Abstract
Ocean acidification (OA) and its associated decline in calcium carbonate saturation states is one of the major threats that tropical coral reefs face this century. Previous studies of the effect of OA on coral reef calcifiers have described a wide variety of outcomes for studies using comparable partial pressure of CO2 (pCO2) ranges, suggesting that key questions remain unresolved. One unresolved hypothesis posits that heterogeneity in the response of reef calcifiers to high pCO2 is a result of regional-scale variation in the responses to OA. To test this hypothesis, we incubated two coral taxa (Pocillopora damicornis and massive Porites) and two calcified algae (Porolithon onkodes and Halimeda macroloba) under 400, 700 and 1000 μatm pCO2 levels in experiments in Moorea (French Polynesia), Hawaii (USA) and Okinawa (Japan), where environmental conditions differ. Both corals and H. macroloba were insensitive to OA at all three locations, while the effects of OA on P. onkodes were location-specific. In Moorea and Hawaii, calcification of P. onkodes was depressed by high pCO2, but for specimens in Okinawa, there was no effect of OA. Using a study of large geographical scale, we show that resistance to OA of some reef species is a constitutive character expressed across the Pacific.
Keywords: ocean acidification, coral, calcifying alga, regional scale, calcification, Pacific Ocean
1. Introduction
The oceans have absorbed approximately 30% of the CO2 released by anthropogenic activities since the eighteenth century, and therefore represent a major sink for atmospheric CO2 [1]. In turn, the dissolution of CO2 leads to modification of the seawater carbonate chemistry, which includes an increase in bicarbonate ions and a decrease in carbonate ion concentration, causing a decrease in pH in a process known as ocean acidification (OA) [2]. Decreasing carbonate ion concentration leads to a decrease in calcium carbonate saturation state (Ω), which is the product of the concentration of calcium and carbonate ions divided by the stoichiometric solubility product for calcium carbonate (aragonite, calcite or high-magnesium calcite, depending on which crystal form is deposited by the organism of interest). Of the three crystal forms of calcium carbonate, calcite is the least soluble, followed by aragonite, whereas the solubility of high-magnesium calcite is linked to the magnesium content (i.e. skeletal solubility increases with magnesium content). Over the last decade, numerous studies have shown that OA can have negative effects on many marine organisms, and particularly on those that calcify (reviewed in [3,4]).
Most empirical studies of the effects of OA on coral reefs have reported a positive correlation between calcification rates and aragonite saturation state (Ωarag) [5], supporting pessimistic projections forecasting the disappearance of most coral reefs before the end of the current century [6–8]. However, recent studies now indicate more nuanced responses to OA for select reef calcifiers [9], with a compilation of laboratory studies of corals suggesting that coral calcification will decline approximately 10–20% (rather than ceasing) for a doubling of present-day partial pressure of CO2 (pCO2) [10]. More subtle responses to OA have also been shown in recent studies reporting signs of resistance to OA for some reef calcifiers [11–13]. Field observations at underwater CO2 vents in Papua New Guinea and sites with high seawater pCO2 in Palau have also shown that some reef calcifiers can persist in naturally acidified conditions [14,15]. For calcifying algae, the responses to increasing pCO2 are diverse, with some studies reporting a decrease in calcification and an increase in bleaching in acidified conditions [16,17], and others detecting resistance to OA [12,18]. The mechanistic basis of the differing responses to OA exhibited by multiple species of corals and calcified algae remains unknown, although several causes have been proposed. These include resistance to OA mediated by strong control of pH at the site of calcification in corals [19,20], the use of bicarbonate ions in the light by both corals and algae [21,22] or the use of corallum morphology to enhance mass-transfer-mediated efflux of protons from the site of calcification to the surrounding seawater [23]. Independent of the underlying mechanism, it now seems clear that at the individual scale, the responses of reef calcifiers to OA are not described effectively by a universal functional relationship between calcification and Ω.
Tropical coral reefs have developed in regions where the mean Ωarag generally is more than 3.4 [24], although there is large variation in Ωarag among locations and on diel basis. At present, coral reefs are located in waters with Ωarag ranging from 2.8 to 4.2, but model projections describing the chemistry of seawater in future seas suggest that the proportion of reefs located in regions with Ωarag > 3.0 will decline to 50% when atmospheric pCO2 reaches 550 μatm, and 0% when it reaches 900 μatm [25]. While geographical disparities in the response of tropical corals to thermal stress have been demonstrated [26], there have been no broad spatial comparisons of the response of tropical corals and calcified algae to OA. The effects of OA on coral reefs have been tested in experiments at laboratories around the world, but explicit syntheses of the results are complex given the diversity of treatments employed (i.e. pCO2 levels) and the differing ancillary incubation conditions (e.g. light intensity, flow, etc.) [10,27]. These problems can be reduced by conducting identical OA experiments at multiple locations, but such work remains technically challenging, not least because the environmental conditions to which organisms have been exposed (i.e. saturation states, temperature) on the reef prior to the experiment also are likely to vary among locations, and potentially can confound the contrast of interest [28,29]. In this study, we compared the response of reef calcifiers to OA at multiple locations on a regional scale and used among-location differences in physical conditions to our advantage. Incubations were performed in Moorea (French Polynesia) where Ωarag varies seasonally from approximately 3.5 to 4.0 (S. Comeau 2013, unpublished data), in Hawaii where Ωarag varies from 2.7 to 3.3 [30], and in Okinawa (Japan) where Ωarag varies from 2.4 to 4.3 (table 1).
Table 1.
