageous. Our approach, in which leg kinematics are modelled by computing only the largest volume of velocities, and hence maximum perception distance, is analogous to the study of analytically unsolvable differential equations in applied mathematics: super- and sub-solutions are obtained to yield encompassing solutions without taking into account the complex dynamics between them [22]. We did not use statistical approaches, such as AIC and allied summaries of goodness-of-fit, to compare models, because our approach was clearly not statistical in nature [23].
3. Results
The spider is at the greatest disadvantage with respect to its prey when the legs are maximally extended, as this maximal extension and the high speed of the legs increase flow disturbances. Flow velocities therefore peak at this point in the leg movement cycle, maximizing the information available to the cricket prey.
The Reynolds number of attacking spiders can be estimated from spider size (3.6 mm) and velocity (from 1 to 40 cm s−1). The Reynolds number of attacking spiders is therefore approximately 2–90, for the highest speeds.
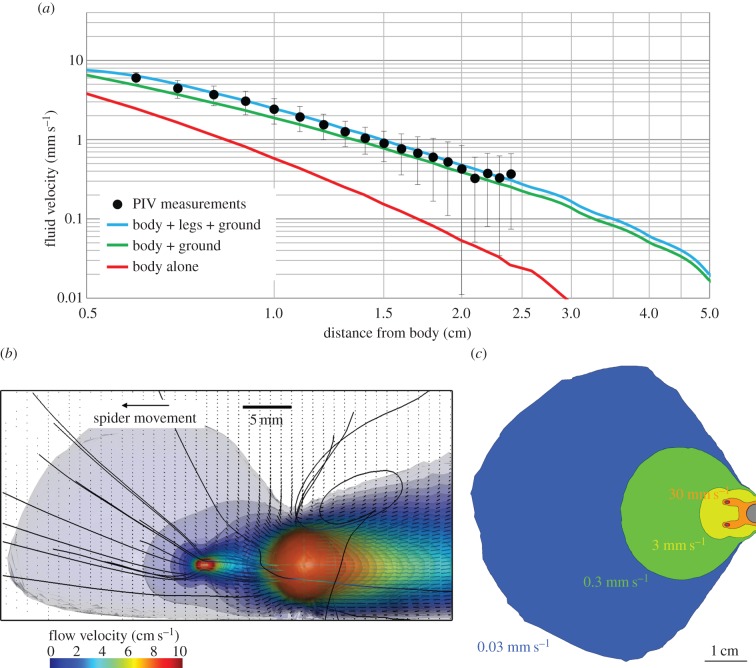
Our CFD–FEM computational solution of the full Navier–Stokes equation for a simplified three-sphere spider model reproduced flow velocities similar to those observed for spiders running at the mean speed (figure 2a). However, it was not sufficient to take only the body into account. The ground effect had to be incorporated to achieve a good fit. The further addition of the front legs improved the fit, albeit to a much lesser degree. The strong ground effect was best assessed by extracting the flow disturbances perpendicular to the ground and considering the asymmetry of both the streamlines and the isosurfaces (figure 2b). At the observed mean speed, the threshold velocity of 30 μm s−1 for air flow disturbances was reached at a distance of up to 5 cm from the spider (figure 2c). Furthermore, thresholds two orders of magnitude above the nominal threshold are reached at a distance equivalent to about one body length of the predator. Such distances are still relatively large and allow escape.
Figure 2.
Maximal flow disturbances upstream from an attacking spider moving at a speed of 9.5 cm s−1. (a) Contributions of the body trunk, legs and ground effect to flow speed at spider mid-height, as calculated from the CFD model. The red line is the flow computed for the body alone, the green line corresponds to the body plus the ground effect and the blue line corresponds to the body plus the ground effect and the legs. The measurement points and their confidence intervals, in black, are taken from Casas et al. [18]. (b) The isosurfaces on the side view represent the external surface of a volume of velocities over a given velocity threshold level. The lowest level, in light blue, corresponds to velocities over 2.1 mm s−1. The vector field represents the direction and speed of flow. The asymmetry of the streamlines and the contours of the velocity volumes indicates the large impact of the ground effect. (c) Five isosurfaces of different flow velocities in the horizontal plane at leg height. Each isosurface, from dark blue to orange, represents a surface with internal velocities greater than 30 µm s−1, 300 µm s−1, 3 mm s−1, 3 cm s−1 and 9 cm s−1, respectively. All parameters were set as in figure 1b.
