Chronic lymphocytic leukemia (CLL) is a B-cell disease representing the most common leukemia in the Western world. Unlike other cancers which are commonly driven by uncontrolled proliferation, CLL cells are explicitly accumulated through defective apoptosis. High levels of Bcl-2 family anti-apoptotic proteins are primarily responsible for resistance to apoptosis and are major targets for therapy development. Sustained levels of Mcl-1 and Bcl-2 appear to be protective mechanisms in response to cytotoxic agents and are found to be associated with poor prognosis in CLL [1]. Levels of Mcl-1 inversely correlated with in vitro and in vivo responsiveness to chemotherapy in CLL [2, 3]. In contrast, strategies lowering the levels of Mcl-1 in leukemia and lymphoma cells triggered apoptosis. Overexpression of anti-apoptotic proteins observed in relapsed leukemias has been associated with failure to achieve complete responses to initial therapy with fludarabine. Collectively, these reports endorse anti-apoptotic proteins as pro-survival factors and reveal the requisite to fine-tune the pro-survival and pro-death proteins in restoring normal apoptosis in CLL.
One of the earliest strategies was to use Bcl-2 antisense oligonucleotide (oblimersen) to knock down the Bcl-2 mRNA [4]. Oblimersen displayed only modest activity as a single agent in a Phase I/II trial [5]. Further insights on to Bcl-2 family proteins as critical regulators of the mitochondria–mediated apoptosis, led to a mechanistic approach of targeting the hydrophobic cleft of Bcl-2 with small molecule mimetics in turn to modulate the function of Bcl-2 [6]. Proof-of-concept of this approach was demonstrated with peptides corresponding to the BH3 domain of the pro-apoptotic protein BAD [7]. Naturally occurring polyphenols (gossypol) are shown as potent inhibitor of all six anti-apoptotic proteins, Bcl-2, Bcl-xl, Bcl-2-A1, Mcl-1, Bcl-B and Bcl-W, giving the molecular explanation for the anticancer activity of this agent [8, 9]. Given that Bcl-xl and Mcl-1 are highly expressed in several hematological malignancies, gossypol was able to overcome the apoptotic resistance [10]. While having the broad spectrum of functionalities, toxic properties associated with hypokalemia and its effect on permanent infertility limited gossypol in clinical use. However, gossypol was considered a lead compound for a new class of antineoplastic agents. Structural modification of gossypol guided by a model of multidimensional nuclear magnetic resonance based structural analysis led to the development of additional analogues of gossypol with improved efficacy.
Apogossypolone (ApoG2) an analogue of gossypol was synthesized with improved efficacy and reduced toxicity by the removal of hydroxyl from 1 and 1′ positions and two reactive aldehyde groups from 8 and 8′ positions [11]. It demonstrated a higher binding affinity to its targets (Ki, 35, 660, and 25 nM for Bcl-2, Bcl-xl, and Mcl-1, respectively) [12]. Preclinical studies of apogossypolone in follicular lymphoma exhibited growth inhibitory effects in vitro and in vivo through activation of intrinsic and extrinsic caspases and release of AIF [13]. In tumor xenograft models, ApoG2 was more stable and better tolerated by mice than was racemic gossypol, with no toxicity on peripheral blood lymphocytes [14]. The mechanism of action of BH3 mimetic is to bind to the anti-apoptotic proteins to facilitate the dissociation pro-apoptotic BH3 only proteins which then activate Bax/Bak to induce apoptosis [15]. Therefore, activation and mitochondrial translocation of multidomain pro-apoptotic Bax/Bak are essentially needed for apoptosis induction [16]. In concert, MEFs derived from Bax and Bak DKO mice were significantly more resistant to drug-induced apoptosis [17]. With this background, we tested apogossypolone in CLL primary cells and demonstrate that ApoG2 induce apoptosis in CLL primary cells and Bax/Bak are necessary for this biological action.