Parameters of the carbonate chemistry during incubations in Moorea, Hawaii and Okinawa, and range of carbonate chemistry conditions at the study sites. (The partial pressure of CO2 (pCO2), and the saturation states of aragonite (Ωarag) and calcite (Ωcalc) were calculated from pHT, total alkalinity (AT), salinity (S) and temperature (T) using the R package seacarb. Experimental values correspond to mean ± s.e. (n = 56). s.e. for the experimental pHT in Moorea as well as temperature and salinity at the three locations were less than 0.01. Ranges of field values were estimated using MCR-LTER data [31] and personal observations in Moorea, data available in the literature [30,32] for Hawaii, and field pCO2 observations using a portable pCO2 system modified from [33] and environmental parameters from [34] for Okinawa.)
| location | targeted pCO2/field | pHT | AT (μmol kg−1) | pCO2 (μatm) | Ωarag | Ωcalc | T (°C) | S |
|---|---|---|---|---|---|---|---|---|
| Moorea | 400 | 8.05 | 2323 ± 10 | 391 ± 3 | 3.73 ± 0.02 | 5.61 ± 0.02 | 27.1 | 36.2 |
| 700 | 7.83 | 2320 ± 9 | 726 ± 8 | 2.50 ± 0.02 | 3.76 ± 0.03 | 27.1 | 36.2 | |
| 1000 | 7.71 | 2320 ± 11 | 993 ± 11 | 1.99 ± 0.02 | 2.99 ± 0.02 | 27.0 | 36.2 | |
| field | 8.02–8.09 | 2345–2385 | 347–437 | 3.5–4.1 | 5.2–6.2 | 26–30 | 35.8–36.4 | |
| Hawaii | 400 | 8.01 ± 0.01 | 2207 ± 1 | 430 ± 11 | 3.32 ± 0.06 | 5.00 ± 0.09 | 27.0 | 35.4 |
| 700 | 7.83 ± 0.01 | 2210 ± 1 | 696 ± 15 | 2.39 ± 0.04 | 3.59 ± 0.06 | 27.0 | 35.5 | |
| 1000 | 7.71 ± 0.01 | 2215 ± 1 | 959 ± 16 | 1.87 ± 0.03 | 2.81 ± 0.04 | 26.9 | 35.5 | |
| field | 7.90–8.10 | 2170–2260 | 400–620 | 2.7–3.3 | 4.0–4.9 | 22–28 | 33.8–35.2 | |
| Okinawa | 400 | 7.99 ± 0.02 | 2216 ± 2 | 449 ± 25 | 3.20 ± 0.08 | 4.82 ± 0.12 | 26.9 | 35.5 |
| 700 | 7.84 ± 0.01 | 2219 ± 2 | 679 ± 24 | 2.41 ± 0.06 | 3.63 ± 0.10 | 26.9 | 35.5 | |
| 1000 | 7.73 ± 0.02 | 2216 ± 2 | 921 ± 37 | 1.95 ± 0.08 | 2.94 ± 0.11 | 26.9 | 35.5 | |
| field | 7.93–8.15 | 2170–2240 | 280–500 | 2.4–4.3 | 3.7–6.5 | 22–28 | 35.2–35.5 |
To test for contrasting responses to OA over a regional scale, we measured the response to OA of four calcifiers that are common on coral reefs throughout the Pacific. Incubations were conducted in three locations using two coral taxa (massive Porites spp. and Pocillopora damicornis) and two species of calcified algae (Porolithon onkodes and Halimeda macroloba) (figure 1). We chose locations exhibiting dissimilar seawater dissolved inorganic carbon (DIC) chemistry to examine effects of geographical origin on the response to OA. Net calcification was quantified at the three locations, and close attention was paid to employing similar experimental protocols employing standardized irradiance (ca 700 μmol quanta m−2 s−1), seawater temperature (27°C) and pCO2 levels (400, 700 and 1000 μatm).
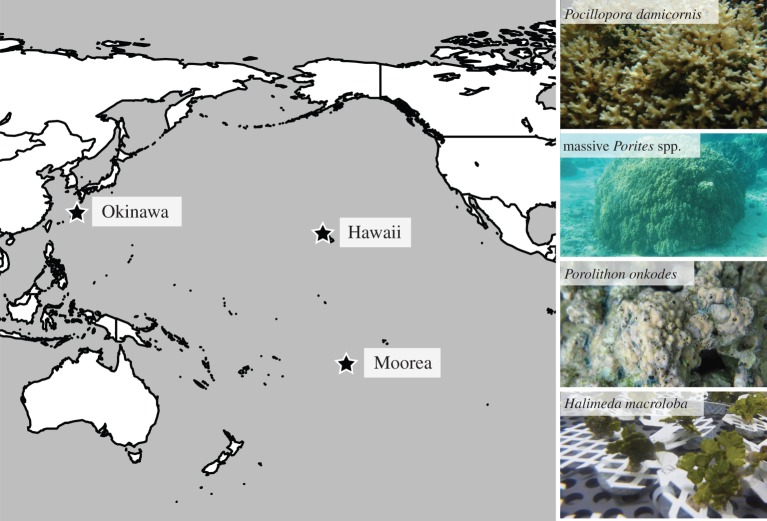
Figure 1.
Map showing the study locations and photos of the four organisms investigated. Studies were performed under similar conditions of temperature (27°C), light (approx. 600–700 μmol quanta m−2 s−1) and pCO2 (400, 700 and 1000 μatm) in Moorea (French Polynesia), Okinawa (Japan) and Hawaii (USA), respectively. At the three locations, the response of calcification to pCO2 was investigated in massive Porites spp., P. onkodes and H. macroloba.
2. Material and methods
(a). Study organisms, organism collection and experimental design
For both coral taxa, ongoing taxonomic revisions combined with the subtle skeletal features required to distinguish taxa made it unreliable to identify the study corals to species, and therefore they were considered as two functional groups. In the Pacific, massive Porites spp. consist of several species that are difficult to distinguish underwater [35]. Therefore, we considered these corals as members of one functional group; in Moorea, analyses of corallites (after [36]) indicated that 85% of these corals were P. lutea and 15% P. lobata. Similarly, recent genetic analyses of P. damicornis have detected variation throughout the Pacific that could reflect the presence of cryptic taxa [37,38].
Three out of the four study organisms precipitate calcium carbonate as aragonite (massive Porites spp., P. damicornis and H. macroloba) and one (P. onkodes) precipitates high-magnesium calcite. As calculation of the saturation state for high-magnesium calcite is challenging and depends on the proportion of magnesium in the skeleton, which was not measured in this study, we calculated saturation states only for aragonite and calcite, which is an overestimation of the actual high-magnesium calcite saturation state.