The monotonous increase in the maximal distance at which spiders are perceived by crickets as a function of spider speed is a consequence of increasing spider attack velocity, resulting in greater flow disturbances (figure 3). The speed of the spider is considered to be constant during the attack, but differs between attacks.
Figure 3.

Maximal danger perception distances in crickets, as a function of spider attack velocity. These distances depend on the aerodynamic conspicuousness of spiders and the sensory abilities of crickets. The maximal flow velocities created by the three-sphere spider model were first extracted from the volume of flow velocities according to a procedure described in the main text. A cutoff value of 30 µm s−1, corresponding to the cricket flow perception threshold, was applied, to define the maximal danger perception distance. The relationship between the attack speed and the maximal danger perception distance splits the space defined by these two variables into two regions differing in their values for the ratio of attack to danger perception distances. For a given attack speed, spiders launching their attack from a distance below the curve will be sensed from onset of the attack. By contrast, the spider has a head-start over its prey if it launches its attack from further away. This is illustrated for a low and a high attack speed. (Online version in colour.)
4. Discussion
Spiders attack crickets at intermediate flow regimes, characterized by Reynolds numbers of approximately 2–90. This implies that both inertial and viscous forces must be taken into account when modelling upstream flow disturbances and that analytical approximations break down. CFD–FEM is the tool of choice for calculating flow disturbances for such intermediate regimes. This numerical method is also routinely used to compute the flow disturbances of copepods, which swim at similar Reynolds numbers [24].
Our computational results demonstrate that the upstream flow generated by running spiders can be faithfully represented with a small number of factors—body size, speed and height above ground—providing information about the role of all body parts in generating flow disturbances. Our findings are consistent with previous studies of swimming copepods, which also identified body shape, body speed and orientation as the principal factors determining the flow field around the body [25–29]. By contrast, the complex motion patterns of beating appendages are of major importance in copepods, whereas the legs of spiders make only a minor contribution to upstream air velocity. Spider legs have marked influence only when fully extended, resulting in an increase in effective body size. This difference in the importance of the contribution of appendages to flow disturbances between spiders and copepods cannot be explained by Reynolds numbers, which are similar for both species. Instead, it may reflect differences in the number of appendages (greater in copepods), their plane of motion with respect to that of the body and the absence of a ground effect in a water column. Indeed, the relatively large ground effect may decrease the relative importance of leg movements in the production of air flow disturbances by spiders. The precise and complete tracking of leg kinematics and more sophisticated models are required to improve our understanding of the role of legs in generating disturbances to air flow in front of moving animals.
The parabolic relationship between spider speed and flow perception explains the benefits of the bimodal—‘sit-and-wait’ and ‘sit-and-pursue’—attack strategy of the spider [17,19]. Moving at very low speed results in the generation of a negligible signal. Running at high speed enables the spider to overcome the warning and escape system of the prey even if highly conspicuous. Running at low and intermediate speeds does not render the spider less conspicuous to its prey and may be highly disadvantageous, allowing the prey more time to escape. These implications are consistent with previous findings [17], despite the erroneous assumption used in this previous study, and by Kant & Humphrey [30]. The lack of estimation and simulation of leg and ground effects in these two papers, two elements magnifying flow disturbances, resulted in a need to double the body size with respect to observations to achieve an appropriate fit. Both papers tended to overestimate the rate of decrease in flow velocity disturbance in front of the spider due to the explicit [30] or implicit [17] assumption that the flow was a potential flow, whatever the spider attack velocities. As a result, these two papers underestimated both the maximal distance at which crickets were able to sense attacking spiders of realistic sizes and the continuous increase in this distance with increasing speed.