Apogossypolone (ApoG2) was obtained from Ascenta Therapeutics (San Diego, CA) and dissolved in DMSO. Our study was carried out in leukemic lymphocytes isolated from peripheral blood samples obtained from patients with CLL. All patients signed written informed consent forms in accordance with the Declaration of Helsinki, and the laboratory protocol was approved by the institutional review board at the University of Texas MD Anderson Cancer Center. Freshly obtained CLL lymphocytes (n=3) [18] were incubated for 24 hrs with varying concentrations of ApoG2 and apoptosis was measured by DiOC6 staining (Fig. 1A) and annexin/PI binding (Fig. 1B). Time-matched control samples were used to determine endogenous cell death. Though there was heterogeneity, there was a time- and dose- dependent apoptosis in all samples tested. The endogenous levels of viable cells after 24 hrs in culture (DiOC6; Fig. 1A) varied between patients, ranging from 56–81%, which upon treatment with apogossypolone reduced to median 65% at 3 μM, 22% at 10 μM, 17% at 20 μM and 10% at 30 μM (p = 0.0177 unpaired 2-tailed t-test comparing control with all concentrations). In particular, the apoptosis was significantly higher when measured by DiOC6 method compared to Annexin/PI binding (Fig. 1B; p = 0.2678) suggesting that the apoptosis pathway involves mitochondria.
Figure 1.
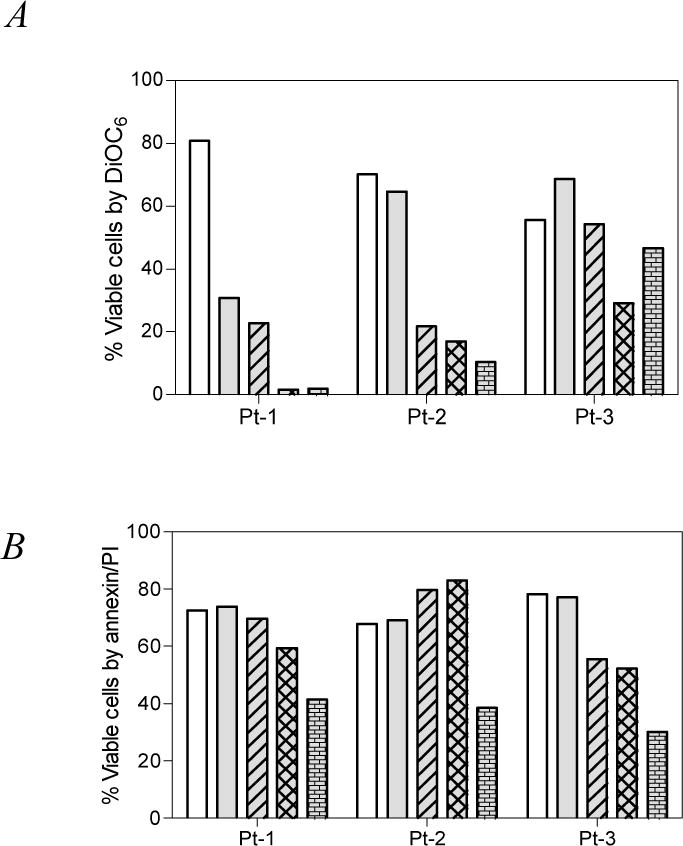
Freshly obtained CLL lymphocytes from patients (n=3) were incubated with 0 – 30 μM (0, 3, 10, 20, 30 μM) of apogossypolone and the viability of cells was tested by two standard apoptosis assays. A. DiOC6 assay. B. Annexin/PI binding assay. Time matched control samples were used in all experiments.
Given that several studies have shown Bax/Bak are necessary for the execution of intrinsic (mitochondria) cell death pathway, next we investigated if apogossypolone-induced apoptosis was dependent on these proteins. Exponentially growing Bax/Bak wild type (WT) or double knock out (DKO) cell lines (obtained from John C. Reed, Sanford, Burnham Institute, San Diego, CA) were incubated with different concentrations of apogossypolone and the apoptosis was measured after 24 hrs. In wild-type Bax/Bax expressing cells, the median values for time-matched untreated samples were 97% with a dose-dependent decrease in viability with apogossypolone (median 90% at 3 μM, 71% at 10 μM, 9% at 20 μM and 14% at 30 μM; (Fig. 2A; DiOC6 method)). In contrast, Bax/Bak DKO cells demonstrated significant resistance to apoptosis even at high concentrations of apogossypolone (p= 0.0014 paired two-tailed t-test comparing wild type with DKO). The trend was similar in experiments carried out with Annexin/PI binding assay (Fig. 2B; p = 0.0005) suggesting that Bax/Bak are necessary for apogossypolone-induced apoptosis.
Figure 2.