In Moorea, French Polynesia, the study was conducted in two different years at the Richard B. Gump South Pacific Research Station using organisms from approximately 1 to 2 m depth on the back reef of the north shore. Net calcification was measured in August–October 2011 for P. damicornis, P. onkodes and H. macroloba and in August–October 2012 for massive Porites spp. In Hawaii, the study was conducted in June 2012 at the Hawaii Institute of Marine Biology (HIMB) on Coconut Island. Organisms were collected from Kaneohe Bay, at 1–2 m depth for P. damicornis and P. onkodes, and 3–4 m depth for massive Porites spp. and H. macroloba. The experiment in Okinawa, Japan, was performed in May–June 2013 at the Sesoko Station, Tropical Biosphere Research Center, using specimens collected around Sesoko Island and at Bise (6 km northeast of Sesoko) at 0–3 m depth. In all experiments and for each organism, calcification was measured in duplicate trials [12] or duplicate tanks (for Hawaii and Okinawa). For each organism and at each location, 36 replicate pieces were collected, allowing for 12 replicates (six for each duplicate trial or tank) per organism for each of the three pCO2 levels. While it would have been preferable to conduct the incubations during the same season using an identical protocol in all locations, this was not possible given constraints of time and logistics. In Hawaii and Okinawa, studies were performed at the end of the spring-early summer (i.e. May/June), and at the end of the austral winter-early spring in Moorea (August/September). Similar irradiances, temperatures and pCO2 levels were used at the three locations, and the organisms were allowed to recover prior to incubations to limit collection stress and reduce bias associated with recent history.
Following collection and attachment to plastic supports, samples remained in a seawater table to recover from preparation for 2 d before being transferred for acclimatization in a separate tank, or in the incubation tanks (for two weeks in Moorea, 10 days in Hawaii and 7 days in Okinawa). Acclimatization took place at 27°C and an irradiance of 600–700 μmol quanta m−2 s−1, both of which are representative of the average temperature and light intensities encountered in the back reef of these locations. During acclimatization and incubation, 75 W Light Emitting Diode (LED) modules (Sol White LED Module; AquaIllumination) provided light on a 12 L : 12 D photoperiod. After acclimatization, 36 individuals of each organism were allocated randomly to the incubation tanks in which light and temperature were identical to those experienced during acclimatization (27°C and 600–700 μmol quanta m−2 s−1). Positions of the organisms in all tanks were changed randomly every 2 days to eliminate position effects. Seawater, filtered through a sand filter, was replaced continuously in the acclimatization and incubation tanks at approximately 100–150 ml min−1.
CO2 treatments consisted of three pCO2 levels corresponding to present-day conditions (approx. 400 μatm), an average value expected by the end of the century (approx. 700 μatm, representative concentration pathway (RCP) scenario 6.0, [39]) and a pessimistic value expected by the end of the twenty-first century (approx. 1000 μatm, RCP scenario 8.5) (table 1). Treatments were created by bubbling the tanks with CO2-manipulated air that was created with solenoid-controlled gas regulation systems (Model A352, Qubit Systems) in Moorea, mass-flow controllers (Model C100L, Sierra Instruments Inc.) in Hawaii and a direct pCO2 control system in Okinawa (developed with the cooperation of Kimoto Electric Co., Ltd., Osaka, Japan). The flow of CO2-manipulated air and seawater was adjusted in each tank to correct for deviations from target pCO2 detected by pH measurements made twice daily.
(b). Carbonate chemistry
At the three locations, pH was measured twice daily using a portable pH meter (Orion 3-stars) fitted with a DG 115-SC pH probe (Mettler) calibrated every other day with Tris/HCl buffers (A. Dickson, San Diego, CA, USA). In Moorea and in Hawaii, pH also was measured spectrophotometrically once a week using m-cresol dye [40]. The two methods provided similar results with a mean difference less than or equal to 0.009 pH. Analysis of total alkalinity (AT) was conducted by open-cell potentiometric titrations using 50 ml samples of seawater collected every 2–3 days on the day of seawater sampling [40]. AT was calculated using a modified Gran function applied to pH values ranging from 3.5 to 3.0 [40]. Parameters of the carbonate system in seawater were calculated from salinity, temperature, AT and pHT using the R package seacarb [41].
(c). Net calcification
To quantify net calcification, the difference between the initial and final buoyant weight [42] after 14 days of incubation was converted to dry weight using an aragonite density of 2.93 g cm−3 for corals and H. macroloba, and a calcite density of 2.71 g cm−3 for P. onkodes. Net calcification was normalized to surface area, obtained using the foil technique for corals, and image analysis (ImageJ, US NIH) of aerial photographs for P. onkodes. Net calcification was normalized to ash-free dry mass for H. macroloba, because determination of surface area was not possible for this species. For corals, biomass (tissue dry mass) was also used to normalize calcification to take into account the effects of pCO2 on tissue growth. Biomass was not measured in P. onkodes, but previous observations have shown no effects of pCO2 on tissue growth of this species (S. Comeau 2012, personal observation). To quantify biomass, corals were fixed in approximately 5% formalin in seawater, their skeletons dissolved in 5% HCl and the remaining formalin-fixed tissue dried for 24–48 h at 60°C prior to weighing (±1 mg).
(d). Statistical analysis
The assumptions of normality and equality of variance were evaluated through graphical analyses of residuals. All analyses were performed using R software (R Foundation for Statistical Computing). Differences in seawater carbonate chemistry within treatments between locations were compared with a two-way ANOVA, with treatment and sites as fixed effects. Area-normalized and biomass-normalized calcification rates were analysed using a three-way ANOVA, where treatments (pCO2 levels) and locations were fixed effects, and duplicate tanks were a nested effect. When there was no statistical effect of the duplicate tanks (p > 0.25), the tank effect was dropped from the analysis. Data were deposited in the PANGAEA database (http://doi.pangaea.de/10.1594/PANGAEA.832834).