Expanding the framework of predator sensory and control distances to predator–prey pairs highlights differences in the degree of overlap between predator attack and prey escape distances [17,31]. In terms of the dichotomous classification of attack modes described in the Introduction, the ‘sit-and-wait’ mode may be considered to be an extreme case in which the attack distance is almost zero, as the prey approaches a stationary predator. As a corollary, the attack speed is also almost zero, implying that there is no danger perception distance. By contrast, the ‘active search’ mode can generate a whole range of ratios of attack to danger perception distances, as a function of the speed of the spider. We previously showed that spiders attack their cricket prey at a distance of 3–6 cm, depending on the substrate [16]. We show here that the cricket is able to perceive the danger over distances of approximately 3 cm for low attack speeds and up to 6 cm for the highest running speeds. For a given spider running speed, the ratio is thus usually about or slightly below one. Ratios greater than one are typical of situations in which the spider launches a targeted attack from a large distance on an as yet unwary prey. Such ratios are not common in our biological system for two reasons. First, the densities of wood crickets and wolf spiders are often high (up to 400 and 50 individuals per square metre, respectively, [32]), so the mean distance between the predator and its prey is often no more than a few centimetres. Furthermore, the architectural complexity of the leaf litter makes long-range attacks almost impossible: leaf fragments in the leaf litter are only about 3 cm long [16]. Thus, even at low cricket densities, the architecture of the leaf litter habitat makes it very difficult for the spider to launch a long-distance attack. In another study in a different system, in grassland vegetation, ranging spiders and their grasshopper prey were often found at short distances from each other, only a few centimetres apart [19]. We therefore conclude that the ratio of predator attack distance to prey danger perception distance is often about one or lower, in both systems. This conclusion may also apply to the myriad of other predator–prey systems within the soil and in highly structured habitats, such as vegetation.
The implications of these findings are twofold. First, there are ecomechanical implications concerning a large range of unrelated characteristics pertaining to the manoeuverability and capture capabilities of spiders in the final instants of the interaction, the subjugation of the prey. Very little is known about these aspects, and the kinematics of spider leg movements during prey capture are almost entirely unknown. This contrasts strongly with the situation for similar studies on mouth movements in suction feeding fishes during close-range prey capture [33]. For prey crickets, our findings suggest that future studies should focus on the hitherto neglected aspects of danger identification and motor control during escape, echoing recent neurophysiological studies on the cercal response to stimuli with various spatial and temporal patterns [34,35]. These aspects have been little studied in most invertebrate predator–prey systems. Second, our findings have ecological implications concerning the importance of the post-encounter steps within a predation sequence in predator–prey interactions. Indeed, for ratios greater than one, attention should be focused on the foraging predator, with the prey considered to be non-interacting. The probability of prey capture may then be considered to be either constant, depending on some phenotypic characteristic of the prey [36,37], or dependent purely on the state of the predator, with satiated predators often less successful [38]. By contrast, a strong bidirectional coupling of the behaviour of the two antagonists would be expected, from the start of the attack, for ratios close to and below one. Another example of this is provided by the attacks of plankton-feeding fish on evasive copepods [39]. These fish, which detect their prey visually, launch attacks at distances of a couple of millimetres from the prey. The danger perception distances of the prey are much larger, owing to the bow wave generated by the moving fish. However, these fish mostly succeed in capturing their prey by generating ‘compensatory suction’. They suck water into their mouths, thereby decreasing the unintentional signal emitted in the plane of the prey: a hydrodynamic stealth mode of prey capture. In situations characterized by a ratio no greater than one, capture probability is a function of a larger number of variables. Not only are the ecomechanical properties of the two antagonists important, as in the former case, but the bidirectional coupling of the behaviour of predator and prey must also be taken into account. Wood crickets and wolf spiders on the forest floor, grasshoppers and spiders in grassland vegetation and plankton-feeding fishes and copepods in water constitute predator–prey systems in which sensory ecology during attack is heavily based on flow sensing. They are also characterized by the unintentional production of aero- and hydrodynamic signals by the predator from the onset of an attack. A systematic survey of other predator–prey interactions based on different sensory ecologies would give insight into their positioning in terms of the degree of coupling during attack.
Supplementary Material
Acknowledgements
We thank A. Landres, O. Dangles, S. Pincebourde and S. Sane for their numerous comments on a previous version of the manuscript and the subject matter editor and the referees for their incisive comments.