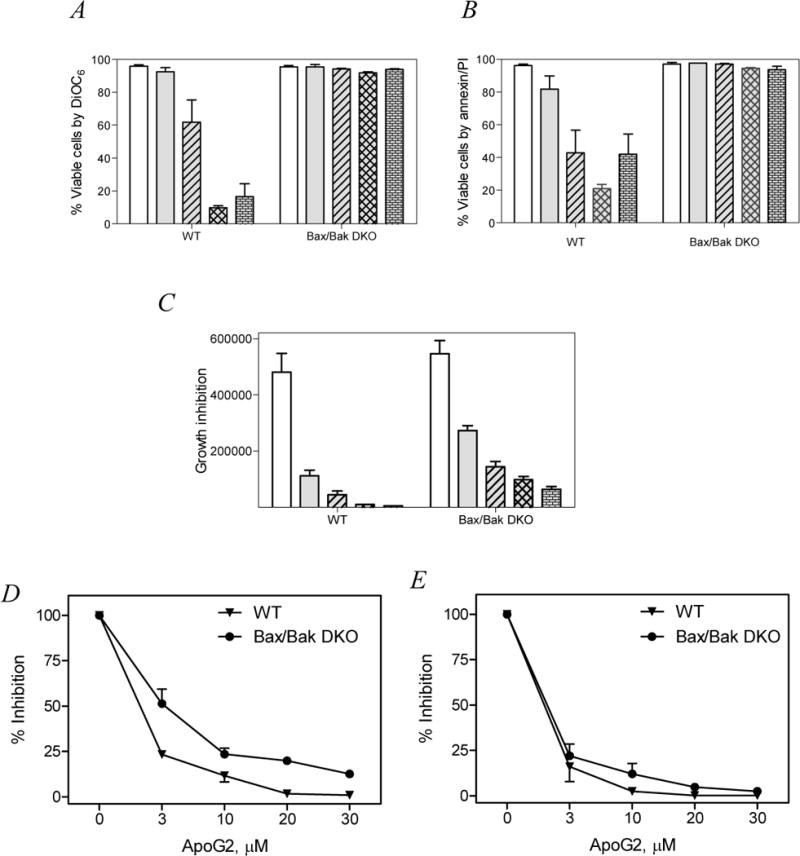
Bax and Bak expressing (WT) and double knockout (DKO) mouse embryo fibroblasts were incubated with 0 – 30 μM of apogossypolone and the viability of cells was tested by two standard apoptosis assays. A. DiOC6 method. B. Annexin/PI binding assay. Growth inhibition was measured by cell count method. C. Bar graph representing decline in total number of cells at the end of 24 hrs incubation with apogossypolone. D and E. Line graph representing dose dependent percent decrease in cell number upon incubation with apogossypolone for 24 hrs (D) or 48 hrs (E). All experiments were representatives of triplicates and the data are mean ±SEM of three similar sets of experiments.
We carried out similar set of experiments to test if Bax/Bak are required for growth inhibition in these cells. Interestingly, both WT and DKO cells were equally sensitive to growth inhibition upon incubation with ApoG2. This further strengthens the notion that Bax/Bak are primarily important in the execution of intrinsic cell death pathway but exhibit functional redundancy on proliferation and growth [16]. At 24hrs, with 10 μM there was 90% growth inhibition in wild type (Bax/Bak) cells, while 70% inhibition of growth was observed in DKO MEFs (Fig. 2C; bar graph and Fig. 2D; line graph). Inhibition of growth was identical after longer (48 hrs) incubation period (Fig. 2E).
Overexpression of Bcl-2 family anti-apoptotic proteins is considered a standard mechanism of resistance to therapeutic response in malignant cells. Several efforts are undertaken to target these proteins in cellular milieu. Among multiple approaches, the mechanistic strategy of inhibiting Bcl-2 family anti-apoptotic proteins by targeting the hydrophobic cleft of anti-apoptotic proteins has been fairly successful. Natural product gossypol and its derivatives are also proven classical prototypes of BH3 mimetics in modulating the function of Bcl-2 family proteins [10]. Several BH3 mimetics such as true BAD like (ABT-737; Navitoclax), putative pan-BH3 only mimetics (GX15-070, Obatoclax), or polyphenols (AT-101) are tested in preclinical and clinical studies. Importantly, both inherent defects in the apoptotic machinery as well as impaired susceptibility to apoptosis due to excessive survival signals delivered by their extrinsic microenvironment are circumvented by BH3 mimetics [18].
