Abstract
Glucocorticoid hormones (CORT) are predicted to promote adaptation to variable environments, yet little is known about the potential for CORT secretion patterns to respond to selection in free-living populations. We assessed the heritable variation underlying differences in hormonal phenotypes using a cross-foster experimental design with nestling North American barn swallows (Hirundo rustica erythrogaster). Using a bivariate animal model, we partitioned variance in baseline and stress-induced CORT concentrations into their additive genetic and rearing environment components and estimated their genetic correlation. Both baseline and stress-induced CORT were heritable with heritability of 0.152 and 0.343, respectively. We found that the variation in baseline CORT was best explained by rearing environment, whereas the variation in stress-induced CORT was contributed to by a combination of genetic and environmental factors. Further, we did not detect a genetic correlation between these two hormonal traits. Although rearing environment appears to play an important role in the secretion of both types of CORT, our results suggest that stress-induced CORT levels are underlain by greater additive genetic variance compared with baseline CORT levels. Accordingly, we infer that the glucocorticoid response to stress has a greater potential for evolutionary change in response to selection compared with baseline glucocorticoid secretion patterns.
Keywords: animal model, barn swallow, cross-foster, environmental variation, glucocorticoids, heritability
1. Introduction
Physiological systems enable animals to adaptively respond to the challenges of life in fluctuating environments. There is often remarkable variation in hormonal responses within and among individuals (e.g. [1]), but in order to assess the potential for evolutionary change of fitness-related physiological mechanisms, some of the phenotypic variation in hormone function must be heritable. Secretion of glucocorticoid hormones—primarily corticosterone (CORT) in birds—is regulated through the hypothalamic–pituitary–adrenal (HPA) axis and is released in a circadian rhythm to facilitate metabolic activity and maintain energy levels. At heightened plasma concentrations, CORT also functions as a crucial component of the stress response [2]. Selection on the responsiveness of the HPA axis has been widely proposed to promote adaptation to fluctuating environments, and a rapidly growing literature documents the links between individual variation in CORT concentration and measures of fitness [3–7]. Yet, while selection requires heritable variation on which to act, the heritability of variation in HPA axis activity patterns in free-living populations remains largely unexplored. Even though the ultimate genomic and non-genomic effects on HPA axis activity are probably influenced by a multitude of factors (e.g. releasing hormones, receptor density, tissue sensitivity), individual variation in plasma CORT levels has been shown to predict survival rate [2–5], reproductive performance [6–8] and behavioural syndromes [9,10]. Therefore, although selection may act on multiple levels involved in the HPA axis response to life's challenges, estimating the genetic variation underlying circulating glucocorticoid levels will provide a deeper understanding of the potential for evolutionary processes to shape variation in an important aspect of the stress response phenotype.
There is a large body of evidence supporting the role of the environment in glucocorticoid secretion (e.g. [1,2,11]), and this dynamic relationship between environmental fluctuations and glucocorticoid responses is considered crucial for modulating life-history strategies to maximize an individual's fitness [12–14]. Population-level latitudinal patterns in glucocorticoid profiles are often related to life-history traits including the optimization of breeding strategies to match breeding season length [15–17]. Plasma CORT concentrations also vary between seasons [18,19] and within a season [6], which probably supports changes in metabolic demands during different life-history stages (e.g. territory acquisition, breeding and nestling provisioning, moult, migration). Inclement weather [2], food availability [20] and the threat of predation [21] are additional ecological factors shown to induce changes in circulating CORT that initiate appropriate physiological and behavioural responses. Although CORT secretion patterns exhibit some dependence on environmental context, inter-individual variation in these responses could also be derived from genetic variation.
Standing genetic variation in a population is subject to evolutionary change that may lead to observable changes in a trait across generations. The degree to which phenotypic variation responds to evolutionary change depends, in part, upon the heritability of a trait. Heritability in the broadest sense is defined as the proportion of the phenotypic variation in a trait that is owing to genetic variation and is determined by the collective contributions of additive, epistatic and dominant processes of gene expression [22]. Narrow-sense heritability focuses on the additive genetic variance underlying observable differences in a trait, and studies that assess the heritability of CORT responses typically estimate narrow-sense heritability. For example, the heritability of the glucocorticoid response to stress is supported by selection studies in captive settings, which confirm that steroid hormone levels are responsive to selection [23–26], and selection solely targeting either circulating CORT or hormone-mediated traits can cause correlated changes in the other trait [27–30]. Further, it is worth noting that epigenetic modifications that alter gene expression are another source of heritable variation that can impact CORT secretion patterns throughout life [31,32]. However, the epigenetic regulation of glucocorticoid responses is difficult to measure in wild populations and particularly in long-term studies, such as ours, where individual fitness and survivorship outcomes are being measured.
Previous studies that examine the role of selection in divergent glucocorticoid phenotypes have focused on stress-induced CORT [23–28], but less is known about the contribution of genetic variation to among-individual variability in baseline CORT concentrations. Moreover, to our knowledge, all studies that have investigated the heritability of the glucocorticoid response have been conducted in captivity (e.g. [23–26]), where environmental variation is probably artificially reduced. Assessing the heritability of both baseline and stress-induced CORT levels in a free-living population will lend greater insight into the importance of genetic and ecological factors in influencing CORT secretion patterns under natural environmental settings.
We conducted a cross-foster experiment with nestlings in a wild population of North American barn swallows (Hirundo rustica erythrogaster). Genotypes were allocated across different nest environments in order to partition the phenotypic variance in baseline and stressed-induced plasma CORT concentrations into genetic (based on genetic relatedness) and environmental (nest in which altricial offspring develop) parameters with the aim to discern the relative roles these factors play in circulating hormone variation. Using a cross-foster design to create variable levels of genetic relatedness within and among nests in a wild avian population allowed us to tease apart the effects of common genes and nest environment, which are not easily distinguishable in captive studies, but are necessary to understand the potential for selection to shape natural variation in glucocorticoid responses.