3. Results
(a). Treatment conditions
At the different locations, the chemical conditions within the tanks were regulated precisely (table 1). The pCO2 levels did not differ among locations (F2, 1494 = 2.67, p = 0.069), but the Ωarag did (F2, 1494 = 68.17, p < 0.001). Exploratory Tukey HSD post hoc analyses revealed that the aragonite saturation state was significantly higher in Moorea than in Hawaii (p < 0.001) and Okinawa (p < 0.001), but there was no difference between Hawaii and Okinawa (p = 0.983). Temperature was significantly different among locations (p < 0.001), although the magnitude of the effect was probably biologically trivial (means differing between locations by less than 0.2°C).
(b). Effects of ocean acidification on corals
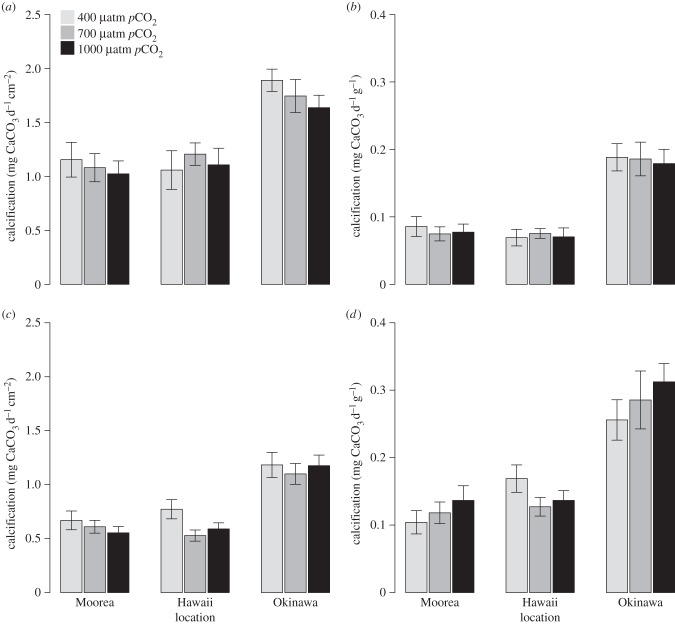
For massive Porites spp., the maximum area-normalized net calcification was measured in Okinawa in the control treatment (1.89 mg CaCO3 d−1 cm−2) and the minimum in Moorea in the 1000 μatm treatment (1.02 mg CaCO3 d−1 cm−2) (figure 2a). For P. damicornis, maximum area-normalized net calcification rate was found in Okinawa in the control and at 1000 μatm (1.18 mg CaCO3 d−1 cm−2), and the minimum in Hawaii at 700 μatm (0.53 mg CaCO3 d−1 cm−2) (figure 2c).
Figure 2.
Calcification of the corals massive Porites spp. and P. damicornis maintained in three pCO2 levels (400, 700 and 1000 μatm) in Moorea, Okinawa and Hawaii, respectively. The first row represents: (a) the area-normalized calcification and (b) the biomass-normalized calcification of massive Porites spp. The second row shows: (c) the area-normalized calcification and (d) the biomass-normalized calcification of P. damicornis. The bars correspond to the mean calcification and the vertical error bars show the s.e. in the measurement of calcification (n = 12).
For both massive Porites spp. and P. damicornis, there was no significant effect of pCO2 on area-normalized net calcification (p = 0.561 and p = 0.149, respectively; table 2), but net calcification differed among locations (p < 0.001 for both species; table 2) with highest area-normalized net calcification among pCO2 treatments in Okinawa compared with Moorea and Hawaii. Area-normalized calcification pooled among pCO2 treatments was approximately 37% lower in Hawaii and Moorea compared with Okinawa for massive Porites spp., and approximately 45% and approximately 47% lower in Hawaii and Moorea compared with Okinawa for P. damicornis. Also there was no significant interaction between pCO2 treatment and location on area-normalized calcification of both massive Porites spp. and P. damicornis (p < 0.798 and p < 0.690; table 2).
Table 2.
Comparison of area-normalized and biomass-normalized calcification for the corals massive Porites spp. and P. damicornis. (Analyses were performed using a two-way ANOVA in which treatment and location were fixed effects. MS, mean square.)
| organism | dependent variable | effect | d.f. | MS | F | p-value |
|---|---|---|---|---|---|---|
| massive Porites spp. | area-normalized calcification (mg CaCO3 d−1 cm−2) | treatment | 2 | 0.32 | 0.58 | 0.561 |
| location | 2 | 5.08 | 22.43 | <0.001 | ||
| treatment × location | 4 | 0.09 | 0.41 | 0.798 | ||
| residuals | 99 | 0.27 | ||||
| biomass-normalized calcification (mg CaCO3 d−1 g−1) | treatment | 2 | 2.9 × 10−4 | 0.10 | 0.910 | |
| location | 2 | 0.14 | 45.72 | <0.001 | ||
| treatment × location | 4 | 2.6 × 10−4 | 0.08 | 0.987 | ||
| residuals | 99 | 3.1 × 10−3 | ||||
| P. damicornis | area-normalized calcification (mg CaCO3 d−1 cm−2) | treatment | 2 | 0.16 | 1.94 | 0.149 |
| location | 2 | 3.40 | 41.84 | <0.001 | ||
| treatment × location | 4 | 0.05 | 0.56 | 0.690 | ||
| residuals | 99 | 0.08 | ||||
| biomass-normalized calcification (mg CaCO3 d−1 g−1) | treatment | 2 | 3.8 × 10−3 | 0.54 | 0.586 | |
| location | 2 | 0.28 | 39.90 | <0.001 | ||
| treatment × location | 4 | 7.2 × 10−3 | 1.01 | 0.405 | ||
| residuals | 99 | 7.1 × 10−3 |
Biomass-normalized net calcification of both massive Porites spp. and P. damicornis was not significantly affected by pCO2 (p = 0.910 and p = 0.586, respectively; table 2) or the interaction between pCO2 and location (p < 0.987 and p = 0.405, respectively; table 2). However, biomass-normalized net calcification of massive Porites spp. differed among locations (p < 0.001; table 2), because of higher biomass-normalized calcification among pCO2 treatments in Okinawa compared with Moorea and Hawaii (figure 2b,d). Biomass-normalized net calcification of massive Porites spp. was approximately 59% lower in Hawaii and Moorea compared with Okinawa and approximately 50% and approximately 57% lower in Hawaii and Moorea compared with Okinawa for P. damicornis.