References
- 1.Endler JA. 1991. Interactions between predators and prey. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 169–196. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 2.Brechbühl R, Casas J, Bacher S. 2011. Diet choice of a predator in the wild: missed opportunities along the prey capture sequence. Ecosphere 2, art 133 ( 10.1890/ES11-00323.1) [DOI] [Google Scholar]
- 3.Reader T, Higginson AD, Barnard CJ, Gilbert FS, and The Behavioral Ecology Field Course. 2006. The effects of predation risk from crab spiders on bee foraging behavior. Behav. Ecol. 17, 933–939. ( 10.1093/beheco/arl027) [DOI] [Google Scholar]
- 4.Morse DH. 1979. Prey capture by the crab spider Misumena calycina (Araneae: Thomisidae). Oecologia 39, 309–319. ( 10.1007/BF00345442) [DOI] [PubMed] [Google Scholar]
- 5.Curio E. 1976. The ethology of predation. Berlin, Germany: Springer. [Google Scholar]
- 6.Patek SN, Korff WL, Caldwell RL. 2004. Biomechanics: deadly strike mechanism of a mantis shrimp. Nature 428, 819–820. ( 10.1038/428819a) [DOI] [PubMed] [Google Scholar]
- 7.Stewart WJ, Cardenas GS, McHenry MJ. 2013. Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 216, 388–398. ( 10.1242/jeb.072751) [DOI] [PubMed] [Google Scholar]
- 8.Domenici P, Blagburn JM, Bacon JP. 2011. Animal escapology I: theoretical issues and emerging trends in escape trajectories. J. Exp. Biol. 214, 2463–2473. ( 10.1242/jeb.029652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny M, Helmuth B. 2009. Confronting the physiological bottleneck: a challenge from ecomechanics. Integr. Comp. Biol. 49, 197–201. ( 10.1093/icb/icp070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrams PA. 2000. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Evol. Syst. 31, 79–105. ( 10.2307/221726) [DOI] [Google Scholar]
- 11.Lima SL. 2002. Putting predators back into behavioral predator–prey interactions. Trends Ecol. Evol. 17, 70–75. ( 10.1016/S0169-5347(01)02393-X) [DOI] [Google Scholar]
- 12.Combes SA, Rundle DE, Iwasaki JM, Crall JD. 2012. Linking biomechanics and ecology through predator–prey interactions: flight performance of dragonflies and their prey. J. Exp. Biol. 215, 903–913. ( 10.1242/jeb.059394) [DOI] [PubMed] [Google Scholar]
- 13.Snyder JB, Nelson ME, Burdick JW, MacIver MA. 2007. Omnidirectional sensory and motor volumes in electric fish. PLoS Biol. 5, e301 ( 10.1371/journal.pbio.0050301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson AM, Lowe JC, Roskilly K, Hudson PE, Golabek JKA, McNutt JW. 2013. Locomotion dynamics of hunting in wild cheetahs. Nature 498, 185–189. ( 10.1038/nature12295) [DOI] [PubMed] [Google Scholar]
- 15.Wisniewska DM, Johnson M, Beedholm K, Wahlberg M, Madsen PT. 2012. Acoustic gaze adjustments during active target selection in echolocating porpoises. J. Exp. Biol. 215, 4358–4373. ( 10.1242/jeb.074013) [DOI] [PubMed] [Google Scholar]
- 16.Morice S, Pincebourde S, Darboux F, Kaiser W, Casas J. 2013. Predator–prey pursuit–evasion games in structurally complex environments. Integr. Comp. Biol. 53, 767–779. ( 10.1093/icb/ict061) [DOI] [PubMed] [Google Scholar]
- 17.Dangles O, Ory N, Steinmann T, Christides J-P, Casas J. 2006. Spider's attack versus cricket's escape: velocity modes determine success. Anim. Behav. 72, 603–610. ( 10.1016/j.anbehav.2005.11.018) [DOI] [Google Scholar]
- 18.Casas J, Steinmann T, Dangles O. 2008. The aerodynamic signature of running spiders. PLoS ONE 3, e2116 ( 10.1371/journal.pone.0002116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JRB, Ament JM, Schmitz OJ. 2014. Fear on the move: predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J. Anim. Ecol. 83, 214–222. ( 10.1111/1365-2656.12111) [DOI] [PubMed] [Google Scholar]
- 20.Shimozawa T, Murakami J, Kumagai T. 2003. Cricket wind receptors: thermal noise for the highest sensitivity known. In Sensors and sensing in biology and engineering (eds Barth F, Humphrey JC, Secomb T.), pp. 145–157. Berlin, Germany: Springer. [Google Scholar]