2. Material and methods
(a). Cross-fostering experiment and sampling
We studied barn swallows that nest mainly in horse barns and culverts located throughout Boulder, Jefferson and Weld Counties, CO, during their breeding season from May through to July 2010. We sampled across 16 sites, ranging in size from 1 to 50 breeding pairs. To allocate genotypes across different nest environments, we designed a non-reciprocal, cross-fostering experiment between pairs of nests that had the same hatch date (determined as the date on which the first egg in a clutch hatched), and in which at least three nestlings had hatched by the following day (day 1). Two randomly chosen nestlings were moved between paired nests on day 1 such that one brood experienced an increase and the other a decrease in brood size. Control nests were maintained in which nestlings were handled at the same frequency as those in manipulated nests but no nestling swaps occurred. Brood size prior to the manipulation ranged from three to six nestlings (mean brood size 4) and after swapping ranged from one seven nestlings (mean brood size 4) for the nests included in this study. To maintain the identity of cross-fostered versus host nestlings, we applied non-toxic marker to the entire leg of swapped nestlings every other day until they were big enough to band with aluminium US Geological Survey (USGS) rings.
We collected blood samples from nestlings on day 12 to measure plasma CORT concentrations. Full broods were removed from 38 nests, but owing to sampling time constraints for baseline samples, we were only able to collect blood samples from a subset of nestlings for some nests (mean total brood size = 4 ± 1.31, mean collected brood size = 2.95 ± 1.11). To estimate baseline CORT concentration, blood samples were taken within 3 min of initial disturbance of the nest [33]. Stress-induced CORT concentration was measured following a standardized restraint stress protocol [1,18] in which we collected a second blood sample 15 min after the initial disturbance. Nestlings were handled between baseline and stress-induced blood sample collections during which we recorded mass and took other morphometric measurements. Among avian studies that assess stress-induced CORT concentrations, sampling time typically takes place between 15 and 60 min; we chose 15 min as a standardized time because capture for blood sampling in order to capture heightened plasma CORT levels while minimizing any adverse effects caused by extended handling stress. We bled and measured nestlings in a location far enough from other active nests at the site to minimize disturbance to other nestlings.
Because barn swallows exhibit moderately high rates of extra-pair fertilization (hereafter, ‘EPF’; approx. 40% of all young are due to EPFs each breeding season in our study population, R. J. Safran, B. R. Jenkins, J. K. Hubbard 2008–2011, unpublished data), some natural nests contain a mix of full and half siblings. Thus, to accurately assign the genetic relationship among nestlings, we conducted genetic paternity analyses (see §2c) by collecting blood samples from all parents associated with the cross-foster experiment. Adult barn swallows were captured by mist net or by hand off the nest and blood samples were taken. We banded adults with a USGS aluminium ring and used a combination of coloured plastic leg bands and assorted colours of non-toxic permanent markers on the white spots of tail feathers to identify the social parents of experimental nests. The sex of adults was assigned by using both morphological (i.e. ventral plumage coloration, tail streamer length, presence/absence of a brood patch) and behavioural (parental behaviour such as incubating) observations.
All blood samples were collected from the brachial vein using heparinized microhematocrit capillary tubes. For nestling plasma collection, blood was transferred to microcentrifuge tubes and kept on ice until plasma could be separated from whole blood by centrifugation. Plasma samples were stored at −70°C until assayed, and the remaining blood cells were stored in lysis buffer at room temperature. Adult blood samples were placed in lysis buffer immediately after collection in the field.
(b). Corticosterone assay
We quantified total CORT concentrations for 102 baseline and 108 stress-induced plasma samples using enzyme immunoassay kits (catalogue number ADI-901–097, Enzo Life Sciences, Plymouth Meeting, PA, USA). We optimized our assay protocol for barn swallow plasma as described in [34,35]. A six-standard curve was used in each of eight assay plates to measure CORT concentration, and both baseline and stress-induced plasma samples collected from an individual were assayed on the same plate. Standards and samples were run in duplicate with an inter-assay variation of 9.81% and an average intra-assay variation of 8.69%. Concentrations for baseline CORT ranged from 0.35 to 19.06 ng ml−1 (mean ± s.d.: 3.85 ± 3.58), and stress-induced CORT ranged from 9.86 to 56.22 ng ml−1 (mean ± s.d.: 26.81 ± 10.17). Most plasma samples were above the detection threshold (0.38 ± 0.09 ng ml−1 across plates), but in order to reduce bias we replaced eight baseline plasma samples that fell below the detection threshold with the plate-specific detection threshold, rather than rejecting these samples entirely.
(c). Paternity analysis and genetic methods to identify sex in nestlings
We used paternity analyses to verify relatedness among brood-mates owing to the high rates of EPFs in our population, but not all experimental brood pairs are represented in our dataset as we were unable to verify paternity for some nestlings. Nestlings were only included in our analyses if we had collected either a baseline blood sample taken within 3 min of initial disturbance, a stress-induced blood sample taken between 15 and 18 min, or both, and we were able to obtain blood samples from both social parents. DNA was extracted from nestling and adult blood samples that were stored in lysis buffer using DNeasy Blood & Tissue Extraction kits (Qiagen, MD, USA). Polymerase chain reaction (PCR) was used to amplify six microsatellite loci previously developed for assessing parentage, and PCR reaction conditions are described in the electronic supplementary material.