(c). Effects of ocean acidification on algae
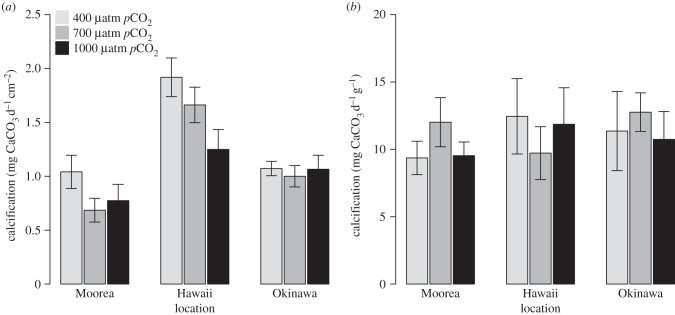
The algal species exhibited contrasting results compared with corals for the effects of OA among locations. For P. onkodes, area-normalized net calcification was highest in Hawaii in the control (1.92 mg CaCO3 d−1 cm−2), and lowest in Moorea at 700 μatm (0.69 mg CaCO3 d−1 cm−2). Area-normalized net calcification was affected by pCO2 (p = 0.024; table 3) and location (p < 0.001; table 3 and figure 3a), but the interaction between these effects was not significant (p = 0.148; table 3 and figure 3a). The effect of pCO2 was striking in Hawaii, where P. onkodes calcified faster than in the other locations under all three pCO2 treatments.
Table 3.
Comparison of area-normalized and biomass-normalized calcification for the algae P. onkodes and H. macroloba, respectively. (Analyses were performed using a two-way ANOVA in which treatment and location were fixed effects. MS, mean square.)
| species | dependent variable | effect | d.f. | MS | F | p-value |
|---|---|---|---|---|---|---|
| P. onkodes | area-normalized calcification (mg CaCO3 d−1 cm−2) | treatment | 2 | 0.95 | 3.88 | 0.024 |
| location | 2 | 5.80 | 23.72 | <0.001 | ||
| treatment × location | 4 | 0.42 | 1.73 | 0.148 | ||
| residuals | 99 | 0.24 | ||||
| H. macroloba | biomass-normalized calcification (mg CaCO3 d−1 g−1) | treatment | 2 | 5.62 | 0.11 | 0.900 |
| location | 2 | 16.33 | 0.31 | 0.737 | ||
| treatment × location | 4 | 29.02 | 0.54 | 0.704 | ||
| residuals | 99 | 53.33 |
Figure 3.
Calcification of the algae P. onkodes and H. macroloba maintained in three pCO2 levels (400, 700 and 1000 μatm) in Moorea, Okinawa and Hawaii. (a) Area-normalized calcification of P. onkodes. (b) Biomass-normalized calcification of H. macroloba. The bars correspond to the mean calcification and the vertical error bars show the s.e. in the measurement of calcification (n = 12).
For H. macroloba, biomass-normalized net calcification was highest in Okinawa at 700 μatm (12.76 mg CaCO3 d−1 g−1), and lowest in Moorea in the control (9.36 mg CaCO3 d−1 g−1) (figure 3b). Biomass-normalized net calcification was unaffected by pCO2 (p = 0.900), location (p = 0.737) or the interaction between the two (p = 0.704; table 3).
4. Discussion
This study tested the response to OA for four reef calcifiers from three locations characterized by dissimilar seawater DIC chemistry. In contrast to our hypothesis—that geographical origin, or local acclimatization, may drive dissimilar responses to OA—location was not related to the response to OA in either of the corals and in only one of the algae.
Our results confirm the more nuanced effects of OA that have been recorded for several tropical coral and algal taxa exposed in laboratory experiments to elevated pCO2 [12,27], as well as for temperate corals and molluscs transplanted into naturally acidified waters [43]. Moreover, they suggest that a linear relationship between calcification and Ωarag for corals [5] is unlikely to be universal for the diversity of organisms responsible for reef construction, although this does not exclude the possibility that it applies at a whole reef scale [24]. Organisms from Moorea, where Ωarag remains high year-round (approx. 4.0), were no more affected by OA than organisms from regions where Ωarag is seasonally low (as in Kaneohe Bay, Hawaii [30] and Okinawa (table 1)). In Hawaii, Ωarag is depressed to approximately 2.7 in winter because of cold seawater (22–23°C) and high pCO2 caused by calcification and long residence times of seawater over the reef (at least in Kaneohe Bay [30]). Similarly, low winter temperature in Okinawa [44] leads to low saturation states (i.e. Ωarag 2.4; table 1). Our results suggest that responses to winter conditions, perhaps through seasonal acclimatization (sensu [45]) or perhaps by local adaptation to low Ωarag [15], does not appear to drive the response of these reef calcifiers to OA. However, this outcome does not exclude the possibility that reef calcifiers are acclimatized or adapted to other physical conditions unique to each location (e.g. differing nutrient concentrations, temperature, etc.). Although corals are well known to alter their biomass and allocation of resources to reproduction throughout the year [46], these effects are unlikely to have biased our analyses as experiments were carried out in spring (Hawaii and Okinawa) or at the end of the austral winter (Moorea), when corals typically have large food reserves [46]. Potentially, the presence of high biomass in our study corals may have enhanced resistance to OA treatments in all three locations [46]. In terms of allocation of resources to reproduction, P. damicornis releases larvae year round [47,48] and massive Porites spp. were likely to be at a similar stage of their reproductive cycle [47,48]. Therefore, reproduction is unlikely to have an interactive effect with the timing of our experiments.
Resistance of massive Porites spp. to short-term OA conditions has been demonstrated in Moorea where corals showed no response of area-normalized net calcification to OA [13,49]. The resistance of this taxon to OA has also been reported in one location in Papua New Guinea where naturally acidified waters occur at a shallow volcanic CO2 seep where massive Porites ecologically is dominant in the reef community [14]. Similar to massive Porites spp., previously we have shown that calcification of P. damicornis in Moorea is resistant to pCO2, at least over 15 days of incubations [12]. It has also been reported from mesocosm experiments that OA does not affect settlement of P. damicornis larvae released from adult colonies maintained in acidified conditions [50].