Given that CLL is primarily a disease representing impaired programmed cell death, rather than proliferation, and the accumulation is primarily due to high levels of anti-apoptotic proteins, it is a quintessential model system to test BH3 mimetics. Consistently, our results show that CLL cells are sensitive to apogossypolone, a novel pan-inhibitor of Bcl-2 family antiapoptotic members (Fig. 1). The concept of mimicking BH3 is based on the discovery that the BH3-only proteins specifically antagonize pro-survival molecules, whose functional activity is to sequester the pro-apoptotic members Bax and Bak. Once activated, BH3-only proteins insert its α-helical BH3 domain into the hydrophobic groove of an anti-apoptotic protein and neutralize its anti-apoptotic property. The design of small molecules capable of mimicking the BH3 domain thus potentially attacks the initial molecular events responsible for triggering the mitochondrial apoptosis pathway.
The expression, function and ability of Bcl-2 family anti-apoptotic proteins to associate as binding partners with other proteins are tissue specific and vary widely. As the interactions between BH3-only proteins and the anti-apoptotic proteins are primarily selective (for example, Noxa binds to only Mcl-1 and A1, and BAD binds only to Bcl-2, Bcl-xl and Bcl-W, whereas Bim and Puma bind to all five pro-survival proteins), the activity of BH3 mimetics is often context-dependent[19]. Identifying the right inhibitor for the right model system is critically important. Given that Mcl-1 is crucial for impaired apoptosis in CLL, inhibitors that can specifically bind to Mcl-1 and neutralize its function may prove beneficial for CLL cells. Two novel BH3 peptides and a small molecule antagonist capable of specifically binding to Mcl-1 have been recently identified. First, a novel Bim-BH3 variant (BimS2A) that is highly specific for Mcl-1 [20] and second,Mcl-1 SAHB, a Mcl-1-specific antagonist capable of inducing apoptosis through a Noxa-like mechanism [21]. Small molecule, maritoclax, selectively binds to Mcl-1, and disrupts the interaction between Bim/Mcl-1 by inducing Mcl-1 degradation via proteasomal pathway [22]. Testing these inhibitors in CLL could reveal further insights onto inhibiting selectively Mcl-1 protein.
Acknowledgments
This work is supported in part by a CLL Consortium grant (CA81534) and a CLL Global Research Foundation grant.
Footnotes
Aapogossypolone induced apoptosis in CLL
References
- 1.Pepper C, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112(9):3807–17. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 2.Kitada S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91(9):3379–89. [PubMed] [Google Scholar]
- 3.Zhou P, et al. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89(2):630–43. [PubMed] [Google Scholar]
- 4.Pepper C, et al. Antisense-mediated suppression of Bcl-2 highlights its pivotal role in failed apoptosis in B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999;107(3):611–5. doi: 10.1046/j.1365-2141.1999.01726.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien SM, et al. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23(30):7697–702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 6.Muchmore SW, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381(6580):335–41. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 7.Kitada S, et al. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46(20):4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 8.Zhai D, et al. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13(8):1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 9.Becattini B, et al. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L) Chem Biol. 2004;11(3):389–95. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Balakrishnan K, et al. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112(5):1971–80. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhan Y, et al. Design and synthesis of a gossypol derivative with improved antitumor activities. Arch Pharm (Weinheim) 2009;342(4):223–9. doi: 10.1002/ardp.200800185. [DOI] [PubMed] [Google Scholar]
- 12.Mohammad RM, YD, Chen B, Aboukameel A, Chen J, Nikolovska-Coleska Z, Al-Katib A, Wang S. ApoG2, a potent, non-toxic small-molecule inhibitor of Bcl-2 family: A preclinical trial in lymphoma. Proc Amer Assoc Cancer Res. 2006 Abstract #1335 (Volume 47) [Google Scholar]
- 13.Arnold AA, et al. Preclinical studies of Apogossypolone: a new nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-XL and Mcl-1 proteins in Follicular Small Cleaved Cell Lymphoma model. Mol Cancer. 2008;7:20. doi: 10.1186/1476-4598-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, et al. Apogossypolone, a nonpeptidic small molecule inhibitor targeting Bcl-2 family proteins, effectively inhibits growth of diffuse large cell lymphoma cells in vitro and in vivo. Cancer Biol Ther. 2008;7(9):1418–26. doi: 10.4161/cbt.7.9.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hockenbery D, et al. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348(6299):334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt K, et al. Bax and Bak independently promote cytochrome C release from mitochondria. J Biol Chem. 2002;277(16):14127–34. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent. Cancer Res. 2005;65(5):2035–43. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 18.Balakrishnan K, et al. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113(1):149–53. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai D, et al. Differential regulation of Bax and Bak by anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem. 2008;283(15):9580–6. doi: 10.1074/jbc.M708426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EF, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180(2):341–55. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart ML, et al. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6(8):595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi K, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J Biol Chem. 2012;287(13):10224–35. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]