Using GeneMapper software (v. 4.0, Applied Biosystems), we assigned genotypes for 111 nestlings and 38 pairs of social parents at all six loci. Additional adult males were included in our paternity analysis to increase the probability of assigning paternity to offspring sired by extra-pair males. Genotypes from adults and offspring were incorporated into a paternity analysis using CERVUS software (v. 2.0) to calculate exclusion probabilities for assessing parentage. With a combined first-parent exclusion probability of 0.922 for all six loci, we were able to assign paternity for 68 nestlings that had the same social and genetic father as well as 20 nestlings that were sired by an extra-pair male. Paternity exclusion was conducted using similar parameters described in [36].
To assess any potential differences in CORT profiles between male and female nestlings, sex was determined using molecular tools. We conducted a PCR protocol with sex-linked markers as described by Griffiths et al. [37] with the exception that 0.25 units of JumpStart Taq DNA polymerase (Sigma) was used with a modified amplification protocol: initial denaturation step at 94°C for 60 s, followed by 34 cycles of 94°C for 45 s, 48°C for 45 s and 72°C for 45 s, and 72°C for 3 min for the final extension. PCR products were visualized from a benchtop ultraviolet transilluminator using Sybr Green (Invitrogen) on a 3% agarose gel.
(d). Statistical analyses for quantitative genetics
The cross-fostering design in conjunction with a high EPF rate created a mosaic of genetic and rearing environment relationships among nestlings: full and half siblings reared in the same and different nests, and the genetically unrelated nest-mates of swapped nestlings. The pedigree consisted of 107 offspring, 38 mothers and 39 fathers (189 identities total). Of these offspring, 65 were within-pair young, and of the 42 extra-pair young we were able to identify the extra-pair father for 19. Note that there were four nestlings removed from our statistical models that were included in the paternity analysis owing to missing mass or sampling time data. We do not have any information regarding the relatedness of mothers and fathers in this pedigree; consequently, all adults were assumed to be unrelated. This assumption is safe given our long-term study of marked individuals in our population showing that natal site fidelity is quite low; between the years 2009 and 2012 only 65 banded nestlings returned to our study area as breeding adults (out of 2360 total banded nestlings), and among them were only two unrelated nest-mates and three maternal half siblings.
We estimated the variance components for phenotypic variance of untransformed baseline and stress-induced CORT concentrations by fitting a bivariate animal model using a Bayesian Markov Chain Monte Carlo (MCMC) technique implemented in MCMCglmm [38]. The bivariate model allowed us to partition total phenotypic variance (VP) into additive genetic variance (VA), common environmental variance (VE) and residual variance (VR). From these variance components, we calculated narrow-sense heritability (h2) and the effect of common rearing environment (e2) for both baseline and stress-induced CORT concentrations separately, as well as estimating the genetic correlation between them. We calculated h2 as VA/VP; e2 was calculated as VE/VP; the genetic correlation was calculated as 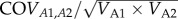 (where A1 and A2 refer to additive genetic variance for baseline CORT and stress-induced CORT, respectively).
(where A1 and A2 refer to additive genetic variance for baseline CORT and stress-induced CORT, respectively).
In the model, pedigree and rearing environment were included as random effects. Rearing environment is the stationary nest location where nestling development took place and was represented by one term that included both the geographical location of the breeding site and the nest location within that site. The variance estimate from the pedigree term represents the additive genetic variance, whereas the variance estimate from the rearing environment term represents the common environmental variance. We specified the priors for VA, VE and VR by splitting the observed phenotypic variance evenly between these three variance components (e.g. [39]). We varied the priors by adjusting the proportion of variance specified to each component. While h2, e2 and the genetic correlation estimates are somewhat sensitive to the specified priors, the overall conclusions regarding relative proportions of phenotypic variance are not (see the electronic supplementary material, table S1). All models were run for 1 000 000 iterations, with a burn-in of 50 000 iterations and every 200th iteration was stored (autocorrelations were weaker than 0.05 for all variance components). For both random effects, we had effective-samples sizes between 4157 and 4967.
We initially explored including the following fixed effects into our model: sample latency time, nestling body mass, brood size, sex and sampling date. The variable ‘sample latency time’ is the elapsed time from initial disturbance (when removing nestlings from their nest for sampling was initiated) to baseline blood sample collection, and it has been previously shown that there is a significant positive relationship between sample latency time and baseline CORT concentration but no relationship with stress-induced CORT concentration [34]. In our sample, we found an effect of latency time on baseline CORT concentration such that CORT levels increased as time from initial disturbance to blood sample collection advanced (Spearman's ρ = 0.414, p < 0.01) but did not find a relationship between latency time and stress-induced CORT concentration (Spearman's ρ = −0.036, p = 0.712). There is considerable developmental variation (e.g. body mass and feather development) within broods, potentially owing to variable parental feeding rates or ectoparasite exposure. Baseline CORT concentration is known to fluctuate with mass in our study population [34], therefore we included mass to control for within-brood variation, as we are interested in the variation explained by shared rearing environment. As brood size was manipulated (increased/decreased) for some nests in this study as part of a separate experiment, we also ran models that included brood size as a fixed effect. However, controlling for brood size probably results in an underestimation of e2 as this is an environmental factor that naturally varies among nests and may contribute to phenotypic variation in CORT measures. We used R to assess correlations among all potential fixed effects and found that there was a significant positive correlation between sample latency time and brood size (Spearman's ρ = 0.417, p < 0.01; all other Spearman's ρ between −0.160 and −0.036, all p > 0.1). Only nestling body mass and sample latency time have statistical effects on the model as the posterior distributions for sampling date, brood size and sex terms overlap zero (see the electronic supplementary material, table S2); consequently, our final model includes only nestling body mass and sample latency time as fixed effects. Here, we report the results of both the maximal and final models (table 2; for models including various combinations of fixed effects see the electronic supplementary material, table S3), and although the overall conclusions are qualitatively similar, we interpret and discuss results based on the model without sampling date, brood size and sex.
Table 2.