Previous studies of P. onkodes have reported deleterious effects of OA of differing magnitudes that consist of decreased net calcification [12], higher sensitivity to grazing [51], depressed net productivity and increased net dissolution [52] and bleaching [16]. Different responses of P. onkodes to high pCO2 in different locations in this study (as well as among previous studies [12,52]) suggest that in contrast to H. macroloba, massive Porites and P. damicornis, the response of P. onkodes to OA may be influenced by the environmental history prior to collection. At local scale, coralline algae experiencing naturally diel pH variations are acclimatized to variable pH [29], and some temperate species found in habitats exposed to large pH changes (i.e. tidal pools) are more resistant to OA than species living in habitats characterized by more stable pH [28]. However, more pronounced negative effects of OA were found on the temperate coralline alga Arthrocardia corymbosa exposed to fluctuating pH [53], which suggests a limit to the capacity of coralline algae to acclimatize to OA. Interestingly, P. onkodes was the most sensitive to OA in Hawaii (this study), where calcification was the highest, which is consistent with the hypothesis that organisms characterized by rapid calcification are more affected by OA that those that calcify slowly [13].
In addition, recent observations in the North Pacific have shown that coralline algae forming a thick thallus have been more affected during the past 30 years by changes in seawater carbonate chemistry than species with thinner thalli [54]. While thickness of the thallus was not measured in our study, it is possible that P. onkodes was thicker in Hawaii where calcification rates were the highest, which would be consistent with the higher sensitivity to OA of coralline algae with thick thalli and rapid calcification. Aside from growth rates as a means to explain varied responses to OA, differential sensitivities to OA among sites in the calcified algae might reflect different mineral compositions of the skeletons. For example, a varying proportion of magnesium, or the presence of dolomite and magnesite that are less sensitive to dissolution, could underlie differential responses of algae to OA [55].
Using comparable manipulative experiments conducted at three locations spanning an unprecedented large spatial scale, our study demonstrates resistance to OA for two corals and one alga, with a second alga (P. onkodes) resistant to OA in one of the three locations. This alone is an important discovery as it demonstrates that local examples of resistance of corals to aspects of OA [11,12] may have general application over a scale of thousands of kilometres. While it was beyond the scope of our study to identify the mechanistic basis of the resistance of these two corals to OA, there are three hypotheses with potential to explain this pattern. First, the tissue of corals could function as a protective barrier isolating the site of calcification from the external seawater [23,43], thereby protecting it from decreasing pH attributed to OA [19,20]. Of the two corals studied, massive Porites is well known for an unusually thick tissue layer that permeates deeply into the skeleton [49]. In this study, a thick tissue layer could have played an important role in protecting massive Porites from OA at the three locations. Second, the ability of corals to use bicarbonate ions in the light, which increases under OA conditions [21,22], could mitigate negative responses driven by Ωarag alone. Such capacity has been demonstrated in Madracis auretenra and Porites rus [21,22], and this could be a common feature of tropical scleractinians. Third, calcification in corals is driven by their ability to maintain high pH at the site of CaCO3 deposition by the export of protons from the site of calcification, but proton export might become more energetically costly under OA conditions [19,20,23]. This hypothesis could explain the resistance of P. damicornis to OA, because it exhibits a highly branched corallum [56] that favours efflux of protons from the site of calcification to the surrounding seawater [23].
As described above for two corals, for H. macroloba there was no effect of pCO2 on net calcification. Previous studies on the response of net calcification to pCO2 in Halimeda report variable results of exposure to high pCO2, including decreased calcification [57], a threshold relationship [18], no effect (in H. macroloba) and a linear decrease in H. minima [12]. The variation in responses reported in previous studies is probably caused both by species-specific responses to OA as well as methodological artefacts. These might include experiments conducted at different irradiances or varying water motion that could affect rates of photosynthesis and calcification in Halimeda.
The mechanisms underlying the resistance of H. macroloba to OA are unlikely to be the same as those for corals (described above). It has been demonstrated using microsensors that calcification in H. discoidea is not controlled actively by the alga, but rather is linked directly to the pH in intercellular spaces that is controlled by the ratio of photosynthesis to respiration [58]. It is possible that in our experiment, increases in pCO2 and HCO3− in seawater favoured photosynthesis during daylight [59] leading to an increase in pH in intercellular spaces that increased calcification. By contrast, at night, respiration and the absence of photosynthesis leads to a decrease in pH in intercellular spaces, which could result in night-time dissolution of CaCO3. As a result, net calcification was not affected by OA.
The resistance of three of four corals and calcified algae to short-term exposure to OA conditions, with a consistent effect across a very large spatial scale, suggests that this ability may represent a constitutive and geographically conserved capacity to resist some of the effects of OA. However, while our study suggests some reef calcifiers will persist in a more acidic future, it remains to be determined whether they can endure the challenges of rising temperature that are likely to occur at a more rapid pace than declining seawater pH, and moreover, whether they can survive if the carbonate framework beneath them begins to dissolve [60]. Our results, as well as past studies demonstrating species-specific responses to pCO2 in reef calcifiers [12,13], suggest that OA may function as a taxonomic filter, favouring the persistence of species that are resilient to OA. As a result, OA could drive the formation of less diverse coral reefs populated across the Pacific with resistant species exemplified by massive Porites, P. damicornis and H. macroloba.
Acknowledgements
We thank K. Nishida and A. Suzuki for field measurement of pCO2 and alkalinity at Sesoko Station and N. Spindel for laboratory assistance in Moorea and Hawaii.
Funding statement
We thank NSF for financial support (OCE 10-41270) and the Moorea Coral Reef Long Term Ecological Research site (OCE 04-17413 and 10-26852) for logistic support. This is contribution 218 of the California State University, Northridge Marine Biology Program, HIMB contribution 1591 and SOEST contribution no. 9172. K.S. and Y.N. were supported by JSPS KAKENHI grant no. 23241017 for the experimental study at the Sesoko Station.