- 21.Löhner R. 2008. Applied computational fluid dynamics techniques: an introduction based on finite element methods. New York, NY: Wiley. [Google Scholar]
- 22.Cantrell RS, Cosner C. 2003. Spatial ecology via reaction–diffusion equations. New York, NY: Wiley. [Google Scholar]
- 23.White JW, Rassweiler A, Samhouri JF, Stier AC, White C. 2014. Ecologists should not use statistical significance tests to interpret simulation model results. Oikos 123, 385–388. ( 10.1111/j.1600-0706.2013.01073.x) [DOI] [Google Scholar]
- 24.Visser A. 2001. Hydromechanical signals in the plankton. Mar. Ecol. Prog. 222, 1–24. ( 10.3354/meps222001) [DOI] [Google Scholar]
- 25.Jiang H, Kiørboe T. 2011. The fluid dynamics of swimming by jumping in copepods. J. R. Soc. Interface 8, 1090–1103. ( 10.1098/rsif.2010.0481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Duren LA, Stamhuis EJ, Videler JJ. 2003. Copepod feeding currents: flow patterns, filtration rates and energetics. J. Exp. Biol. 206, 255–267. ( 10.1242/jeb.00078) [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Osborn TR, Meneveau C. 2002. The flow field around a freely swimming copepod in steady motion. Part I: theoretical analysis. J. Plankt. Res. 24, 167–189. ( 10.1093/plankt/24.3.167) [DOI] [Google Scholar]
- 28.Malkiel E, Sheng J, Katz J, Strickler JR. 2003. The three-dimensional flow field generated by a feeding calanoid copepod measured using digital holography. J. Exp. Biol. 206, 3657–3666. ( 10.1242/jeb.00586) [DOI] [PubMed] [Google Scholar]
- 29.Catton KB, Webster DR, Brown J, Yen J. 2007. Quantitative analysis of tethered and free-swimming copepodid flow fields. J. Exp. Biol. 210, 299–310. ( 10.1242/jeb.02633) [DOI] [PubMed] [Google Scholar]
- 30.Kant R, Humphrey JAC. 2009. Response of cricket and spider motion-sensing hairs to airflow pulsations. J. R. Soc. Interface 6, 1047–1064. ( 10.1098/rsif.2008.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riechert SE. 2009. Spiders as representative ‘sit-and-wait’ predators. In Natural enemies (ed. Crawlay M.), pp. 313–328. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 32.Gabbutt PD. 1959. The bionomics of the wood cricket, Nemobius sylvestris (Orthoptera: Gryllidae). J. Anim. Ecol. 28, 15–42. ( 10.2307/2011) [DOI] [Google Scholar]
- 33.Skorczewski T, Cheer A, Cheung S, Wainwright PC. 2014. Use of computational fluid dynamics to study forces exerted on prey by aquatic suction feeders. J. R. Soc. Interface 44, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulder-Rosi J, Cummins GI, Miller JP. 2010. The cricket cercal system implements delay-line processing. J. Neurophys. 103, 1823–1832. ( 10.1152/jn.00875.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupuy F, Steinmann T, Pierre D, Christidès J-P, Cummins G, Lazzari C, Miller J, Casas J. 2012. Responses of cricket cercal interneurons to realistic naturalistic stimuli in the field. J. Exp. Biol. 215, 2382–2389. ( 10.1242/jeb.067405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeschke JM, Kopp M, Tollrian R. 2002. Predator functional responses: discriminating between handling and digesting prey. Ecol. Monogr. 72, 95–112. ( 10.1890/0012-9615(2002)072[0095:PFRDBH]2.0.CO;2) [DOI] [Google Scholar]
- 37.Metz JAJ, van Batenburg FHD. 1985. Holling's ‘hungry mantid’ model for the invertebrate functional response considered as a Markov process. Part I. The full model and some of its limits. J. Math. Biol. 22, 209–223. ( 10.1007/BF00275716) [DOI] [Google Scholar]
- 38.Van Rijn PC, Bakker FM, Van der Hoeven WA, Sabelis MW. 2005. Is arthropod predation exclusively satiation-driven? Oikos 109, 101–116. ( 10.1111/j.0030-1299.2005.12987.x) [DOI] [Google Scholar]
- 39.Gemmell BJ, Adhikari D, Longmire EK. 2014. Volumetric quantification of fluid flow reveals fish's use of hydrodynamic stealth to capture evasive prey. J. R. Soc. Interface 11, 20130880 ( 10.1098/rsif.2013.0880) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.