Estimates of heritability (h2) (calculated as VA/VP), effect of common rearing environment (e2) (calculated as VE/VP) for baseline and stress-induced CORT concentration and the genetic correlation (calculated as 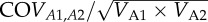 ) between them estimated by: (i) the maximum model with sampling date, mass, sex, brood size and sample latency time as fixed effects, and (ii) the final model with mass and sample latency time as fixed effects.
) between them estimated by: (i) the maximum model with sampling date, mass, sex, brood size and sample latency time as fixed effects, and (ii) the final model with mass and sample latency time as fixed effects.
| DIC | baseline CORT |
stress-induced CORT |
genetic correlation (95% BCI) | |||
|---|---|---|---|---|---|---|
| h2 (95% BCI) | e2 (95% BCI) | h2 (95% BCI) | e2 (95% BCI) | |||
| intercept + date + mass + sex + BS + latency time | 1037.216 | 0.183 (0.061–0.462) | 0.617 (0.311–0.769) | 0.366 (0.125–0.588) | 0.492 (0.268–0.712) | −0.252 (−0.709–0.315) |
| intercept + mass + latency time | 1039.088 | 0.152 (0.062–0.458) | 0.555 (0.309–0.771) | 0.343 (0.128–0.598) | 0.491 (0.251–0.693) | −0.297 (−0.688–0.333) |
3. Results
Phenotypic variance was much greater for stress-induced CORT concentration compared with baseline CORT concentration among nestling barn swallows in our population (table 1). From the variance component estimates, we calculated h2 (posterior mode ±95% Bayesian credible interval (BCI)) for baseline CORT concentration to be 0.152 (0.062–0.458) and e2 (95% BCI) to be 0.555 (0.309–0.771). For stress-induced CORT concentration with the same 95% BCI parameters described for baseline CORT, we calculated an h2 estimate of 0.343 (0.128–0.598) and an e2 estimate of 0.491 (0.251–0.693) (table 2 and figure 1). The final model deviance information criterion (DIC) with pedigree and rearing environment as random effects was 1039.088 and revealed a better fit compared with the same model with one of the random effects removed (without pedigree DIC: 1159.542; without nest DIC: 1069.69). Thus, using a model comparison approach, we can conclude that including both pedigree and rearing environment as variance components is statistically meaningful (see the electronic supplementary material, figure S1). These results suggest that the variation in baseline CORT is best explained by rearing environment, whereas variation in stress-induced CORT is best explained by a combination of additive genetic effects and rearing environment. The genetic correlation for these two traits was not significantly different from zero as the 95% BCI overlapped with zero (table 2 and the electronic supplementary material, figure S2), which parallels the lack of within-individual phenotypic correlation observed between these traits (Spearman's ρ = 0.114, p > 0.1).
Table 1.
Posterior modes (and 95% Bayesian credible interval (BCI)) of variance components for baseline and stress-induced CORT concentrations for the final model with mass and latency time as fixed effects.
| baseline CORT | stress-induced CORT | |
|---|---|---|
| additive genetic variance (VA) | 1.958 (0.757–5.194) | 37.354 (14.240–59.457) |
| common environmental variance (VE) | 5.45 (2.411–12.154) | 40.25 (16.396–89.74) |
| residual variance (VR) | 1.998 (0.922–4.178) | 14.309 (5.265–29.54) |
| total phenotypic variance (VP) | 11.075 (7.909–17.126) | 95.278 (67.921–141.756) |
Figure 1.
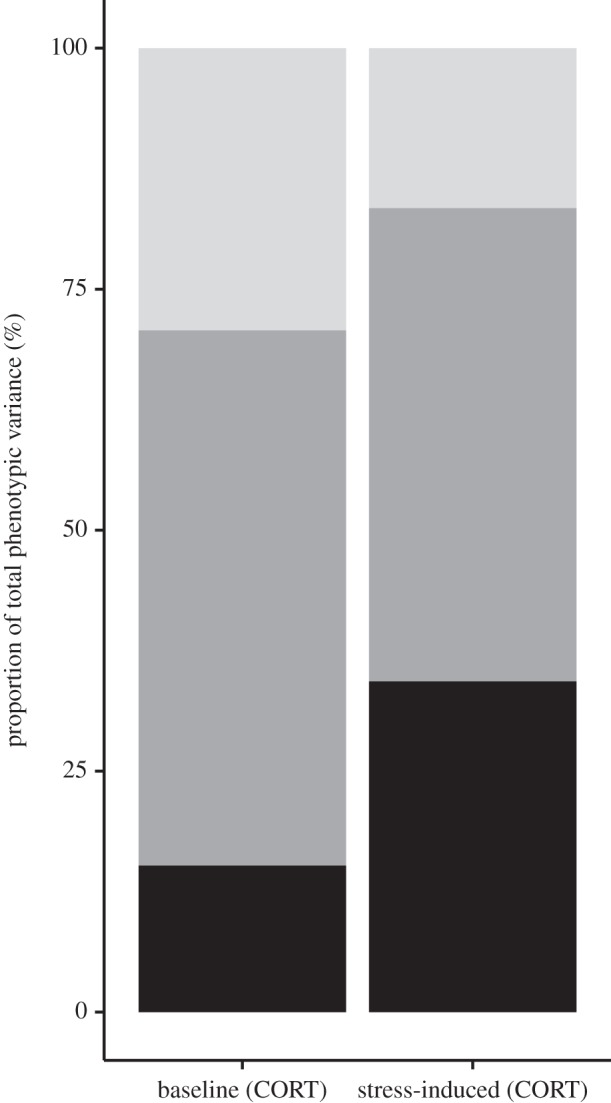
Summary of how phenotypic variance is partitioned across additive genetic variance (black bars), common environmental variance (dark grey bars) and residual variance (light grey bars) for baseline and stress-induced CORT concentrations.