References
- 1.Sabine CL, et al. 2004. The oceanic sink for anthropogenic CO2. Science 305, 367–371. ( 10.1126/science.1097403) [DOI] [PubMed] [Google Scholar]
- 2.Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366. ( 10.1126/science.1097329) [DOI] [PubMed] [Google Scholar]
- 3.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte C, Gattuso J-P. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattuso J-P, Hansson L. 2011. Ocean acidification. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Langdon C, Atkinson MJ. 2005. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 110, C09S07 ( 10.1029/2004JC002576) [DOI] [Google Scholar]
- 6.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 7.Silverman J, Lazar B, Cao L, Caldeira K, Erez J. 2009. Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys. Res. Lett. 36, C09S07.. ( 10.1029/2008GL036282) [DOI] [Google Scholar]
- 8.Van Hooidonk R, Maynard JA, Manzello D, Planes S. 2014. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Change Biol. 20, 103–112. ( 10.1111/gcb.12394) [DOI] [PubMed] [Google Scholar]
- 9.Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422. ( 10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 10.Chan NCS, Connolly SR. 2013. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol. 19, 282–290. ( 10.1111/gcb.12011) [DOI] [PubMed] [Google Scholar]
- 11.Takahashi A, Kurihara H. 2013. Ocean acidification does not affect the physiology of the tropical coral Acropora digitifera during a 5-week experiment. Coral Reefs 32, 305–314. ( 10.1007/s00338-012-0979-8) [DOI] [Google Scholar]
- 12.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2013. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58, 388–398. ( 10.4319/lo.2013.58.1.0388) [DOI] [Google Scholar]
- 13.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol. Oceanogr. 59, 1081–1091. ( 10.4319/lo.2014.59.3.1081) [DOI] [Google Scholar]
- 14.Fabricius KE, et al. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165–169. ( 10.1038/nclimate1122) [DOI] [Google Scholar]
- 15.Shamberger KEF, Cohen AL, Golbuu Y, McCorkle DC, Lentz SJ, Barkley HC. 2014. Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys. Res. Lett. 41, 499–504. ( 10.1002/2013GL058489) [DOI] [Google Scholar]
- 16.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17 442–17 446. ( 10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT. 2008. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114–117. ( 10.1038/ngeo100) [DOI] [Google Scholar]
- 18.Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134. ( 10.1130/G30210A.1) [DOI] [Google Scholar]
- 19.Ries JB. 2011. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064. ( 10.1016/j.gca.2011.04.025) [DOI] [Google Scholar]
- 20.McCulloch M, Falter J, Trotter J, Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2, 623–627. ( 10.1038/nclimate1473) [DOI] [Google Scholar]
- 21.Jury CP, Whitehead RF, Szmant AM. 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (= Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 16, 1632–1644. ( 10.1111/j.1365-2486.2009.02057.x) [DOI] [Google Scholar]
- 22.Comeau S, Carpenter RC, Edmunds PJ. 2013. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. R. Soc. B 280, 20122374 ( 10.1098/rspb.2012.2374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jokiel PL. 2011. The reef coral two compartment proton flux model: a new approach relating tissue-level physiological processes to gross corallum morphology. J. Exp. Mar. Biol. Ecol. 409, 1–12. ( 10.1016/j.jembe.2011.10.008) [DOI] [Google Scholar]
- 24.Kleypas JA, Buddemeier RW, Archer D, Gattuso J-P, Langdon C, Opdyke BN. 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120. ( 10.1126/science.284.5411.118) [DOI] [PubMed] [Google Scholar]
- 25.Ricke KL, Orr JC, Schneider K, Caldeira K. 2013. Risks to coral reefs from ocean carbonate chemistry changes in recent earth system model projections. Environ. Res. Lett. 8, 034003 ( 10.1088/1748-9326/8/3/034003) [DOI] [Google Scholar]
- 26.Berkelmans R, van Oppen MJH. 2006. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 273, 2305–2312. ( 10.1098/rspb.2006.3567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmunds PJ, Brown D, Moriarty V. 2012. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Glob. Change Biol. 18, 2173–2183. ( 10.1111/j.1365-2486.2012.02695.x) [DOI] [Google Scholar]
- 28.Noisette F, Egilsdottir H, Davoult D, Martin S. 2013. Physiological responses of three temperate coralline algae from contrasting habitats to near-future ocean acidification. J. Exp. Mar. Biol. Ecol. 448, 179–187. ( 10.1016/j.jembe.2013.07.006) [DOI] [Google Scholar]
- 29.Johnson MD, Moriarty V, Carpenter RC. 2014. Acclimatization to variable pCO2 in the crustose coralline alga Porolithon onkodes. PLoS ONE 9, e87678 ( 10.1371/journal.pone.0087678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagan KE, Mackenzie FT. 2007. Air–sea CO2 exchange in a subtropical estuarine-coral reef system, Kaneohe Bay, Oahu, Hawaii. Mar. Chem. 106, 174–191. ( 10.1016/j.marchem.2007.01.016) [DOI] [Google Scholar]
- 31.Alldredge A. 2012. MCR LTER: Coral reef: water column: offshore ocean acidification: water profiles, CTD, and chemistry. knb-lter-mcr.1037.1 See http://metacat.lternet.edu/knb/metacat/knb-lter-mcr.1037.1/lter.