4. Discussion
In this study, we assessed the potential for evolutionary processes to shape the variation in circulating glucocorticoid levels by estimating the heritability of variation in baseline and stress-induced plasma CORT concentrations. We used a cross-foster design with a wild population of nestling North American barn swallows to tease apart genetic differences associated with glucocorticoid secretion phenotypes being expressed under variable rearing environments. We held developmental stage constant by comparing same-age nestlings rather than incorporating parent CORT measurements into the animal model pedigree or examining parent–offspring regressions in circulating CORT, which are probably sensitive to ontogenetic stage and the different ecological contexts experienced by nestlings and their parents. Our analyses revealed relatively low heritability and a greater effect of rearing environment on baseline CORT levels compared with stress-induced CORT levels. For stress-induced plasma CORT concentrations, we detected considerable phenotypic variation that was moderately heritable but also largely influenced by rearing environment. It is also worth mentioning that the error term captured a notable amount of phenotypic variation for these two traits, which may account for differences among nest-mates in dominance effects, gene by environment interactions, exposure to ectoparasites, or variation in feeding rates. Further, we did not detect a statistical genetic association between circulating concentrations of baseline and stress-induced CORT. The lack of a genetic correlation suggests that the physiological mechanisms underlying the secretion of glucocorticoids during basal activity and stressful events differ and that selection may shape variation in these phenotypically distinctive traits independently.
(a). Baseline corticosterone
Baseline CORT influences fitness by regulating metabolic function both during and in transition between different life-history stages (e.g. [11,18,40]). Physiological traits that are important for mediating life-history trade-offs and fitness are often shown to have relatively low heritability [41]. Consistent with this, we observed that only a small proportion of the variation in baseline CORT concentration among nestlings was explained by their genetic relationship. A low heritability estimate and relatively low phenotypic variation compared with stress-induced CORT might suggest that selection has reduced overall genetic variation among nestlings in order to cope with metabolic needs in a similar fashion (stronger stabilizing selection). Alternatively, the amount of additive genetic variation explaining phenotypic variation may be reduced if genetically related nestlings raised in different nests express different baseline CORT secretion patterns owing to variable environmental parameters (phenotypic plasticity). Other sources of variation in baseline CORT levels could stem from the plasticity in physiological processes regulating HPA function such as adjustments in glucocorticoid receptor densities (reviewed in [12]) or alterations in the circadian release of the hypothalamic neurohormone corticotropic-releasing hormone (e.g. [42]). Although the additive genetic variance was notably lower than the rearing environment variance, there was overlap in the 95% BCIs for h2 and e2, suggesting that genetic differences among nestlings still contribute, to some degree, to the observable differences in baseline CORT levels.
In natural populations, baseline CORT can vary dramatically within individuals over time [6,40,43] in response to fluctuating environments and changes in metabolic demands [11,44]. The longitudinal variation within individuals in baseline CORT levels among different environmental contexts [18,19] as well as within-individual variation among different life-history stages [6] exemplify the flexibility of baseline glucocorticoid response patterns and underscore the importance of the environmental influences on such patterns. Similar ecological factors experienced by nest-mates, which are probably represented by the rearing environment variance component in the animal model, may include weather variability [45], fluctuations in food availability [46] and possibly the variability in parental effort. Although some research suggests that brood size can influence glucocorticoid levels and related fitness measures [46,47], we did not find an effect of brood size on CORT concentration similarly to several other studies [45,48,49]. Moreover, during the year of our study, changes in brood size did not influence parental provisioning behaviour (Vitousek et al. [50]). Nonetheless, there could be indirect effects of enlarged or reduced brood sizes on nestling CORT that we were unable to account for. Additionally, changes in temporal patterns of predation could impact nestling CORT levels indirectly through changes in parental behaviour ([21], Vitousek et al. [51]). The influence of these seasonal factors on individual differences in baseline CORT secretion patterns, including temporal changes in weather, competition, food availability, predation risk and parental behaviour linked to the timing of breeding remain largely unexplored and merit further study.
(b). Stress-induced corticosterone
There was a considerable amount of phenotypic variation in stress-induced CORT concentration in our population of nestling barn swallows. Because there was overlap in the 95% BCIs for each variance component estimate and the heritability estimate fell within the 95% BCI for the rearing environment, we conclude that both genetic factors and environmental context are important for understanding the mechanisms underlying variation in measures of the physiological stress response we presented here.
That natural variation in nestling stress-induced CORT concentration is moderately heritable supports the role of genetic differences in mediating variability in the hormonal response to stress among individuals. This also suggests that there is a similarity in genotypic expression underlying glucocorticoid responses to stress among genetically related nestlings regardless of the nest environment they were raised in. The maintenance of standing genetic variation underlying the glucocorticoid stress response could serve to promote differential physiological or behavioural coping strategies under unpredictable environmental conditions [9,18,19] or to optimize life-history strategies that are most favourable to current survival or reproductive needs [13,14,16]. Overall, our results confirm that individual variation in stress-induced CORT concentration—a trait that plays an important role in initiating appropriate physiological and behavioural responses to challenges [11,12]—is underlain by an appreciable amount of standing additive genetic variation that supports the potential for evolutionary processes to shape the physiological response to stress.
A large proportion of the total phenotypic variance in stress-induced CORT was also explained by variance owing to rearing environment. Some ecological factors that have been shown to influence individual stress responses include food availability [21,52], weather [53] and both previous and prolonged exposure to stressors (i.e. repeated handling or disturbance, populations with high predator prevalence) [21,31,54]. There is a critical window, typically throughout early development, during which the HPA axis is especially susceptible to permanent organizational changes in stress reactivity that could cause alterations in glucocorticoid secretion profiles and behavioural coping styles in subsequent years [10,55,56]. Because heritability estimates can be sensitive to capricious environmental parameters [41], captive studies provide a meaningful estimate of standing additive genetic variation underlying phenotypic variation within a narrow range of environmental contexts. However, our study may provide a more realistic estimate of environmental influences on CORT secretion patterns given the backdrop of genetic variation expressed under natural ecological settings.
(c). The role of parental effects on corticosterone secretion patterns
The regulation of glucocorticoid responses has been shown to be particularly vulnerable to environmental conditions during early development. Because we swapped nestlings the day after hatching, siblings shared early parental effects and nest environment during incubation and immediately after hatching, which could inflate our estimate of heritability by increasing similarities in CORT profiles among siblings. Although we were not able to account for maternal effects prior to laying in our current study, there are many ways in which mothers influence their offspring's HPA axis activity. In mammals, prenatal CORT exposure and postnatal maternal care (such as grooming and time spent with offspring) can induce long-lasting changes in offspring HPA responsiveness mediated through epigenetic processes [31,55,56]. In avian species, variation in the amount of CORT deposited in egg yolks may have similar effects on offspring phenotype [57–59]. Nest environment prior to hatching such as incubation temperature can also alter nestling baseline and stress-induced CORT levels [60]. Epigenetic modifications on the glucocorticoid response in avian species is not as well documented as in mammals, but a study comparing wild and domesticated chickens showed that total epigenetic modifications of gene expression, although different in modification profiles, is heritable [61]. Epigenetics may therefore be an important factor influencing HPA responsiveness to variable environments in birds, and it is possible that some of the phenotypic variation in CORT levels in our population may be owing to epigenetic modifications or other parental effects. Nevertheless, we could not account for epigenetic effects as this typically requires terminal sampling that would be detrimental to a population that is the subject of long-term study. Here, genetically related siblings were raised in different nest environments at the earliest age possible for tracking identification as one way to address potential parental (and overall environmental) effects on the plasticity of developing nestling glucocorticoid responses.
(d). Conclusion
This study provides what is to our knowledge the first estimate of the heritability of variation in glucocorticoid concentrations in a free-living population and yields new insights into the relative contribution of genetic and environmental factors in influencing individual variation in hormonal phenotypes. We found that for nestling barn swallow baseline CORT, the phenotypic variance was best explained by rearing environment, whereas a combination of genetic and environmental factors contributed to the variation in stress-induced CORT levels. Circulating hormone levels are known to exhibit high plasticity and phenotypic flexibility (e.g. during the course of the stress response), and this flexibility may itself confer an adaptive benefit. But because CORT concentrations display great temporal variation, it is difficult to fully assess an individual's ability to respond to stressors and to accurately interpret heritability estimates based on a single sample. Future challenges include assessing the potential for heritability in temporal patterns of CORT secretion across reaction norms, and linking these patterns with the behaviours that drive fitness.
Supplementary Material
Acknowledgements
We would like to thank Andrew Flynn, Rachel Wildrick-Bradley, Kate Gloeckner, Tessa Warner, Haley Biddle and Katarzyna Chmiel for assistance with data collection in the field, Monica Brandhuber for sample organization in the laboratory, and the Nevada Genomics Center staff for microsatellite genotyping. Recognition goes to Maxwell Joseph for guidance with the statistical modelling, and we are grateful to three anonymous reviewers and Dr Julien Martin for thorough reviews of previous drafts of this manuscript.
Our research protocols were approved by the University of Colorado's IACUC (permit no. 1004.01), the Colorado Division of Wildlife and the United States Federal Bird Banding Laboratory.
Data accessibility
Data supporting this study can be accessed through Dryad using the doi:10.5061/dryad.h14kk.
Funding statement
The funding for this study was provided by the University of Colorado to both B.R.J. and R.J.S., and Sigma Xi to B.R.J. M.N.V. was supported by a University of Colorado Chancellor's Fellowship.
References
- 1.Cockrem JF. 2013. Individual variation in glucocorticoid stress response in animals. Gen. Comp. Endocr. 181, 45–58. ( 10.1016/j.ygcen.2012.11.025) [DOI] [PubMed] [Google Scholar]
- 2.Wingfield JC. 2003. Control of behavioural strategies for capricious environments. Anim. Behav. 66, 807–816. ( 10.1006/anbe.2003.2298) [DOI] [Google Scholar]
- 3.Blas J, Bortolotti GR, Tella JL, Baos R, Marchant TA. 2007. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884. ( 10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelier F, Holberton RL, Marra PP. 2009. Does stress response predict return rate in a migratory bird species? A study of American redstarts and their non-breeding habitat. Proc. R. Soc. B 276, 3545–3551. ( 10.1098/rspb.2009.0868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero LM, Wikelski M. 2010. Stress physiology as a predictor of survival in Galapagos marine iguanas. Proc. R. Soc. B 277, 3157–3162. ( 10.1098/rspb.2010.0678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang JQ, Hau M, Bonier F. 2011. Within seasons and among years: when are corticosterone levels repeatable? Horm. Behav. 60, 559–564. ( 10.1016/j.yhbeh.2011.08.004) [DOI] [PubMed] [Google Scholar]
- 7.Ouyang JQ, Quetting M, Hau M. 2012. Corticosterone and brood abandonment in a passerine bird. Anim. Behav. 84, 261–268. ( 10.1016/j.anbehav.2012.05.006) [DOI] [Google Scholar]