- 32.Shamberger KEF, Feely RA,, Sabine CL, Atkinson MJ, DeCarlo EH, Mackenzie FT, Drupp PS, Butterfield DA. 2011. Calcification and organic production on a Hawaiian coral reef. Mar. Chem. 127, 64–75. ( 10.1016/j.marchem.2011.08.003) [DOI] [Google Scholar]
- 33.Saito H, Tamura N, Kitano H, Mito A, Takahashi C, Suzuki A, Kayanne H. 1995. A compact seawater pCO2 measurement system with membrane equilibrator and nondispersive infrared gas analyzer. Deep Sea Res. I 42, 2025–2033. ( 10.1016/0967-0637(95)00090-9) [DOI] [Google Scholar]
- 34.Tada K, Sakai K, Nakano Y, Takemura A, Montani S. 2003. Size-fractionated phytoplankton biomass in coral reef waters off Sesoko Island, Okinawa, Japan. J. Plankton Res. 25, 991–997. ( 10.1093/plankt/25.8.991) [DOI] [Google Scholar]
- 35.Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ. 2009. Shape-shifting corals: molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol. Biol. 9, 45 ( 10.1186/1471-2148-9-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edmunds PJ. 2009. Effect of acclimatization to low temperature and reduced light on the response of reef corals to elevated temperature. Mar. Biol. 156, 1797–1808. ( 10.1007/s00227-009-1213-2) [DOI] [Google Scholar]
- 37.Schmidt-Roach S, Lundgren P, Miller KJ, Gerlach G, Noreen AME, Andreakis N. 2013. Assessing hidden species diversity in the coral Pocillopora damicornis from Eastern Australia. Coral Reefs 32, 161–172. ( 10.1007/s00338-012-0959-z) [DOI] [Google Scholar]
- 38.Marti-Puig P, Forsman ZH, Haverkort-Yeh RD, Knapp IS, Maragos JE, Toonen RJ. 2014. Extreme phenotypic polymorphism in the coral genus Pocillopora; micro-morphology corresponds to mitochondrial groups, while colony morphology does not. Bull. Mar. Sci. 90, 211–231. ( 10.5343/bms.2012.1080) [DOI] [Google Scholar]
- 39.Moss RH, et al. 2010. The next generation of scenarios for climate change research and assessment. Nature 463, 747–756. ( 10.1038/nature08823) [DOI] [PubMed] [Google Scholar]
- 40.Dickson AG, Sabine CL, Christian JR. 2007. Guide to best practices for ocean CO2 measurements. PICES Special Publication, 3. [Google Scholar]
- 41.Lavigne H, Gattuso J-P. 2011. Seacarb: seawater carbonate chemistry with R, R package v. 2.4.1. See http://CRAN.Rproject.org/package=seacarb. [Google Scholar]
- 42.Spencer-Davies P. 1989. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–95. ( 10.1007/BF00428135) [DOI] [Google Scholar]
- 43.Rodolfo-Metalpa R, et al. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312. ( 10.1038/nclimate1200) [DOI] [Google Scholar]
- 44.Yamashiro H, Mikame Y, Suzuki H. 2012. Localized outbreak of attached diatoms on the coral Montipora due to low-temperature stress. Nat. Sci. Rep. 2, 552 ( 10.1038/srep00552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkelmans R, Willis BL. 1999. Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18, 219–228. ( 10.1007/s003380050186) [DOI] [Google Scholar]
- 46.Thornhill DJ, et al. 2011. A connection between colony biomass and death in Caribbean reef-building corals. PLoS ONE 6, e29535 ( 10.1371/journal.pone.0029535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edmunds PJ, Leichter JJ, Adjeroud M. 2010. Landscape-scale variation in coral recruitment in Moorea, French Polynesia. Mar. Ecol. Prog. Ser. 414, 75–89. ( 10.3354/meps08728) [DOI] [Google Scholar]
- 48.Harrison PL. 2011. Sexual reproduction of scleractinian corals. In Coral reefs: an ecosystem in transition (eds Dubinsky Z, Stambler N.), pp. 151–176. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 49.Edmunds PJ. 2011. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 56, 2402–2410. ( 10.4319/lo.2011.56.6.2402) [DOI] [Google Scholar]
- 50.Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT. 2008. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483. ( 10.1007/s00338-008-0380-9) [DOI] [Google Scholar]
- 51.Johnson MD, Carpenter RC. 2012. Ocean acidification and warming decrease calcification in the crustose coralline alga Hydrolithon onkodes and increase susceptibility to grazing. J. Exp. Mar. Biol. Ecol. 434–435, 94–101. ( 10.1016/j.jembe.2012.08.005) [DOI] [Google Scholar]
- 52.Diaz-Pulido G, Anthony KRN, Kline DI, Dove S, Hoegh-Guldberg O. 2012. Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae. J. Phycol. 48, 32–39. ( 10.1111/j.1529-8817.2011.01084.x) [DOI] [PubMed] [Google Scholar]
- 53.Cornwall CE, Hepburn CD, McGraw CM, Currie KI, Pilditch CA, Hunter KA, Boyd PW, Hurd CL. 2013. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proc. R. Soc. B 280, 20132201 ( 10.1098/rspb.2013.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCoy SJ, Pfister CA. 2014. Historical comparisons reveal altered competitive interactions in a guild of crustose coralline algae. Ecol. Let. 17, 475–483. ( 10.1111/ele.12247) [DOI] [PubMed] [Google Scholar]
- 55.Nash MC, et al. 2013. Dolomite-rich coralline algae in reefs resist dissolution in acidified conditions. Nat. Clim. Change 3, 268–272. ( 10.1038/nclimate1760) [DOI] [Google Scholar]
- 56.Lesser MP, Weis VM, Patterson MR, Jokiel PL. 1994. Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Biol. Ecol. 178, 153–179. ( 10.1016/0022-0981(94)90034-5) [DOI] [Google Scholar]
- 57.Sinutok S, Hill R, Doblin MA, Wuhrer R, Ralph PJ. 2011. Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol. Oceanogr. 56, 1200–1212. ( 10.4319/lo.2011.56.4.1200) [DOI] [Google Scholar]
- 58.De Beer D, Larkum AWD. 2001. Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors. Plant Cell Environ. 24, 1209–1217. ( 10.1046/j.1365-3040.2001.00772.x) [DOI] [Google Scholar]
- 59.Johnson VR, Russell BD, Fabricius KE, Brownlee C, Hall-Spencer JM. 2012. Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob. Change Biol. 18, 2792–2803. ( 10.1111/j.1365-2486.2012.02716.x) [DOI] [PubMed] [Google Scholar]
- 60.Cyronak T, Santos IR, Eyre BD. 2013. Permeable coral reef sediment dissolution driven by elevated pCO2 and pore water advection. Geophys. Res. Lett. 40, 4876–4881. ( 10.1002/grl.50948) [DOI] [Google Scholar]