- 8.Angelier F, Wingfield JC, Weimerskirch H, Chastel O. 2010. Hormonal correlates of individual quality in a long-lived bird: a test of the ‘corticosterone-fitness hypothesis’. Biol. Lett. 6, 846–849. ( 10.1098/rsbl.2010.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carere C, Caramaschi D, Fawcett TW. 2010. Covariation between personalities and individual differences in coping with stress: converging evidence and hypotheses. Curr. Zool. 56, 728–740. [Google Scholar]
- 10.Schoech SJ, Rensel MA, Heiss RS. 2011. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr. Zool. 57, 514–530. [Google Scholar]
- 11.Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocr. 148, 132–149. ( 10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 12.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions . Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 13.Bókony V, Lendvai AZ, Liker A, Angelier F, Wingfield JC, Chastel O. 2009. Stress response and the value of reproduction: are birds prudent parents?. Am. Nat. 173, 589–598. ( 10.1086/597610) [DOI] [PubMed] [Google Scholar]
- 14.Schultner J, Kitaysky AS, Gabrielsen GW, Hatch SA, Bech C. 2013. Differential reproductive responses to stress reveal the role of life-history strategies within a species. Proc. R. Soc. B 280, 20132090 ( 10.1098/rspb.2013.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goymann W, Geue D, Schwabl I, Flinks H, Schmidl D, Schwabl H, Gwinner E. 2006. Testosterone and corticosterone during the breeding cycle of equatorial and European stonechats (Saxicola torquata axillaris and S. t. rubicola). Horm. Behav. 50, 779–785. ( 10.1016/j.yhbeh.2006.07.002) [DOI] [PubMed] [Google Scholar]
- 16.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212. ( 10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eikenaar C, Husak J, Escallón C, Moore IT. 2012. Variation in testosterone and corticosterone in amphibians and reptiles: relationships with latitude, elevation, and breeding season length. Am. Nat. 180, 642–654. ( 10.1086/667891) [DOI] [PubMed] [Google Scholar]
- 18.Wingfield JC, Vleck CM, Moore MC. 1992. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. J. Exp. Zool. 264, 419–428. ( 10.1002/jez.1402640407) [DOI] [PubMed] [Google Scholar]
- 19.Wingfield JC, Deviche P, Sharbaugh S, Astheimer LB, Holberton R, Suydam R, Hunt K. 1994. Seasonal changes of the adrenocortical responses to stress in redpolls, Acanthis flammea, in Alaska. J. Exp. Zool. 270, 372–380. ( 10.1002/jez.1402700406) [DOI] [Google Scholar]
- 20.Herring G, Cook MI, Gawlik DE, Call EM. 2011. Food availability is expressed through physiological stress indicators in nestling white ibis: a food supplementation experiment. Funct. Ecol. 25, 682–690. ( 10.1111/j.1365-2435.2010.01792.x) [DOI] [Google Scholar]
- 21.Clinchy M, Zanette L, Charlier TD, Newman AEM, Schmidt KL, Boonstra R, Soma KK. 2011. Multiple measures elucidate glucocorticoid responses to environmental variation in predation threat. Oecologia 166, 607–614. ( 10.1007/s00442-011-1915-2) [DOI] [PubMed] [Google Scholar]
- 22.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 23.Satterlee DG, Johnson WA. 1988. Selection of Japanese quail for contrasting blood corticosterone response to immobilization. Poultry Sci. 67, 25–32. ( 10.3382/ps.0670025) [DOI] [PubMed] [Google Scholar]
- 24.Pottinger TG, Carrick TR. 1999. Modification of the plasma cortisol response to stress in rainbow trout by selective breeding. Gen. Comp. Endocr. 116, 122–132. ( 10.1006/gcen.1999.7355) [DOI] [PubMed] [Google Scholar]
- 25.Odeh FM, Cadd GG, Satterlee DG. 2003. Genetic characterization of stress responsiveness in Japanese quail. 2. Analyses of maternal effects, additive sex linkage effects, heterosis, and heritability by diallel crosses. Poultry Sci. 82, 31–35. ( 10.1093/ps/82.1.31) [DOI] [PubMed] [Google Scholar]
- 26.Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. 2006. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J. Evol. Biol. 19, 343–352. ( 10.1111/j.1420-9101.2005.01034.x) [DOI] [PubMed] [Google Scholar]
- 27.Touma C, et al. 2008. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology 33, 839–862. ( 10.1016/j.psyneuen.2008.03.013) [DOI] [PubMed] [Google Scholar]
- 28.Hodgson ZG, Meddle SL, Roberts ML, Buchanan KL, Evans MR, Metzdorf R, Gahr M, Healy SD. 2007. Spatial ability is impaired and hippocampal mineralcorticoid receptor mRNA expression reduced in zebra finches (Taeniopygia guttata) selected for acute high corticosterone response to stress. Proc. R. Soc. B 274, 239–245. ( 10.1098/rspb.2006.3704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stöwe M, Rosivall B, Drent PJ, Möstl E. 2010. Selection for fast and slow exploration affects baseline and stress-induced corticosterone excretion in great tit nestlings, Parus major. Horm. Behav. 58, 864–871. ( 10.1016/j.yhbeh.2010.08.011) [DOI] [PubMed] [Google Scholar]
- 30.Baugh AT, Schaper SV, Hau M, Cockrem JF, de Goede P, van Oers K. 2012. Corticosterone responses differ between lines of great tits (Parus major) selected for divergent personalities. Gen. Comp. Endocr. 175, 488–494. ( 10.1016/j.ygcen.2011.12.012) [DOI] [PubMed] [Google Scholar]
- 31.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854. ( 10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 32.Darnaudéry M, Maccari S. 2008. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 57, 571–585. ( 10.1016/j.brainresrev.2007.11.004) [DOI] [PubMed] [Google Scholar]
- 33.Romero LM, Reed JM. 2005. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Phys. A 140, 73–79. ( 10.1016/j.cbpb.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 34.Jenkins BR, Vitousek MN, Safran RJ. 2013. Signaling stress? An analysis of phaeomelanin-based plumage color and individual corticosterone levels at two temporal scales in North American barn swallows, Hirundo rustica erythrogaster. Horm. Behav. 64, 665–672. ( 10.1016/j.yhbeh.2013.08.006) [DOI] [PubMed] [Google Scholar]
- 35.Wada H, Hahn TP, Breuner CW. 2007. Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. Gen. Comp. Endocr. 150, 405–413. ( 10.1016/j.ygcen.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 36.Neuman CR, Safran RJ, Lovette IJ. 2007. Male tail streamer length does not predict paternity in a population of North American barn swallows. J. Avian Biol. 38, 28–36. ( 10.1111/j.2007.0908-8857.03713.x) [DOI] [Google Scholar]
- 37.Griffiths R, Double MC, Orr K, Dawson RJG. 1998. A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. ( 10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 38.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 39.Taylor RW, Boon AK, Dantzer B, Réale D, Humphries MM, Boutin S, Gorrell JC, Coltman DW, McAdam AG. 2012. Low heritabilities, but genetic and maternal correlations between squirrel behaviours. J. Evol. Biol. 25, 614–624. ( 10.1111/j.1420-9101.2012.02456.x) [DOI] [PubMed] [Google Scholar]
- 40.Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocr. 128, 1–24. ( 10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 41.Charmantier A, Grant D. 2005. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425. ( 10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kino T. 2012. Circadian rhythms of glucocorticoid hormone actions in target tissues: potential clinical implications. Sci. Signal 5(pt4) ( 10.1126/scisignal.2003333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cockrem JF, Silverin B. 2002. Variation within and between birds in corticosterone responses of great tits (Parus major). Gen. Comp. Endocr. 125, 197–206. ( 10.1006/gcen.2001.7750) [DOI] [PubMed] [Google Scholar]
- 44.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. ( 10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 45.Bize P, Stocker A, Jenni-Eiermann S, Gasparini J, Roulin A. 2010. Sudden weather deterioration but not brood size affects baseline corticosterone levels in nestling alpine swifts. Horm. Behav. 58, 591–598. ( 10.1016/j.yhbeh.2010.06.020) [DOI] [PubMed] [Google Scholar]
- 46.Kitaysky AS, Wingfield JC, Piatt JF. 2001. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 12, 619–625. ( 10.1016/j.yhbeh.2007.10.010) [DOI] [Google Scholar]
- 47.Saino N, Suffritti C, Martinelli R, Rubolini D, Møller AP. 2003. Immune response covaries with corticosterone plasma levels under experimentally stressful conditions in nestling barn swallows (Hirundo rustica). Behav. Ecol. 14, 318–325. ( 10.1093/beheco/14.3.318) [DOI] [Google Scholar]
- 48.Gil D, Bulmer E, Celis P, Puerta M. 2008. Increased sibling competition does not increase testosterone or corticosterone levels in nestlings of the spotless starling (Sturnus unicolor). Horm. Behav. 54, 238–243. ( 10.1016/j.yhbeh.2007.11.013) [DOI] [PubMed] [Google Scholar]
- 49.Kozlowski C, Ricklefs RE. 2011. The effects of brood size on growth and steroid hormone concentrations in nestling eastern bluebirds (Sialia sialis). Gen. Comp. Endocr. 173, 447–453. ( 10.1016/j.ygcen.2011.07.002) [DOI] [PubMed] [Google Scholar]
- 50.Vitousek MN, Jenkins BR, Hubbard JK, Safran RJ. In preparation. Are corticosterone levels flexibly modulated based on brood value? An experimental test in breeding barn swallows.
- 51.Vitousek MN, Jenkins BR, Safran RJ. Submitted. Stress and success: individual differences in the glucocorticoid stress response predict behavior and reproductive success under high predation risk. [DOI] [PubMed]
- 52.Kitaysky AS, Piatt JF, Wingfield JC, Romero M. 1999. The adrenocortical stress-response of black-legged kittiwake chicks in relation to dietary restrictions. J. Comp. Phys. B 169, 303–310. ( 10.1007/s003600050225) [DOI] [Google Scholar]
- 53.Romero LM, Reed JM, Wingfield JC. 2000. Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 118, 113–122. ( 10.1006/gcen.1999.7446) [DOI] [PubMed] [Google Scholar]
- 54.Grissom N, Bhatnagar S. 2009. Habituation to repeated stress: get used to it. Neurobiol. Learn Mem. 92, 215–224. ( 10.1016/j.nlm.2008.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. 2006. Fetal programming of hypothalamo–pituitary–adrenal function: prenatal stress and glucocorticoids. J. Physiol. 572, 31–44. ( 10.1113/jphysiol.2006.105254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. 2009. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm. Behav. 55, 292–298. ( 10.1016/j.yhbeh.2008.11.006) [DOI] [PubMed] [Google Scholar]
- 57.Hayward LS, Wingfield JC. 2004. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and phenotype. Gen. Comp. Endocr. 135, 365–371. ( 10.1016/j.ygcen.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 58.Love OP, Chin EH, Wynne-Edwards KE, Williams TD. 2005. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 166, 751–766. ( 10.1086/497440) [DOI] [PubMed] [Google Scholar]
- 59.Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP. 2005. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J. Exp. Zool. 303A, 998–1006. ( 10.1002/jez.a.224) [DOI] [PubMed] [Google Scholar]
- 60.DuRant SE, Hepp GR, Moore IT, Hopkins BC, Hopkins WA. 2009. Slight differences in incubation temperature affect early growth and stress endocrinology of wood duck (Aix sponsa) ducklings. J. Exp. Biol. 213, 45–51. ( 10.1242/jeb.034488) [DOI] [PubMed] [Google Scholar]
- 61.Nätt D, Rubin CJ, Wright D, Johnsson M, Beltéky J, Andersson L, Jensen P. 2012. Heritable genome-wide variation in gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics 13, 59 ( 10.1186/1471-2164-13-59) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this study can be accessed through Dryad using the doi:10.5061/dryad.h14kk.


